Abstract
LC3-associated phagocytosis (LAP) of Burkholderia pseudomallei by murine macrophage (RAW 264.7) cells is an intracellular innate defense mechanism. Beclin 1, a protein with several roles in autophagic processes, is known to be recruited to phagosomal membranes as a very early event in LAP. We sought to determine whether knockdown of Beclin 1 by small interfering RNA (siRNA) would affect recruitment of LC3 and subsequent LAP of infecting B. pseudomallei. Both starvation and rapamycin treatment can induce Beclin 1-dependent autophagy. Therefore, we analyzed the consequences of Beclin 1 knockdown for LAP in infected cells that had been either starved or treated with rapamycin by determining the levels of bacterial colocalization with LC3 and intracellular survival. Concurrently, we confirmed the location of bacteria as either contained in phagosomes or free in the cytoplasm. We found that both rapamycin and starvation treatment enhanced LAP of B. pseudomallei but that the rapamycin response is Beclin 1 independent whereas the starvation response is Beclin 1 dependent.
INTRODUCTION
Burkholderia pseudomallei is the causative agent of melioidosis, a bacterial disease with high rates of mortality in many regions of the world (1). B. pseudomallei is an intracellular pathogen with the capacity to invade most mammalian cell types, including phagocytic and nonphagocytic cells (2). We have reported that treatment of murine macrophage (RAW 264.7) cells with the pharmacological stimulator of autophagy, rapamycin, increases bacterial colocalization with the autophagy marker protein LC3 and reduces the intracellular survival of B. pseudomallei (3). However, subsequently we showed that intracellular bacteria were either free in the cytosol or sequestered in single-membrane phagosomes but almost never in double-membrane autophagosomes. From these and other observations, we concluded that LC3 can be recruited directly to bacteria-containing, single-membrane phagosomes in a process designated LC3-associated phagocytosis (LAP) (4) and that LAP results in increased killing of intracellular B. pseudomallei. LAP can be clearly distinguished from canonical macroautophagy (hereinafter termed autophagy), which is characterized by the formation of double-membrane vesicles (autophagosomes) for the sequestration of cytoplasmic organelles, proteins, or bacteria for delivery to lysosomes for degradation (5).
Some of the events associated with the recruitment of LC3 to the phagosome membrane in LAP have been described by Sanjuan et al. (6). The activation of Toll-like receptor (TLR) signaling during the phagocytosis of extracellular bacteria leads to very rapid recruitment (within 10 min of infection) of LC3 to the phagosome membrane (6). Proteomic analysis of a purified membrane fraction from latex bead-containing phagosomes has demonstrated the specific enrichment of LC3 on phagosomal membranes (7). The association of LC3 with the phagosome requires the autophagy-related proteins Atg5 and Atg7 but does not require the formation of an autophagosome structure. The recruitment of LC3 is preceded by the association of Beclin 1 and phosphatidylinositol (PI) 3-kinase activity with the phagosome membrane. Collectively, these events are presumed to result in enhanced fusion of phagosomes to lysosomes, using the same mechanisms by which autophagosome-lysosome fusion is facilitated, to promote the destruction of the engulfed bacteria (6).
Beclin 1 is known to have several roles in the regulation of autophagy. It acts in the induction of autophagy (8, 9), in the formation of autophagosomes (10), and in their maturation (9). Reduction in the levels of Beclin 1 by small interfering RNA (siRNA) results in an inhibition of autophagy (11). However, there is little information available concerning the association of Beclin 1 with phagosomal membranes and, by extension, the roles it may play in controlling infections by microbial pathogens. Gutierrez et al. reported that nutrient starvation increased colocalization of Beclin 1 with Mycobacterium-containing phagosomes (12). More recently, it was reported that infection with Coxiella burnetii induces the recruitment of Beclin 1 to the limiting membrane of the large C. burnetii replicative vacuoles (CRVs), where the invading bacteria survive and replicate. Depletion of Beclin 1 alters the development of CRVs, confirming a requirement for the protein in this process (13). Notably, the limiting membrane of the CRV is also decorated with LC3. Another recent study has demonstrated that a member of the signaling lymphocyte activation molecule family (SLAMF1 or CD150) acts as a sensor of Gram-negative bacteria and is incorporated into phagosomes that have sequestered the associated bacteria. SLAMF1 recruits a complex containing Beclin 1, which is responsible for phagosome maturation and eventual killing of the intraphagosomal bacteria (14).
In the context of infection by B. pseudomallei, it is unclear whether the presence of Beclin 1 at the phagosomal membrane is an essential requirement for recruitment of LC3 and subsequent LAP. We asked, therefore, whether knockdown of Beclin 1 by siRNA would affect LAP of B. pseudomallei in RAW 264.7 cells. Furthermore, as both starvation and rapamycin treatment can induce Beclin 1-dependent autophagy (5), we analyzed the level of LAP in Beclin 1 knockdown cells that had been starved or treated with rapamycin. We found that Beclin 1 is required for efficient LAP of B. pseudomallei and that, while both rapamycin and starvation treatments enhanced LAP, the rapamycin response was Beclin 1 independent whereas the starvation response was Beclin 1 dependent. This finding adds to the growing list of autophagy-related processes that have been reported to be Beclin 1 independent and shows a critical difference in the mechanisms of LAP induction in response to starvation and rapamycin treatment.
MATERIALS AND METHODS
Bacterial strains and mammalian cell culture.
B. pseudomallei K96243 was cultured in Luria-Bertani (LB) broth at 37°C. The bipD mutant was generated as described previously (4) and cultured in LB containing 40 μg/ml chloramphenicol. The mouse macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (Manassas, VA). A RAW 264.7 cell line stably expressing green fluorescent protein (GFP)-LC3 (RAWGFP-LC3) was described previously (3). Mammalian cells were maintained at 37°C in 5% (vol/vol) CO2 without antibiotics in RPMI 1640 medium (Gibco Laboratories) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS) (Thermo Electron Australia, Noble Park North, Victoria, Australia).
Transfection with siRNAs.
RAW 264.7 or RAWGFP-LC3 cells were cultured in 24-well or 6-well cell culture plates to ∼70% confluence. Cells were transiently transfected with either mouse Beclin 1 siRNA or nontargeted control siRNA (Thermo Scientific Dharmacon, Inc., Lafayette, CO) at a final concentration of 30 nM using Lipofectamine RNAiMAX (Invitrogen) as the transfection reagent according to the manufacturer's instructions. After gene transfection for 48 h, the medium was changed, and the cells were then treated as described below.
Infection of cell lines with B. pseudomallei and treatments to modulate autophagy.
RAW 264.7 or RAWGFP-LC3 cells treated with siRNAs were infected with B. pseudomallei K96243 or the bipD mutant at a multiplicity of infection (MOI) of 10:1 (unless otherwise stated) and incubated at 37°C for 1 h to allow bacterial invasion. Cells were washed three times with phosphate-buffered saline (PBS), pH 7.2, and then incubated in fresh medium containing kanamycin (800 μg/ml) and ceftazidime (100 μg/ml) to kill extracellular bacteria. To test the effect of starvation on bacterial infection, cells were incubated either in starvation medium lacking amino acids and serum (Earl's balanced salt solution [EBSS]; Gibco) or in complete culture medium (RPMI 1640 supplemented with 10% [vol/vol] FCS). Alternatively, to test the effect of rapamycin treatment, cells were incubated in complete culture medium with or without rapamycin (4 μM; LC Laboratories, Woburn, MA).
Bacterial replication assays.
Infected RAW 264.7 cells were lysed to release intracellular bacteria using PBS containing 0.1% (vol/vol) Triton X-100. Serial dilutions of the cell lysates were plated onto LB agar, and the numbers of intracellular bacteria (expressed as CFU) were enumerated by direct colony counts after 48 h (3). The data are presented as percent survival relative to that of the control (cells transfected with nontargeted control siRNA and not subjected to either rapamycin or starvation treatment). The specific intracellular growth rates of B. pseudomallei were not measured under the different conditions used in this study.
Immunofluorescence.
RAWGFP-LC3 cells, cultured on glass coverslips in 24-well trays, were infected and then subjected to different treatments to assess the colocalization of bacteria with GFP-LC3-labeled structures as described previously (3).
TEM.
RAW 264.7 cells cultured in 6-well trays were infected at an MOI of 15:1 and then subjected to starvation or rapamycin treatment as described above. Sample preparation and image acquisition for transmission electron microscopy (TEM) were described previously (4).
Immunoblotting.
Whole-cell lysates were prepared from infected cells subjected to different treatments. Proteins in the lysates were separated by polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Pall Life Sciences) as previously described (3). The membranes were probed at 4°C for 16 h with primary antibody mouse anti-Beclin 1 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) (1:500 dilution), mouse anti-LC3 antibody (NanoTools, Teningen, Germany) (1:500 dilution), or rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Sigma) (1:10,000 dilution), followed by extensive washing and incubation for 1 h with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody, either sheep anti-mouse IgG-HRP (GE Healthcare, United Kingdom) (1:1,000 dilution) or goat anti-rabbit IgG-HRP (Thermo Fisher Scientific, Rockford, IL) (1:50,000 dilution). The signals were generated using SuperSignal West Femto maximum sensitivity substrate (Thermo Fisher Scientific) according to the manufacturer's instructions and recorded using photographic film. The film was scanned and band intensities quantified using ImageQuant TL software (GE Healthcare).
Statistical analyses.
Data are expressed as means ± standard errors of the means (SEM) of three independent experiments. For quantification of colocalization studies, at least 100 bacteria were counted for each condition in each experiment, unless otherwise indicated. For single comparisons, P values were calculated by using the 2-tailed, 2-sample, unpaired Student's t test. A P value of <0.05 was considered to be statistically significant. A P value of <0.005 was reported where applicable.
RESULTS
Beclin 1 is critical for starvation-induced LAP but not for rapamycin-induced LAP.
We have shown previously that B. pseudomallei is susceptible to LAP in RAW 264.7 cells and that the level of LAP can be enhanced by rapamycin treatment. To determine the importance of Beclin 1 for the development of LAP, we measured the colocalization of B. pseudomallei with GFP-LC3 in Beclin 1 knockdown cells following either starvation or rapamycin treatment. RAWGFP-LC3 cells were transfected with either an siRNA construct targeted against Beclin 1 or a nontargeted control siRNA and then infected with B. pseudomallei at an MOI of 10:1. Infected cells were either left untreated, treated with rapamycin, or starved at 1 h postinfection (p.i.). Western blot analysis indicated that Beclin 1 production was reduced by approximately 80% in Beclin 1 siRNA-treated cells compared with the level in control siRNA-treated cells (Fig. 1A). The colocalization of B. pseudomallei (red) with GFP-LC3 (green) was evaluated using fluorescence microscopy (Fig. 1B). In untreated cells transfected with control siRNA, the percentage of bacteria colocalized with GFP-LC3 was 5.9% ± 0.2% (mean ± SEM). However, the colocalization was reduced to 2.8% ± 0.4% in cells transfected with the Beclin 1 siRNA (Fig. 1C) (P < 0.05). Thus, in untreated cells, knockdown of Beclin 1 suppresses the association of B. pseudomallei with LC3.
Fig 1.
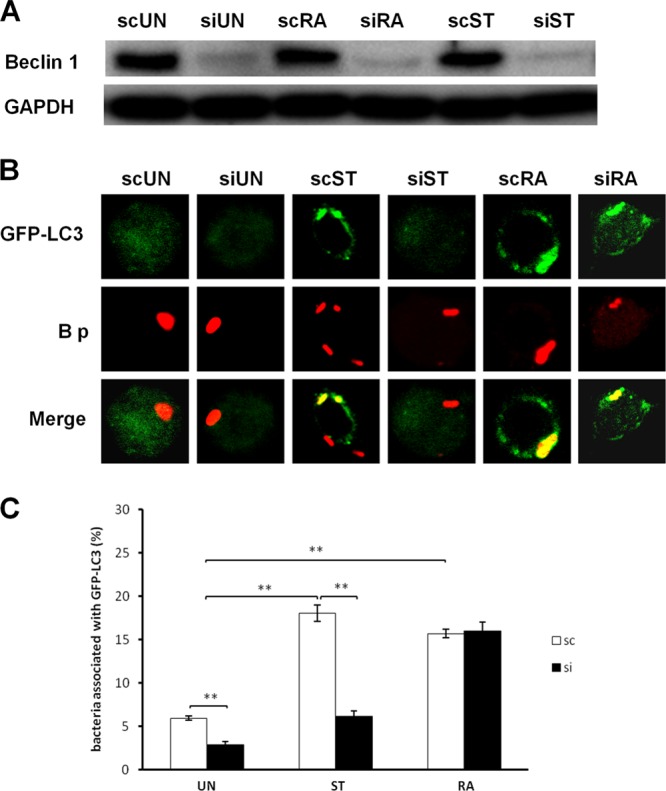
Colocalization of B. pseudomallei and GFP-LC3 in Beclin 1 knockdown cells following starvation or rapamycin treatment. RAW 264.7 cells stably expressing GFP-LC3 were transfected with either a nontargeted control (sc) or Beclin 1 (si) siRNA and then infected with B. pseudomallei at an MOI of 10:1. At 1 h p.i., the cells were incubated in EBSS medium (starvation; ST), RPMI medium containing 4 μM rapamycin (RA), or RPMI medium only (untreated; UN) for a further 2 h and then analyzed by confocal laser scanning microscopy. (A) Western immunoblotting was used to evaluate the efficiency of Beclin 1 knockdown in infected RAW 264.7 cells transfected with a Beclin 1-targeted siRNA compared with that in cells transfected with a nontargeted control (sc). A GAPDH loading control is shown. (B) Representative images of colocalization (yellow; merge) of bacteria (red; B p) with GFP-LC3 (green). Colocalization in 3 dimensions was confirmed by analysis of images acquired along the z axis (data not shown). The original magnification was 180×. (C) Quantitative analysis of colocalization of wild-type bacteria with GFP-LC3 in control (sc) or Beclin 1 (si) siRNA-transfected cells under different treatment conditions. Data represent the means ± SEM of three separate experiments (n = 100 bacteria). *, P < 0.05; **, P < 0.005.
When B. pseudomallei-infected RAW 264.7 cells transfected with nontargeted control siRNA were starved, the percentage of bacteria colocalized with GFP-LC3 increased to 18.0% ± 1.0%. In contrast, the percentage of colocalization observed in starved cells transfected with the Beclin 1 siRNA construct was only 6.2% ± 0.6% (Fig. 1C) (P < 0.005). Thus, the increase in colocalization of B. pseudomallei with GFP-LC3 in response to starvation is primarily Beclin 1 dependent. Conversely, following rapamycin treatment, control and Beclin 1 knockdown siRNA infected cells showed similar levels of bacterial colocalization with GFP-LC3 (16.0% ± 0.5% and 15.7% ± 1.0%, respectively) (Fig. 1C), indicating that the rapamycin-dependent increase in B. pseudomallei colocalization is Beclin 1 independent.
Knockdown of Beclin 1 expression results in the decreased colocalization of a B. pseudomallei bipD mutant with GFP-LC3.
We next carried out infections using a B. pseudomallei mutant lacking expression of the BipD protein. BipD is a component of the needle tip complex of the B. pseudomallei type III secretion system apparatus (15). BipD, like BopA, is required for evasion of LAP (4). Our rationale for using a bipD mutant rather than a bopA mutant was that a bipD mutant is highly susceptible to LAP in RAW 264.7 cells, showing significantly greater colocalization with GFP-LC3 than wild-type or bopA bacteria at 2 h p.i. and no escape from phagosomes up to 4 h p.i (4). While the loss of BopA does lead to a delay in escape from phagosomes, this is not as pronounced as the delay observed for the bipD mutant. Therefore, we reasoned that the use of the bipD mutant would allow us to directly assess the requirement of Beclin 1 for recruitment of LC3 to B. pseudomallei-containing phagosomes and, thus, would provide the best scenario for determining the effect of starvation or rapamycin treatment on LAP in Beclin 1 knockdown cells. RAWGFP-LC3 cells transfected with either the Beclin 1 siRNA or the nontargeted control siRNA were infected with either wild-type B. pseudomallei or the bipD mutant at an MOI of 10:1, followed by starvation or nonstarvation treatment for another 2 h. The colocalization of bacteria with GFP-LC3 was evaluated using fluorescence microscopy (Fig. 2A).
Fig 2.
bipD mutant bacteria show decreased colocalization with GFP-LC3 in Beclin 1 knockdown cells. RAW 264.7 cells stably expressing GFP-LC3 were transfected with either a nontargeted control (sc) or Beclin 1 (si) siRNA and then infected with bipD mutant bacteria at an MOI of 10:1. At 1 h p.i., the cells were incubated in EBSS medium (starvation; ST) or RPMI medium only (untreated; UN) for a further 2 h and then analyzed by confocal microscopy. (A) Representative images of colocalization (yellow; merge) of bacteria (red; B p) with GFP-LC3 (green). The representative images are presented at 120× magnification. (B) Quantitative analysis of colocalization of bipD mutant with GFP-LC3 in nontargeted control (sc) or Beclin 1 (si) siRNA-transfected cells under different treatment conditions. Data represent the means ± SEM of three separate experiments (n = 100 bacteria). **, P < 0.005.
The percentage of colocalization of bipD mutant bacteria with GFP-LC3 in nonstarved control siRNA cells was 34.0% ± 1.2%, but it was only 16.0% ± 1.5% in nonstarved Beclin 1 siRNA cells (Fig. 2B). Thus, Beclin 1 plays an important role in LC3 recruitment directly to B. pseudomallei-containing phagosomes. Furthermore, following starvation, the percentage of bipD mutant bacteria colocalized with GFP-LC3 in cells transfected with the nontargeted control RNA increased to 58.7% ± 1.8%, but it was only 30.0% ± 2.6% in starvation-treated Beclin 1 knockdown cells (Fig. 2B). These data confirm our previous observations that the bipD mutant, which is unable to escape from phagosomes at up to 4 h p.i., shows increased susceptibility to LAP compared to that of wild-type B. pseudomallei. Furthermore, they confirm that starvation stimulates LAP and that the recruitment of LC3 to B. pseudomallei-containing phagosomes under starvation conditions is primarily Beclin 1 dependent.
The intracellular survival of B. pseudomallei in RAW 264.7 cells following starvation treatment is reduced in a Beclin 1-dependent manner.
Since both starvation and rapamycin treatment of RAW 264.7 cells increased colocalization of B. pseudomallei with GFP-LC3, we analyzed the intracellular survival of bacteria following starvation or rapamycin treatment in control or Beclin 1 knockdown cells. RAW 264.7 cells were transfected with either Beclin 1 siRNA or the nontargeted control siRNA and then infected as described above. In cells transfected with nontargeted control siRNA, starvation reduced the survival of intracellular B. pseudomallei by 68.6% ± 3.0% (Fig. 3). Therefore, starvation can stimulate the killing of B. pseudomallei by LAP in RAW 264.7 cells. Furthermore, the survival of B. pseudomallei was increased in untreated cells containing the Beclin 1 siRNA (Fig. 3, compare scUN with siUN), indicating that Beclin 1 plays a role in the killing of intracellular B. pseudomallei. However, the survival of B. pseudomallei in starved Beclin 1 knockdown cells, although slightly lower than that observed in unstarved Beclin 1 siRNA cells, was not significantly different from that observed in unstarved control cells. These data are consistent with the colocalization data and clearly show that killing of B. pseudomallei is highly Beclin 1 dependent (Fig. 3).
Fig 3.
Intracellular survival of wild-type B. pseudomallei in Beclin 1 knockdown cells. RAW 264.7 cells were transfected with either nontargeted control (sc) or Beclin 1 (si) siRNA and then infected with B. pseudomallei. At 1 h p.i., cells were incubated in fresh RPMI medium alone (untreated; UN) or with 4 μM rapamycin (RA) or in EBSS (starvation; ST) for a further 2 h, and then bacterial survival was determined by direct plate counts. The data were normalized to the results for the untreated, nontargeted control (scUN; 100%) and represent the means ± SEM of three separate experiments. **, P < 0.005.
Following rapamycin treatment, we observed a 52.2% ± 2.2% reduction in intracellular survival (Fig. 3, compare scUN and scRA), confirming our earlier observations (3). Notably, this reduction in intracellular survival was similar in both control cells and Beclin 1 knockdown cells, indicating that the rapamycin-induced killing of intracellular B. pseudomallei is Beclin 1 independent.
Knockdown of Beclin 1 suppresses the retention of B. pseudomallei in phagosomes in starved cells but not in rapamycin-treated cells.
We have shown that at 2 h p.i., virtually all B. pseudomallei bacteria within RAW 264.7 cells exist either free in the cytosol or in single-membrane phagosomes (4). To evaluate the intracellular location of bacteria in Beclin 1 knockdown cells, we analyzed infected cells using transmission electron microscopy (TEM) (Fig. 4A). Cells were transfected with either the Beclin 1 siRNA construct or control siRNA, infected with B. pseudomallei at an MOI of 15:1, and then either starved or treated with rapamycin.
Fig 4.
Transmission electron microscopy analysis of B. pseudomallei-containing vacuoles in Beclin 1 knockdown cells. RAW 264.7 cells stably expressing GFP-LC3 were transfected with either a nontargeted control (sc) or Beclin 1 (si) siRNA and then infected with B. pseudomallei at an MOI of 15:1. At 1 h p.i., the cells were incubated in EBSS medium (starvation; ST), RPMI medium containing 4 μM rapamycin (RA), or RPMI medium only (untreated; UN) for a further 2 h and then subjected to transmission electron microscopy (TEM) analysis. (A) Representative TEM micrographs of intracellular bacteria (a to c). Boxed areas are shown as magnified images below each panel. (a) A bacterium free in the cytosol. (b and c) Phagosome membrane-bound bacteria. (B) Quantification of the percentages of membrane-bound bacteria in RAW 264.7 cells under different conditions. **, P < 0.005 relative to the results for control cells.
In cells transfected with control siRNA, the percentage of B. pseudomallei-containing phagosomes increased from 35.7% ± 1.5% to 59.0 ± 2.5% following starvation treatment (Fig. 4B, scUN versus scST) and to 60.3% ± 3.0% following rapamycin treatment (Fig. 4B, scUN versus scRA). Therefore, starvation and rapamycin treatment resulted in similar increases in the number of bacteria contained within phagosomes in RAW 264.7 cells. In untreated Beclin 1 knockdown cells, 24.0% ± 1.5% of bacteria were contained in phagosomes, in comparison to 35.0% ± 1.5% for the control cells (Fig. 4B), indicating that Beclin 1 plays an important role in the uptake and/or retention of B. pseudomallei within phagosomes. While the number of bacteria within phagosomes increased when the cells containing the nontargeted siRNA were starved (Fig. 4B, scUN versus scST), there was no significant increase observed in Beclin 1 knockdown-treated cells (Fig. 4B, scUN versus siST), clearly indicating that the starvation results in an increase in retention of bacteria within phagosomes and that this is Beclin 1 dependent. However, for the rapamycin-treated cells, both the Beclin 1 knockdown cells and the nontargeted control cells showed a similar increase in the percentage of bacteria within phagosomes (Fig. 4B). Therefore, the rapamycin-induced increase in B. pseudomallei-containing phagosomes is Beclin 1 independent.
Knockdown of Beclin 1 reduces the number of green puncta in starved, but not in rapamycin-treated, B. pseudomallei-infected cells.
Collectively, our data show that both rapamycin treatment and starvation lead to increased colocalization of B. pseudomallei with GFP-LC3 and that this is due almost exclusively to LAP and results in decreased survival of intracellular bacteria. Importantly, Beclin 1 is critical for the starvation-induced LAP response but not for the rapamycin-induced LAP response. Both starvation and rapamycin treatment of cells can result in increased levels of autophagy, which can be estimated by quantifying the number of green puncta arising through the formation of GFP-LC3-II and its association with autophagosome membranes. However, within cells that have been infected with B. pseudomallei, green puncta include autophagosomes and phagosomal membranes associated with LC3-II (due to LAP). We therefore counted the number of green puncta to determine whether Beclin 1 knockdown alters the ability of cells to form GFP–LC3-II-labeled membranes. We determined the number of green puncta per cell following starvation or rapamycin treatment first in noninfected cells as a measure of canonical autophagy alone and then in infected cells as a measure of both canonical autophagy and LAP events.
The number of green puncta per uninfected cell was 3.0 ± 0.4 for Beclin 1 knockdown cells and 3.5 ± 0.4 for control cells (Fig. 5B). Following starvation, the number of green puncta per cell increased to 5.7 ± 0.4 for Beclin 1 knockdown cells, and 8.9 ± 0.4 for control cells. Rapamycin treatment resulted in even greater increases in the number of green puncta per cell compared to the number in untreated cells, 12.8 ± 0.5 for Beclin 1 knockdown cells and 15.9 ± 0.7 for control cells. Beclin 1 knockdown decreased the formation of green puncta in starved cells (P < 0.01) and in rapamycin-treated cells (P < 0.05), indicating that Beclin 1 knockdown plays a role in the formation of canonical autophagosomes under both conditions.
Fig 5.
The effect of Beclin 1 knockdown on the number of green puncta in starved and rapamycin-treated cells. Cells stably expressing GFP-LC3 were transfected with either nontargeted control (sc) or Beclin 1 (si) siRNA and then infected with wild-type B. pseudomallei (A) or left uninfected (B). At 1 h p.i., the cells were incubated in fresh RPMI medium with or without rapamycin (4 μM) or in EBSS (starvation) for another 2 h, and then they were visualized by confocal microscopy and the number of green puncta per infected cell determined. A total of 100 cells was counted for each condition. Data represent the means ± SEM of at least three separate experiments. *, P < 0.05; **, P < 0.005.
For B. pseudomallei-infected cells, similar results for the formation of green puncta were observed (Fig. 5A). Under each condition, untreated or starvation or rapamycin treatment, the number of green puncta was higher for infected cells than for uninfected cells (Fig. 5A versus B). We interpret this increase as representing LAP events. The number of green puncta per infected cell was 4.5 ± 0.3 for Beclin 1 knockdown cells and 5.7 ± 0.3 for control cells (Fig. 5B). Following starvation, the number of green puncta per infected cell increased to 9.2 ± 0.7 for Beclin 1 knockdown cells and 13.6 ± 0.5 for control cells, and Beclin 1 knockdown again led to decreased formation of green puncta in starved cells. In contrast, rapamycin treatment of infected cells resulted in a similar number of green puncta per cell for control and Beclin 1 knockdown cells, 19.1 ± 0.7 and 17.0 ± 1.2, respectively (P > 0.05) (Fig. 5A), indicating that in infected and rapamycin-treated cells, Beclin 1 knockdown does not impair the production of LC3-II and formation of green puncta.
DISCUSSION
Beclin 1 plays a role in the killing of B. pseudomallei in RAW 264.7 cells.
In this study, we have shown that knockdown of Beclin 1 resulted in increased intracellular survival of B. pseudomallei in RAW 264.7 cells. This increased survival was observed in untreated cells but was most marked following starvation. We propose that this increased survival is due to a reduced level of LAP, a conclusion supported by the decreased colocalization of intracellular B. pseudomallei with GFP-LC3 and by TEM analysis showing reduced numbers of bacteria trapped in phagosomes. Furthermore, increased colocalization of the bipD mutant (which cannot escape from phagosomes during the course of the experiment) with GFP-LC3 was also Beclin 1 dependent, indicating that Beclin 1 is required for LC3 recruitment to B. pseudomallei-containing phagosomes. These data clearly indicate that Beclin 1 is involved in LAP and the killing of B. pseudomallei in RAW 264.7 cells.
Starvation- and rapamycin-induced responses to infection by B. pseudomallei are differentially dependent on the expression of Beclin 1.
Our results demonstrate that starvation stimulates the association of GFP-LC3 with bacteria-containing phagosomes, presumably through increased recruitment of LC3 to the phagosomal membrane. This LC3 recruitment is Beclin 1 dependent, as the effect of starvation on LC3 recruitment and bacterial killing was significantly decreased in Beclin 1 knockdown cells. As we have previously observed (3), rapamycin treatment also leads to increased colocalization of bacteria with GFP-LC3 and reduced bacterial survival. However, Beclin 1 does not appear to play a significant role in rapamycin-dependent LAP (Fig. 3). Therefore, we conclude that rapamycin-induced LAP is largely Beclin 1 independent. One possibility is that in infected cells, different pools of LC3 are mobilized under starvation and rapamycin treatments; this will be a focus of ongoing investigations. However, our data cannot exclude a role for the remaining pool(s) of Beclin 1 in rapamycin-induced LAP in the Beclin 1 knockdown cells.
Very recently, Martinez et al. demonstrated that LAP is required for the efficient clearance of dead SVEC (saphenous vein endothelial cells) by macrophages (16). In that report, a reduction of about 70% in Beclin 1 levels was achieved by siRNA treatment in bone marrow-derived macrophages expressing GFP-LC3. Following treatment for 24 h with rapamycin, the wild-type macrophages but not the Beclin 1-deficient macrophages displayed GFP-LC3 punctum formation. Furthermore, the Beclin 1-deficient macrophages, in contrast to wild-type macrophages, showed no GFP-LC3 translocation to the dead-cell-containing phagosomes. Consistent with these observations was the finding that upon engulfment of dead cells, significant conversion of LC3-I to LC3-II could not be detected by Western blotting. This is in contrast to our observation that LAP of B. pseudomallei in response to rapamycin is largely Beclin 1 independent. The reasons these observations contrast with those made in our study may relate to the nature of the phagosomal cargo, i.e., dead cells compared to live bacteria.
The role of Beclin 1 in autophagy and LAP.
We used the number of green puncta per cell as a measure of the recruitment of LC3 to phagosomes and/or autophagosomes following starvation or rapamycin treatment. In infected control cells, both starvation and rapamycin led to a significant increase in the average number of puncta per cell, consistent with their documented roles as inducers of autophagy. In Beclin 1 knockdown cells that had undergone starvation, the number of green puncta per cell was reduced, consistent with the inhibition of the recruitment of LC3 to both phagosomes and autophagosomes. These data are consistent with earlier observations showing that reduction in the levels of Beclin 1 by siRNA results in an inhibition of autophagy in starved HeLa cells (11). However, in Beclin 1 knockdown cells treated with rapamycin, there was no significant reduction in the numbers of green puncta for infected cells. Reduction in the levels of Beclin 1 by siRNA has been shown to result in an inhibition of autophagy (11); such a reduction would necessarily decrease the overall numbers of green puncta because the recruitment of GFP-LC3 to autophagosomal membranes is inhibited. Therefore, it can be concluded that the smaller decrease in numbers of green puncta for rapamycin treatment compared to the results for starvation in infected cells and, also, in comparison to the results for uninfected cells treated with rapamycin occurs because only recruitment of GFP-LC3 to autophagosomes, and not recruitment to phagosomes, is affected.
Beclin 1-independent autophagy pathways.
Several reports of Beclin 1-independent autophagy pathways have been made recently (17–23). However, the responsiveness of these pathways to rapamycin has not been addressed. Notably, these pathways have been identified largely in cancer cells subjected to treatment with chemical agents in the context of induction of apoptotic and/or autophagic cell death.
A very recent report indicated that ∼75% knockdown of Beclin 1 (comparable to the ∼80% knockdown achieved here) did reduce rapamycin-induced autophagy efficiently (24), albeit in cortical neurons. This finding contrasts with our observations that rapamycin can stimulate autophagy in uninfected cells, as well as stimulate LAP of B. pseudomallei in infected cells, apparently independently of Beclin 1. Moreover, this suggests that mobilization of LC3 for recruitment to phagosome membranes uses machinery similar to that used for autophagy. The Beclin 1 complex is critical for the formation of phosphatidylinositol 3 phosphate (PI3P) at membranes, a process which underpins the formation of autophagosome membranes. Therefore, there must be alternative pathways for the formation of PI3P at autophagosome membranes. This suggests that a better understanding of the need for PI3P at phagosome membranes in the context of LAP is required, together with the recruitment of other host components to the phagosomal membrane.
Additional evidence in support of Beclin 1-independent LAP.
A very recent study by Abdulrahman et al. (25) reported the outcome of infection of macrophages by Burkholderia cenocepacia, an opportunistic pathogen that is particularly significant in cystic fibrosis (CF) patients (25). CF is caused by mutation within the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR). A common mutation is deletion of the three nucleotides that comprise the codon for phenylalanine (F) at position 508. In CFTR ΔF508 (ΔF508) macrophages of both mice and humans, cross-linking of Beclin 1 by the tissue enzyme transglutaminase results in sequestration of Beclin 1 as a component of phosphatidylinositol-3-kinase complex III within aggresomes. Consequently, these cells have compromised autophagic activity (26). The level of reduction of Beclin 1 observed was comparable to that which we achieved by siRNA knockdown in our study. Thus, it is of interest to consider the fate of B. cepacia in the Beclin 1-depleted ΔF508 macrophages.
In ΔF508 macrophages, B. cepacia showed increased survival, with bacteria residing in vacuoles that did not fuse with lysosomes. Of note was the observation that treatment with rapamycin markedly enhanced the clearance of B. cepacia by induced autophagy. These observations mirror those made in our study, although the nature of the induced autophagy was not fully characterized. Analysis of electron microscopy (EM) images by Abdulrahman et al. showed that in ΔF508 macrophages, most bacteria remained inside single-membrane vacuoles not labeled with GFP-LC3 (25). However, upon treatment of infected ΔF508 macrophages with rapamycin, enhanced colocalization of bacteria with LC3 was observed. Confirmation by EM of the nature of the compartment, whether single or double membrane, was not reported. The question remains as to how rapamycin overcomes the autophagy deficiency arising from loss of Beclin 1 in ΔF508 macrophages. Either the residual pool of Beclin 1 is sufficient for the induction of macroautophagy by rapamycin or, if insufficient, then the induced autophagy observed is Beclin 1 independent. Clearly, it would be of interest to determine unequivocally whether B. cepacia undergoes LAP or canonical autophagy upon treatment of ΔF508 macrophages with rapamycin.
ACKNOWLEDGMENTS
This work was supported by grants from the Australian Research Council and the National Health and Medical Research Council, Australia.
We thank Georg Ramm, Lan Gong, and Joan Clark for assistance with TEM.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. White NJ. 2003. Melioidosis. Lancet 361:1715–1722 [DOI] [PubMed] [Google Scholar]
- 2. Jones AL, Beveridge TJ, Woods DE. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cullinane M, Gong L, Li X, Lazar-Adler N, Tra T, Wolvetang E, Prescott M, Boyce JD, Devenish RJ, Adler B. 2008. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy 4:744–753 [DOI] [PubMed] [Google Scholar]
- 4. Gong L, Cullinane M, Treerat P, Ramm G, Prescott M, Adler B, Boyce JD, Devenish RJ. 2011. The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS One 6:e17852 doi:10.1371/journal.pone.0017852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. 2010. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 90:1383–1435 [DOI] [PubMed] [Google Scholar]
- 6. Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. 2007. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450:1253–1257 [DOI] [PubMed] [Google Scholar]
- 7. Shui W, Sheu L, Liu J, Smart B, Petzold CJ, Hsieh TY, Pitcher A, Keasling JD, Bertozzi CR. 2008. Membrane proteomics of phagosomes suggests a connection to autophagy. Proc. Natl. Acad. Sci. U. S. A. 105:16952–16957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, Gruss P, Piacentini M, Chowdhury K, Cecconi F. 2007. Ambra1 regulates autophagy and development of the nervous system. Nature 447:1121–1125 [DOI] [PubMed] [Google Scholar]
- 9. Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, Noda T, Yoshimori T. 2010. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J. Cell Biol. 190:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, Pledger WJ, Wang HG. 2007. Bif interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 9:1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. 2005. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 25:1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119:753–766 [DOI] [PubMed] [Google Scholar]
- 13. Vázquez CL, Colombo MI. 2010. Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell Death Differ. 17:421–438 [DOI] [PubMed] [Google Scholar]
- 14. Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, Boes M, Regueiro JR, Reinecker HC, Terhorst C. 2010. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat. Immunol. 11:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson S, Roversi P, Espina M, Olive A, Deane JE, Birket S, Field T, Picking WD, Blocker AJ, Galyov EE, Picking WL, Lea SM. 2007. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J. Biol. Chem. 282:4035–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. 2011. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. U. S. A. 108:17396–17401 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Chu CT, Zhu J, Dagda R. 2007. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy 3:663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao P, Bauvy C, Souquère S, Tonelli G, Liu L, Zhu Y, Qiao C, Bakula D, Proikas-Cezanne T, Pierron G, Codogno P, Chen Q, Mehrpou M. 2010. The BH3-mimetic gossypol induces both beclin1-dependent and beclin 1-independent cytoprotective autophagy in cancer cells. J. Biol. Chem. 285:25570–25581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. 2008. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 15:1318–1329 [DOI] [PubMed] [Google Scholar]
- 20. Seo G, Kim SK, Byun YJ, Oh E, Jeong SW, Chae GT, Lee SB. 2011. Hydrogen peroxide induces Beclin 1-independent autophagic cell death by suppressing the mTOR pathway via promoting the ubiquitination and degradation of Rheb in GSH-depleted RAW 264.7 cells. Free Radic. Res. 45:389–399 [DOI] [PubMed] [Google Scholar]
- 21. Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. 2010. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ. 17:1867–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tian S, Lin J, Zhou J, Wang X, Li Y, Ren X, Yu W, Zhong W, Xiao J, Sheng F, Chen Y, Jin C, Li S, Zheng Z, Xia B. 2010. Beclin 1-independent autophagy induced by a Bcl-XL/Bcl-2 targeting compound, Z18. Autophagy 6:1032–1041 [DOI] [PubMed] [Google Scholar]
- 23. Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. 2010. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One 5:e9996 doi:10.1371/journal.pone.0009996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. 2011. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 7:1115–1131 [DOI] [PubMed] [Google Scholar]
- 25. Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DHA, Newland C, Rosales-Reyes RR, Kopp B, McCoy K, Montione R, Schlesinger LS, Gavrilin MA, Wewers MD, Valvano MA, Amer A. 2011. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy 7:1359–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. 2010. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 12:863–875 [DOI] [PubMed] [Google Scholar]


