Abstract
Francisella tularensis is a Gram-negative bacterium and the causative agent of the disease tularemia. Escape of F. tularensis from the phagosome into the cytosol of the macrophage triggers the activation of the AIM2 inflammasome through a mechanism that is not well understood. Activation of the AIM2 inflammasome results in autocatalytic cleavage of caspase-1, resulting in the processing and secretion of interleukin-1β (IL-1β) and IL-18, which play a crucial role in innate immune responses to F. tularensis. We have identified the 5-formyltetrahydrofolate cycloligase gene (FTL_0724) as being important for F. tularensis live vaccine strain (LVS) virulence. Infection of mice in vivo with a F. tularensis LVS FTL_0724 mutant resulted in diminished mortality compared to infection of mice with wild-type LVS. The FTL_0724 mutant also induced increased inflammasome-dependent IL-1β and IL-18 secretion and cytotoxicity in macrophages in vitro. In contrast, infection of macrophages with a F. tularensis LVS rluD pseudouridine synthase (FTL_0699) mutant resulted in diminished IL-1β and IL-18 secretion from macrophages in vitro compared to infection of macrophages with wild-type LVS. In addition, the FTL_0699 mutant was not attenuated in vivo. These findings further illustrate that F. tularensis LVS possesses numerous genes that influence its ability to activate the inflammasome, which is a key host strategy to control infection with this pathogen in vivo.
INTRODUCTION
Francisella tularensis is a virulent Gram-negative bacterium that infects phagocytic cells of the innate immune system (1). Both F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B) can cause disease in healthy humans (1). Cutaneous infection with F. tularensis results in an ulceroglandular form of tularemia following the bite of a vector, such as a tick or deer fly, carrying the bacteria or from direct contact with blood of an infected animal through an abrasion in the skin (1, 2). A pneumonic form of tularemia can be acquired through inhalation of as few as 10 type A organisms and is associated with high mortality if untreated (1, 3). The F. tularensis subsp. holarctica live vaccine strain (LVS) is attenuated in humans but still causes fatal disease in mice and retains many features of virulent type A and type B organisms in vitro (4); for this reason, F. tularensis LVS is widely studied as a model of the virulent type A and type B strains.
F. tularensis is recognized by pattern recognition receptors (PRRs), including Toll-like receptor 2 (TLR2) and the PYHIN family member absent in melanoma 2 (AIM2), both of which are critical for defense against infection (5–9). Sensing of F. tularensis by TLR2 at the macrophage surface leads to the elaboration of a number of proinflammatory cytokines (7). Following phagocytosis by the macrophage, F. tularensis escapes the phagosome into the cytosol of the cell, where it replicates (10, 11). Additionally, phagosomal escape of F. tularensis triggers the activation of the AIM2 inflammasome through the recognition of cytosolic bacterial double-stranded DNA (dsDNA) (6–9). AIM2 inflammasome activation in turn results in the processing and secretion of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 as well as the induction of a pyroptotic death pathway in the infected macrophage (6–9).
Evasion or modulation of inflammasome activation is a survival strategy that is employed by a number of bacteria (12–16). We, and others, have previously described F. tularensis mutants that were capable of inducing increased activation of the AIM2 inflammasome, leading to enhanced production of IL-1β and IL-18 as well as increased macrophage cell death (12, 13, 16–18). In this report, we identify two novel F. tularensis LVS mutants that are capable of modulating inflammasome activation. Mutation of the F. tularensis LVS FTL_0724 gene, which is annotated to encode 5-formyltetrahydrofolate cycloligase, results in enhanced inflammasome activation and cytotoxicity upon infection of macrophages. The F. tularensis LVS FTL_0724 mutant is also markedly attenuated in vivo. In contrast, a mutation in the FTL_0699 gene, which is annotated to encode the ribosomal large subunit pseudouridine synthase D (RluD), inhibits macrophage inflammasome activation without affecting bacterial virulence in vivo.
MATERIALS AND METHODS
Bacterial strains, plasmid construction, and growth conditions.
F. tularensis subsp. holarctica LVS was obtained from ATCC (ATCC 29684). LVS containing a chromosomal insertion mutation in FTL_0724 and FTL_0699 was obtained using Tn5 delivery plasmid pBB109 as previously described (19). To confirm that the Tn5 insertion was affecting FTL_0724 and FTL_0699 expression, genomic DNA sequencing was performed as previously described (19). Complementation of the FTL_0724::Tn5 and FTL_0699::Tn5 mutant strains with wild-type FTL_0699 and FTL_0724 or catalytically inactive FTL_0724 R127A containing the ribosomal binding site was performed as previously described (20). Briefly, the wild-type gene and the ribosomal binding site were amplified from LVS genomic DNA by PCR and ligated into the pBB103 plasmid (20). The plasmid containing the wild-type gene was incorporated into the bacteria via cryotransformation (19). The R127A mutation in FTL_0724 was prepared by using a QuikChange II site-directed mutagenisis kit (Agilent Technologies). LVSs were grown on Difco cysteine heart agar supplemented with 9% sheep red blood cells (SRBC) for 48 h at 37°C; 25 μg/ml spectinomycin was added to plates for growth of the FTL_0699, FTL_0724, and FTL_0724 R127A-complemented strains. For in vivo studies, bacteria were grown overnight in modified Mueller-Hinton (MMH) broth (Becton, Dickinson) supplemented with 1% (wt/vol) glucose, 0.025% ferric pyrophosphate, and 2% IsoVitaleX. For in vitro growth curve experiments, bacteria were grown over a time course in MMH or Chamberlain's defined media (CDM) (21).
Mice and in vivo F. tularensis LVS infections.
The generation of ASC-, caspase-1-, NLRP3-, NLRC4-, AIM2-, IL-1RI-, and IL-18-deficient mice has been described previously (9, 22–26). Age- and sex-matched C57BL/6 mice purchased from NCI were used as wild-type controls. For in vivo infections, 6-to-8-week-old mice were injected intraperitoneally (i.p.) with the indicated dose of F. tularensis LVS or the FTL_0724::Tn5 and FTL_0699::Tn5 mutants. Mice were monitored every 12 h for lethality; mice found to be in a moribund state for more than 4 h were considered terminal and euthanized. Bacterial burdens in the spleen and liver of infected mice were determined at day 3 postinfection by dilution plating of tissue homogenates onto Difco cysteine heart agar supplemented with 9% SRBC. The University of Iowa Institutional Animal Care and Use Committee approved all protocols used in this study.
Macrophage infections.
Bone marrow-derived macrophages (BMMϕ) were generated as previously described (27). Unless otherwise indicated, BMMϕ were primed with 50 ng/ml lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 (Invivogen) for 4 h prior to infection. BMMϕ were infected with F. tularensis LVS, the FTL_0724::Tn5 mutant, and the FTL_0699::Tn5 mutant at a multiplicity of infection (MOI) of 50:1. At 9 h postinfection, or at the indicated time, supernatants were collected and assayed for IL-1β, IL-18, IL-12 p40, and keratinocyte-derived cytokine (KC) by enzyme-linked immunosorbent assay (ELISA). Antibody pairs for the IL-1β and IL-18 ELISAs were from R&D Systems and MBL, respectively. Antibody pairs for the IL-12 p40 and KC ELISAs were from eBiosciences. BMMϕ cytotoxicity was determined by measuring lactate dehydrogenase (LDH) release using a cytotoxicity detection kit (Promega). Immunoblotting for caspase-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were performed as previously described (28).
Macrophage challenge with bacterial lysates and DNA.
For challenge with crude cell lysates, LVS, FTL_0699::Tn5, or FTL_0724::Tn5 bacteria were sonicated and filtered through a 0.22-μm-pore-size cellulose acetate filter. Protein levels were determined by a bicinchoninic acid (BCA) protein assay. LPS-primed BMMϕ were transfected with 1 μg of protein using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Supernatants were collected 12 h posttransfection. To challenge cells with DNA from bacteria, DNA was first isolated using a Qiagen DNeasy blood and tissue kit according to the manufacturer's protocol; 750 ng was then transfected into cells using Lipofectamine 2000 according to the manufacturer's protocol. Supernatants were harvested 6 h posttransfection.
Statistical analysis.
All results were analyzed using a two-tailed unpaired Student's t test, with the exception of bacterial burdens, which were analyzed by a two-tailed Mann-Whitney U test using Prism software. Values of P < 0.05 were considered statistically significant.
RESULTS
Mutations in F. tularensis LVS FTL_0699 and FTL_0724 modulate Francisella-induced IL-1β and IL-18 secretion and cytotoxicity.
We, and others, have previously identified Francisella mutants, in both F. tularensis LVS and F. novicida, that induce enhanced AIM2 inflammasome activation (12, 13, 16, 17). To identify the F. tularensis genes involved in AIM2 inflammasome activation, we screened a F. tularensis LVS transposon library for mutants that either enhanced or diminished IL-1β secretion from LPS-primed BMMϕ. We focused on two mutants (FTL_0699 and FTL_0724) that had not previously been characterized as influencing inflammasome activation and that had no known defects in replication or survival.
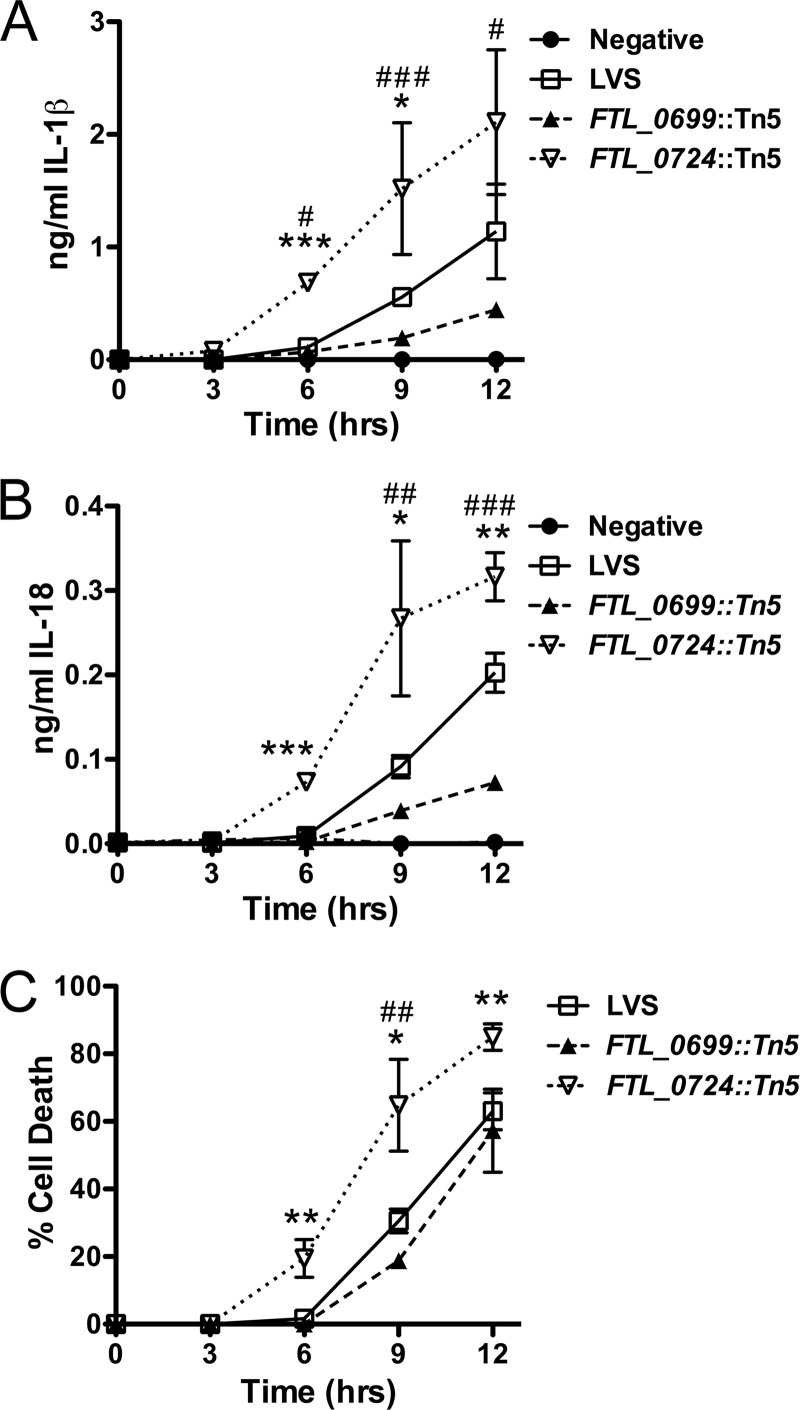
FTL_0724 is annotated to encode a highly conserved enzyme, 5-formyltetrahydrofolate cycloligase (also known as 5,10-methenyltetrahydrofolate synthetase), involved in folate metabolism (29). Infection of LPS-primed BMMϕ with the F. tularensis LVS FTL_0724::Tn5 mutant resulted in increased secretion of both IL-1β and IL-18 in comparison to LPS-primed BMMϕ infected with wild-type LVS (Fig. 1A and B). In contrast, infection of LPS-primed BMMϕ with the F. tularensis LVS FTL_0699::Tn5 mutant resulted in diminished secretion of both IL-1β and IL-18 compared to infection with wild-type LVS (Fig. 1A and B). FTL_0699 is annotated to encode the ribosomal large subunit pseudouridine synthase D (RluD). Consistent with the enhanced and diminished secretion of IL-1β and IL-18 induced by the FTL_0724::Tn5 and FTL_0699::Tn5 mutants, respectively, infection of LPS-primed BMMϕ with the FTL_0724::Tn5 mutant resulted in increased cytotoxicity, whereas infection with the FTL_0699::Tn5 mutant caused a minimal but consistent decrease in cytotoxicity (Fig. 1C).
Fig 1.
F. tularensis LVS FTL_0699::Tn5 and FTL_0724::Tn5 transposon mutants modulated Francisella-induced IL-1β and IL-18 secretion and cytotoxicity. (A to C) LPS-primed BMMϕ were infected with wild-type F. tularensis LVS or the indicated transposon mutant at an MOI of 50:1. Supernatants and cell lysates were collected at the indicated times postchallenge. Secretion of IL-1β (A) and IL-18 (B) into the supernatants was assessed by ELISA. (C) Cytotoxicity was assessed by measuring LDH release into the supernatant and expressed as a percentage of LDH release by Triton X-100 detergent. Determinations were performed in triplicate, and data are expressed as means ± standard deviations (SD). Data are representative of the results of three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (for FTL_0724::Tn5 compared to LVS); #, P ≤ 0.05; ##, P ≤ 0.01; ###, P ≤ 0.001 (for FTL_0699::Tn5 compared to LVS).
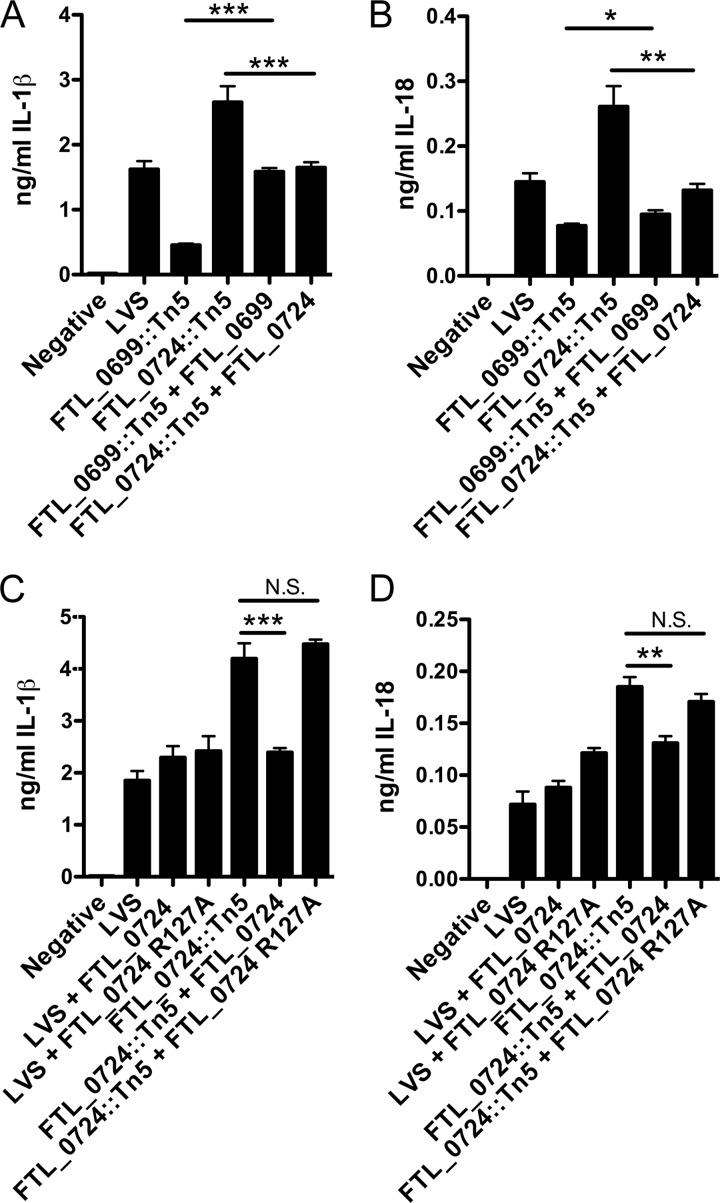
Complementation of both the FTL_0699::Tn5 mutant and the FTL_0724::Tn5 mutant with a wild-type copy of FTL_0699 and FTL_0724, respectively, restored their induction of IL-1β and IL-18 production from infected macrophages to levels close to that of wild-type LVS (Fig. 2A and B). The function of 5-formyltetrahydrofolate cycloligase has previously been examined in Mycoplasma pneumoniae, and a conserved catalytic residue (R115) necessary for enzymatic function was identified (30). Hancock et al. also demonstrated that mutation of R115 to an alanine rendered the resulting protein catalytically inactive (30). The R115 catalytic residue in M. pneumonia is conserved in F. tularensis LVS (see Fig. S1 in the supplemental material). To determine if catalytic activity of 5-formyltetrahydrofolate cycloligase was required for limiting F. tularensis LVS-induced IL-1β and IL-18 secretion, we mutated the corresponding arginine in FTL_0724 to an alanine (R127A). Whereas complementation of the FTL_0724::Tn5 mutant with a plasmid carrying the wild-type FTL_0724 gene abrogated the enhanced IL-1β and IL-18 secretion induced by the FTL_0724::Tn5 mutant, complementation with FTL_0724 R127A failed to restore the ability of the FTL_0724::Tn5 mutant to elicit normal levels of IL-1β or IL-18 relative to wild-type LVS (Fig. 2C and D).
Fig 2.
Complementation of FTL_0699::Tn5 and FTL_0724::Tn5 mutants with FTL_0699 and FTL_0724, respectively, restored normal induction of IL-1β and IL-18 secretion. (A and B) LPS-primed BMMϕ were challenged at an MOI of 50:1 with wild-type LVS, the FTL_0699::Tn5 mutant, the FTL_0724::Tn5 mutant, or the complemented mutant bacterium (FTL_0699::Tn5 + FTL_0699, FTL_0724::Tn5 + FTL_0724). (C and D) LPS-primed BMMϕ were challenged at an MOI of 50:1 with wild-type LVS, the FTL_0724::Tn5 mutant, or bacteria complemented with either FTL_0724 or catalytically inactive FTL_0724 R127A. IL-1β and IL-18 release into the supernatant 9 h postinfection was determined by ELISA. Determinations were performed in triplicate, and the results are expressed as means ± SD. Data are representative of the results of three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; N.S., nonsignificant.
Mutations in F. tularensis LVS FTL_0699 and FTL_0724 modulate Francisella-induced caspase-1 activation.
The processing and secretion of IL-1β and IL-18 as well as pyroptotic cell death are dependent on the activation of the cysteine protease caspase-1. Activation of caspase-1 results in the autocatalytic processing of the 45-kDa pro-caspase-1 to generate two subunits, p20 and p10. This is a two-step process that requires both priming and activation steps. Priming with an agent such as LPS results in the generation of pro-IL-1β as well as in readying the inflammasome for subsequent activation through an as-yet-unidentified mechanism (31, 32). The phagosomal escape of F. tularensis into the macrophage cytoplasm, with the concurrent release of bacterial DNA into the cytosol, is thought to act as the second signal for the AIM2 inflammasome, leading to activation of caspase-1 (6, 8, 9).
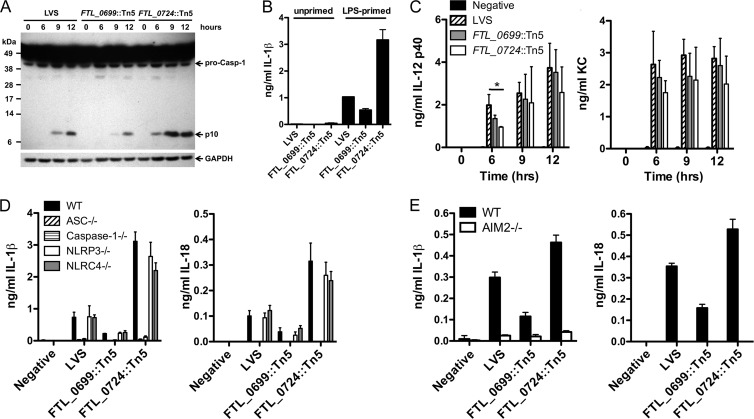
LPS-primed BMMϕ infected with wild-type LVS activated caspase-1 by 9 h postinfection, as judged by immunoblot detection of the p10 cleavage product (Fig. 3A). In contrast, those infected with the FTL_0724::Tn5 mutant induced caspase-1 activation more rapidly (by 6 h postinfection) and to a greater extent than those infected with the wild-type LVS (Fig. 3A). Consistent with the diminished IL-1β and IL-18 secretion, the FTL_0699::Tn5 mutant induced diminished activation of caspase-1 compared to wild-type LVS (Fig. 3A).
Fig 3.
F. tularensis LVS FTL_0699::Tn5 and FTL_0724::Tn5 transposon mutants modulated Francisella-induced caspase-1 activation. (A) Lysates from LPS-primed BMMϕ infected with LVS, the FTL_0699::Tn5 mutant, or the FTL_0724::Tn5 mutant (50:1 MOI) for the indicated times were immunoblotted with antibodies against the p10 subunit of caspase-1 or GAPDH. Data are representative of the results of two separate experiments. (B) Unprimed or LPS-primed BMMϕ were infected as described for panel A. IL-1β and IL-18 release into the supernatant 12 h postinfection was determined by ELISA. (C) Unprimed BMMϕ were infected as described for panel A; supernatants were collected at the indicated times postinfection, and IL-12 p40 and KC levels were assessed by ELISA. (D and E) LPS-primed BMMϕ from wild-type (WT), ASC−/−, caspase-1−/−, NLRP3−/−, NLRC4−/−, and AIM2−/− mice were infected as described for panel A. IL-1β and IL-18 release into the supernatant 9 h postinfection was determined by ELISA. (B, D, and E) Determinations were performed in triplicate, and the results are expressed as means ± SD. Data are representative of the results of two (E) or three (B and D) independent experiments. (C) Data are expressed as means ± standard errors of the means (SEM) of the results of three independent experiments. *, P ≤ 0.05.
Enhanced IL-1β secretion induced by the FTL_0724::Tn5 mutant was not secondary to it bypassing the need for a priming step, as neither wild-type LVS nor the FTL_0724::Tn5 mutant was capable of inducing IL-1β secretion from unprimed macrophages (Fig. 3B). In contrast to the changes in IL-1β and IL-18 release observed following infection of LPS-primed macrophages with either the FTL_0724::Tn5 mutant or the FTL_0699::Tn5 mutant, the secretion of IL-12 p40 and KC by unprimed BMMϕ infected with F. tularensis LVS was largely unaffected by the absence of FTL_0724 or FTL_0699 (Fig. 3C). Infection of BMMϕ with the FTL_0724::Tn5 mutant induced less IL-12 p40 production at 6 h postinfection than infection with the wild-type LVS; however, this reduction in cytokine production was not observed at later time points (Fig. 3C). Taken together, these data suggest that F. tularensis LVS expression of FTL_0724 limits caspase-1 activation and the subsequent cytotoxicity and secretion of IL-1β and IL-18. In contrast, expression of FTL_0699 by F. tularensis LVS is required for its efficient caspase-1 activation in LPS-primed macrophages.
In addition to AIM2, the NLR family members NLRP3 and NLRC4 can form functional inflammasomes in response to specific NLRP3 and NLRC4 agonists (33). To determine if NLRP3 or NLRC4 contributed to IL-1β and IL-18 secretion induced by the FTL_0724::Tn5 or FTL_0699::Tn5 mutant, LPS-primed BMMϕ from wild-type, caspase-1−/−, ASC−/−, NLRP3−/−, and NLRC4−/− mice were infected with LVS, the FTL_0724::Tn5 mutant, or the FTL_0699::Tn5 mutant (Fig. 3D). Secretion of IL-1β and IL-18 in response to infection with wild-type LVS, the FTL_0724::Tn5 mutant, and the FTL_0699::Tn5 mutant was dependent on the presence of caspase-1 and ASC but independent of the presence of NLRP3 and NLRC4 (Fig. 3D). To directly test if AIM2 contributed to IL-1β and IL-18 secretion during challenge with FTL_0724::Tn5 or the FTL_0699::Tn5 mutants, LPS-primed BMMϕ from AIM2−/− and wild-type mice were challenged with LVS, the FTL_0724::Tn5 mutant, or the FTL_0699::Tn5 mutant. Secretion of both IL-1β and IL-18 by macrophages challenged with LVS and the FTL_0724::Tn5 and FTL_0699::Tn5 mutants was dependent on the presence of AIM2 (Fig. 3E). These data suggest that the augmented caspase-1 activation induced by the FTL_0724::Tn5 mutant did not reflect activation of a different inflammasome pathway, such as NLRP3 or NLRC4, but was dependent on AIM2 inflammasome activation.
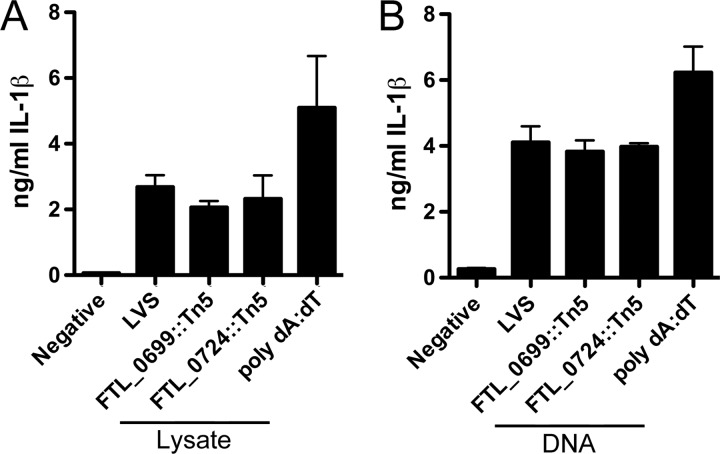
DNA from wild-type LVS and the FTL_0699::Tn5 and FTL_0724::Tn5 mutants induce comparable levels of IL-1β secretion.
Activation of the AIM2 inflammasome occurs in response to recognition of cytosolic dsDNA (34–36). Given that both 5-formyltetrahydrofolate cycloligase and the RluD pseudouridine synthase, annotated to be encoded by FTL_0724 and FTL_0699, respectively, indirectly play roles in nucleotide synthesis and modification, we investigated whether an intrinsic modification of bacterial components was responsible for the alteration in IL-1β secretion induced by the FTL_0724::Tn5 and FTL_0699::Tn5 mutants (29, 37, 38). LPS-primed BMMϕ were transfected with equal amounts of bacterial crude lysates of wild-type LVS and the FTL_0724::Tn5 and FTL_0699::Tn5 mutants (Fig. 4A). As expected, transfection with the synthetic dsDNA analogue poly(dA-dT) induced the secretion of IL-1β; however, no significant differences in IL-1β secretion between BMMϕ transfected with crude lysates of either wild-type LVS or the mutant were observed (Fig. 4A). Similarly, transfection of purified DNA from wild-type LVS or mutant bacteria resulted in comparable levels of IL-1β secretion (Fig. 4B). Taken together, these data suggest that modification of bacterial components or DNA in the FTL_0724::Tn5 and FTL_0699::Tn5 mutants is not responsible for the observed alterations in macrophage IL-1β secretion.
Fig 4.
Intrinsic modification of a bacterial component was not responsible for the altered IL-1β secretion induced by the FTL_0699::Tn5 and FTL_0724::Tn5 mutants. LPS-primed BMMϕ were challenged with equivalent amounts of crude bacterial lysate (1 μg) (A) or purified bacterial DNA (750 ng) (B) from LVS, the FTL_0699::Tn5 mutant, or the FTL_0724::Tn5 mutant or the AIM2 agonist poly(dA-dT) in the presence of the Lipofectamine transfection agent. At 12 (A) or 6 (B) h postchallenge, IL-1β release into the supernatant was determined by ELISA. Determinations were performed in triplicate, and the results are expressed as means ± SD. Data are representative of the results of three independent experiments.
FTL_0724::Tn5 and FTL_0699::Tn5 mutants have decreased growth rates compared to wild-type LVS in vitro.
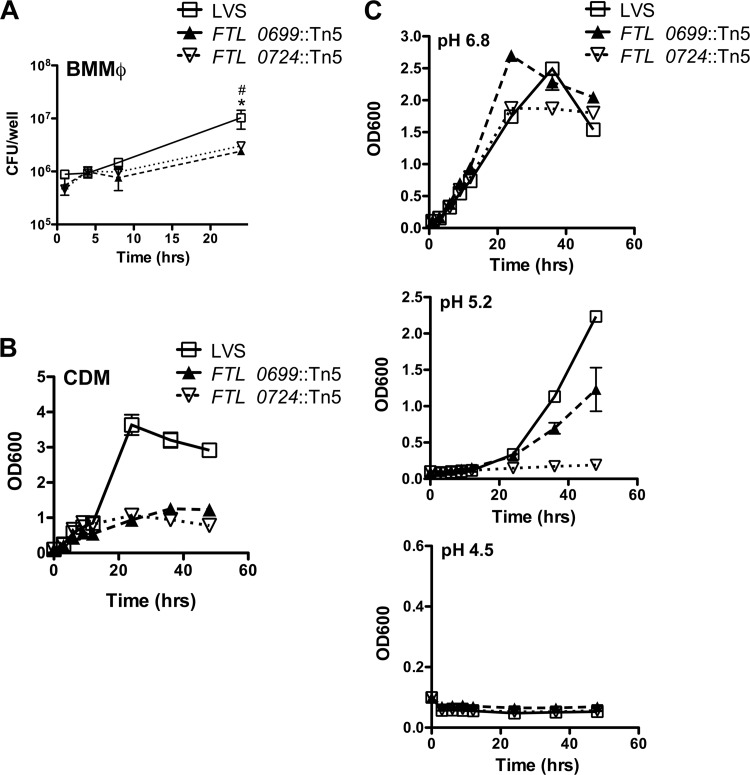
In order to assess whether the observed differences in the ability of the FTL_0724::Tn5 and FTL_0699::Tn5 mutants to activate the inflammasome were due to different growth characteristics in vitro, we assessed the growth of the mutants and wild-type LVS within primed BMMϕ as well as growth in broth culture. The FTL_0724::Tn5 and FTL_0699::Tn5 mutants displayed reduced intracellular replication within LPS-primed BMMϕ (Fig. 5A). Consistent with this, both mutants exhibited decreased growth in Chamberlain's defined media (CDM) (Fig. 5B). To determine if the FTL_0724::Tn5 and FTL_0699::Tn5 mutants were more susceptible than LVS to various stressors, the bacteria were grown in modified Mueller-Hinton (MMH) broth at acidic pH. At pH 6.8, FTL_0724::Tn5 and FTL_0699::Tn5 mutants grew with growth kinetics similar to those of LVS (Fig. 5C). However, at pH 5.2, the FTL_0699::Tn5 mutant had a decreased growth rate compared to LVS and the FTL_0724::Tn5 mutant had profoundly impaired growth (Fig. 5C). At pH 4.5, none of the bacterial strains were able to grow (Fig. 5C). To test if FTL_0724::Tn5 and FTL_0699::Tn5 mutants were more susceptible than LVS to osmotic stress, the bacterial strains were grown in MMH broth supplemented with 3% or 4% NaCl. Although the bacterial strains grew more slowly than when no additional salt was provided, there were no differences between LVS and the two mutants in growth (data not shown). These data suggest that under optimal conditions, the FTL_0724::Tn5 and FTL_0699::Tn5 mutants grow with kinetics similar to those of wild-type LVS; however, when nutrients are limited or under pH stress conditions, the FTL_0724::Tn5 and FTL_0699::Tn5 mutants exhibit decreased growth compared to wild-type LVS.
Fig 5.
Growth characteristics of the FTL_0699::Tn5 and FTL_0724::Tn5 mutants. (A) LPS-primed BMMϕ were infected with LVS, the FTL_0699::Tn5 mutant, or the FTL_0724::Tn5 mutant (50:1 MOI); 1 h later, BMMϕ monolayers were washed to remove extracellular bacteria. At the indicated times postinfection, BMMϕ were lysed with 0.5% saponin and plated on bacterial media to determine numbers of CFU/well. (B and C) F. tularensis LVS and the FTL_0699::Tn5 and FTL_0724::Tn5 mutants were grown in Chamberlain's defined media (CDM; pH 6.8) (B) or modified Mueller-Hinton (MMH) broth at pH 4.5, 5.2, or 6.8 (C) at 37°C; at the indicated times, growth was determined by measuring absorbance at 600 nm. Data are representative of the results of three (A) or two (B and C) independent experiments. Determinations were performed in triplicate, and the results are expressed as means ± SD. *, P ≤ 0.05 for FTL_0724::Tn5 compared to LVS; #, P ≤ 0.05 for FTL_0699::Tn5 compared to LVS.
The FTL_0724::Tn5 mutant is attenuated in vivo, whereas the FTL_0699::Tn5 mutant retains its virulence.
To determine if FTL_0724 and FTL_0699 were important for F. tularensis LVS virulence in vivo, wild-type mice were challenged i.p. with either LVS or the FTL_0724::Tn5 or FTL_0699::Tn5 mutant and monitored for survival. Infection with 3 × 104 CFU of LVS resulted in 88% mortality by day 10 postinfection (Fig. 6A). In contrast, all mice infected with 3 × 104 CFU of the FTL_0724::Tn5 mutant survived through day 14 postinfection (Fig. 6A). Infection with 3 × 104 CFU of the FTL_0699::Tn5 mutant, similar to infection with wild-type LVS, resulted in 70% mortality by day 5 (Fig. 6A). The increased survival of mice infected with the FTL_0724::Tn5 mutant relative to those infected with wild-type LVS was also reflected in significantly lower bacterial burdens in infected organs 3 days postinfection (P < 0.01; Fig. 6B). Collectively, these data demonstrate that the ability of F. tularensis LVS to limit inflammasome activation is critical for its virulence in vivo. In contrast, the FTL_0699::Tn5 mutant, which is inefficient in its ability to induce inflammasome activation, retained full virulence in vivo.
Fig 6.
The FTL_0724::Tn5 mutant was attenuated in vivo, whereas the FTL_0699::Tn5 mutant remained virulent. (A and B) WT C57BL/6 mice were infected i.p. with 3 × 104 CFU of the indicated strain of bacteria. (A) Mice were monitored for survival (n ≥ 10 mice per group). Results were pooled from two independent experiments. (B) At 3 days postinfection, bacterial burdens in liver and spleen were assessed (n = 5 mice per group). **, P ≤ 0.01; N.S., nonsignificant. (C and D) WT, ASC−/−, or caspase-1−/− mice (n ≥ 10 mice per group) were infected i.p. with either 3 × 104 CFU or 5 × 105 CFU of LVS or the FTL_0724::Tn5 mutant and monitored for survival. Results were pooled from two independent experiments. (E and F) IL-1RI−/− or IL-18−/− mice were infected i.p. with 5 × 105 CFU of LVS or the FTL_0724::Tn5 mutant and monitored for survival (n = 6 to 8 mice per group).
To further evaluate if enhanced inflammasome activation was responsible for the attenuation of the FTL_0724::Tn5 mutant in vivo, wild-type, ASC−/−, and caspase-1−/− mice were challenged i.p. with either LVS or the FTL_0724::Tn5 mutant and survival was monitored. ASC−/− and caspase-1−/− mice infected with 3 × 104 CFU of the FTL_0724::Tn5 mutant had increased mortality (30% and 60% mortality at day 14 postinfection, respectively) compared to wild-type mice (0% and 10% mortality at day 14 postinfection, respectively; Fig. 6C and D). Infection with 5 × 105 CFU of the FTL_0724::Tn5 mutant increased mortality in the ASC−/− and caspase-1−/− mice to 60% and 70%, respectively, compared to that of wild-type mice (0% and 10% mortality at day 14 postinfection, respectively; Fig. 6C and D).
Inflammasome activation leads to processing and secretion of IL-1β and IL-18. To determine if cytokines IL-1β and IL-18 were important for survival following challenge with the FTL_0724::Tn5 mutant, we infected IL-1RI−/− and IL-18−/− mice with 5 × 105 CFU of LVS or the FTL_0724::Tn5 mutant. IL-1RI−/− and IL-18−/− mice infected with either LVS or the FTL_0724::Tn5 mutant succumbed to disease by day 7 postinfection, showing the importance of IL-1RI and IL-18 for protection against challenge with the FTL_0724::Tn5 mutant (Fig. 6E and F). Together, these data suggest that the attenuation of the FTL_0724::Tn5 mutant in vivo was due at least in part to increased inflammasome activation. However, the partial resistance of ASC−/− and caspase-1−/− mice to low-dose infection with the FTL_0724::Tn5 mutant suggests that additional factors may contribute to its attenuation in vivo.
DISCUSSION
Activation of the AIM2 inflammasome in response to infection with the facultative intracellular pathogen F. tularensis is critical for host defenses. As such, mice specifically deficient in components of the AIM2 inflammasome are particularly susceptible to infection with both F. tularensis LVS and F. novicida (6, 8). In this study, we demonstrated that the F. tularensis LVS gene FTL_0724 is required for its in vivo virulence. The attenuation of infection with the FTL_0724::Tn5 mutant in vivo is in part due to the hyperactivation of the AIM2 inflammasome, as demonstrated by the susceptibility of ASC−/− and caspase-1−/− mice to infection with the FTL_0724::Tn5 mutant. In contrast, the FTL_0699::Tn5 mutant remained virulent in vivo despite having a defect in its ability in vitro to effectively activate caspase-1 in macrophages following infection. Taken together, these data highlight the critical function of caspase-1 in controlling F. tularensis LVS infection and demonstrate that mutant organisms that are unable to restrict their ability to activate this inflammatory pathway are markedly attenuated in vivo.
The AIM2 inflammasome is activated upon sensing of Francisella dsDNA within the cytosol of the macrophage (34–36, 39). Escape of F. tularensis from the phagosome into the macrophage cytosol precedes AIM2 inflammasome activation, as demonstrated by the finding that F. tularensis mutants that fail to escape the phagosome also do not induce caspase-1 activation (40–42). However, it remains unclear if bacterial damage and DNA leakage occur prior to or after phagosomal escape. Recently, we and others described a number of F. tularensis LVS and F. novicida mutants that induce increased caspase-1 activation and pyroptosis in infected macrophages (12, 13, 16–18). It appears that, rather than having direct inflammasome inhibitory properties, these genes play roles in the maintenance of bacterial membrane stability. All of the reported mutants display aberrant membrane morphologies and undergo increased lysis within the macrophage (13, 16). Consistent with these findings, we did not observe intrinsic differences in the ability of crude lysates or bacterial DNA from the FTL_0724::Tn5 mutant to induce IL-1β secretion when directly transfected into the macrophage.
As folate metabolism is important for numerous cellular functions, including the biosynthesis of nucleic acids and proteins, it is likely that mutations within the 5-formyltetrahydrofolate cycloligase (FTL_0724) gene may influence bacterial membrane stability. The FTL_0724::Tn5 mutant exhibited reduced intracellular growth in macrophages compared to wild-type LVS. This reduced intracellular growth of the FTL_0724::Tn5 mutant might have been due to either increased pyroptosis of the macrophages or increased intracellular lysis of the FTL_0724::Tn5 mutant. The FTL_0724::Tn5 mutant exhibited a similar reduction in growth in Chamberlain's defined media, suggesting decreased fitness of the FTL_0724::Tn5 mutant compared to wild-type LVS. Interestingly, the FTL_0724::Tn5 mutant was unable to grow at an acidic pH of 5.2, suggesting that pH sensitivity led to increased bacterial damage within the phagosome.
In contrast to FTL_0724, mutation of FTL_0699, encoding RluD pseudouridine synthase, resulted in the diminished induction of caspase-1 in infected macrophages. Surprisingly, unlike other Francisella mutants that have been described to be defective in activating caspase-1, the FTL_0699::Tn5 mutant was capable of phagosomal escape and intracellular replication and, more importantly, was virulent in vivo (41, 42). Further studies are required to determine why the FTL_0699::Tn5 mutant does not effectively activate caspase-1; however, it does not appear to be due to a lack of functional AIM2 agonists, as crude lysates and DNA from the FTL_0699::Tn5 mutant directly transfected into macrophages were fully capable of inducing IL-1β secretion.
Through the use of F. tularensis mutant strains, we, and others, have identified several genes that are important for F. tularensis to evade recognition by specific PRRs. Identifying pathways that are required for F. tularensis to evade inflammasome activation would provide potential novel targets for therapeutic use. In addition, specific F. tularensis mutants that alter innate immune responses are also likely to affect the subsequent adaptive immune response that is generated. By integrating knowledge of both the innate and adaptive immune responses triggered by attenuated F. tularensis mutants, future studies may lead to the identification of improved vaccine strain candidates.
Supplementary Material
ACKNOWLEDGMENTS
We thank Millennium Pharmaceuticals and Richard Flavell (Yale University) for providing knockout mice, Katherine Fitzgerald and Vijay Rathinam (University of Massachusetts) for providing AIM2-deficient femurs, and Vickie Knepper-Adrian for assistance with animal husbandry. We also thank William Nauseef, Michael Apicella, and Lee-Ann Allen for helpful discussion and critical review of the manuscript.
A Merit Review Grant from the Veterans Administration (IBX000167A) (F.S.S.), NIH grant P01 AI44642 (F.S.S.), and the Midwest Regional Center of Excellence project grant U54 AI057160 (B.D.J. and F.S.S.) supported this work.
Footnotes
Published ahead of print 31 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00991-12.
REFERENCES
- 1. McLendon MK, Apicella MA, Allen LA. 2006. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 60:167–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saslaw S, Eigelsback HT, Wilson HE, Prior JA, Carhart S. 1961. Tularemia vaccine study I. Intracutaneous challenge. Arch. Intern. Med. 107:121–133 [DOI] [PubMed] [Google Scholar]
- 3. Saslaw S, Eigelsback HT, Prior JA, Wilson HE, Carhart S. 1961. Tularemia vaccine study II. Respiratory challenge. Arch. Intern. Med. 107:134–146 [DOI] [PubMed] [Google Scholar]
- 4. Sjöstedt A. 2003. Virulence determinants and protective antigens of Francisella tularensis. Curr. Opin. Microbiol. 6:66–71 [DOI] [PubMed] [Google Scholar]
- 5. Cole LE, Shirley KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, Vogel SN. 2007. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect. Immun. 75:4127–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hong K, Wickstrum JR, Yeh H, Parmely MJ. 2007. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect. Immun. 75:5338–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O'Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U. S. A. 107:9771–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. U. S. A. 103:14578–14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clemens DL, Lee BY, Horwitz MA. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang MT, Mortensen BL, Taxman DJ, Craven RR, Taft-Benz S, Kijek TM, Fuller JR, Davis BK, Allen IC, Brickey WJ, Gris D, Wen H, Kawula TH, Ting JP. 2010. Deletion of ripA alleviates suppression of the inflammasome and MAPK by Francisella tularensis. J. Immunol. 185:5476–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng K, Broz P, Jones J, Joubert L, Monack D. 2011. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell. Microbiol. 13:1586–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Gotz F, Liu GY, Underhill DM. 2010. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1β secretion. Cell Host Microbe 7:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. 2007. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204:3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ulland TK, Buchan BW, Ketterer MR, Fernandes-Alnemri T, Meyerholz DK, Apicella MA, Alnemri ES, Jones BD, Nauseef WM, Sutterwala FS. 2010. Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J. Immunol. 185:2670–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jayakar HR, Parvathareddy J, Fitzpatrick EA, Bina XR, Bina JE, Re F, Emery FD, Miller MA. 2011. A galU mutant of Francisella tularensis is attenuated for virulence in a murine pulmonary model of tularemia. BMC Microbiol. 11:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss DS, Brotcke A, Henry T, Margolis JJ, Chan K, Monack DM. 2007. In vivo negative selection screen identifies genes required for Francisella virulence. Proc. Natl. Acad. Sci. U. S. A. 104:6037–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buchan BW, McLendon MK, Jones BD. 2008. Identification of differentially regulated Francisella tularensis genes by use of a newly developed Tn5-based transposon delivery system. Appl. Environ. Microbiol. 74:2637–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchan BW, McCaffrey RL, Lindemann SR, Allen LA, Jones BD. 2009. Identification of migR, a regulatory element of the Francisella tularensis live vaccine strain iglABCD virulence operon required for normal replication and trafficking in macrophages. Infect. Immun. 77:2517–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chamberlain RE. 1965. Evaluation of live tularemia vaccine prepared in a chemically defined medium. Appl. Microbiol. 13:232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 159:3364–3371 [PubMed] [Google Scholar]
- 23. Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267:2000–2003 [DOI] [PubMed] [Google Scholar]
- 24. Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galán JE. 2006. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J. Exp. Med. 203:1407–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317–327 [DOI] [PubMed] [Google Scholar]
- 26. Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383–390 [DOI] [PubMed] [Google Scholar]
- 27. Sutterwala FS, Noel GJ, Clynes R, Mosser DM. 1997. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J. Exp. Med. 185:1977–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. U. S. A. 105:9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grimshaw CE, Henderson GB, Soppe GG, Hansen G, Mathur EJ, Huennekens FM. 1984. Purification and properties of 5,10,-methenyltetrahydrofolate synthetase from Lactobacillus casei. J. Biol. Chem. 259:2728–2733 [PubMed] [Google Scholar]
- 30. Hancock AN, Coleman RS, Johnson RT, Sarisky CA, Johann TW. 2008. Investigations of the roles of arginine 115 and lysine 120 in the active site of 5,10-methenyltetrahydrofolate synthetase from Mycoplasma pneumoniae. Protein J. 27:303–308 [DOI] [PubMed] [Google Scholar]
- 31. Leemans JC, Cassel SL, Sutterwala FS. 2011. Sensing damage by the NLRP3 inflammasome. Immunol. Rev. 243:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroder K, Sagulenko V, Zamoshnikova A, Richards AA, Cridland JA, Irvine KM, Stacey KJ, Sweet MJ. 27 July2012. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 33. Pedra JH, Cassel SL, Sutterwala FS. 2009. Sensing pathogens and danger signals by the inflammasome. Curr. Opin. Immunol. 21:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bürckstümmer T, Baumann C, Blüml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10:266–272 [DOI] [PubMed] [Google Scholar]
- 35. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458:514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323:1057–1060 [DOI] [PubMed] [Google Scholar]
- 37. Gutgsell NS, Deutscher MP, Ofengand J. 2005. The pseudouridine synthase RluD is required for normal ribosome assembly and function in Escherichia coli. RNA 11:1141–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leppik M, Peil L, Kipper K, Liiv A, Remme J. 2007. Substrate specificity of the pseudouridine synthase RluD in Escherichia coli. FEBS J. 274:5759–5766 [DOI] [PubMed] [Google Scholar]
- 39. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barker JR, Chong A, Wehrly TD, Yu J, Rodriguez SA, Liu J, Celli J, Arulanandam BP, Klose KE. 2009. The Francisella tularensis pathogenicity island encodes a secretion system that is required for phagosome escape and virulence. Mol. Microbiol. 74:1459–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. 2006. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc. Natl. Acad. Sci. U. S. A. 103:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mariathasan S, Weiss DS, Dixit VM, Monack DM. 2005. Innate immunity against Francisella tularensis is dependent on the ASC/caspase 1 axis. J. Exp. Med. 202:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.