Abstract
Candida albicans is a normal resident of the human gastrointestinal and urogenital tracts and also a prevalent fungal pathogen. During both commensalism and infection, it must match the immunological defenses of its host while adapting to environmental cues and the local nutrient status. C. albicans regularly colonizes glucose-poor niches, thereby depending upon alternative carbon sources for growth. However, most studies of host immune responses to C. albicans have been performed on fungal cells grown on glucose, and the extent to which alternative physiologically relevant carbon sources impact innate immune responses has not been studied. The fungal cell wall is decorated with multifarious pathogen-associated molecular patterns and is the main target for recognition by host innate immune cells. Cell wall architecture is both robust and dynamic, and it is dramatically influenced by growth conditions. We found that growth of C. albicans cells on lactate, a nonfermentative carbon source available in numerous anatomical niches, modulates their interactions with immune cells and the resultant cytokine profile. Notably, lactate-grown C. albicans stimulated interleukin-10 (IL-10) production while decreasing IL-17 levels, rendering these cells less visible to the immune system than were glucose-grown cells. This trend was observed in clinical C. albicans isolates from different host niches and from different epidemiological clades. In addition, lactate-grown C. albicans cells were taken up by macrophages less efficiently, but they were more efficient at killing and escaping these phagocytic cells. Our data indicate that carbon source has a major impact upon the C. albicans interaction with the innate immune system.
INTRODUCTION
Fungal infections are characterized by the large number of microenvironments occupied during disease establishment and progression. In general Candida albicans is relatively harmless, as this fungus is carried by 40 to 80% of healthy individuals in the population. When the balance between colonization and host defense is disrupted, infections occur, with vaginal, esophageal, and oropharyngeal candidiasis being some of the most frequent mucosal infections in humans. A significant proportion of these infections are recurrent (1). In immunocompromised individuals, C. albicans can proliferate unimpeded, invade the bloodstream and the tissues, and cause a variety of infections, including pneumonia, septicemia, endocarditis, and systemic candidiasis. Nearly 40% of disseminated candidiasis cases are fatal (2).
C. albicans can shift from harmless commensal to opportunistic pathogen, and this shift requires the ability to evade the defenses of the host immune system, among which the innate immunity is paramount (3). The first step in mounting protective immunity is the recognition of the fungal pathogen by cells of the innate immune system. Pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs), and this is followed by activation of intracellular signaling cascades and the release of chemokines and cytokines, as well as the accumulation of inflammatory cells at the site of infection (3).
The cell wall is the main protective barrier for C. albicans and is critical in host-pathogen interactions as the initial target for immune recognition. Several receptor families recognize different components of the cell wall, with the major structural polysaccharides chitin and β-glucan generally being recognized at bud scars, while mannans and mannoproteins are recognized at the fungal cell surface (4, 5). These structures are sensed by two main classes of PRRs. First, Toll-like receptors (TLRs) recognize phospholipomannan (6) and O-linked mannan (7). Second, C-type lectin receptors (CLRs) recognize β-glucan and other types of glycosylated mannan (7, 8). Cell wall glycosylation is critical for the recognition and uptake of C. albicans, as defects in phosphomannan biosynthesis decrease the phagocytosis of fungal cells by macrophages (9). Additionally, proteins covalently associated with mannose polymers on the outer cell wall layer constitute major antigens (9, 10, 11).
The fungal cell wall is a highly dynamic structure, its architecture being modulated in response to changes in cell morphology and growth conditions. Given the variety and dynamism of the niches that C. albicans inhabits in the human host, the fungus must constantly tune its physiology to the nutrient conditions. Phenotypic switching (yeast-to-hyphal and white-to-opaque) and variations in growth conditions are likely scenarios in the variety of niches that C. albicans inhabits in the human host. In addition, environmental cues, such as changes in ambient pH or carbon source, drive changes in the C. albicans cell wall proteome (12, 13) and the thickness and architecture of the different cell wall layers (14, 15, 16).
These cell wall changes are thought to be highly relevant in vivo because sugars such as glucose, fructose, or galactose are only transiently available to C. albicans during colonization of the gastrointestinal (GI) tract, for example (17). In many other niches, such as mucosal or skin surfaces and regions of the GI, sugars are not available or present at low concentrations. In these niches alternative carbon sources, such as amino and organic acids, provide vital nutrients that support the growth of the infecting fungus (18, 19).
C. albicans displays considerable metabolic flexibility, which allows it to assimilate the variety of nutrients available in the diverse microenvironments it can occupy within the host. Comparisons of C. albicans with its benign relative Saccharomyces cerevisiae have revealed that significant transcriptional rewiring has taken place during their evolution as well as significant divergence in the regulators that control carbohydrate and lipid metabolism (20, 21), reflecting the contrasting lifestyles of these yeasts. Unlike S. cerevisiae, C. albicans continues to respire in the presence of glucose (22). Furthermore, there is considerable evidence for niche-specific metabolic regulation in C. albicans (18, 23, 24, 25, 26, 27). For example, the glyoxylate cycle is essential for the survival of C. albicans in some host environments (19), especially in sugar-limited niches. Genes encoding carboxylic acids transporters or metabolizing enzymes are significantly induced in vivo (28). Growth on carboxylic acids such as lactate involves the catabolism of some of this carbon source via the tricarboxylic cycle to generate the metabolic energy to drive anabolic processes, such as gluconeogenesis. Gluconeogenesis is required to synthesize the hexose sugars essential for cell wall biogenesis and the pentoses required for nucleic acid biosynthesis (via the pentose phosphate cycle). A lower proportion of energy-rich carbon sources such as glucose is required for energy production, leaving more of this hexose for cell wall biosynthesis, for example. Therefore, growth on alternative, nonfermentative carbon sources like lactate leads to the biosynthesis of a thinner, structurally different cell wall (14). Given the significant impact of carbon source on the architecture of the C. albicans cell wall (14) and the importance of the cell surface in immune recognition, we reasoned that changes in carbon source are likely to affect the recognition of C. albicans cells by the immune system.
Therefore, in this study, we tested whether growth on lactate influences the immune response and recognition of C. albicans by cells of the innate immune system. We used lactic acid as an alternative carbon source to glucose because of its physiological relevance. Lactic acid is found in ingested foods, generated by lactic acid bacteria in the GI and urogenital tracts (29), and is essential for the proliferation of Candida glabrata in the intestinal tract (30). Lactic acid is also a component of isotonic solutions used in surgery or burn injury (e.g., lactated Ringer's solutions, Hartmann's solution), factors that increase the risk of systemic candidiasis (1). We show that growth on this alternative carbon source significantly affects cytokine production by peripheral blood mononuclear cells (PBMCs) and macrophages, the phagocytosis of C. albicans by these cells, and the ability of C. albicans to escape and kill macrophages. Our studies underline the importance of carbon source for fungal virulence, indicating that in addition to modulating the physiological status of the fungus (12, 14, 30, 31), carbon source has a distinct effect on the host-fungus interaction.
MATERIALS AND METHODS
Strains and growth conditions.
Strains used in this study (see Table S3 in the supplemental material) were grown at 30°C in minimal medium containing a carbon source (2% glucose, 2% lactate, or glucose plus lactate each at 1%), 0.67% yeast nitrogen base without amino acids (YNB), and supplemented with 10 μg/ml of the appropriate auxotrophic requirements. Cells were grown overnight at 30°C, 200 rpm, diluted to an optical density at 600 nm (OD600) of 0.1 in fresh medium, and harvested at mid-exponential phase (OD600, 0.5) for analyses and sensitivity assays. All experiments were performed with yeast cells grown at a pH 5.2 to 5.6.
Volunteers.
Blood samples were collected from healthy, nonsmoking volunteers. After written informed consent was obtained, venipuncture was performed to collect blood into 10-ml EDTA tubes (Monoject).
Isolation and stimulation of PBMCs.
Separation and stimulation of PBMCs was performed as previously described (7) from buffy coats obtained from healthy blood donors at the Bloodbank Nijmegen. Cells were adjusted to a concentration of 5 × 106 cells/ml and incubated at 37°C in round-bottom 96-well plates (volume, 100 μl/well) with either UV-killed C. albicans (105 or 106 cells/ml) or culture medium (7). After 24 h, 48 h, or 7 days, supernatants were collected and stored at −20°C until assayed.
Cytokine measurements.
IL-6, IL-10 (Sanquin, Amsterdam, The Netherlands), gamma interferon (IFN-γ; Pelikine Compact, CLB, Amsterdam, The Netherlands), tumor necrosis factor alpha (TNF-α), and IL-17 (R&D, The Netherlands) concentrations from the culture supernatant were measured by using commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. For all cytokine measurements, the cytokine levels without yeast challenge were around or below the level of detection. Detection limits for individual cytokines were as follows: TNF-α, 39 pg/ml; IL-6, 312 pg/ml; IFN-γ, 7.8 pg/ml; IL-10, 4.68 pg/ml; IL-17, 31.2 pg/ml.
Antifungal drug susceptibility.
Antifungal drug susceptibility was analyzed by treating mid-exponential-phase C. albicans cells grown on the specified carbon source with tunicamycin (4 μg/ml), caspofungin (0.08 μg/ml), amphotericin B (Ambisome; 10 μg/ml), or miconazole (25 μg/ml) for 1 h at 30°C and 200 rpm. Cells were then serially diluted and plated onto YPD agar. CFU were quantified, and antifungal drug sensitivities were calculated relative to those observed for untreated control cells. Means ± standard errors of the means (SEM) for at least three independent experiments are presented.
Macrophage cell cultures.
J774.1 murine macrophages (European Collection of Cell Cultures) were cultured in Dulbecco's modified Eagle's medium (DMEM; Lonza Group, Ltd., Braine-l'Alleud, Belgium), supplemented with 10% (vol/vol) fetal calf serum (FCS; Biosera, Ringmer, United Kingdom), 2% (wt/vol) penicillin and streptomycin antibiotics (Invitrogen, Ltd., Paisley, United Kingdom), and 1% l-glutamine (Invitrogen) in tissue culture flasks (Nagle Nunc, Int., Hereford, United Kingdom) at 37°C and 5% (vol/vol) CO2.
Yeast cell phagocytosis assay.
Uptake of C. albicans by macrophages was assessed using a standard phagocytosis assay (9), with the only difference that macrophages and live fungal cells were incubated for 2 h at C. albicans:macrophage ratios of 3:1 or 1:1. UV-killed cells were incubated for 2 h using a C. albicans:macrophage ratio of 3:1. Data were obtained in triplicate from four independent experiments by analyzing at least 100 macrophages per well.
Macrophage killing assay.
The macrophage killing assay was conducted as previously described (9) and under the same conditions described above for the phagocytosis assay. Macrophages and fungal cells were incubated for 2 h at 3:1 C. albicans/macrophage ratios. Data were obtained in triplicate from at least four separate experiments by analyzing at least 400 macrophages per well.
Statistical analyses.
Results from at least three independent sets of experiments are expressed as means ± SEM. Wilcoxon tests (IBM SPSS Statistics 20) and t tests (Excel) were used to determine statistical significance. Dunnett's tests and one way analyses of variance (ANOVA) were performed using IBM SPSS Statistics 20 for each carbon source (glucose or lactate) to determine differences between or within groups. Coefficients of variation (CV) were calculated in Excel between donors for each of the C. albicans isolates examined. The level of significance was set at a P value of <0.05.
RESULTS
Growth of C. albicans cells on lactate dampens their stimulation of PBMCs and macrophages.
We showed previously that growth of C. albicans cells on lactate instead of glucose significantly alters the conformation of mannan fibrils on the cell surface as well as the expression of cell wall and secreted proteins (12, 14). As cell surface mannoproteins are the first point of direct contact between the pathogen and innate immune cells, we examined the impact of alternative carbon source assimilation upon host immune responses. First, we tested the ability of cells grown on lactate, glucose, or a mixture of glucose plus lactate to stimulate PBMCs, as these cells are a critical component of the immune system due to their ability to induce cytokine production.
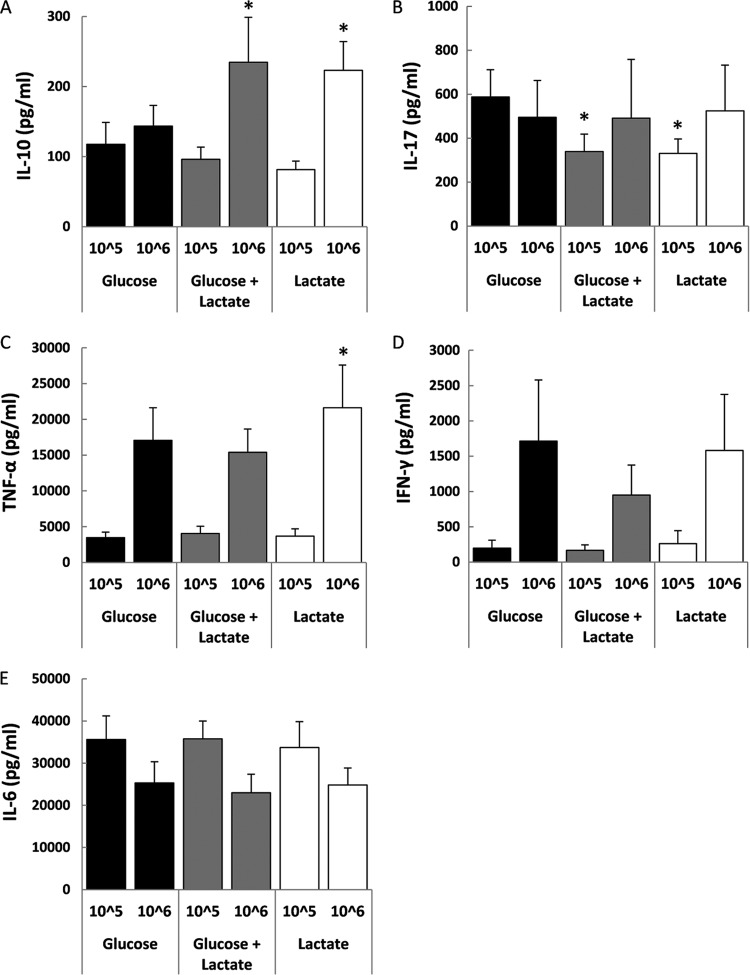
PBMCs are a mixed population of different cell types whose ratio of monocytes to leukocytes varies between different donors. Therefore, two different C. albicans inocula that are commonly used in the literature (105 and 106) (32, 33, 34) were used in the initial experiments (Fig. 1). We found that growth on lactate altered the PBMC cytokine profile induced by UV-killed C. albicans yeast cells (Fig. 1). In particular, compared to glucose-grown cells, lactate-grown cells stimulated increased production of IL-10 (Fig. 1A) and TNF-α (Fig. 1C), along with decreased levels of IL-17 (Fig. 1B). These changes in cytokine levels were also observed in PBMCs stimulated with cells grown on a mix of glucose and lactate (Fig. 1A and B), suggesting that the complex mixtures of carbon sources present in host niches might induce alterations in cytokine profiles. No significant changes were observed in the levels of IFN-γ or IL-6 under these conditions (Fig. 1D and E). IL-10 induction was observed more clearly at the higher C. albicans concentration (106). IL-10 was measured after at 48 h, because after this point IL-10 decreases to undetectable levels by 72 h (34). Therefore, the higher C. albicans concentration gave better resolution of the carbon source differences at 48 h. In contrast, the IL-17 assays were performed after 7 days of incubation, as this cytokine increases steadily within this interval (34). At this time point, the higher C. albicans inoculum probably saturated the IL-17 production, resulting in a clearer difference at the lower 105 dilution. Those dilutions that gave a clearer resolution between the glucose and lactate samples were used for further experiments (106 for IL-10 and 105 for IL-17).
Fig 1.
Growth of C. albicans cells on lactate affects cytokine production by host PBMCs. Human PBMCs were incubated with UV-killed C. albicans RM1000 cells grown on glucose, lactate, or glucose plus lactate. The supernatant was collected for ELISA measurements of IL-10 (48-h stimulation) (A), IL-17 (7-day stimulation) (B), TNF-α (24-h stimulation) (C), IFN-γ (48-h stimulation) (D), or IL-6 (24-h stimulation) (E). The cytokine levels without yeast challenge were all below the level of detection (TNF-α, 39 pg/ml; IL-6, 312 pg/ml; IFN-γ, 7.8 pg/ml; IL-10, 4.68 pg/ml; IL-17, 31.2 pg/ml). The data are cumulative results from 9 to 12 different donors and are expressed as means ± SEM. *, P < 0.05 relative to PBMCs incubated with glucose-grown C. albicans.
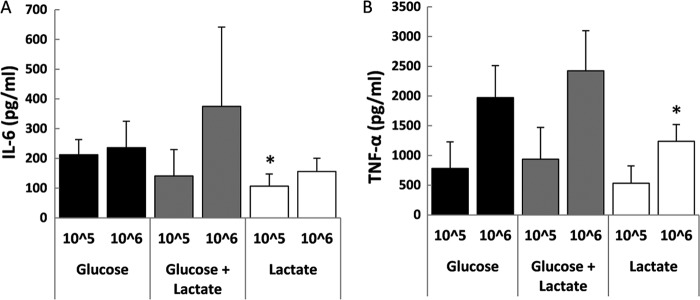
In addition to circulating cells, an even more relevant population is human macrophages, due to their local interaction with colonizing microorganisms. Upon incubation with human macrophages, lactate-grown C. albicans failed to induce the same levels of IL-6 and TNF-α as glucose-grown cells (Fig. 2A and B), suggesting that lactate-grown cells induce a dampened immune response. These results reinforced the notion that the assimilation of carbon sources other than glucose by C. albicans cells in host niches significantly impacts their ability to induce a host immune response.
Fig 2.
Growth of C. albicans cells on lactate affects cytokine production by macrophages. Human macrophages were incubated with UV-killed C. albicans RM1000 cells grown on glucose, lactate, or glucose plus lactate. The supernatant was collected after 24 h of stimulation for IL-6 (A) and TNF-α (B) ELISA measurements. The cytokine levels without yeast challenge were below the level of detection (IL-10, 4.68 pg/ml; IL-17, 31.2 pg/ml). The data are cumulative results from 8 to 12 different donors and are expressed as means ± SEM. *, P < 0.05 relative to cytokine production induced by glucose-grown C. albicans.
The anti-inflammatory response of PBMCs to lactate-grown cells from the SC5314 C. albicans strain lineage.
The above-described experiments were performed with the C. albicans strain RM1000. RM1000 is a member of a strain lineage that has been derived from the clinical isolate SC5314 (see Table S3 in the supplemental material), which belongs to the first C. albicans epidemiological clade (35). This lineage includes the strains CAI4, RM1000, and BWP17 (see Table S3), which are widely used for molecular dissection for C. albicans. Some of these strains have accumulated aneuploidies, which can arise during the production of recombinant strains (36, 37). Therefore, we analyzed the impact of carbon source on the ability of these strains to induce cytokine production by PBMCs. Similar trends of increased IL-10 were observed across this strain lineage (Fig. 3). However, the differences in cytokine production were more dramatic for BWP17 than the other strains. This suggests that some chromosomal rearrangements or mutations that have arisen during the construction of this strain lineage might have exerted subtle effects upon PAMP structure or expression and, consequently, upon the host immune response.
Fig 3.
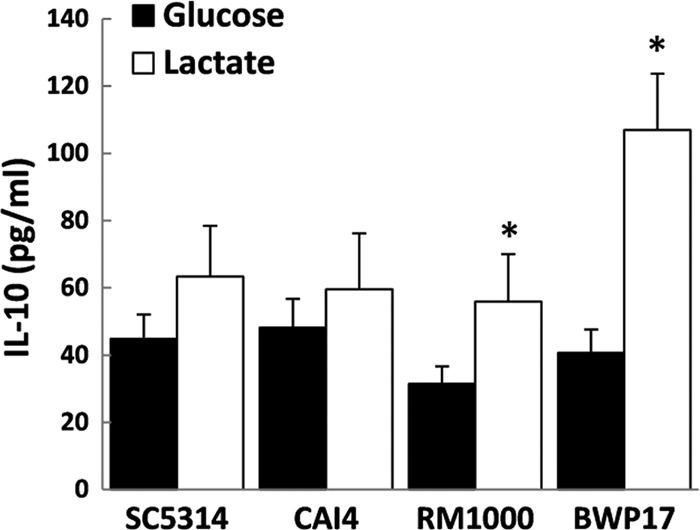
Impact of carbon source on the induction of cytokine production by C. albicans strains from the SC5314 lineage (see Table S3 in the supplemental material). Human PBMCs were incubated with UV-killed C. albicans cells grown on glucose or lactate. The supernatant was collected for IL-10 ELISA measurements after 48 h. The IL-10 levels without yeast challenge were below the limit of detection (4.68 pg/ml). The data are cumulative results from nine different donors and are expressed as means ± SEM. *, P < 0.05 relative to PBMCs incubated with the glucose-grown respective C. albicans strain.
The impact of carbon source on C. albicans-induced cytokine production varies among clinical isolates and between donors.
Clinical C. albicans isolates were initially classified into five major clades based on DNA fingerprinting of the moderately repetitive sequence Ca3 (38). Further typing based on more than 400 isolates revealed a more complex population structure comprising four major and eight minor clades that display differences in drug resistance and their tendencies to infect or colonize different niches (35). These studies revealed the need to analyze the pathogenic characteristics of representative strains from all clades, rather than one classical strain, such as SC5314.
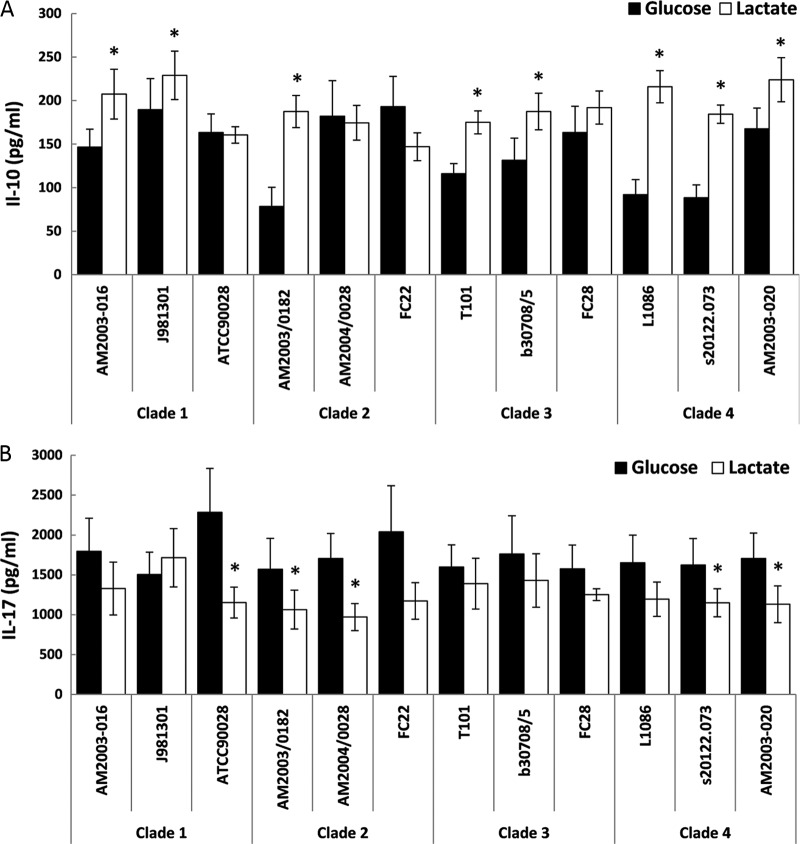
In this context, we examined the effects of C. albicans carbon source upon immune responses for clinical isolates from different epidemiological clades. We focused on IL-10 and IL-17 for these experiments, as we had observed significant differences in the induction of these influential cytokines (Fig. 1A and B). We looked at IL-10 and IL-17 production by PBMCs exposed to 12 clinical isolates belonging to the four major C. albicans clades, selecting isolates from the blood, oropharynx, vaginal mucosa, or a wound (see Table S3). The majority of these isolates displayed IL-10 and IL-17 trends that were similar to those observed for the RM1000 strain (Fig. 4A and B). However, there were variations in the PBMC responses induced by these clinical isolates, and no correlation was observed with either the epidemiological clade or the tissue from which the strain was isolated. We found no significant difference in the way in which carbon source modulated cytokine production between the four clades (see Table S1 in the supplemental material). The impact of carbon source on cytokine production varied for some isolates within clades (e.g., the isolates from clade 2). However, for the majority of isolates, growth on lactate significantly shifted the cytokine profile toward increased IL-10 and decreased IL-17 production (Fig. 4A and B; see also Table S1). In addition, donors differed significantly with respect to the strength of their cytokine responses. However, they displayed similar trends to glucose- and lactate-grown C. albicans cells (see Table S1). Overall, the data indicated that host-fungus interactions are both carbon source and strain dependent, even for strains within the same Candida clade or lineage.
Fig 4.
Impact of carbon source on the induction of cytokine production by clinical C. albicans isolates from the four major clades. The strains examined from each clade were isolated from different host niches (see Table S3 in the supplemental material). Human PBMCs were incubated with UV-killed C. albicans cells grown on glucose or lactate. The supernatant was collected for ELISA measurements after 48 h for IL-10 (A) or after 7 days for IL-17 (B). The cytokine levels without yeast challenge were close to or below the level of detection (IL-10, <15 pg/ml; IL-17, <39 pg/ml). The data are cumulative results from six different donors and are expressed as means ± SEM. *, P < 0.05 relative to PBMCs incubated with the corresponding C. albicans strain grown on glucose.
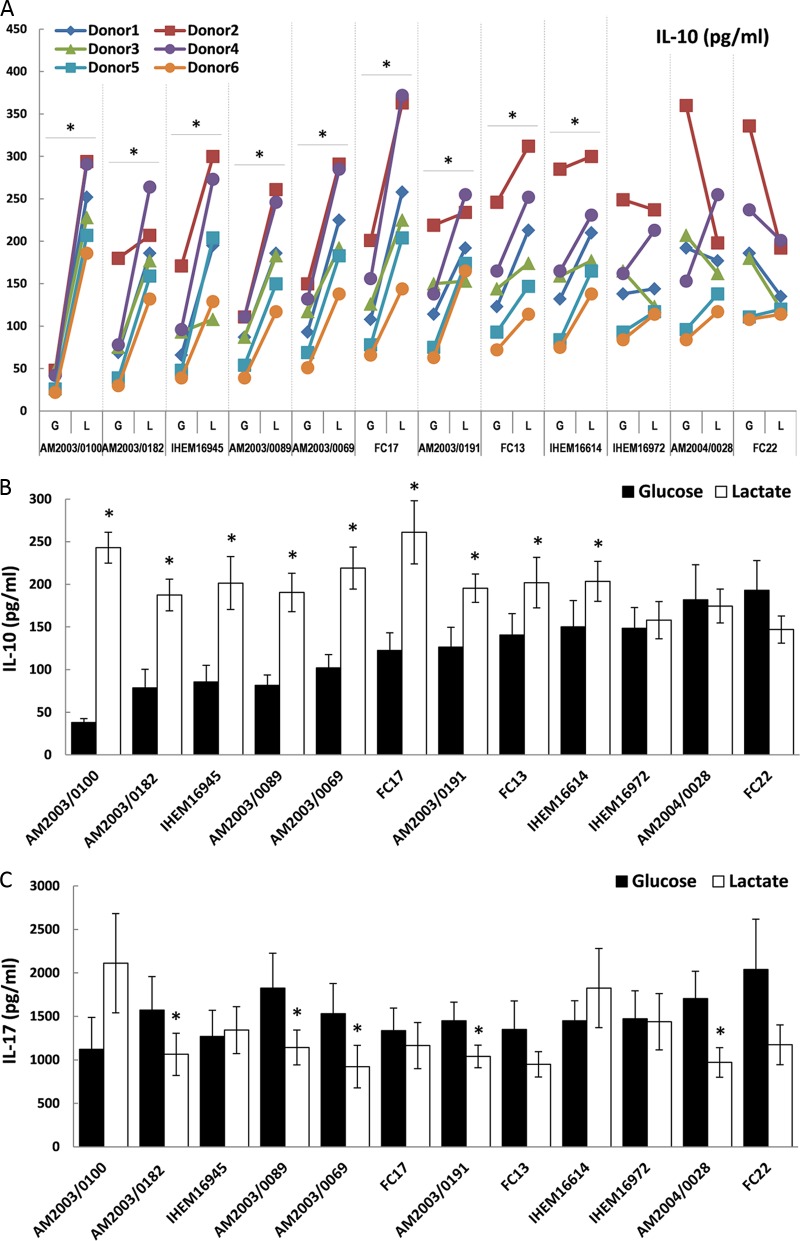
In particular, isolates from clade 2 displayed differences in the impact of carbon source on the PBMC response (Fig. 4A). Therefore, we extended our analyses to include nine additional pathogenic strains isolated from the blood, oropharynx, semen, or vaginal mucosa (see Table S3). Once again, we observed significant variability between donors with respect to their cytokine responses (Fig. 5A; see also Table S2 in the supplemental material). The six donors differed in the strengths of responses elicited, but most displayed similar trends with respect to the impact of carbon source upon their cytokine response (Fig. 5A). We noted a strong tendency toward increased IL-10 production and decreased IL-17 levels for lactate-grown C. albicans cells among these individuals.
Fig 5.
Impact of carbon source on C. albicans-induced cytokine production in 12 clinical isolates from clade 2. The selected strains were originally isolated from a range of host niches (see Table S3 in the supplemental material). Human PBMCs were incubated with UV-killed C. albicans cells grown on glucose (G) or lactate (L). The supernatant was collected for ELISA measurements after 48 h for IL-10 (A and B) and after 7 days for IL-17 (C). The cytokine levels without yeast challenge were close to or below the level of detection (IL-10, <15 pg/ml; IL-17, <39 pg/ml). (A) The data represent IL-10 levels for individual donors, and asterisks denote significant differences (P < 0.05) relative to PBMCs incubated with the corresponding C. albicans strain grown on glucose. (B and C) Panels represent cumulative results from six different donors and are expressed as means ± SEM. *, P < 0.05 relative to PBMCs incubated with the corresponding C. albicans strain grown on glucose.
The growth of more than half of the clade 2 clinical isolates on lactate rather than glucose led to significantly increased IL-10 production and decreased IL-17 production by PBMCs (Fig. 5B and C). However, other clinical isolates from this clade did not display this phenotype, as the carbon source had no significant impact on these strains. These strain differences did not correlate with the site of infection from which they originated. We suggest that these findings represent sporadic strain differences that have arisen relatively recently in evolutionary terms. C. albicans clinical strains are known to display considerable karyotypic variation, and the types of stress that are encountered by the fungus as it passages through the host are known to induce chromosomal rearrangements (39). Indeed, variability between clinical isolates has been reported, and correlations with their virulence or type of infection have remained difficult to identify (40, 41, 42).
The relationship between IL-10 and the Th17 response to C. albicans is not fully understood. C. albicans cells or the fungal cell wall component zymosan can promote IL-17 differentiation but also increased production of IL-10 (43). Anti-IL-10 causes a reduction in the proportion of T cells that were able to produce IL-17 (44), but it was also shown that IL-10 is inhibitory to Th17 cell generation (45). In general, when comparing the effects of growth on lactate and glucose for the C. albicans clade 2 isolates, there was an inverse correlation between IL-10 and IL-17 production. In other words, increased IL-10 levels were generally associated with reduced IL-17 production (Fig. 4; see also Fig. S1 in the supplemental material). Taken together, these results indicate that growth of C. albicans cells on lactate significantly impacts cytokine production, and in more than half of the strains tested this effect was consistent with a shift in the balance from a Th17 response toward a Th2 response. However, the high level of immunological variability between donors and genomic differences between clinical isolates may mask some effects in vitro.
Impact of carbon source on C. albicans antifungal resistance.
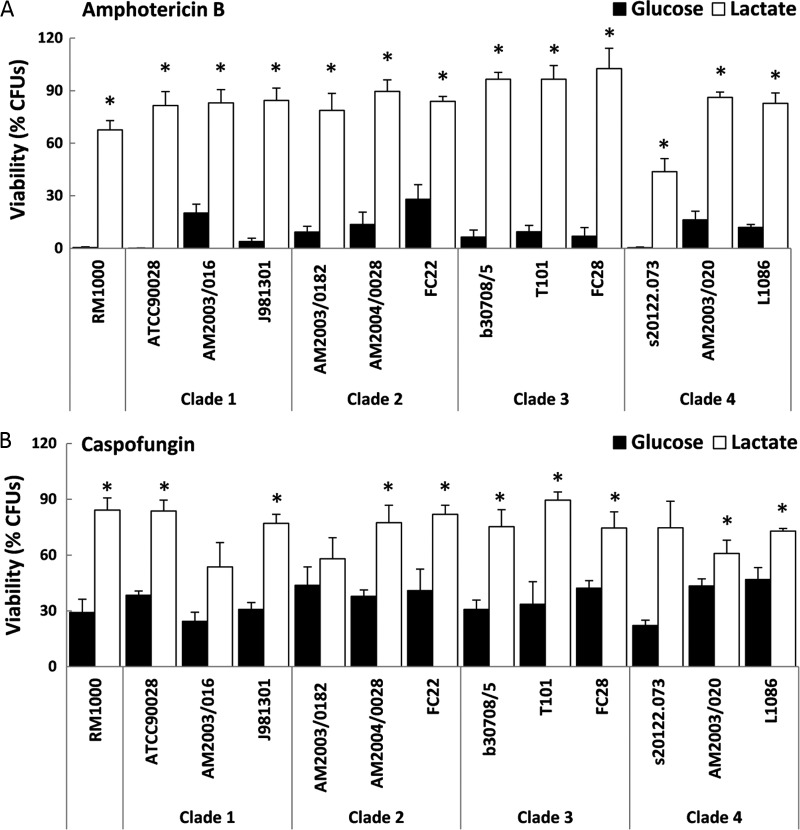
We previously reported that growth on carbon sources other than glucose affects resistance to antifungal drugs in a derivative of a clinical isolate from clade 1 (RM1000) (14). However, in this study we found that growth on lactate altered the response of PBMCs to C. albicans stimulation but that this effect was subject to strain variations which were not associated with epidemiological clade or clinical source. Therefore, we tested whether the effect of carbon source upon antifungal resistance was also subject to strain variation. We examined the sensitivity of strains from the four major clades (see Table S3 in the supplemental material) to amphotericin B, caspofungin, miconazole, and tunicamycin. Growth on lactate instead of glucose had a significant effect upon the antifungal resistance of C. albicans, and this effect was observed throughout all of the pathogenic isolates and clades tested (Fig. 6; see also Fig. S3 in the supplemental material). We concluded that the effects of carbon source upon drug resistance are not overridden by strain phylogeny, anatomical source, or strain variations (genome alterations) that might follow host passage.
Fig 6.
Impact of carbon source on the antifungal sensitivity of clinical C. albicans isolates from the four major clades. The selected C. albicans isolates were originally isolated from a range of host niches (see Table S3 in the supplemental material). Sensitivities were assayed using the antifungal drugs amphotericin B (Ambisome; 10 μg/ml) (A) and caspofungin (6.4 ng/ml) (B). Means ± SEM for at least three independent experiments are presented. P values are relative to results with glucose-grown cells: *, P < 0.05.
Carbon source alters the interaction of C. albicans with macrophages.
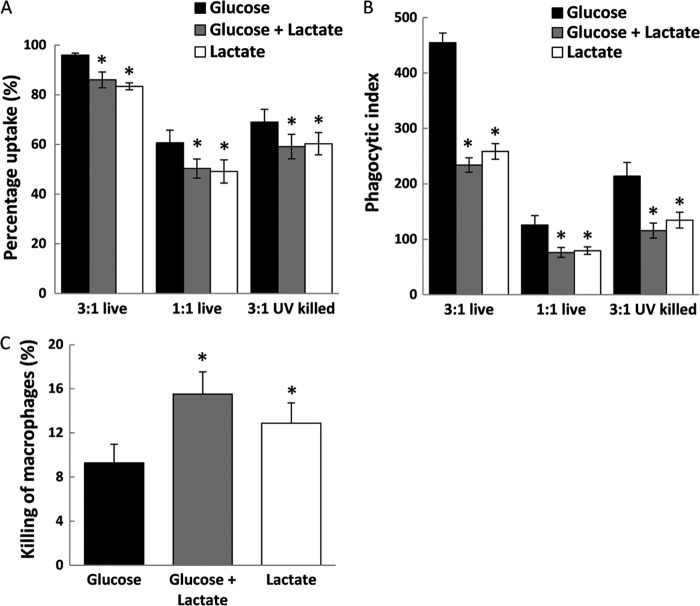
Next, we assessed the effects of carbon source on fungus-macrophage interactions. We reasoned that given the lowered immune response of macrophages and PBMCs to lactate-grown C. albicans cells, the rate of recognition and phagocytosis by macrophages might also be affected by carbon source. Therefore, we challenged a murine macrophage cell line (J774.1) with live fungal cells grown on glucose, lactate, or glucose plus lactate, incubating C. albicans cells with macrophages in either a 3:1 or 1:1 ratio. With both dilutions and under all three growth conditions, J774.1 macrophages efficiently took up C. albicans cells within the 2-h interaction assay (Fig. 7A). The majority of macrophages (>80%) ingested at least one fungal cell when cells were incubated in a 3:1 ratio. Coincubation experiments revealed that C. albicans cells grown on lactate or a mix of glucose plus lactate were taken up by macrophages less efficiently than glucose-grown cells (Fig. 7B). This was observed at both incubation ratios (3:1 and 1:1), but the biggest differences between growth conditions occurred at the 3:1 ratio (Fig. 7B).
Fig 7.
Impact of carbon source on phagocytosis of C. albicans cells by murine macrophages. (A and B) Percent uptake (the percentage of macrophages that took up at least one fungal cell) (A) and phagocytic index (the number of fungal cells taken up per 100 macrophages) for J774.1 macrophages incubated with live or UV-killed C. albicans RM1000 plus Clp20 cells (B) grown on glucose, lactate, or glucose plus lactate. Fungal cells and macrophages were incubated at 3:1 or 1:1 C. albicans/macrophage ratios. Values represent means ± SEM for four independent experiments. P values are relative to phagocytosis of glucose-grown cells: *, P < 0.05. (C) Killing of macrophages by C. albicans RM1000 cells grown on glucose, lactate, or glucose plus lactate. Results are expressed as the percentage of J774.1 macrophages killed in 400 macrophages. The C. albicans/macrophage ratio was 3:1. Values represent means ± SEM for four independent experiments. P values are relative to killing induced by glucose-grown C. albicans: *, P < 0.05.
The decreased uptake of live lactate-grown cells was consistent with the dampened immune response of human macrophages to lactate-grown UV-killed C. albicans (Fig. 2). To exclude the possibility that UV killing was confounding the effects of carbon source upon C. albicans-macrophage interactions, we compared the behavior of live and UV-killed cells with the murine macrophage cell line. Fewer UV-killed cells than live cells were ingested by the macrophages (Fig. 7A and B). Nevertheless, the impact of carbon source on these cells was similar to that of live cells, strengthening the concordance between the human cytokine data set (Fig. 2) and these interaction assays.
Although they were less efficiently phagocytosed than glucose-grown cells, cells grown on lactate, and in particular cells grown on the mixed medium, were more efficient at killing macrophages (Fig. 7C). These observations confirmed the importance of carbon source in the recognition and phagocytosis of C. albicans cells by macrophages, further reinforcing the idea that differential nutrient availability in host niches significantly affects host-fungus interactions.
DISCUSSION
Host defenses against C. albicans infection represent a dynamic interplay between the activation of immune responses and the ability of the pathogen to modulate these responses. A first stage in triggering host innate immunity involves the recognition of various PAMPs displayed by fungal cells. A large number of receptors (TLRs and C-type lectin receptors) recognize components of the fungal cell wall, such as chitin, β-glucan, mannan, and covalently attached proteins, and these recognition events are followed by the release of proinflammatory cytokines and phagocytosis (3, 5).
We previously showed that growth on alternative carbon sources induced fungal cell wall remodelling and modulation of the cell wall proteome and secretome (12, 14). This subsequently affected important virulence parameters, such as stress and drug resistance, adherence, biofilm formation, and infection outcome (12, 14). In this study, we have now shown that growth of C. albicans cells on lactate rather than glucose alters the resultant PBMC and macrophage cytokine profiles (Fig. 1 and 2) and, in particular, leads to increased IL-10 production and decreased IL-17 levels.
We observed similar effects upon IL-10 levels when we challenged PBMCs with a congenic set of C. albicans strains derived from the clinical isolate SC5314 (Fig. 3). However, we observed clade and strain variations when we extended these analyses to include clinical isolates from the four major epidemiological clades (Fig. 4 and 5; see also Tables S1 and S2 in the supplemental material). There was no obvious correlation between the effects of carbon source upon the PBMC response and the clade or the anatomical site from which the isolates were obtained. This was not surprising, as strains isolated from one anatomical site are capable of infecting other sites. Also, donors displayed significant differences in the strength of their cytokine responses to C. albicans cells grown on different carbon sources (Fig. 5A; see also Tables S1 and S2). This was expected, as individuals display considerable variability with regard to their immunoreactivity. Nevertheless, for the majority of these C. albicans clinical isolates, anti-inflammatory cytokine responses were observed when cells were grown on lactate rather than glucose. Moreover, we observed that increased IL-10 production generally correlated with decreased IL-17 production (Fig. 4; see also Fig. S1 in the supplemental material).
The observed strain variation is not surprising. Strains from different clades display a large frequency of sequence polymorphisms (42, 46). The existing typing systems, such as multilocus sequence typing, measure the properties of a few genes, compared with the thousands present in the genome. Furthermore, a major nutritional shift from a fermentative carbon source such as glucose to a nonfermentative carbon source such as lactate affects a large proportion of the genome (47) and hence is likely to result in the revelation of many genetic differences in the phenotype. Given the major impact of carbon source on the cell surface (12, 14), some of these strain differences are likely to be reflected in subtly altered PAMP structure or expression at the cell surface.
Both IL-10 and IL-17 represent key immune cytokines for the host defense against C. albicans infection. The Th17 response plays an important role in the protection against extracellular bacteria and fungi by producing IL-17. IL-17 is one of the key cytokines in stimulating host immunity in response to Candida infection, inducing granulopoiesis (48) and neutrophil recruitment (49). C. albicans mannan directly induces prostaglandin E2 (PGE2) via the macrophage mannan receptor (MR) (50), a strong proinflammatory mediator essential for the Th17 response. Lactate-grown cells display a significant reduction in the amount of mannan in the cell wall as well as in the organization of the mannan fibrils (14). The amount of β-glucan is also decreased in the lactate-grown cell wall, but its recognition might be masked by the altered architecture of the mannan fibrils and the different proteins attached to it (12, 14). β-Glucan recognition via the dectin-1 receptor synergistically induces the production of PGE2 (50). Thus, both mannan and β-glucan contribute to activation of the Th17 response via the MR and the dectin-1/TLR2 pathways, respectively. An early stop codon mutation in dectin-1, which recognizes β-glucan, leads to defective IL-17 production and recurrent vulvovaginal candidiasis and onychomycosis (33), indicating the importance of the Th17 response in mucosal and skin infections. Hence, the downregulation of IL-17 production promotes fungal infection.
Th2-derived anti-inflammatory cytokines such as IL-10 also play an important role in the host defense against disseminated candidiasis (51). Increased IL-10 production, modulated through different TLRs and dectin-1, shifts the balance toward anti-inflammatory cytokine responses (52). On the other hand, the upregulation of IL-10 also exacerbates Candida infection in mice (53). IL-10-deficient mice are more resistant to Candida infection, due to an upregulated Th1 antifungal response (54). Hence, increased IL-10 production might predispose the host to Candida infection.
We have shown that the cell wall architecture of C. albicans is dramatically altered after cells are grown on lactate rather than glucose, with both the thickness and architecture of the different cell wall polysaccharides being modulated in response to carbon source (14). These alterations are accompanied by significant changes in the cell wall proteome and secretome (12). The major polysaccharides of the Candida cell wall play key roles in the recognition by host immune cells and the induction of cytokine production. Candida mannan is recognized by the MR, which drives Th17 differentiation and IL-17 production (55). Phospholipomannan is recognized by TLR2 (6), and the production of IL-10 is TLR2 and dectin-1 dependent (32, 56). Therefore, the alterations in cell wall architecture might either have a direct effect on downstream IL-10 and IL-17 production by PBMCs or an indirect effect through the upregulation of IL-10, leading to lower IL-17 production as a result. Overall, the observed upregulation of IL-10 and downregulation of IL-17 might suggest that lactate-grown C. albicans cells are more virulent than glucose-grown cells. This is in close agreement with in vivo studies showing that lactate-grown cells are more virulent in both vaginal and systemic infections (14).
We also showed that the carbon source significantly affects the interaction between C. albicans cells and macrophages, which are key immune cells during mucosal infection. Lactate-grown cells were taken up less efficiently by macrophages, and those Candida cells that were phagocytosed were more effective at escaping the macrophages and killing them (Fig. 7). C. albicans cells grown on a mix of glucose and lactate behaved similarly to lactate-grown cells, underlining the importance of these findings for in vivo niches which are complex and generally offer mixtures of nutrients rather than a single carbon source. We hypothesize that the decreased uptake of lactate-grown C. albicans cells is mediated by changes in the structure of mannan fibrils (14) as well as differences in the glycophosphatidylinositol-linked component (12), leading to a lower level of immune recognition.
The surfaces colonized by C. albicans differ widely with respect to nutrient availability. The skin and the oropharyngeal and vaginal mucosae differ significantly in terms of their microbial flora, ambient pH, carbon source, and the types of immune cells present. In particular, colonization of the vagina implies sharing of this mucosal environment with lactic acid-producing Lactobacillus spp., which are the main colonizing flora. Interestingly, while older studies have suggested a protective effect of lactobacilli on vaginal colonization with fungi (57), more recent research has revealed that Lactobacillus colonization of the vagina is a strong risk for vulvovaginal candidiasis (58). Our study provides the first evidence for the mechanisms by which lactate supplied by the Lactobacillus vaginal flora could contribute to this process.
In conclusion, the in vitro observations we have described here are of direct relevance to C. albicans infections. These infections involve the colonization by the pathogen of complex and dynamic in vivo niches that contain mixtures of carbon sources and often lack sugars such as glucose. Several lines of evidence indicate that assimilation of alternative carbon sources increases the fitness of Candida in certain host niches (18, 19, 28, 30, 59). Our study reinforces these observations by indicating that changes in local carbon source also have a significant impact on Candida-host interactions. Considering the different niches in which Candida grows in the body, these findings have the potential to fundamentally change our understanding of the interactions between the fungus and the human host.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the FINSysB Network. A.J.P.B. is supported by grants from the European Commission (PITN-GA-2008-214004 and ERC-2009-AdG-249793), the BBSRC (BB/D009308/1 and BB/F00513X/1), and the Wellcome Trust (080088). M.G.N. is supported by an ERC Consolidator Grant (310372).
We thank members of the Aberdeen Fungal Group and FINSysB Network for insightful discussions, as well as Donna MacCallum for strains, Frank C. Odds for advice, and Susan Budge for excellent technical support.
Footnotes
Published ahead of print 31 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01092-12.
REFERENCES
- 1. Pfaller M, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 36:1–53 [DOI] [PubMed] [Google Scholar]
- 2. Dongari-Bagtzoglou A, Fidel PL., Jr 2005. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 84:966–977 [DOI] [PubMed] [Google Scholar]
- 3. Netea MG, Marodi L. 2010. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 31:346–353 [DOI] [PubMed] [Google Scholar]
- 4. Gazi U, Martinez-Pomares L. 2009. Influence of the mannose receptor in host immune responses. Immunobiology 214:554–561 [DOI] [PubMed] [Google Scholar]
- 5. Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. 2012. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D. 2003. Candida albicans phospholipomannan is sensed through toll-like receptors. J. Infect. Dis. 188:165–172 [DOI] [PubMed] [Google Scholar]
- 7. Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116:1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196:407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKenzie CG, Koser U, Lewis LE, Bain JM, Mora-Montes HM, Barker RN, Gow NA, Erwig LP. 2010. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect. Immun. 78:1650–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Filler SG. 2011. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot. Cell 10:168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. 2010. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J. Infect. Dis. 201:1718–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ene IV, Heilmann CJ, Sorgo AG, Walker LA, Koster CG, Munro CA, Klis FM, Brown AJ. 2012. Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans. Proteomics 12:3164–3179 doi:10.1002/pmic.201200228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sosinska GJ, de Koning LJ, de Groot PW, Manders EM, Dekker HL, Hellingwerf KJ, de Koster CG, Klis FM. 2011. Mass spectrometric quantification of the adaptations in the wall proteome of Candida albicans in response to ambient pH. Microbiology 157:136–146 [DOI] [PubMed] [Google Scholar]
- 14. Ene IV, Adya AK, Wehmeier S, Brand AC, Maccallum DM, Gow NA, Brown AJ. 2012. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell. Microbiol. 14:1319–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lowman DW, Ensley HE, Greene RR, Knagge KJ, Williams DL, Kruppa MD. 2011. Mannan structural complexity is decreased when Candida albicans is cultivated in blood or serum at physiological temperature. Carbohydr. Res. 346:2752–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kruppa MD, Greene RR, Noss I, Lowman DW, Williams DL. 2011. C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum and cultivation at physiological temperature. Glycobiology 21:1173–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khatib R, Riederer KM, Ramanathan J, Baran J., Jr 2001. Faecal fungal flora in healthy volunteers and inpatients. Mycoses 44:151–156 [DOI] [PubMed] [Google Scholar]
- 18. Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. 2006. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell. Microbiol. 8:961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorenz MC, Fink GR. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83–86 [DOI] [PubMed] [Google Scholar]
- 20. Lavoie H, Hogues H, Whiteway M. 2009. Rearrangements of the transcriptional regulatory networks of metabolic pathways in fungi. Curr. Opin. Microbiol. 12:655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martchenko M, Levitin A, Hogues H, Nantel A, Whiteway M. 2007. Transcriptional rewiring of fungal galactose-metabolism circuitry. Curr. Biol. 17:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niimi M, Kamiyama A, Tokunaga M. 1988. Respiration of medically important Candida species and Saccharomyces cerevisiae in relation to glucose effect. J. Med. Vet. Mycol. 26:195–198 [PubMed] [Google Scholar]
- 23. Fradin C, Hube B. 2003. Tissue infection and site-specific gene expression in Candida albicans. Adv. Appl. Microbiol. 53:271–290 [DOI] [PubMed] [Google Scholar]
- 24. Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. 2005. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 56:397–415 [DOI] [PubMed] [Google Scholar]
- 25. Rubin-Bejerano I, Fraser I, Grisafi P, Fink GR. 2003. Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 100:11007–11012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, Hube B. 2007. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol. Microbiol. 63:1606–1628 [DOI] [PubMed] [Google Scholar]
- 27. Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. 2007. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell. Microbiol. 9:2938–2954 [DOI] [PubMed] [Google Scholar]
- 28. Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buchalter SE, Crain MR, Kreisberg R. 1989. Regulation of lactate metabolism in vivo. Diabetes Metab. Rev. 5:379–391 [DOI] [PubMed] [Google Scholar]
- 30. Ueno K, Matsumoto Y, Uno J, Sasamoto K, Sekimizu K, Kinjo Y, Chibana H. 2011. Intestinal resident yeast Candida glabrata requires Cyb2p-mediated lactate assimilation to adapt in mouse intestine. PLoS One 6:e24759 doi:10.1371/journal.pone.0024579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vylkova S, Carman AJ, Danhof HA, Collette JR, Zhou H, Lorenz MC. 2011. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio 2(3):e00055–11 doi:10.1128/mBio.00055-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. 2008. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 10:2058–2066 [DOI] [PubMed] [Google Scholar]
- 33. Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. 2009. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361:1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, Netea MG. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329–340 [DOI] [PubMed] [Google Scholar]
- 35. Tavanti A, Davidson AD, Fordyce MJ, Gow NA, Maiden MC, Odds FC. 2005. Population structure and properties of Candida albicans, as determined by multilocus sequence typing. J. Clin. Microbiol. 43:5601–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arbour M, Epp E, Hogues H, Sellam A, Lacroix C, Rauceo J, Mitchell A, Whiteway M, Nantel A. 2009. Widespread occurrence of chromosomal aneuploidy following the routine production of Candida albicans mutants. FEMS Yeast Res. 9:1070–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Selmecki A, Bergmann S, Berman J. 2005. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol. Microbiol. 55:1553–1565 [DOI] [PubMed] [Google Scholar]
- 38. Soll DR, Pujol C. 2003. Candida albicans clades. FEMS Immunol. Med. Microbiol. 39:1–7 [DOI] [PubMed] [Google Scholar]
- 39. Forche A, Abbey D, Pisithkul T, Weinzierl MA, Ringstrom T, Bruck D, Petersen K, Berman J. 2011. Stress alters rates and types of loss of heterozygosity in Candida albicans. mBio 2(4):e00129–11 doi:10.1128/mBio.00129-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJ, Gow NA, Odds FC. 2009. Property differences among the four major Candida albicans strain clades. Eukaryot. Cell 8:373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Odds FC, Bougnoux ME, Shaw DJ, Bain JM, Davidson AD, Diogo D, Jacobsen MD, Lecomte M, Li SY, Tavanti A, Maiden MC, Gow NA, D'Enfert C. 2007. Molecular phylogenetics of Candida albicans. Eukaryot. Cell 6:1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Odds FC. 2010. Molecular phylogenetics and epidemiology of Candida albicans. Future Microbiol. 5:67–79 [DOI] [PubMed] [Google Scholar]
- 43. Du Z, Kelly E, Mecklenbrauker I, Agle L, Herrero C, Paik P, Ivashkiv LB. 2006. Selective regulation of IL-10 signaling and function by zymosan. J. Immunol. 176:4785–4792 [DOI] [PubMed] [Google Scholar]
- 44. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179–189 [DOI] [PubMed] [Google Scholar]
- 45. Inatsu A, Kogiso M, Jeschke MG, Asai A, Kobayashi M, Herndon DN, Suzuki F. 2011. Lack of Th17 cell generation in patients with severe burn injuries. J. Immunol. 187:2155–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jacobsen MD, Bougnoux ME, d'Enfert C, Odds FC. 2008. Multilocus sequence typing of Candida albicans isolates from animals. Res. Microbiol. 159:436–440 [DOI] [PubMed] [Google Scholar]
- 47. Rodaki A, Bohovych IM, Enjalbert B, Young T, Odds FC, Gow NA, Brown AJ. 2009. Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol. Biol. Cell 20:4845–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. 1998. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 161:6383–6389 [PubMed] [Google Scholar]
- 49. Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG. 2010. The Candida Th17 response is dependent on mannan- and beta-glucan-induced prostaglandin E2. Int. Immunol. 22:889–895 [DOI] [PubMed] [Google Scholar]
- 51. Romani L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1–23 [DOI] [PubMed] [Google Scholar]
- 52. Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. 2007. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 26:605–616 [DOI] [PubMed] [Google Scholar]
- 53. Tonnetti L, Spaccapelo R, Cenci E, Mencacci A, Puccetti P, Coffman RL, Bistoni F, Romani L. 1995. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur. J. Immunol. 25:1559–1565 [DOI] [PubMed] [Google Scholar]
- 54. Del Sero G, Mencacci A, Cenci E, d'Ostiani CF, Montagnoli C, Bacci A, Mosci P, Kopf M, Romani L. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1:1169–1180 [DOI] [PubMed] [Google Scholar]
- 55. Van de Veerdonk FL, Kullberg BJ, van der Meer JW, Gow NA, Netea MG. 2008. Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr. Opin. Microbiol. 11:305–312 [DOI] [PubMed] [Google Scholar]
- 56. Van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. 2005. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 73:7458–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Auger P, Joly J. 1980. Microbial flora associated with Candida albicans vulvovaginitis. Obstet. Gynecol. 55:397–401 [DOI] [PubMed] [Google Scholar]
- 58. McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, Mandaliya K, Jaoko W, Ndinya-Achola JO, Holmes KK. 2009. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J. Infect. Dis. 199:1883–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramirez MA, Lorenz MC. 2007. Mutations in alternative carbon utilization pathways in Candida albicans attenuate virulence and confer pleiotropic phenotypes. Eukaryot. Cell 6:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.