Abstract
Mycobacterium avium subsp. paratuberculosis is the causative agent of Johne's disease in cattle. The complex, multifaceted interaction of M. avium subsp. paratuberculosis with its host includes dampening the ability of infected cells to respond to stimuli that promote M. avium subsp. paratuberculosis clearance. By disrupting host defenses, M. avium subsp. paratuberculosis creates an intracellular environment that favors the establishment and maintenance of infection. Toll-like receptors (TLRs) are important sensors that initiate innate immune responses to microbial challenge and are also immunotherapeutic targets. For example, TLR9 contributes to host defense against M. avium subsp. paratuberculosis, and its agonists (CpG oligodeoxynucleotides [ODNs]) are under investigation for treatment of Johne's disease and other infections. Here we demonstrate that M. avium subsp. paratuberculosis infection changes the responsiveness of bovine monocytes to TLR9 stimulation. M. avium subsp. paratuberculosis inhibits classical TLR9-mediated responses despite a 10-fold increase in TLR9 expression and maintained uptake of CpG ODNs. Other TLR9-mediated responses, such as oxidative burst, which occur through noncanonical signaling, remain functional. Kinome analysis verifies that classic TLR9 signaling is blocked by M. avium subsp. paratuberculosis infection and that signaling instead proceeds through a Pyk2-mediated mechanism. Pyk2-mediated signaling does not hinder infection, as CpG ODNs fail to promote M. avium subsp. paratuberculosis clearance. Indeed, Pyk2 signaling appears to be an important aspect of M. avium subsp. paratuberculosis infection, as Pyk2 inhibitors significantly reduce the number of intracellular M. avium subsp. paratuberculosis bacteria. The actions of M. avium subsp. paratuberculosis on TLR9 signaling may represent a strategy to generate a host environment which is better suited for infection, revealing potential new targets for therapeutic intervention.
INTRODUCTION
Johne's disease (JD), a chronic inflammatory disorder of the gastrointestinal tract of ruminants, is caused by Mycobacterium avium subsp. paratuberculosis (1, 2). JD is responsible for the highest average production losses among five production-limiting diseases of the dairy industry and is therefore of considerable economic importance (3). The realized economic impact, and potential zoonotic threat, of M. avium subsp. paratuberculosis has energized efforts to develop effective disease management strategies to reduce colonization of cattle by this pathogen. Understanding the mechanisms by which M. avium subsp. paratuberculosis subverts host immune responses could form the basis for rational development of a vaccine and/or immunotherapeutics. From a broader perspective, certain aspects of M. avium subsp. paratuberculosis infection may enable JD to serve as an animal model of human mycobacterial diseases, such as tuberculosis, as well as other enteric disorders such as Crohn's disease and irritable bowel disorder.
M. avium subsp. paratuberculosis colonization of the bovine host includes its eventual localization to phagosomes of intestinal macrophages (4, 5). Commandeering of these cells requires subversion of the normal cellular functions of the macrophage that would result in destruction of the internalized bacteria (6, 7). Various pathogenic mycobacteria, including M. avium subsp. paratuberculosis, have been shown to disrupt cellular processes to ensure their intracellular survival. The subversions of host processes by mycobacteria are complex, involving a number of mechanisms, including secreted effectors, altered calcium signaling, and iron metabolism (8, 9, 10, 11). Mycobacteria, including M. avium subsp. paratuberculosis, are perhaps best characterized for their ability to block phagosomal acidification and endosomal fusion (12, 13, 14); however, M. avium subsp. paratuberculosis also targets other host processes to optimize intracellular survival. For example, emerging evidence suggests that M. avium subsp. paratuberculosis desensitizes infected cells to stimuli that promote clearance of intracellular pathogens. This strategy of overriding host defensive signaling responses is utilized by many pathogens, in particular those causing chronic infections (15).
Clearance of intracellular pathogens is classically associated with gamma interferon (IFN-γ). Not surprisingly, a number of intracellular pathogens, including mycobacteria, disrupt the ability of infected cells to respond to IFN-γ. M. avium subsp. paratuberculosis-infected cattle produce elevated levels of IFN-γ (16) but are unable to successfully resolve the infection. This suggests an inability of cells in the infected animals to respond to, rather than produce, IFN-γ. We have confirmed that M. avium subsp. paratuberculosis infection of bovine monocytes limits their responsiveness to IFN-γ through the induced expression of suppressors of cytokine signaling (SOCS) repressor proteins and decreased expression of the IFN-γ receptor (17). M. avium subsp. paratuberculosis may exert similar influence over other host defense systems. In particular, emerging evidence suggests that immune evasion by mycobacteria may involve modulation of Toll-like receptor (TLR) function (18).
Toll-like receptors are a family of pathogen recognition receptors responsible for sensing and responding to microbial challenge through recognition of specific pathogen-associated molecular patterns (19). Mutations of the TLR system are associated with increased sensitivity to infection by M. avium subsp. paratuberculosis (20, 21). Altered levels of TLR expression during natural M. avium subsp. paratuberculosis infection suggest that M. avium subsp. paratuberculosis influences TLR signaling as part of its strategy to facilitate infection (22). A number of mammalian TLRs, including TLR9, have roles in mycobacterial infection (23, 24, 25). The specific nature of the contributions of the TLRs to host-pathogen interaction with M. avium subsp. paratuberculosis is complex and controversial; mycobacterial activation of certain TLRs initiates responses that are beneficial to the host (26), while activation of other TLRs, such as TLR2, appears to suppress macrophage antimicrobial responses (27). In addition to the generic TLR-mediated antimicrobial responses, there are additional phenotypes associated with activation of these receptors. In particular, phagosomal maturation can be triggered through TLR receptors, making them logical targets for mycobacterial subversion (28).
In addition to their contribution to the host-pathogen interaction, TLRs also represent important immunotherapeutic targets. Of the TLRs, the greatest efforts for development of immunotherapeutics have been devoted toward TLR9. TLR9 is activated by microbial DNA containing unmethylated CpG motifs, a ligand which can be effectively mimicked by synthetic CpG oligodeoxynucleotide (ODN) analogs (29). These agonists have been applied in a range of animal models as prophylactic therapies, vaccine adjuvants, and antimicrobial therapies, including treatment of a variety of intracellular pathogens (30, 31). A number of studies also indicate the potential to use CpG ODNs for treatment of mycobacterial infections. Pretreatment of human monocyte-derived macrophages with CpG ODNs, but not other TLR agonists, promotes maturation of the phagolysosome and inhibits the growth of M. tuberculosis (32). CpG ODNs have also been shown to offer protective effects in a number of mouse models of mycobacterial infection (33). Collectively, these results have been interpreted to indicate that CpG ODNs induce effective antimycobacterial responses in a variety of cell types of a variety of species. While this offers hope that CpG ODNs might have therapeutic significance in the treatment of mycobacterial diseases, it is critical to consider the ability of these sophisticated pathogens to alter the CpG ODN responsiveness in relevant cells of relevant species.
Classically, signaling through the Toll-like systems involves an intracellular cascade that is initiated by the direct interaction of myeloid differentiation primary response gene 88 (MyD88) with the dimerized, ligand-bound TLR. This initiates the recruitment and activation of interleukin 1 (IL-1) receptor-activated kinase 4 (IRAK-4), which phosphorylates IRAK-1, resulting in IRAK-1 association with tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) for eventual activation of nuclear factor kappa B (NF-κB). Activation of NF-κB occurs via the IkB kinase (IKK) complex through the phosphorylation-triggered degradation of IkB, which allows nuclear translocation of NF-κB, with subsequent induction of various inflammatory genes associated with a proinflammatory innate immune response (34). Variations on this signaling pathway have been identified, including links between TLR signaling and activation of mitogen-activated protein kinases (MAPKs) (35) as well as, in the case of TLR3/4, MyD88-independent signaling through Toll/interleukin 1 receptor domain-containing adapter inducing IFN-beta (TRIF) (36, 37). Despite these exceptions, MyD88 remains a key transducer of TLR signals. Interestingly, knockout studies with mice have shown that Myd88−/− mice are highly susceptible to infection by aerosolized M. tuberculosis (38), and this has been linked to a direct association of MyD88 with the IFN receptor (39), hinting that MyD88 could be a common target for pathogen evasion of host responses.
In this investigation, we examined whether infection of bovine monocytes, physiologically relevant cells of the host organism, with M. avium subsp. paratuberculosis alters their ability to respond to CpG ODN stimulation. TLR9 responsiveness is evaluated through functional assays of IL-10 induction and oxidative burst as well as through global kinome analysis utilizing a bovine-specific peptide array. Inhibition of IL-10 induction is observed while other phenotypes, such as oxidative burst, remain functional in M. avium subsp. paratuberculosis-infected cells. Kinome analysis verifies that M. avium subsp. paratuberculosis blocks classical TLR signaling to the activation of NF-κB, offering an explanation for the inability of the infected cells to respond to CpG ODNs in spite of increased expression of the TLR9 receptor and maintained ability to take up CpG ODNs. Kinome analysis also reveals that secondary signaling pathways from TLR9 are specifically activated in M. avium subsp. paratuberculosis-infected cells. These pathways are initiated from Pyk2, a downstream effector of MyD88, through MAP kinase signaling to activate functional responses such as oxidative burst. Signaling through these tertiary TLR9-mediated signaling pathways appears insufficient to initiate a protective response, as treatment of M. avium subsp. paratuberculosis-infected monocytes with CpG ODNs fails to reduce bacterial loads. Instead, activation of Pyk2 signaling appears to benefit the pathogen, as treatment of M. avium subsp. paratuberculosis-infected bovine monocytes with a Pyk2 inhibitor promotes a dramatic reduction of intracellular M. avium subsp. paratuberculosis. Collectively, these results reveal a potential mechanism that M. avium subsp. paratuberculosis uses to evade host immune responses by altering the TLR9 signaling normally elicited by CpG ODNs, highlighting the complex interplay between M. avium subsp. paratuberculosis and its bovine host, and the plasticity of TLR9 signaling. The ability of Pyk2 inhibitors to promote clearance of M. avium subsp. paratuberculosis may also form the basis for rationale development of therapeutics for JD.
MATERIALS AND METHODS
Isolation of bovine blood monocytes.
Blood was collected from 3 cattle (9-month-old Charolais cross steers) by venipuncture using tubes containing EDTA as an anticoagulant. Blood was transferred to 50-ml polypropylene tubes and centrifuged at 1,400 × g for 20 min at 20°C. Mononuclear leukocytes were isolated from the buffy coat and mixed with PBSA (Ca2+- and Mg2+-free phosphate-buffered saline [PBS]) to a final volume of 35 ml. The cell suspension was layered onto 15 ml of 54% isotonic Percoll (Amersham Biosciences, GH Healthcare) and centrifuged at 2,000 × g for 20 min at 20°C. Peripheral blood mononuclear cells (PBMCs) from the Percoll-PBSA interface were collected and washed three times with cold PBSA. Monocytes were purified from isolated PBMCs by magnetically activated cell sorting (MACS) purification using anti-CD14 microbeads (Miltenyi Biotec Inc., Auburn, CA). Monocytes (>95% CD14+) were plated at 5 × 106 cells/well in 6-well plates in RPMI 1640 medium (GIBCO) supplemented with 10% fetal bovine serum (FBS; GIBCO). Isolated monocytes were rested overnight prior to stimulation.
Infection of bovine monocytes with M. avium subsp. paratuberculosis.
M. avium subsp. paratuberculosis K10 culture was incubated at 37°C on Middlebrook 7H10 agar (Difco Laboratories, Detroit, MI) with oleic acid-albumin-dextrose-catalase (OADC) enrichment medium (Difco Laboratories) and mycobactin J (Allied Monitor Inc., Fayette, MO). After 3 to 4 weeks of growth, colonies were transferred to Middlebrook 7H9 broth (Difco Laboratories) containing 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO), OADC enrichment medium, and mycobactin J and incubated at 37°C for 5 days to achieve log-phase growth. CFU were determined using the pelleted wet-weight method whereby 1 mg of M. avium subsp. paratuberculosis pellet is equal to 107 CFU (40). A 50-ml centrifuge tube was weighed prior to the addition of 50 ml of a 5-day liquid M. avium subsp. paratuberculosis culture. The culture was centrifuged at 3,400 × g for 30 min. Supernatant was decanted and the pellet dried for 30 min. Tube weight was then recorded and pellet weight determined. The M. avium subsp. paratuberculosis pellet was then resuspended in the appropriate volume of cell culture medium to achieve a 5:1 multiplicity of infection (MOI). Appropriate bacterial loads were added to each well which contained five million monocytes. Plates were spun at 300 × g for 2 min to promote interaction between M. avium subsp. paratuberculosis and the monocytes. All infected plates were incubated for 3 h at 37°C. Medium was removed, and cells were washed three times with warm RPMI 1640 medium.
Cytospins.
Cells were harvested using a trypsin-EDTA (0.05% trypsin) solution. The cells were prepped for cytospins by centrifugation at 325 × g for 5 min. Cells were resuspended in 200 μl of PBSA plus 0.1% EDTA. Cytospins were performed by adding 100 μl of cell suspension to the cytospin (Cytospin 4 [ThermoShandon]) and spinning at 300 × g for 3 min to deposit cells onto a glass slide. Slides were allowed to dry overnight in a fume hood. Cells were heat fixed to slides by briefly passing the slides through a flame. Slides were placed over boiling water, stained with carbol fuchsin for 5 min, and rinsed, and acid destain was briefly added to each slide before rinsing with water. Slides were counterstained using methylene blue (Sigma) for 1 min and rinsed with water. Slides were allowed to dry overnight in a fume hood and then cover slipped using Entellan New rapid mounting medium (Electron Microscopy Sciences). Cells were observed on a light microscope under oil immersion (100×). Over three replicate experiments, an infection efficiency of 93% ± 4% was observed.
Stimulation with TLR agonists.
MACS-purified CD14+ bovine monocytes were stimulated with 100 ng/ml of lipopolysaccharide (LPS) (Escherichia coli O111:B4) (Sigma-Aldrich), 100 ng/ml of flagellin (purified from Salmonella enterica serovar Typhimurium [Alexis Biochemicals]), 5 μg/ml of CpG 2007 (Merial), or medium for 3 h at 37°C. This quantity and this type of LPS were previously shown to induce cellular responses in bovine monocytes (41). CpG ODNs are often species specific in their ability to induce innate immune responses, and ODN 2007 (TCGTCGTTGTCGTTTTGTCGTT) was shown to activate bovine monocytes (41). Cells were pelleted and stored at −80°C before use with the peptide arrays. Unless otherwise specified, investigations of cellular responses to TLR stimulation were conducted after incubation of the cells with the TLR agonist for 3 h at 37°C.
RNA extraction.
Quantitative reverse transcription-PCR (qRT-PCR) to determine the impact of M. avium subsp. paratuberculosis infection on TLR expression was performed 5 h postinfection. Total RNA extraction was performed as per the RNeasy minikit protocol (Qiagen). Briefly, 1 ml of buffer RLT plus beta-mercaptoethanol was added to each well for 5 min. Cells were collected in a 2-ml tube, vortexed briefly, and stored at −80°C until further processing. Homogenization of samples was achieved by running samples through a QIAshredder (Qiagen). Molecular-grade ethanol was added to each sample before running the sample through an RNeasy minispin column. An optional DNase treatment was performed on each sample by adding a DNase solution (Qiagen) to the column and allowing the solution to sit for 15 min. Three washes were performed, followed by elution in nuclease-free water. Each sample was quantified and checked for purity using a 2100 Bioanalyzer (Agilent Technologies, Inc.).
Preparation of cDNA library.
RNA (200 ng) was converted to cDNA by adding 8 μl of 2× RT buffer and 2 μl of RT enzyme (Invitrogen) to a total volume of 10 μl. A master mixture of buffer and enzyme was made to eliminate pipetting error. Samples were placed in a thermocycler under the following conditions: 25°C for 5 min, 50°C for 60 min, and 70°C for 15 min. RNA was removed by adding 1 μl of E. coli RNase H for 20 min. cDNA was stored at −20°C.
qRT-PCR.
Each reaction mixture for qRT-PCR included 9 μl of iQ SYBR green master mix (Bio-Rad), 3 μl of primer mix (3.3 μM), 2 μl of nuclease-free water, and 1μl of cDNA for a total of 15 μl of reaction mixture. Thermocycler conditions were as follows: cycle 1, 55°C for 2 min; cycle 2, 95°C for 8.5 min; cycle 3, step 1 at 95°C for 15 s, step 2 at 55°C for 30 s, and step 3 at 72°C for 30 s; and cycle 4, 55°C for 10 s. The set point temperature was increased after cycle 2 by 1°C. Results were analyzed using the threshold cycle (2−ΔΔCT) method described in Applied Biosystems user bulletin no. 2 (part no. 4303859). This primer set and the PCR conditions used have been previously validated for specificity and efficiency of characterization of bovine TLR expression (42).
Oxidative-burst assays.
Superoxide production was measured by chemiluminescence by established protocols, with the exception that 4 × 106 bovine monocytes were used without phorbol myristate acetate activation (41). Cells were treated with 5 μg/ml of CpG ODN for 3 h. Before luminescence was read, the 96-well plate was wrapped in foil and incubated at 37°C and 5% CO2 for 20 min. Luminescence was measured at 30-s intervals by using a VICTOR3V 1420 multilabel counter (PerkinElmer).
Western blotting.
Purified bovine monocytes, both infected and uninfected, were stimulated with TLR agonists as described above for 3 h at 37°C. Stimulated cells were then pelleted, lysed with 100 μl of SDS sample buffer (62.5 mM Tris-HCl [pH 6.8 at 25°C], 2% [wt/vol] SDS, 10% glycerol, 50 mM dithiothreitol [DTT], 0.01% [wt/vol] bromophenol blue, 1 mM phenylmethylsulfonyl fluoride [PMSF]), and denatured (5 min at 95°C), and 30 μl of lysate was loaded on a 10% SDS gel and transferred to a nitrocellulose membrane. Phospho-Pyk2(Y402) and Pyk antibody (New England BioLabs) and a secondary fluorescent antibody (LI-COR Biosciences) were used in accordance with the supplier's protocol. An image of the blot was captured with an Odyssey scanner (LI-COR Biosciences).
ELISA.
Purified bovine monocytes were treated as indicated above for the infection and TLR agonist experiments. For the Pyk2 inhibitor experiment, 5 μM, 15 μM, or 30 μM PP2 (Sigma) was added to 5 × 105 cells in a 24-well plate which had been incubated overnight. LPS was added to appropriate wells as indicated above. Cells were left for 3 h at 37°C with 5% CO2. Following this incubation, 5 μg/ml of CpG was added to the appropriate wells. Cells were incubated at 37°C with 5% CO2 for 48 h before supernatant was collected for enzyme-linked immunosorbent assay (ELISA). For the TLR and infection experiments, plates were incubated overnight. Supernatant was collected from each well, and IL-10 secretion was measured using a capture ELISA specific for bovine IL-10 (43).
Viable counts.
Purified monocytes (1 × 106) were added to each well of a 6-well plate in 3 ml of RPMI plus 10% FBS. Cells were incubated overnight at 37°C with 5% CO2. PP2 at either 5 μM, 10 μM, 15 μM, or 20 μM was added to appropriate wells for 1 h. Infection with M. avium subsp. paratuberculosis was done as described above. Medium was replaced, and replacement PP2 was added. Cells were then incubated for 18 h at 37°C with 5% CO2. Following incubation, medium was removed and 1 ml of 0.1% Triton-X (Sigma) in 0.1 M PBS was added to wells to lyse monocytes. Cells were incubated for 30 min at 37°C, and dilutions were made in 0.1 M PBS and plated onto Difco Middlebrook 7H10 agar (BD). Plates were incubated for approximately 4 weeks at 37°C, until colonies were visible for counting.
Cellular uptake of ODNs.
Isolated bovine monocytes (1 × 106) were treated with 4 μg of fluorescently labeled CpG 1826 ODN (Eurofins MWG Operon, Huntsville, AL), in the presence or in the absence of M. avium subsp. paratuberculosis infection. Cells were incubated with each treatment for 30 min at 37°C with 5% CO2. Cells were removed and transferred to 15-ml polypropylene tubes, washed with PBSA, and spun down at 1,200 rpm for 10 min. After the final wash, cells were resuspended in 400 μl of PBSA and analyzed by fluorescence-activated cell sorter (FACS) analysis.
Peptide arrays.
Design, construction, and application of the peptide arrays was based upon a previously reported protocol, with modifications (44). Notably, the kinome arrays were all performed within a single experiment to minimize technical variance. Briefly, approximately 10 × 106 cells were pelleted and lysed by addition of 100 μl of lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 mM NaF, 1 μg/ml of leupeptin, 1 g/ml of aprotinin, 1 mM PMSF) (all products from Sigma-Aldrich unless otherwise indicated). Cells were incubated on ice for 10 min and spun in a microcentrifuge for 10 min at 4°C. A 70-μl aliquot of the supernatant was mixed with 10 μl of activation mix (50% glycerol, 500 μM ATP [New England BioLabs, Pickering, Ontario, Canada], 60 mM MgCl2, 0.05% [vol/vol] Brij 35, 0.25 mg/ml of BSA) and then incubated on the peptide array for 2 h at 37°C. Arrays were then washed with PBS–1% Triton.
Slides were submerged in phospho-specific fluorescent ProQ Diamond phosphoprotein stain (Invitrogen) with agitation for 1 h. Arrays were then washed three times in destain containing 20% acetonitrile (EMD Biosciences, VWR distributor, Mississauga, Ontario, Canada) and 50 mM sodium acetate (Sigma) at pH 4.0 for 10 min. A final wash was done with distilled deionized H2O. Arrays were air dried for 20 min and then centrifuged at 300 × g for 2 min to remove any remaining moisture from the array. Arrays were read using a GenePix Professional 4200A microarray scanner (MDS Analytical Technologies, Toronto, Ontario, Canada) at 532 to 560 nm with a 580-nm filter to detect dye fluorescence. Images were collected using GenePix 6.0 software (MDS). Spot intensity signals were collected as the mean pixel intensity using local feature background intensity background calculation with the default scanner saturation level.
Data analysis. (i) Data sets.
The data set contains the signal intensities associated with each of 300 peptides for the monocytes under the different treatment conditions. Those treatments are labeled “CpG” (CpG treatment alone), “MAP” (M. avium subsp. paratuberculosis infection alone), “MAP + CpG” (M. avium subsp. paratuberculosis infection followed by CpG treatment), and “Mono” (no treatment). For each animal and each treatment, there are three intra-array replicates. All data processing and analyses were done through previously described approaches (45), with the following study specifics.
(ii) Animal-animal variability analysis.
For each of the 300 peptides, an F test was used to determine whether there are significant differences among the three animals under the same treatment condition. Therefore, 300 F tests were carried out for a single treatment on the three animals, with 1,200 tests in total for all four treatments (i.e., 300 peptides × 4 treatments).
(iii) Treatment-treatment variability analysis.
Peptides identified by the F test as having consistent patterns of response to the various treatments across the three animals were subjected to a paired t test to compare their signal intensities under a treatment condition with those under control conditions. For each animal-independent peptide, the responses from all three animals were pooled to increase the statistical confidence. Three tests were performed for each peptide, specifically CpG versus Mono, MAP + CpG versus Mono, and MAP versus Mono. Peptides with significant (P < 0.1) changes in phosphorylation were identified. This level of significance was chosen to retain as many data as possible and thus facilitate subsequent pathway analysis.
(iv) Pathway analysis of differentially phosphorylated peptides.
InnateDB is a publicly available resource which, based on levels of either differential expression or phosphorylation, predicts biological pathways based on experiment fold change data sets (46). Pathways are assigned a probability value (P) based on the number of proteins present for a particular pathway as well as the degree to which they are differentially expressed or modified relative to a control condition. For our investigation, input data were limited to those peptides selected in the treatment-treatment variability analysis (see above). Since InnateDB requires fold change (FC) values as input (with P values optional), the differences between the variance stabilization-transformed intensities under control and treatment are converted to fold change values by the formula 2d, where d = averagetreatment − averagecontrol, as previously described (45).
RESULTS
CpG-induced IL-10 secretion.
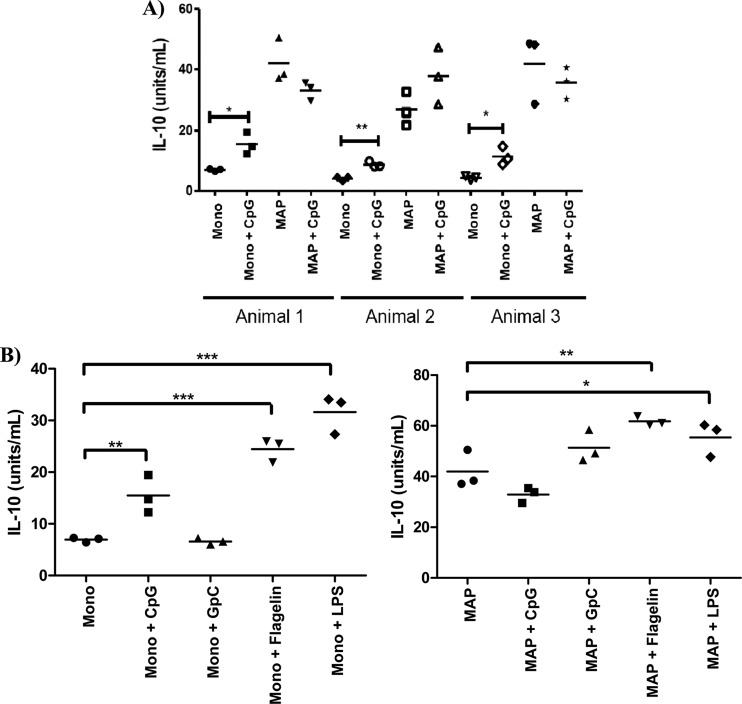
The influence of M. avium subsp. paratuberculosis infection on TLR9 responsiveness was first evaluated through the capacity of M. avium subsp. paratuberculosis-infected and uninfected monocytes to secrete IL-10 in response to CpG ODN stimulation. IL-10 secretion is a well-established biomarker of TLR responsiveness by bovine monocytes (47, 48). Furthermore, as M. avium subsp. paratuberculosis is known to induce IL-10 expression, this may also represent a key host response during persistent M. avium subsp. paratuberculosis infection (49). As M. avium subsp. paratuberculosis infection increases IL-10 production, the induction of IL-10 through TLR stimulation was evaluated relative to the higher baseline of IL-10 within infected cells; induction of IL-10 by CpG ODNs was considered relative to the unstimulated cells of the same infection status. Furthermore, within outbred populations, such as cattle, a natural variance in CpG responsiveness is anticipated (50). Accordingly, monocytes were isolated from three animals and considered individually; IL-10 induction was considered relative to the resting levels of IL-10 production from cells of the same animal.
Monocytes purified from three animals were TLR9 responsive, as indicated by significant increases in IL-10 production following CpG treatment (P < 0.05 for two animals and 0.01 for the third animal) (Fig. 1A). In contrast, relative to the elevated IL-10 secretion level induced by M. avium subsp. paratuberculosis infection alone, treatment of M. avium subsp. paratuberculosis-infected cells with CpG ODNs failed to induce a significant increase in IL-10 production (Fig. 1A).
Fig 1.
TLR-stimulated production of IL-10 in M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes. MACS-purified CD14+ bovine monocytes, either uninfected or infected with Mycobacterium avium subsp. paratuberculosis (MAP) (MOI = 5), were treated with either 100 ng/ml of LPS (Escherichia coli O111:B4), 100 ng/ml of flagellin, 5 μg/ml of CpG 2007, 5 μg/ml of GpC 2007, or medium controls for 48 h. The supernatant was used to perform ELISAs for bovine IL-10. Statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. (A) CpG-stimulated production of IL-10 in M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes. (B) TLR-stimulated production of IL-10 in M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes.
Effect of M. avium subsp. paratuberculosis infection is specific for TLR9.
While TLR9 was the focus of this investigation, other TLR agonists were considered to establish a context, and possible mechanism, for the impact of M. avium subsp. paratuberculosis infection on TLR signaling. Treatment of uninfected bovine monocytes with TLR agonists results in significant increases in IL-10 production (CpG ODNs [P < 0.01], flagellin [P < 0.001], and LPS [P < 0.001]). GpC ODN, a negative-control antagonist of TLR9, fails to increase IL-10 production (Fig. 1B). Consistent with our previous experiment, CpG ODN treatment of M. avium subsp. paratuberculosis-infected bovine monocytes causes no further increase in IL-10 secretion. In contrast, M. avium subsp. paratuberculosis infection does not block the ability of agonists of either TLR4 or TLR5 to promote increased secretion of IL-10 (Fig. 1B). Notably, for each of these other TLR ligands the absolute increase in IL-10, relative to the unstimulated control, is the same for both M. avium subsp. paratuberculosis-infected and uninfected cells. This suggests that M. avium subsp. paratuberculosis infection has minimal impact on the responsiveness of these TLRs and that the mechanisms used to dampen TLR9 responses are relatively specific. Finally, the levels of IL-10 induced by the other TLR ligands from M. avium subsp. paratuberculosis-infected cells are considerably higher than those observed from the M. avium subsp. paratuberculosis-infected cells. This indicates that the inability of M. avium subsp. paratuberculosis-infected bovine monocytes to respond to TLR9 agonists does not reflect the inability of the cells to produce IL-10 above the levels which result from M. avium subsp. paratuberculosis infection alone.
TLR9 expression in M. avium subsp. paratuberculosis-infected monocytes.
There are a number of potential mechanisms through which M. avium subsp. paratuberculosis infection could dampen CpG ODN responsiveness, most simply through decreased expression of TLR9. Previously, our group reported that the decreased responsiveness of M. avium subsp. paratuberculosis-infected bovine monocytes to IFN-γ stimulation reflected in part decreased expression of the IFN-γ receptor (17). Additionally, reports indicate that M. avium subsp. paratuberculosis impacts the levels of TLR expression during natural infection (22, 45).
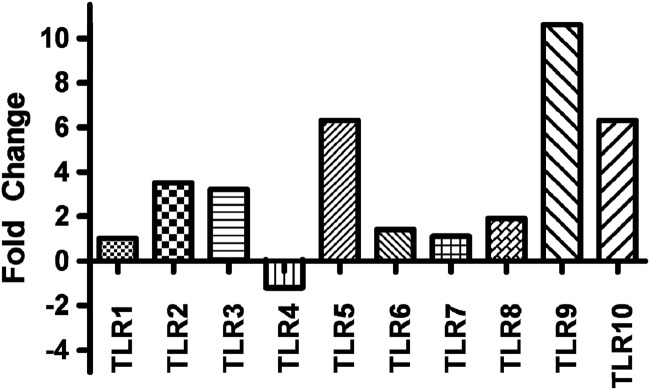
While the primary interest of this investigation was the impact of M. avium subsp. paratuberculosis on TLR9 signaling, patterns of expression of TLR1 through TLR10 were analyzed to determine the specificity and magnitude of impact of M. avium subsp. paratuberculosis infection on TLR expression. M. avium subsp. paratuberculosis infection of purified bovine monocytes was found to dramatically impact levels of expression of several TLRs. Most notably, relative to uninfected cells, M. avium subsp. paratuberculosis infection increased expression of TLR9 greater than 10-fold (Fig. 2). Other TLRs, such as TLR5 and TLR10, also showed increased, although less pronounced, induction following M. avium subsp. paratuberculosis infection. This observation is consistent with previous investigations that found that in vivo expression of TLR9 is increased during natural M. avium subsp. paratuberculosis infection (51). The increased expression of TLR9 following M. avium subsp. paratuberculosis infection is seemingly in functional contradiction to the observed decreased sensitivity of the infected cells to CpG ODN stimulation. However, perhaps the most significant conclusion from these observations is the focused impact of M. avium subsp. paratuberculosis on TLR9, as increased expression of the receptor may represent a compensatory mechanism by the cell to overcome dampening effects mediated by M. avium subsp. paratuberculosis.
Fig 2.
Expression of various TLRs in M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes. Bovine monocytes were isolated from whole blood using CD14+ microbeads and MACS separation columns. Bovine monocytes were infected with a 6-day liquid culture of Mycobacterium avium subsp. paratuberculosis at a 5:1 ratio. Expression of the various TLRs was determined at 5 h postinfection through qRT-PCR and is expressed as fold change relative to uninfected monocytes. Data are presented as mean values for monocytes isolated from (n = 3) animals.
CpG uptake by M. avium subsp. paratuberculosis-infected cells.
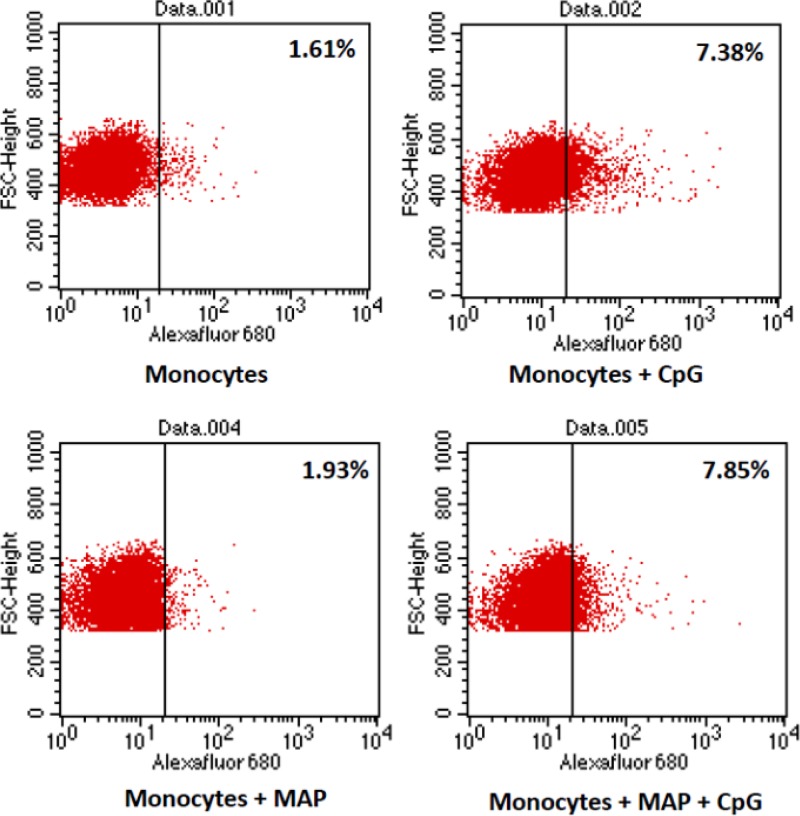
As M. avium subsp. paratuberculosis is known to impact the trafficking and maturation of intracellular organelles (12, 13, 14), the ability to respond to CpG stimulation could reflect an inability to take up ODNs in spite of normal, or even increased, expression of TLR9. This potential disconnect between levels of receptor expression and cellular responsiveness was investigated by quantifying uptake of fluorescently labeled ODNs by uninfected and M. avium subsp. paratuberculosis-infected bovine monocytes. There was no significant difference in ODN uptake between uninfected and M. avium subsp. paratuberculosis-infected cells (Fig. 3). The apparent increased levels of TLR9 receptor expression, and retained ability to take up CpG ODN ligands, indicate that the inability of the M. avium subsp. paratuberculosis-infected cells to respond to CpG stimulation reflects disruption of intracellular signaling events that occur subsequent to ligand binding by TLR9.
Fig 3.
Uptake of CpG ODNs in M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes. MACS-purified CD14+ bovine monocytes were infected with Mycobacterium avium subsp. paratuberculosis (MAP) (MOI = 5) for 3 h before comparison of uptake of Alexafluor-conjugated CpG ODNs in infected and uninfected monocytes.
Kinome analysis.
Kinome analysis was performed to address the hypothesis that the impact of M. avium subsp. paratuberculosis on TLR9 responsiveness occurs at a level of signal transduction rather than ligand binding. Cellular extracts prepared from purified bovine monocytes under the various infection and treatment conditions were subjected to kinome analysis utilizing a novel bovine-specific peptide array developed by our lab (44) and employing a software platform for kinome data analysis developed by our group (45).
Pathway analysis.
Kinome data were subjected to pathway overrepresentation analysis to identify cellular pathways/processes activated under the different treatment conditions. To ensure that the identified pathways represent conserved and consistent biological responses, input data were limited to peptides with significant changes in phosphorylation level relative to the control treatment. These selected data were analyzed through InnateDB (46).
Activation of the TLR pathway in uninfected, but not M. avium subsp. paratuberculosis-infected, monocytes.
As anticipated, and as we have previously demonstrated, treatment of purified bovine monocytes with CpG ODNs resulted in activation of the TLR signaling pathway (41). Pathway overrepresentation analysis identified the pathway associated with TLR signaling as being activated (Table 1). The TLR signaling pathway was identified as the top hit with very high confidence (P < 0.02). This pathway is presented by 18 peptides on the array that correspond to TLR signaling intermediates. Following CpG stimulation, all 18 showed significant (P < 0.10) differential phosphorylation relative to the unstimulated monocytes, 14 with increased phosphorylation and 4 with decreased phosphorylation (Table 1). In contrast, TLR signaling pathways were not activated in M. avium subsp. paratuberculosis-infected cells following CpG treatment. Instead, pathway analysis indicated that the TLR signaling pathway was downregulated under these conditions (P < 0.06).
Table 1.
Pathway analysis of M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes in response to CpG stimulationa
| Signaling type | Pathway | ↕ | Uninfected monocytes |
M. avium subsp. paratuberculosis-infected monocytes |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Upregulated |
Downregulated |
Upregulated |
Downregulated |
|||||||
| ↑ | P | ↓ | P | ↑ | P | ↓ | P | |||
| TLR | Toll-like receptor signaling pathway | 18 | 14 | 0.02 | 4 | 0.99 | 2 | 0.98 | 8 | 0.06 |
| Pyk2 | Links between Pyk2 and MAPKs | 10 | 3 | 0.98 | 7 | 0.07 | 9 | 0.01 | 1 | 1 |
InnateDb is a publicly available pathway analysis tool (31). Based on levels of differential expression or phosphorylation, InnateDB is able to predict pathways which are consistent with the experimental data. Pathways are assigned a probability value (P) based on the number of proteins present for a particular pathway. Output also includes the number of the uploaded pathways associated with a particular pathway as well as the subset of those which are differentially phosphorylated. For our investigation, fold change cutoffs were set at confidence of difference between treatment and monocyte control equal to a P value of <0.1. ↕, number of peptides on the array relating to the pathway; ↑ and ↓, number of peptides with increased and decreased phosphorylation, respectively, with respect to the control condition.
Activation of Pyk2 signaling in M. avium subsp. paratuberculosis-infected monocytes.
As the arrays represent a vast number of signaling events and pathways, they also afford considerable opportunity for validation work as well as novel discovery. Kinome analysis proved an effective tool to verify an absence of canonical TLR signaling in M. avium subsp. paratuberculosis-infected bovine monocytes following treatment with CpG ODNs. Interestingly, under the same conditions, the pattern of peptide phosphorylation observed on the arrays was consistent with activation of the Pyk2 signaling pathway. Specifically, pathway analysis indicated with a high degree of confidence (P < 0.01) that CpG treatment of M. avium subsp. paratuberculosis-infected cells activated Pyk2 signaling (Table 1).
Pyk2 is a major cell adhesion-activated tyrosine kinase and is highly expressed in macrophages and monocytes. Traditionally, Pyk2 is associated with lymphocyte migration but is also involved in macrophage activation and inflammatory responses (52). Pyk2 has recently been linked to TLR signaling, with the observation that Pyk2 is activated through interaction with MyD88, an early signaling intermediate in the TLR pathway (53).
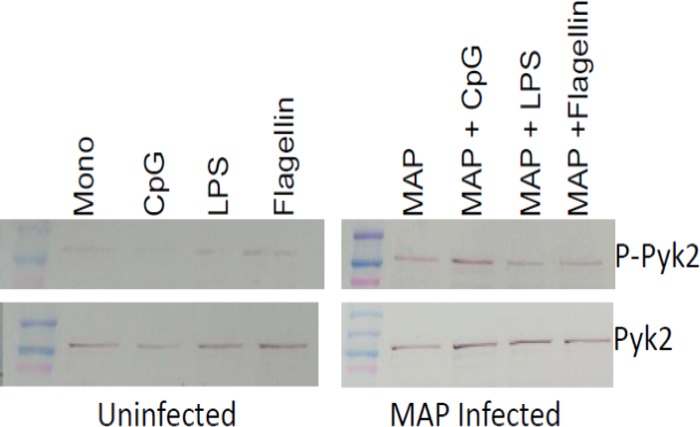
The activation of Pyk2-mediated signaling upon CpG stimulation of M. avium subsp. paratuberculosis-infected bovine monocytes was verified with phosphorylation-specific antibodies (Fig. 4). These experiments included stimulation of M. avium subsp. paratuberculosis-infected monocytes with a number of TLR agonists, including CpG ODNs (TLR9), LPS (TLR4), and flagellin (TLR5). Interestingly, while the MyD88 intermediate is common to all TLR pathways, within the M. avium subsp. paratuberculosis-infected cells, the further activation of Pyk2 appeared to be specific to CpG stimulation (Fig. 4). This may be consistent with the observation that M. avium subsp. paratuberculosis infection has the greatest impact on TLR9 expression and responsiveness, which may indicate that the pathogen prioritizes the TLRs or that particular TLRs are more sensitive to some aspect of, or response to, M. avium subsp. paratuberculosis infection.
Fig 4.
Activation of Pyk2 in M. avium subsp. paratuberculosis-infected and uninfected bovine monocytes. MACS-purified CD14+ bovine monocytes were infected with Mycobacterium avium subsp. paratuberculosis (MAP) (MOI = 5) for 3 h before stimulation with TLR agonists (100 ng/ml of LPS, 5 μg/ml of CpG 2007, or 100 ng/ml of flagellin or medium) for an additional 3 h. Western blotting was performed to compare levels of Pyk2 expression and activation of Pyl2 through phosphorylation with Pyk2 and phospho-Pyk2(Y402) antibodies, respectively.
TLR9-mediated activation of oxidative burst in M. avium subsp. paratuberculosis-infected cells.
Activation of the Pyk2 signaling response is predicted to activate oxidative burst through phosphorylation-mediated control of p40phox and p47phox proteins (41). Consistent with this hypothesis, peptides representing these phosphorylation events on the kinome array underwent patterns of phosphorylation consistent with activation of oxidative burst (data not shown). Activation of oxidative burst was selected as a response for further validation in part because oxidative burst is a readily quantifiable biomarker of cellular response. Validation of CpG ODN-mediated activation of oxidative burst in M. avium subsp. paratuberculosis-infected cells was also of interest to validate the ability of TLR9 to activate a functional response within M. avium subsp. paratuberculosis-infected cells and confirm that M. avium subsp. paratuberculosis functions to redirect, rather than completely inhibit, responses to CpG ODNs.
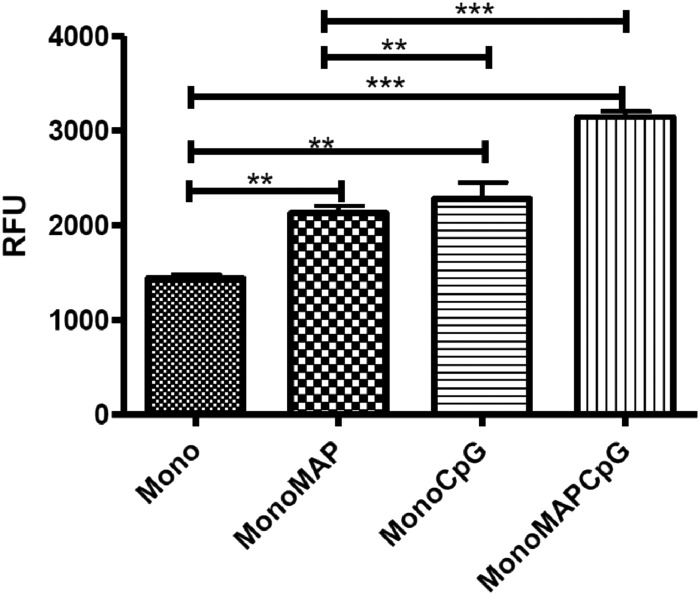
Consistent with previous reports from our group (41), treatment of bovine monocytes with CpG ODNs resulted in a significant (P < 0.01) increase in oxidative burst activity (Fig. 5). A similar level of oxidative burst activity was observed as a consequence of M. avium subsp. paratuberculosis infection. Using the evaluated level of oxidative burst observed in the M. avium subsp. paratuberculosis-infected cells as the new baseline, further increases in this activity were observed following treatment with CpG ODNs (P < 0.001) (Fig. 5). This result indicated that infection of bovine monocytes with M. avium subsp. paratuberculosis did not result in a complete blockage of TLR9 responsiveness and that the dampened CpG ODN responsiveness observed in the IL-10 assays represents a discrete, potentially temporal, impact of M. avium subsp. paratuberculosis on TLR9 responsiveness.
Fig 5.
Activation of oxidative burst. MACS-purified CD14+ bovine monocytes were infected with Mycobacterium avium subsp. paratuberculosis (MAP) (MOI = 5) for 3 h before stimulation with 5 μg/ml of CpG 2007 or medium for an additional 3 h. Data presented represent the mean ± 1 SD of values from monocytes isolated from (n = 3) animals. Statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. The magnitude of response is presented as relative fluorescence units (RFU).
CpG treatment of M. avium subsp. paratuberculosis-infected bovine monocytes.
A number of groups have demonstrated the ability of CpG ODNs to promote clearance of M. avium subsp. paratuberculosis, as well as other mycobacteria (32, 33). These findings have been taken as supportive evidence of the potential to use TLR9 agonists in the treatment of mycobacterial infections. Importantly, many of these investigations utilize ODNs as a pretreatment, which favors ODN action by preceding any inhibitory effects which may be exerted by M. avium subsp. paratuberculosis on TLR9 responses. Additionally, many of these studies look at the ability of CpG ODNs to promote clearance of M. avium subsp. paratuberculosis from cells of species that are not natural targets of M. avium subsp. paratuberculosis infection. In these cellular contexts, M. avium subsp. paratuberculosis may not be able to instigate its normal immune subversion.
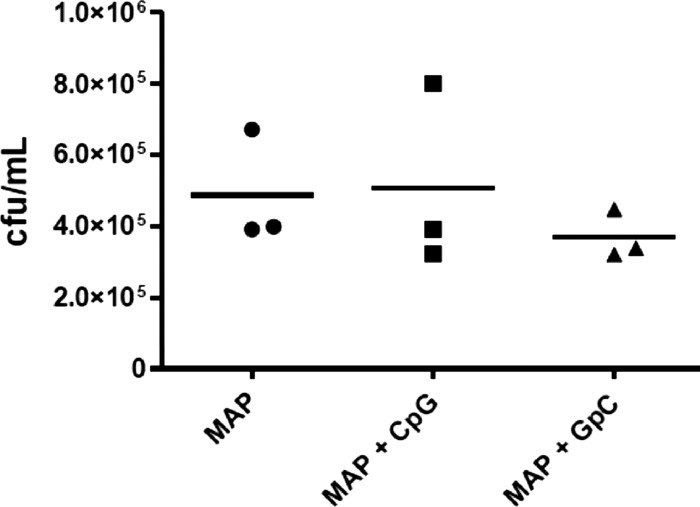
Our results indicated that M. avium subsp. paratuberculosis influences discrete aspects of TLR9-mediated signaling in bovine monocytes, but how this might influence the therapeutic potential of CpG ODNs for the clearance of M. avium subsp. paratuberculosis from bovine cells was not apparent. To address this question, M. avium subsp. paratuberculosis-infected monocytes were treated with CpG ODNs at 3 h postinfection for 18 h and lysed, and CFU of surviving M. avium subsp. paratuberculosis were quantified. Under these conditions, CpG ODN treatment failed to induce a significant reduction in M. avium subsp. paratuberculosis infection levels (Fig. 6). These experiments were conducted three times, with monocytes isolated from three different animals. Similar patterns were observed for the three animals, and the results from a single representative animal are shown. While there was variability in the extent to which monocytes were infected in each trial, presumably due to differences in the number of bacteria per cell rather than the percentage of cells which were infected, treatment with CpG ODNs failed to significantly reduce M. avium subsp. paratuberculosis infection in any of these trials. This is consistent with studies by other groups in which CpG treatment of infected cells failed to promote M. avium subsp. paratuberculosis clearance and is consistent with the hypothesis, and data generated thus far, that M. avium subsp. paratuberculosis may block aspects of TLR9-mediated responses which are required to promote clearance of M. avium subsp. paratuberculosis (32).
Fig 6.
Treatment of M. avium subsp. paratuberculosis-infected bovine monocytes with CpGs ODNs. MACS-purified CD14+ bovine monocytes were infected with Mycobacterium avium subsp. paratuberculosis (MAP) (MOI = 5) for 3 h and then treated with CpG ODN 2007 for 18 h. Treatment efficacy is evaluated through quantification of M. avium subsp. paratuberculosis viable counts determined through plating. These experiments were conducted three times, with monocytes isolated from three different animals. Data from a representative animal are shown.
Treatment of M. avium subsp. paratuberculosis infections with Pyk2 inhibitors.
Activation of Pyk2 during infection by M. avium subsp. paratuberculosis could reflect the priorities of the host to counter M. avium subsp. paratuberculosis infection, in which case inhibition of these responses would be anticipated to promote infections. Alternatively, activation of Pyk2 could represent host actions initiated by the pathogen to enhance intracellular survival, in which case disruption of these processes would be anticipated to favor M. avium subsp. paratuberculosis clearance. These potential outcomes were investigated by treating M. avium subsp. paratuberculosis-infected bovine monocytes with the Pyk2 inhibitor PP2 at levels that do not negatively impact the viability of the monocytes; monocytes maintain greater than 95% viability at the highest doses of inhibitor tested. Treatment of the infected cells with the Pyk2 inhibitor resulted in significant (a P value of <0.01 at 5 μM to a P value of <0.001 at 30 μM) reductions of intracellular loads of M. avium subsp. paratuberculosis (Fig. 7). These findings suggest that activation of a Pyk2-mediated response benefits the pathogen rather than the host. To confirm that the Pyk2 inhibitors were not having an effect on the cells' TLR9 response independent of M. avium subsp. paratuberculosis, we treated monocytes with concentrations of PP2 from 5 to 30 μM along with CpG. Results confirmed that PP2 treatment did not result in any significant decrease in monocyte IL-10 responsiveness when treated with CpG (data not shown).
Fig 7.
Treatment of M. avium subsp. paratuberculosis-infected bovine monocytes with Pyk2 inhibitors. MACS-purified CD14+ bovine monocytes were infected with Mycobacterium avium subsp. paratuberculosis (MAP) (MOI = 5) for 3 h. Cells were then treated with the indicated concentration of Pyk2 inhibitor for 18 h, and then M. avium subsp. paratuberculosis viable counts were determined through plating. Statistical significance is indicated as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Multiple monocyte samples from each animal were infected and assayed for bacterial viability.
Model of M. avium subsp. paratuberculosis impact on TLR9 signaling.
Kinome analysis of bovine monocytes following stimulation with CpG ODNs verified activation of classic TLR signaling (Fig. 8A). In contrast, kinome analysis of M. avium subsp. paratuberculosis-infected bovine monocytes following CpG ODN demonstrated an absence of signaling through the classic TLR pathway. Instead, the evidence suggests that M. avium subsp. paratuberculosis infection results in a diversion of TLR9 signaling to a Pyk2-mediated MAPK pathway (Fig. 8B).
Fig 8.
Pathway representation of canonical and Pyk2-mediated TLR9 signaling. Shown are signaling pathways based on known phosphorylation events of TLR9. A coloring scheme is used to illustrate phosphorylation events that were detected with the peptide array when analyzing lysates from bovine monocytes stimulated with CpG ODNs in the presence (B) and absence (A) of M. avium subsp. paratuberculosis infection. Differential levels of phosphorylation relative to the medium-treated control (P < 0.1) are presented as green for increased phosphorylation, red for decreased phosphorylation, and blue for insignificant phosphorylation or peptide not present on array.
DISCUSSION
Establishment of chronic infections by M. avium subsp. paratuberculosis depends in part on the ability of the bacteria to subvert host responses that promote clearance of the pathogen. The pathogen must prioritize the host responses that represent the greatest threat and modulate many redundant and sometimes overlapping mechanisms that may be spatially or temporally distinct. We have previously investigated how M. avium subsp. paratuberculosis subverts IFN-γ responsiveness at or near the IFN-γ receptor (17). TLR agonists have been suggested as immunotherapeutics for JD, and others have shown that agonists of TLR9, but not other TLRs, have the ability to promote M. avium subsp. paratuberculosis clearance (32). In this study, we examined responses of M. avium subsp. paratuberculosis-infected bovine monocytes to the TLR9 agonist CpG ODN to better understand mechanisms of M. avium subsp. paratuberculosis interaction with its bovine host.
Here we demonstrate that M. avium subsp. paratuberculosis exerts a discrete, but potent, influence on TLR responsiveness. Both functional and kinome data indicate that M. avium subsp. paratuberculosis blocks the ability of TLR9 to activate the classical TLR signaling pathway. This is supported by the inability of M. avium subsp. paratuberculosis to promote IL-10 release from infected cells as well as the absence of TLR9-associated signaling events through kinome analysis. Interestingly, this effect appears to be specific for TLR9, as other TLR agonists retain the ability to induce further IL-10 release from M. avium subsp. paratuberculosis-infected cells. Although a hallmark of M. avium subsp. paratuberculosis infection is induction of IL-10 production to promote anti-inflammatory responses, the kinetics of induction of IL-10 by M. avium subsp. paratuberculosis is quite slow, with significant levels of IL-10 not emerging until at least 12 h after infection (54). It is also known that M. avium subsp. paratuberculosis-induced IL-10 secretion, although a key immunomodulatory mechanism, is not the sole mechanism of M. avium subsp. paratuberculosis immune evasion, as not all (57%) infected monocytes treated with anti-IL-10 antibodies are able to clear M. avium subsp. paratuberculosis infection (55). Here we endeavored to look at early effects of M. avium subsp. paratuberculosis infection on host cell signaling. IL-10 upregulation is critical for M. avium subsp. paratuberculosis pathogenesis but contributes to a temporally distinct secondary response.
Kinome analysis revealed that peptides corresponding to early TLR signaling intermediates are not phosphorylated in M. avium subsp. paratuberculosis-infected cells treated with CpG, suggesting that the pathogen blocks responsiveness at an early stage at, or near, the receptor. Indeed, two peptides corresponding to IRAK-1 phosphorylation sites (T387 and T100) do not undergo phosphorylation in M. avium subsp. paratuberculosis-infected, CpG-stimulated cells. Uninfected cells treated with CpG showed increased phosphorylation of IRAK-1 T387 and T100. Thus, it appears that M. avium subsp. paratuberculosis acts upstream of IRAK-1 activation.
The lack of phosphorylation of early intermediates in the TLR pathway suggested a possible downregulation of TLR9 expression, but we found that TLR9 expression actually increased, suggesting that M. avium subsp. paratuberculosis interferes with TLR9 signaling somewhere downstream of the receptor but upstream of IRAK-1. We also noted that while IL-10 secretion was impaired, CpG-treated M. avium subsp. paratuberculosis-infected bovine monocytes were still able to induce oxidative burst, as we had observed for uninfected monocytes previously (41). This suggested that M. avium subsp. paratuberculosis interferes specifically with TLR9-mediated proinflammatory signaling but not signals that induce oxidative burst. Such a divergence of TLR9 signaling has been previously observed with IRAK-4-deficient human neutrophils that elicit oxidative burst in response to CpG ODN but show impaired cytokine responses (49). Inhibition of phosphatidylinositol 3-kinase (PI3K) prevented all CpG responses of the neutrophils, revealing that oxidative burst occurred by a direct PI3K-mediated pathway independent of IRAK-4.
Kinome analysis also revealed that CpG treatment of M. avium subsp. paratuberculosis-infected cells resulted in activation of MAPK signaling through Pyk2, a FAK-related tyrosine kinase that has been implicated in macrophage activation (53) and inflammation (56). Expressed at very high levels in monocytes and macrophages, Pyk2 is thought to participate in the regulation of inflammatory response through the recruitment of these cells to sites of inflammation. Activation of Pyk2 by the TLRs occurs through direct interaction of Pyk2 with the death domain of MyD88 (53), where interaction has been suggested to negatively regulate Pyk2. Activation of Pyk2-dependent signaling events appears to represent a bifurcation of TLR induced signaling as inhibitors of Src block the Pyk2-mediated responses without influencing IRAK-1-mediated signal transduction (51). Furthermore, Pyk2 has recently been shown to be critical for PI3KB integrin-mediated activation of platelet adhesion (57). However, there is likely cross talk between canonical TLR signaling and Pyk2-mediated signaling because Pyk2-deficient macrophages undergo reduced LPS-induced activation of NF-κB and IL-1 expression (53).
Interestingly, inhibition of Pyk2 signaling in M. avium subsp. paratuberculosis-infected monocytes with PP2 resulted in M. avium subsp. paratuberculosis clearance, suggesting that M. avium subsp. paratuberculosis favors the Pyk2 pathway for its intracellular survival at the expense of the host. How Pyk2-related signaling allows M. avium subsp. paratuberculosis to persist in monocytes is not known but might be related to its antiapoptotic effects (58), as well as attenuating cytokine release that would normally occur through the canonical TLR9 pathway.
The two central findings of this investigation, that M. avium subsp. paratuberculosis infection blocks TLR9 responsiveness and that it potentiates infected cells for signaling through Pyk2, share a signaling intermediate, MyD88. Blocking could occur by many potential mechanisms but in general must occur by preventing MyD88 from activating IRAK-1. Because MyD88 engages both Pyk2 and the canonical TLR9 pathway, it is possible that M. avium subsp. paratuberculosis interferes with an uncharacterized MyD88-related signaling pathway that facilitates divergence of these responses. Significantly, MyD88 is critical for lysosome-phagosome fusion, another well-characterized way that M. avium subsp. paratuberculosis avoids clearance. How M. avium subsp. paratuberculosis alters MyD88 signaling to cause a shift of the TLR9 response away from the canonical pathway could be related to secreted M. avium subsp. paratuberculosis effectors. Studies examining how M. avium subsp. paratuberculosis alters TLR9 and MyD88 signaling in a more clinical context, in addition to how MyD88-mediated pathways in M. avium subsp. paratuberculosis-infected cells are altered in response to other TLR agonists, will help clarify how M. avium subsp. paratuberculosis evades the bovine immune system.
ACKNOWLEDGMENTS
We acknowledge funding support provided by the Beef Cattle Research Council, Alberta Livestock Industry Development Fund, Alberta Livestock and Meat Agency, Saskatchewan Ministry of Agriculture, and Natural Sciences and Engineering Research Council.
We declare that we have no competing interests.
Footnotes
Published ahead of print 31 October 2012
Published with permission of the Director of VIDO as journal series number 645.
REFERENCES
- 1. Tiwari A, VanLeeuwen JA, McKenna SL, Keefe GP, Barkema HW. 2006. Johne's disease in Canada. Part I: clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can. Vet. J. 47:874–882 [PMC free article] [PubMed] [Google Scholar]
- 2. McKenna SL, Keefe GP, Tiwari A, VanLeeuwen J, Barkema HW. 2006. Johne's disease in Canada. Part II: disease impacts, risk factors, and control programs for dairy producers. Can. Vet. J. 47:1089–1099 [PMC free article] [PubMed] [Google Scholar]
- 3. Chi J, VanLeeuwen JA, Weersink A, Keefe GP. 2002. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 55:137–153 [DOI] [PubMed] [Google Scholar]
- 4. Khare S, Nunes JS, Figueiredo JF, Lawhon SD, Rossetti CA, Gull T, Rice-Ficht AC, Adams LG. 2009. Early phase morphological lesions and transcriptional responses of bovine ileum infected with Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 46:717–728 [DOI] [PubMed] [Google Scholar]
- 5. Momotani E, Whipple DL, Thiermann AB, Cheville NF. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131–137 [DOI] [PubMed] [Google Scholar]
- 6. Weiss DJ, Souza CD. 2008. Review paper: modulation of mononuclear phagocyte function by Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 45:829–841 [DOI] [PubMed] [Google Scholar]
- 7. Woo SR, Heintz JA, Albrecht R, Barletta RG, Czuprynski CJ. 2007. Life and death in bovine monocytes: the fate of Mycobacterium avium subsp. paratuberculosis. Microb. Pathog. 43:106–113 [DOI] [PubMed] [Google Scholar]
- 8. Ting LM, Kim AC, Cattamanchi A, Ernst JD. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163:3898–3906 [PubMed] [Google Scholar]
- 9. Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, Miyazaki T, Albrecht I, Massner J, Pieters J. 2007. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell 130:37–50 [DOI] [PubMed] [Google Scholar]
- 10. Li Q, Ding X, Thomas JJ, Harding CV, Pecora ND, Ziady AG, Shank S, Boom WH, Lancioni CL, Rojas RE. 2012. Rv2468c, a novel Mycobacterium tuberculosis protein that costimulates human CD4+ T cells through VLA-5. J. Leukoc. Biol. 91:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma AK, Naithani R, Kumar V, Sandhu SS. 2011. Iron regulation in tuberculosis research: promise and challenges. Curr. Med. Chem. 18:1723–1731 [DOI] [PubMed] [Google Scholar]
- 12. Sweet L, Singh PP, Azad AK, Rajaram MV, Schlesinger LS, Schorey JS. 2010. Mannose receptor-dependent delay in phagosome maturation by Mycobacterium avium glycopeptidolipids. Infect. Immun. 78:518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong D, Bach H, Sun J, Hmama Z, Av-Gay Y. 2011. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. U. S. A. 108:19371–19376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weiss DJ, Souza CD, Evanson OA, Sanders M, Rutherford M. 2008. Bovine monocyte TLR2 receptors differentially regulate the intracellular fate of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. J. Leukoc. Biol. 83:48–55 [DOI] [PubMed] [Google Scholar]
- 15. Finlay BB, McFadden G. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124:767–782 [DOI] [PubMed] [Google Scholar]
- 16. Waters WR, Miller JM, Palmer MV, Stabel JR, Jones DE, Koistinen KA, Steadham EM, Hamilton MJ, Davis WC, Bannantine JP. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arsenault RJ, Yue L, Bell K, Doig K, Potter A, Griebel P, Kusalik A, Napper S. 11 June 2012. Mycobacterium avium subsp. paratuberculosis inhibits interferon gamma-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's disease. Infect. Immun. doi:10.1128/IAI.00406-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krutzik SR, Modlin RL. 2004. The role of Toll-like receptors in combating mycobacteria. Semin. Immunol. 16:35–41 [DOI] [PubMed] [Google Scholar]
- 19. Barton GM, Kagan JC. 2009. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat. Rev. Immunol. 9:535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mucha R, Bhide MR, Chakurkar EB, Novak M, Mikula I., Sr 2009. Toll-like receptors TLR1, TLR2 and TLR4 gene mutations and natural resistance to Mycobacterium avium subsp. paratuberculosis infection in cattle. Vet. Immunol. Immunopathol. 128:381–388 [DOI] [PubMed] [Google Scholar]
- 21. Ruiz-Larrañaga O, Manzano C, Iriondo M, Garrido JM, Molina E, Vazquez P, Juste RA, Estonba A. 2011. Genetic variation of Toll-like receptor genes and infection by Mycobacterium avium ssp. paratuberculosis in Holstein-Friesian cattle. J. Dairy Sci. 94:3635–3641 [DOI] [PubMed] [Google Scholar]
- 22. Taylor DL, Zhong L, Begg DJ, de Silva K, Whittington RJ. 2008. Toll-like receptor genes are differentially expressed at the sites of infection during the progression of Johne's disease in outbred sheep. Vet. Immunol. Immunopathol. 124:132–151 [DOI] [PubMed] [Google Scholar]
- 23. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. 2011. Innate immune recognition of Mycobacterium tuberculosis. Clin. Dev. Immunol. doi:10.1155/2011/405310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher CA, Bhattarai EK, Osterstock JB, Dowd SE, Seabury PM, Whilock RH, Schukken YH, Schnabel RD, Taylor JF, Womack JE, Sebury CM. 2011. Evolution of the TLR gene family and member associations with Mycobacterium avium subspecies paratuberculosis infection. PLoS One 6:e27744 doi:10.1371/journal.pone.0027744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Subharat S, Shu D, de Lisle GW, Buddle BM, Wedlock DN. 2012. Altered patterns of Toll-like receptor gene expression in cull cows infected with Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 145:471–478 [DOI] [PubMed] [Google Scholar]
- 26. Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910–918 [DOI] [PubMed] [Google Scholar]
- 28. Sanjuan MA, Milasta S, Green DR. 2009. Toll-like receptor signaling in the lysosomal pathways. Immunol. Rev. 227:203–220 [DOI] [PubMed] [Google Scholar]
- 29. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546–549 [DOI] [PubMed] [Google Scholar]
- 30. Zimmermann S, Egeter O, Hausmann S, Lipford GB, Rocken M, Wagner H, Heeg K. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627–3630 [PubMed] [Google Scholar]
- 31. Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291–2298 [PubMed] [Google Scholar]
- 32. Wang JP, Hayashi T, Datta SK, Kornbluth RS, Raz E, Guiney DG. 2005. CpG oligonucleotides partially inhibit growth of Mycobacterium tuberculosis, but not Salmonella or Listeria, in human monocyte-derived macrophages. FEMS Immunol. Med. Microbiol. 45:303–310 [DOI] [PubMed] [Google Scholar]
- 33. Juffermans NP, Leemans JC, Florquin S, Verbon A, Kolk AH, Speelman P, van Deventer SJ, van der Poll T. 2002. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect. Immun. 70:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell 132:344–362 [DOI] [PubMed] [Google Scholar]
- 35. Hoarau C, Gerard B, Lescanne E, Henry D, Francois S, Lacapere JJ, El Benna J, Dang PM, Grandchamp B, Lebranchu Y, Gougerot-Pocidalo MA, Elbim C. 2007. TLR9 activation induces normal neutrophil responses in a child with IRAK-4 deficiency: involvement of the direct PI3K pathway. J. Immunol. 179:4754–4765 [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640–643 [DOI] [PubMed] [Google Scholar]
- 37. Hazeki K, Masuda N, Funami K, Sukenobu N, Matsumoto M, Akira S, Takeda K, Seya T, Hazeki O. 2003. Toll-like receptor-mediated tyrosine phosphorylation of paxillin via MyD88-dependent and -independent pathways. Eur. J. Immunol. 33:740–747 [DOI] [PubMed] [Google Scholar]
- 38. Scanga Bafica CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. 2004. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 72:2400–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun D, Ding A. 2006. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat. Immunol. 7:375–381 [DOI] [PubMed] [Google Scholar]
- 40. Hines ME, II, Stabel JR, Sweeney RW, Griffin F, Talaat AM, Bakker D, Benedictus G, Davis WC, de Lisle GW, Gardner IA, Juste RA, Kapur V, Koets A, Pruitt G, Whitlock RH. 2007. Experimental models for Johne's disease: a review and proposed international guidelines. Vet. Microbiol. 122:197–222 [DOI] [PubMed] [Google Scholar]
- 41. Arsenault RJ, Jalal S, Babiuk LA, Potter A, Griebel PJ, Napper S. 2009. Kinome analysis of Toll-like receptor signaling in bovine monocytes. J. Recept. Signal Transduct. Res. 29:299–311 [DOI] [PubMed] [Google Scholar]
- 42. Charavaryamath C, Fries P, Gomis S, Bell C, Doig K, le Guan L, Napper S, Griebel PJ. 2011. Mucosal changes in a long-term bovine intestinal segment model following removal of ingesta and microflora. Gut Microbes 2:134–144 [DOI] [PubMed] [Google Scholar]
- 43. Booth JS, Arsenault R, Napper S, Griebel PJ, Potter AA, Babiuk LA, Mutwiri GK. 2010. TLR9 signaling failure renders Peyer's patch regulatory B cells unresponsive to stimulation with CpG oligodeoxynucleotides. J. Innate Immun. 2:483–494 [DOI] [PubMed] [Google Scholar]
- 44. Jalal S, Arsenault R, Potter AA, Babiuk LA, Griebel PJ, Napper S. 2009. Genome to kinome: species-specific peptide arrays for kinome analysis. Sci. Signal. 2:pl1 doi:10.1126/scisignal.254pl1 [DOI] [PubMed] [Google Scholar]
- 45. Li Y, Arsenault RJ, Trost B, Slind J, Griebel PJ, Napper S, Kusalik A. 2012. A systematic approach for approach analysis of peptide array kinome data. Sci. Signal. 5:pl2 doi:10.1126/scisignal.2002429 [DOI] [PubMed] [Google Scholar]
- 46. Lynn DJ, Winsor GL, Chan C, Richard N, Laird MR, Barsky A, Gardy JL, Roche F, Chan MTH, Shah N, Lo R, Naseer M, Que J, Yau M, Acab M, Tulpan D, Whiteside MD, Chikatamarla A, Mah B, Munzner T, Hokamp K, Hancock RE, Brinkman FS. 2008. InnateDB: facilitating systems-level analyses of the mammalian innate immune response. Mol. Syst. Biol. 4:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waibler Z, Anzaghe M, Konur A, Akira S, Muller W, Kalinke U. 2008. Excessive CpG 1668 stimulation triggers IL-10 production by cDC that inhibits IFN-alpha responses by pDC. Eur. J. Immunol. 38:3127–3137 [DOI] [PubMed] [Google Scholar]
- 48. Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ. 2003. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood 102:4487–4492 [DOI] [PubMed] [Google Scholar]
- 49. Khalifeh MS, Stabel JR. 2004. Upregulation of transforming growth factor-beta and interleukin-10 in cows with clinical Johne's disease. Vet. Immunol. Immunopathol. 99:39–46 [DOI] [PubMed] [Google Scholar]
- 50. Griebel PJ, Brownlie R, Manuja A, Nichani A, Mookherjee N, Popowych Y, Mutwiri G, Hecker R, Babiuk LA. 2005. Bovine Toll-like receptor 9: a comparative analysis of molecular structure, function and expression. Vet. Immunol. Immunopathol. 108:11–16 [DOI] [PubMed] [Google Scholar]
- 51. Nalubamba K, Smeed J, Gossner A, Watkins C, Dalziel R, Hopkins J. 2008. Differential expression of pattern recognition receptors in the three pathological forms of sheep paratuberculosis. Microbes Infect. 10:598–604 [DOI] [PubMed] [Google Scholar]
- 52. Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. 2003. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. U. S. A. 100:10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xi CX, Xiong F, Zhou Z, Mei L, Xiong WC. 2010. PYK2 interacts with MyD88 and regulates MyD88-mediated NF-kappaB activation in macrophages. J. Leukoc. Biol. 87:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss DJ, Evanson OA, Moritz A, Deng MQ, Abrahamsen MS. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556–5561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weiss DJ, Evanson OA, de Souza C, Abrahamsen MS. 2005. A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp. paratuberculosis. Am. J. Vet. Res. 66:721–726 [DOI] [PubMed] [Google Scholar]
- 56. Anand AR, Cucchiarini M, Terwilliger EF, Ganju RK. 2008. The tyrosine kinase Pyk2 mediates lipopolysaccharide-induced IL-8 expression in human endothelial cells. J. Immunol. 180:5636–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Consonni A, Cipolla L, Guidetti G, Canobbio I, Ciraolo E, Hirsch E, Falasca M, Okigaki M, Balduini C, Torti M. 2012. Role and regulation of phosphatidylinositol 3-kinase beta in platelet integrin alpha2beta1 signaling. Blood 119:847–856 [DOI] [PubMed] [Google Scholar]
- 58. Burdick AD, Ivnitski-Steele ID, Lauer FT, Burchiel SW. 2006. PYK2 mediates anti-apoptotic AKT signaling in response to benzo[a]pyrene diol epoxide in mammary epithelial cells. Carcinogenesis 27:2331–2340 [DOI] [PubMed] [Google Scholar]