Abstract
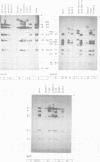
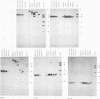
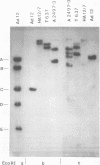
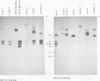
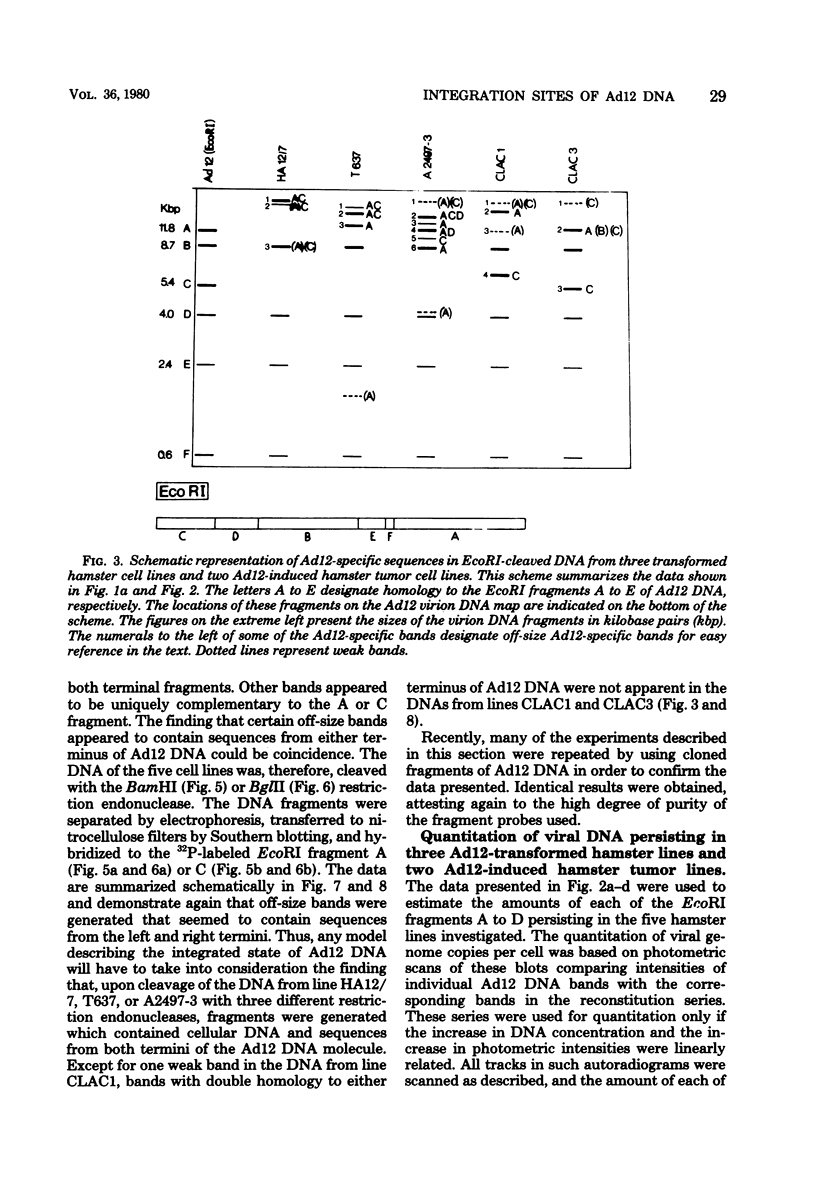
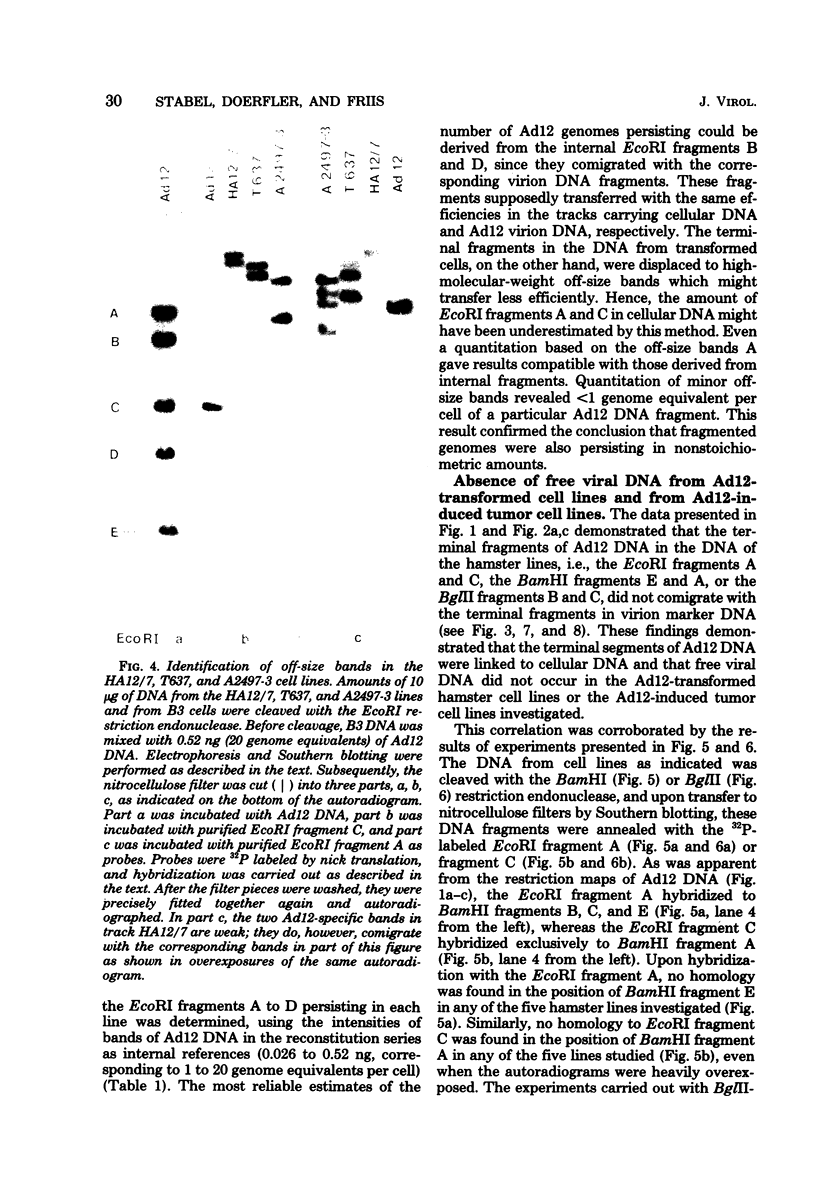
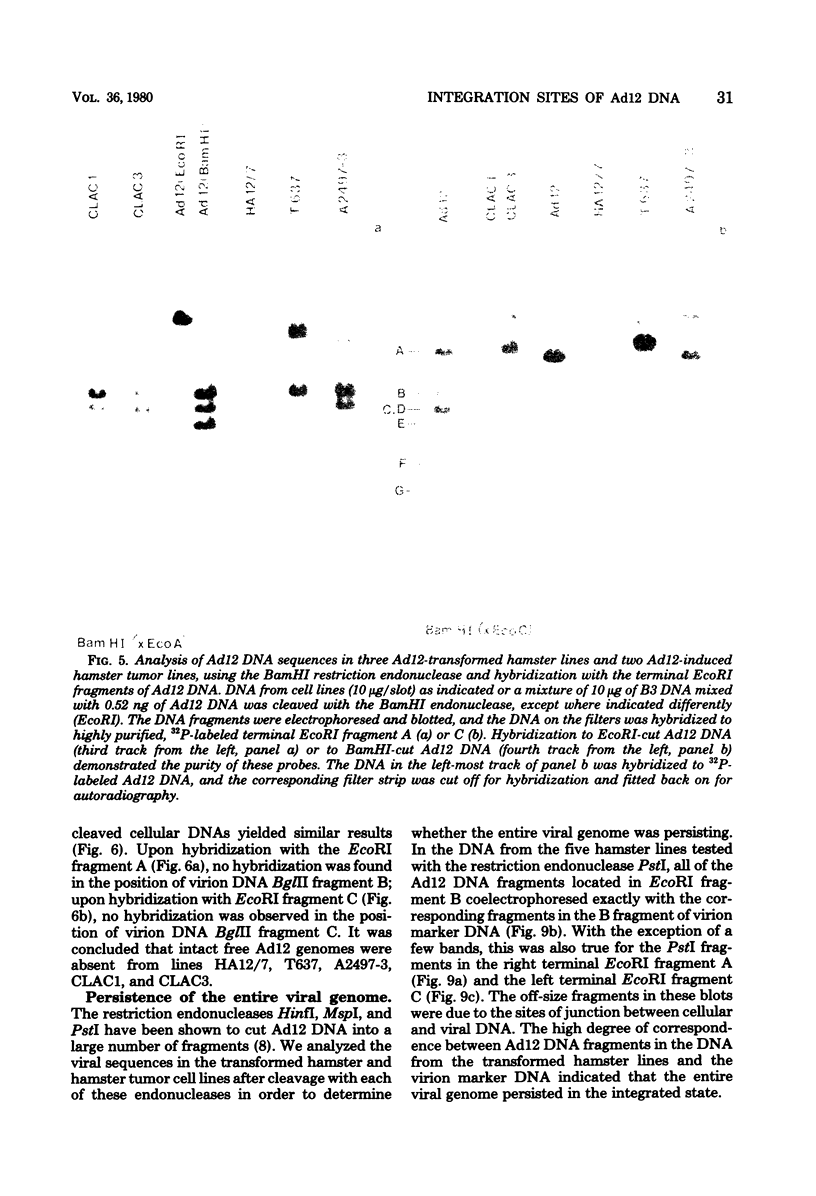
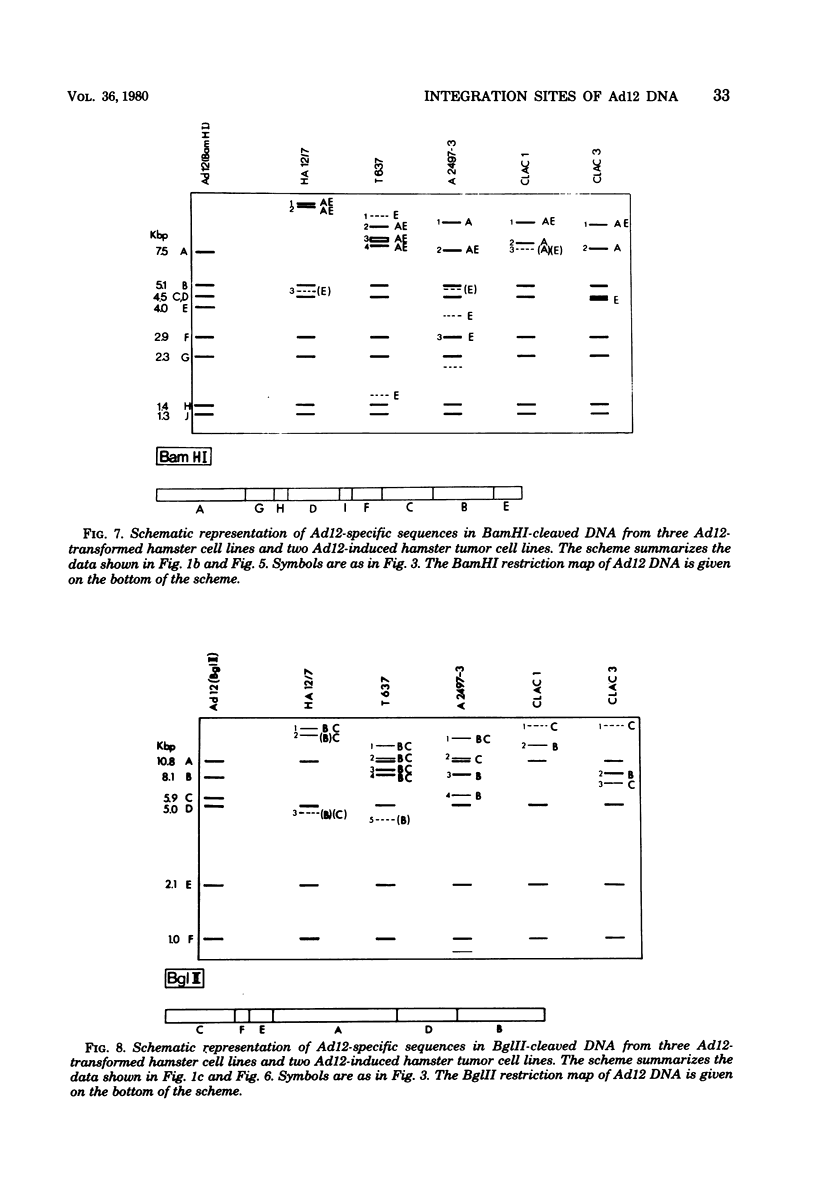
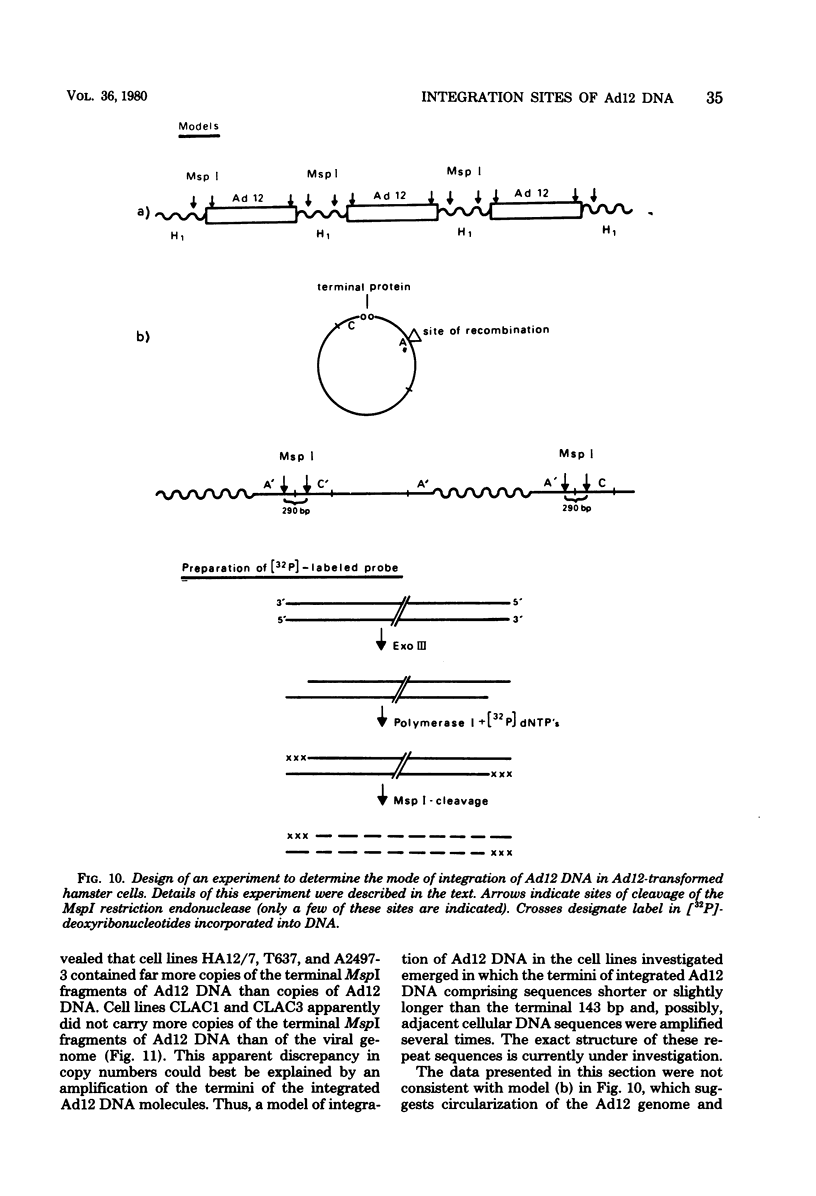
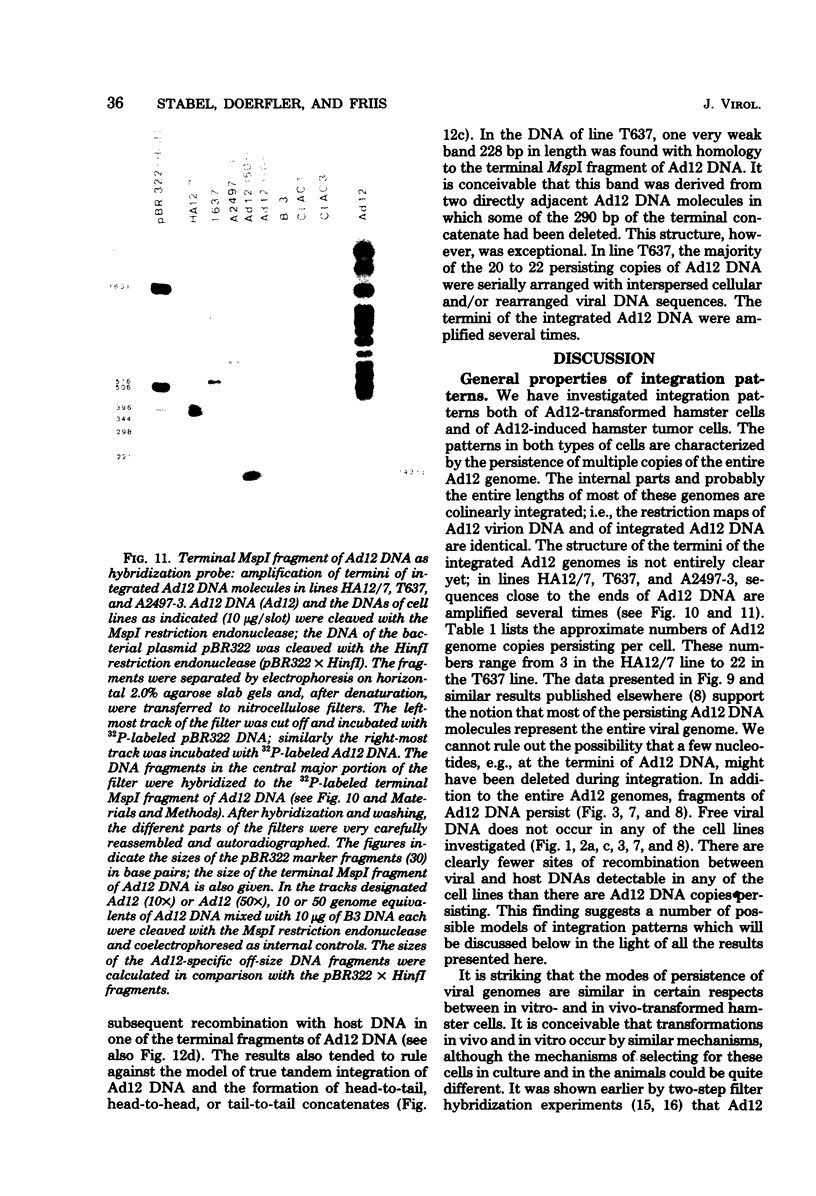
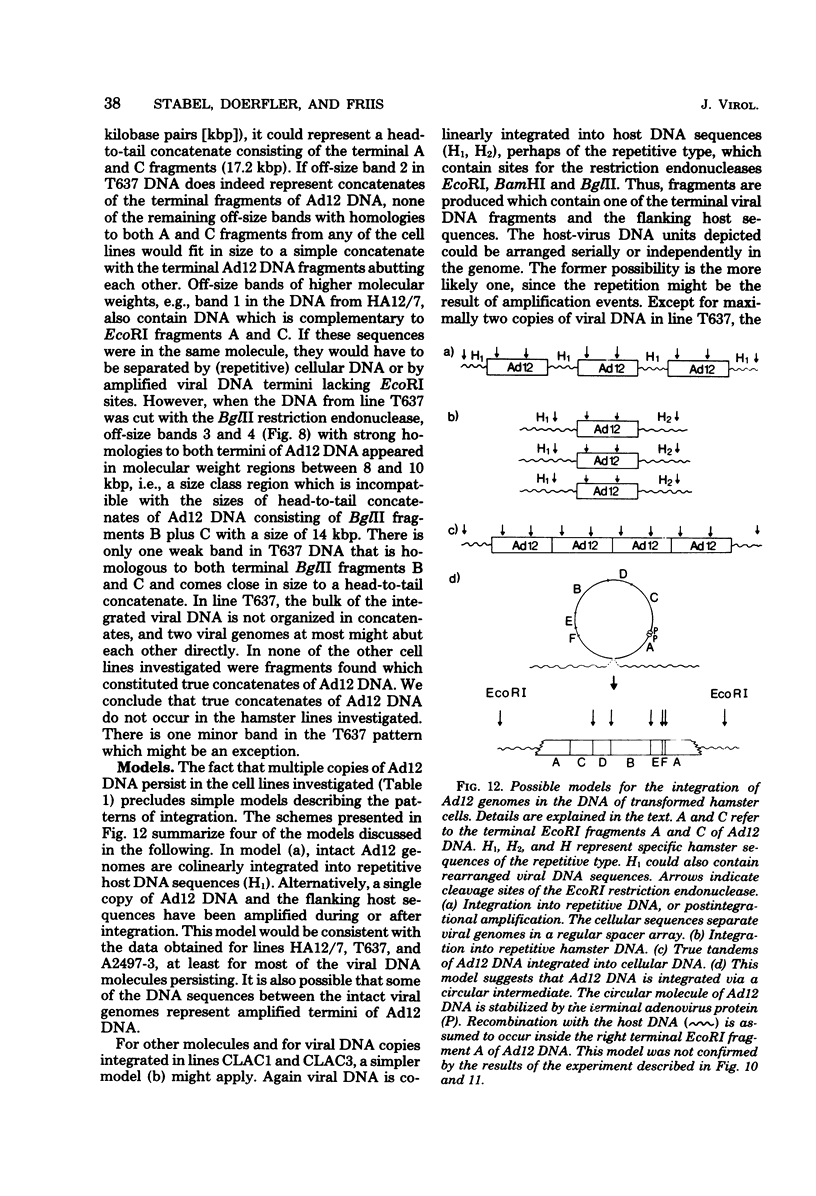
The patterns and sites of integration of adenovirus type 12 (Ad12) DNA were determined in three lines of Ad12-transformed hamster cells and in two lines of Ad12-induced hamster tumor cells. The results of a detailed analysis can be summarized as follows. (i) All cell lines investigated contained multiple copies (3 to 22 genome equivalents per cell in different lines) of the entire Ad12 genome. In addition, fragments of Ad12 DNA also persisted separately in non-stoichiometric amounts. (ii) All Ad12 DNA copies were integrated into cellular DNA. Free viral DNA molecules did not occur. The terminal regions of Ad12 DNA were linked to cellular DNA. The internal parts of the integrated viral genomes, and perhaps the entire viral genome, remained colinear with virion DNA. (iii) Except for line HA12/7, there were fewer sites of integration than Ad12 DNA molecules persisting. This finding suggested either that viral DNA was integrated at identical sites in repetitive DNA or, more likely, that one or a few viral DNA molecules were amplified upon integration together with the adjacent cellular DNA sequences, leading to a serial arrangement of viral DNA molecules separated by cellular DNA sequences. Likewise, in the Ad12-induced hamster tumor lines (CLAC1 and CLAC3), viral DNA was linked to repetitive cellular sequences. Serial arrangement of Ad12 DNA molecules in these lines was not likely. (iv) In general, true tandem integration with integrated viral DNA molecules directly abutting each other was not found. Instead, the data suggested that the integrated viral DNA molecules were separated by cellular or rearranged viral DNA sequences. (v) The results of hybridization experiments, in which a highly specific probe (143-base pair DNA fragment) derived from the termini of Ad12 DNA was used, were not consistent with models of integration involving true tandem integration of Ad12 DNA or covalent circularization of Ad12 DNA before insertion into the cellular genome. (vi) Evidence was presented that a small segment at the termini of the integrated Ad12 DNA in cell lines HA12/7, T637, and A2497-3 was repeated several times. The exact structures of these repeat units remained to be determined. The occurrence of these units might reflect the mechanism of amplification of viral and cellular sequences in transformed cell lines.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Stabel S., Ibelgaufts H., Sutter D., Neumann R., Groneberg J., Scheidtmann K. H., Deuring R., Winterhoff U. Selectivity in integration sites of adenoviral DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):551–564. doi: 10.1101/sqb.1980.044.01.057. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Doerfler W. Intracellular forms of adenovirus DNA. V. Viral DNA sequences in hamster cells abortively infected and transformed with human adenovirus type 12. J Virol. 1976 Nov;20(2):373–383. doi: 10.1128/jvi.20.2.373-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Interactions of adenovirus type 2 with rat embryo cells. Permissiveness, transformation and in vitro characteristics of adenovirus transformed rat embryo cells. J Gen Virol. 1974 Nov;25(2):263–273. doi: 10.1099/0022-1317-25-2-263. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Lukanidin E., Topp W. C., Sambrook J. Transformation of rat cells by the hybrid virus Ad2(2+) HEY. J Gen Virol. 1979 Feb;42(2):339–356. doi: 10.1099/0022-1317-42-2-339. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Green M. R., Chinnadurai G., Mackey J. K., Green M. A unique pattern of integrated viral genes in hamster cells transformed by highly oncogenic human adenovirus 12. Cell. 1976 Mar;7(3):419–428. doi: 10.1016/0092-8674(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Chardonnet Y., Doerfler W. Integrated viral sequences in adenovirus type 12-transformed hamster cells. Cell. 1977 Jan;10(1):101–111. doi: 10.1016/0092-8674(77)90144-1. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Doerfler W. Revertants of adenovirus type-12-transformed hamster cells have lost part of the viral genomes. Int J Cancer. 1979 Jul 15;24(1):67–74. doi: 10.1002/ijc.2910240112. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Sutter D., Soboll H., Doerfler W. Morphological revertants of adenovirus type 12-transformed hamster cells. J Gen Virol. 1978 Sep;40(3):635–645. doi: 10.1099/0022-1317-40-3-635. [DOI] [PubMed] [Google Scholar]
- Ibelgaufts H., Doerfler W., Scheidtmann K. H., Wechsler W. Adenovirus type 12-induced rat tumor cells of neuroepithelial origin: persistence and expression of the viral genome. J Virol. 1980 Jan;33(1):423–437. doi: 10.1128/jvi.33.1.423-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Mak S. Adenovirus type 12 DNA sequences in primary hamster tumors. J Virol. 1977 Oct;24(1):408–411. doi: 10.1128/jvi.24.1.408-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey J. K., Wold W. S., Rigden P., Green M. Transforming region of group A, B, and C adenoviruses: DNA homology studies with twenty-nine human adenovirus serotypes. J Virol. 1979 Mar;29(3):1056–1064. doi: 10.1128/jvi.29.3.1056-1064.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Shiroki K., Shimojo H., Ojima S., Fujinaga K. Transformation of a rat cell line by an adenovirus 7 DNA fragment. Virology. 1978 Jul 1;88(1):1–7. doi: 10.1016/0042-6822(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Handa H., Shimojo H., Yano S., Ojima S., Fujinaga K. Establishment and characterization of rat cell lines transformed by restriction endonuclease fragments of adenovirus 12 DNA. Virology. 1977 Oct 15;82(2):462–471. doi: 10.1016/0042-6822(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated viral DNA sequences in hamster cells transformed by adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):565–568. doi: 10.1101/sqb.1980.044.01.058. [DOI] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Tiemer D., Enquist L., Leder P. Improved derivative of a phage lambda EK2 vector for cloning recombinant DNA. Nature. 1976 Oct 7;263(5577):526–527. doi: 10.1038/263526a0. [DOI] [PubMed] [Google Scholar]
- Tjia S. T., Carstens E. B., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus II. The viral DNA and the kinetics of its replication. Virology. 1979 Dec;99(2):399–409. doi: 10.1016/0042-6822(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb A. J., van Ormondt H., Schrier P. I., Lupker J. H., Jochemsen H., van den Elsen P. J., DeLeys R. J., Maat J., van Beveren C. P., Dijkema R. Structure and function of the transforming genes of human adenoviruses and SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):383–399. doi: 10.1101/sqb.1980.044.01.043. [DOI] [PubMed] [Google Scholar]