Abstract
Mycobacterium tuberculosis persists in the tissues of mammalian hosts despite inducing a robust immune response dominated by the macrophage-activating cytokine gamma interferon (IFN-γ). We identified the M. tuberculosis phosphate-specific transport (Pst) system component PstA1 as a factor required to resist IFN-γ-dependent immunity. A ΔpstA1 mutant was fully virulent in IFN-γ−/− mice but attenuated in wild-type (WT) mice and mice lacking specific IFN-γ-inducible immune mechanisms: nitric oxide synthase (NOS2), phagosome-associated p47 GTPase (Irgm1), or phagocyte oxidase (phox). These phenotypes suggest that ΔpstA1 bacteria are sensitized to an IFN-γ-dependent immune mechanism(s) other than NOS2, Irgm1, or phox. In other species, the Pst system has a secondary role as a negative regulator of phosphate starvation-responsive gene expression through an interaction with a two-component signal transduction system. In M. tuberculosis, we found that ΔpstA1 bacteria exhibited dysregulated gene expression during growth in phosphate-rich medium that was mediated by the two-component sensor kinase/response regulator system SenX3-RegX3. Remarkably, deletion of the regX3 gene suppressed the replication and virulence defects of ΔpstA1 bacteria in NOS2−/− mice, suggesting that M. tuberculosis requires the Pst system to negatively regulate activity of RegX3 in response to available phosphate in vivo. We therefore speculate that inorganic phosphate is readily available during replication in the lung and is an important signal controlling M. tuberculosis gene expression via the Pst-SenX3-RegX3 signal transduction system. Inability to sense this environmental signal, due to Pst deficiency, results in dysregulation of gene expression and sensitization of the bacteria to the host immune response.
INTRODUCTION
Mycobacterium tuberculosis is a persistent pathogen that can survive in the lungs of infected hosts for decades in a subclinical latent state. M. tuberculosis persists despite inducing a robust adaptive immune response. The immune response contains M. tuberculosis in the majority of individuals who become infected and is essential to maintain bacterial latency, since reactivation is associated with factors that reduce immune system function (1). The macrophage-activating cytokine gamma interferon (IFN-γ), produced by CD4+ and CD8+ T cells, is a central component of the immune response to M. tuberculosis. In humans, susceptibility to mycobacterial infections is associated with naturally occurring mutations affecting IFN-γ or the IFN-γ receptor (2) or depletion of T cell populations (1). Mice infected with M. tuberculosis develop a chronic persistent infection of the lungs that is restrained by adaptive immunity. Gene knockout mice that are deficient for either IFN-γ or the IFN-γ receptor fail to control M. tuberculosis replication in the lungs and succumb rapidly (3, 4).
In the lungs, M. tuberculosis survives and replicates within professional phagocytes, including macrophages and dendritic cells (5). Macrophages deploy a diverse set of microbicidal functions to kill invading microbes, some of which are dependent on IFN-γ activation (6). IFN-γ stimulates production of reactive oxygen species (ROS) by phagocyte oxidase (phox) and reactive nitrogen species (RNS) by inducible nitric oxide synthase (NOS2) (7). Although mice lacking the gp91 subunit of phox are no more susceptible than wild-type animals to M. tuberculosis infection (8), the bacteria presumably do encounter ROS because mutants have been identified that are attenuated in a phox-dependent manner (9, 10). In contrast, it is clear that RNS mediate resistance to murine tuberculosis because NOS2-deficient mice fail to control bacterial replication in the lungs and succumb rapidly (11). In mice, IFN-γ also stimulates the immunity-related p47 GTPases that are required to control infections with diverse intracellular pathogens (12). The Irgm1 GTPase (formerly LRG-47) is recruited to the mycobacterial phagosome (13), where it promotes maturation and acidification (14). Irgm1-deficient mice are highly susceptible to M. tuberculosis because they fail to control bacterial replication in the lungs (14).
Despite this arsenal of host defenses, M. tuberculosis can persist indefinitely in the lungs, suggesting the existence of mechanisms to counteract the antimicrobial functions that are activated by IFN-γ. Indeed, M. tuberculosis resists RNS and ROS generated in IFN-γ-activated macrophages by producing enzymes that detoxify these compounds and by expressing factors that repair or degrade damaged cellular components (15). M. tuberculosis also uses specific resistance mechanisms to counteract the acidic pH encountered in maturing phagosomes (16).
In a previous report, we described a strategy to identify M. tuberculosis genes that are required to counteract IFN-γ-dependent host immune responses using differential signature-tagged mutagenesis (17). According to this strategy, we selected transposon insertion mutants that were capable of progressive replication in the lungs of IFN-γ−/− mice but not in the lungs of NOS2−/− mice. These mutants were presumed to have a defect in resisting an IFN-γ-dependent immune mechanism other than RNS generated by NOS2. Here, we report the characterization of M. tuberculosis PstA1, a putative counterimmune factor identified using this approach.
The Pst system is a high-affinity, low-velocity ABC-type transporter that utilizes energy provided by ATP hydrolysis to import inorganic phosphate (Pi) across the cytoplasmic membrane. The Pst system comprises two membrane-spanning proteins (PstA and PstC), an extracellular high-affinity Pi binding protein (PstS), and a cytoplasmic ATPase (PstB) (18). Bacteria use the Pst transporter to scavenge Pi under conditions of Pi limitation, and some pathogens require the Pst system for virulence (19). The M. tuberculosis genome encodes two putative Pst Pi uptake systems and a third paralog of PstS (20). Previous reports demonstrated that the M. tuberculosis Pst system components PstS3, PstC2, PstA1, and PhoT (a PstB ortholog) are required for survival in macrophages (21) and that PstS2 and PstS1 are required for replication in the lungs of mice (22).
In some bacterial species, the Pst system also controls gene expression in response to external Pi concentration by interacting with a two-component signal transduction system (19). Although the molecular mechanisms are not fully understood, the Pst system negatively regulates the expression of Pi-responsive genes during growth in Pi-rich conditions. Null mutations in the Pst system result in constitutive expression of the Pi starvation-responsive regulon (18). In mycobacteria, the SenX3-RegX3 two-component signal transduction system has been implicated in Pi-responsive transcriptional regulation (23, 24), but it is unknown how the activity of this system is controlled. Here, we show that M. tuberculosis PstA1 is essential for virulence in mice and persistence in the face of IFN-γ-dependent host immunity. We also demonstrate a hitherto unappreciated role of the M. tuberculosis Pst system in regulating gene expression in response to Pi availability. We show that aberrant gene expression in ΔpstA1 mutant bacteria is mediated by the response regulator RegX3 and is responsible for hypersensitivity of the ΔpstA1 mutant to stress conditions in vitro and to host immune responses in vivo. Our results implicate Pi as an important signal regulating the expression of bacterial factors that are critical for persistence in an immunocompetent host.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. tuberculosis Erdman and derivative strains were grown at 37°C in Middlebrook 7H9 (Difco) liquid culture medium supplemented with 10% albumin-dextrose-saline (ADS), 0.5% glycerol, and 0.1% Tween 80 or on Middlebrook 7H10 (Difco) solid culture medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) (BD Biosciences) and 0.5% glycerol. Frozen stocks were prepared by growing liquid cultures to mid-exponential phase (optical density at 600 nm [OD600] of 0.6 to 0.8), adding glycerol to 15% final concentration, and storing aliquots at −80°C. For phosphate-limiting (Pi-free) 7H9 broth, a 100× liquid stock of 7H9 base was reconstituted without addition of the Pi-buffering components (Na2HPO4 and KH2PO4). The 1× Pi-free 7H9 was made with 0.5% glycerol, 10% ADS, 0.1% Tween 80, and 50 mM MOPS (morpholinepropanesulfonic acid) buffer, pH 6.6.
Cloning of deletion and complementation constructs.
Constructs for deletion of pstA1 and regX3 or for creating the regX3D52A point mutant (with an Asp-to-Ala change at position 52 encoded by regX3) were generated in the allelic exchange vector pJG1100 (25) or pJG1111b (26), which contain the aph (kanamycin resistance), hyg (hygromycin resistance), and sacB (sucrose sensitivity) markers (see Table S1 in the supplemental material). The pJG1111b vector additionally contains lacZ under the control of the M. tuberculosis antigen 85 promoter. Genomic regions 500 to 800 bp upstream and downstream of the genes to be deleted were PCR amplified from M. tuberculosis Erdman genomic DNA using the oligonucleotides listed in Table S2 in the supplemental material, cloned in pCR2.1-TOPO (Invitrogen), and sequenced. Reverse primers for amplification of the upstream regions were designed with an AvrII restriction site in-frame with the translation start codon. Forward primers for amplification of the downstream regions were designed with an AvrII restriction site in-frame with the stop codon. The upstream and downstream fragments were removed from pCR2.1 by restriction with PacI/AvrII or AvrII/AscI, respectively, and ligated together in pJG1100 or pJG1111b between the PacI and AscI sites to generate in-frame deletion constructs. The pstA1 upstream and downstream regions were ligated together to create the ΔpstA1 construct pMK201, encoding the first 3 amino acids of PstA1 fused to the last 6 amino acids of PstA1 (see Table S1). The regX3 upstream and downstream regions were ligated together to create the ΔregX3 construct pAT205, encoding the first 10 amino acids of RegX3 fused to the last 3 amino acids of RegX3 (see Table S1).
The regX3D52A point mutation was generated using splicing-by-overlap extension PCR (27). DNA fragments 600 bp upstream and downstream of the mutation were PCR amplified from M. tuberculosis Erdman genomic DNA using primers R1 and F2 harboring a single base pair change that converts a GAT codon for aspartate to a GCT codon for alanine (see Table S2 in the supplemental material). These DNA fragments were annealed together by complementary sequences in the R1 and F2 primers and PCR amplified with the F1 and R2 primers. The resulting product was cloned in pCR2.1-TOPO and sequenced. The insert containing the A-to-C point mutation was digested from pCR2.1 with PacI and AscI and ligated to similarly digested pJG1100 to generate the regX3D52A point mutation construct pAT212 (see Table S1 in the supplemental material).
For the ΔpstA1 complementing plasmid, the pstS3C2A1 operon promoter (367 bp 5′ to the translational start site of pstS3) plus the first 34 codons of pstS3 were PCR amplified from M. tuberculosis Erdman genomic DNA using primers pstL1promF and pstL1promR. The last 20 codons of pstC2 and the entire pstA1 gene were PCR amplified from M. tuberculosis Erdman genomic DNA using primers pstA1comF and pstA1comR (see Table S2 in the supplemental material). These PCR products were digested with SalI/EcoRI and EcoRI/AvrII, respectively, and ligated together in the episomal vector pMV261 between the SalI and AvrII restriction sites to generate pMK205 (see Table S1 in the supplemental material). This construct encodes the first 34 amino acids of PstS3 fused in-frame to the last 19 amino acids of PstC2, followed by the complete pstA1 open reading frame; thus, pstA1 expression is controlled by the native transcriptional and translational signals.
For regX3 complementation, the regX3 gene plus all putative regulatory elements, including 210 bp upstream of senX3, were PCR amplified from M. tuberculosis ΔsenX3 genomic DNA using primers SRCF2 and SRCR (see Table S2 in the supplemental material). The PCR product was cloned in pCR2.1-TOPO and sequenced. The insert was removed by digestion with EcoRI and HindIII and cloned in similarly digested pND200, a derivative of the integrative vector pMV361 that lacks the hsp60 promoter, to generate pAT213 (see Table S1 in the supplemental material).
M. tuberculosis strain construction.
M. tuberculosis strains harboring unmarked in-frame ΔpstA1 or ΔregX3 deletions or the regX3D52A point mutation were constructed by a two-step homologous recombination method for allelic exchange (28, 29) using vector pMK201, pAT205, or pAT212, respectively. These plasmids were recombined into the wild-type M. tuberculosis Erdman chromosome (pMK201) or the M. tuberculosis ΔpstA1 chromosome by electroporation with 1 to 2 μg of purified plasmid DNA. Electrocompetent M. tuberculosis was prepared by growing strains to mid-exponential phase (OD600 0.6 to 1.0) in 7H9 broth, adding 0.1 volume of 2 M glycine, incubating for an additional 20 to 24 h at 37°C, washing in 10% glycerol (1× volume twice followed by 0.5× volume once), and resuspending in 1/20 volume 10% glycerol. Transformants were grown for 24 h in 7H9 broth prior to selection of recombinants on 7H10 agar containing kanamycin (15 μg/ml) and hygromycin (50 μg/ml). Kanr Hygr colonies were picked and grown to mid-exponential phase in 7H9 broth without antibiotics. Integration of the constructs at the correct chromosomal locus was confirmed by PCR on heat-inactivated cell lysates using the following primer pairs: for ΔpstA1 upstream, A1F3/A1R4; for ΔpstA1 downstream, A1F4/A1R3; for ΔregX3 upstream, RXF3/RXR4; for ΔregX3 downstream, RXF4/RXR5; for regX3D52A upstream, dRX3F1/pJGR; and for regX3D52A downstream, pJGF/DAEQR4 (see Table S2 in the supplemental material). Clones that contained the allelic exchange vector integrated at the correct locus were plated on 7H10 agar containing 2% sucrose for counterselection of the pJG1111 or pJG1100 vector. Sucrose-resistant clones were grown in 7H9 broth, and isolates in which the deletion replaced the wild-type allele were identified by PCR with the following primer pairs: for ΔpstA1, A1F3/A1R3, and for ΔregX3, RXF3/RXR5 (see Table S2). For the regX3D52A point mutation, excision of the pJG1100 vector was confirmed by PCR with primer pair dRX3F1/DAEQR4 (see Table S2). The presence of the point mutation was confirmed by sequencing of the PCR product.
The ΔpstA1 mutant was complemented by electroporation with pMK205 (pMV-pstA1) and selection of transformants on 7H10 agar containing kanamycin (15 μg/ml). The ΔregX3 mutant was complemented by electroporation with pAT213 (pND-regX3), an integrating plasmid containing regX3 under the control of the senX3-regX3 promoter, and selection of transformants on 7H10 agar containing kanamycin (15 μg/ml). The presence of the complementing plasmid was confirmed by PCR amplification with primer pair D52AF2/SRCR.
Southern hybridization.
Genomic DNA was extracted from M. tuberculosis Erdman and ΔpstA1 strains and digested overnight with restriction enzyme KpnI, PstI, or XhoI. Digested DNA was separated by electrophoresis on a Tris-acetate-EDTA (TAE) agarose gel and transferred to Hybond N+ nylon membrane (Amersham). An ∼200-bp probe downstream from the ΔpstA1 deletion was generated by PCR amplification from M. tuberculosis Erdman genomic DNA using primers pstA1KOF2 and pstA1del2R (see Table S2 in the supplemental material). The probe was labeled with 32P using the Megaprime labeling system (Amersham) and purified on a ProbeQuant G-50 microcolumn (Amersham). The blot was blocked in Rapid-Hyb buffer (Amersham) and probed with the 32P-labeled PCR product for 2.5 h at 65°C. The membrane was washed according to the manufacturer's instructions. Autoradiographic films (Kodak) were exposed to the blot for 1 to 2 h and developed on an automatic film processor.
Analysis of PDIM production.
M. tuberculosis strains grown to mid-exponential phase (OD600 0.5) were labeled with 10 μCi [14C]propionate (specific activity, 54 Ci/mol; Amersham), which is preferentially incorporated into phthiocerol dimycocerosate (PDIM), for 48 h (30). Apolar lipids were extracted from labeled cells essentially as described previously (31). Labeled cells were collected by centrifugation (2,500 × g for 10 min) and resuspended in 5 ml of 10:1 (vol/vol) methanol–0.3% NaCl. Lipids were extracted twice in 5 ml petroleum ether by vigorous vortexing followed by centrifugation (750 × g for 10 min) to separate the phases. After each extraction, the upper layer containing the apolar lipid fraction was collected and remaining bacteria were killed by the addition of an equal volume of chloroform and incubation for 1 h at room temperature. The pooled 14C-labeled apolar lipid fractions were concentrated by evaporation overnight in a chemical fume hood, spotted (20 μl) on an aluminum silica gel 60 F254 thin-layer chromatography (TLC) plate (Merck), and developed in 9:1 petroleum ether–diethyl ether. The plates were air dried, and the 14C-labeled spots were detected using a phosphor screen (Amersham) and PhosphorImager Typhoon 9400 imaging system (Amersham).
Mouse infections.
Male and female C57BL/6, IFN-γ−/−, NOS2−/−, and gp91phox−/− mice 6 to 8 weeks of age were purchased from Charles River Laboratories, France, or Jackson Laboratory, United States. Irgm1−/− mice (32) were bred under specific-pathogen-free conditions at the EPFL Center of Phenogenomics. Mice were infected via the aerosol route with ∼100 CFU using a custom-built aerosol chamber, as described previously (25). Bacteria were grown to mid-exponential phase (OD600 of 0.5) in 7H9 broth, collected by centrifugation (2,500 × g for 10 min), and resuspended in an equal volume of phosphate-buffered saline containing 0.05% Tween 80 (PBS-T). Clumps were removed by low-speed centrifugation (150 × g for 5 min). The declumped supernatant was adjusted to an OD600 of 0.05 in PBS-T (∼5 × 107 CFU/ml), and CFU were enumerated by plating serially diluted aliquots on 7H10 agar and incubating at 37°C for 3 to 4 weeks. Mice were infected via the aerosol route with ∼100 CFU using a custom-built aerosol chamber (University of Wisconsin, Madison, Department of Mechanical Engineering) with a 15-min exposure time as described previously (33). Groups of infected mice (n = 4) were euthanized by CO2 overdose at specified time points postinfection. Lungs were removed aseptically and homogenized in 3 ml PBS-T. Bacterial CFU were enumerated by plating serially diluted lung homogenates on 7H10 agar containing 100 μg/ml cycloheximide and counting colonies after 3 to 4 weeks at 37°C. For survival experiments, groups of infected mice (n = 4 to 8) were monitored closely and those animals showing signs of illness (ruffled fur, immobility, hunched posture, and labored breathing) were sacrificed by CO2 overdose and scored as “died.” The animal protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of The Rockefeller University and by the chief veterinarian of EPFL, the Service de la Consommation et des Affaires Vétérinaires of the Canton of Vaud, and the Swiss Office Vétérinaire Fédéral. All animal experiments were done in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the Swiss Law for the Protection of Animals.
Pi starvation.
Bacteria were grown to mid-exponential phase (OD600 of 0.5) in 7H9 broth, washed twice, diluted to an OD600 of 0.05 in Pi-free 7H9 broth, and incubated at 37°C. CFU were enumerated at 0, 3, 5, 7, and 10 days by plating serially diluted culture aliquots on 7H10 agar.
Cell wall and ROS stress.
Bacteria were grown to mid-exponential phase (OD600 of 0.5) in 7H9 broth, diluted to an OD600 of 0.05 in fresh 7H9 broth, and incubated at 37°C after the addition of SDS or H2O2 at concentrations indicated below. CFU were enumerated at 0 and 24 h by plating serially diluted culture aliquots on 7H10 agar.
RNS stress.
Bacteria were grown to mid-exponential phase (OD600 of 0.5) in 7H9 broth and treated with the NO donor diethylenetriamine-NONOate (DETA-NO; Sigma) essentially as described previously (34) except that complete 7H9 broth was used. DETA-NO was added at 0, 24, and 48 h. CFU were enumerated at 0 and 72 h by plating serially diluted culture aliquots on 7H10 agar.
Oxygen limitation.
Survival of hypoxic conditions was assessed using an unstirred culture model of nonreplicating persistence (35). Bacteria were grown to mid-exponential phase (OD600 of 0.5) in 7H9 broth, diluted to an OD600 of 0.05 in fresh 7H9 broth, and aliquoted in 1.5-ml screw-cap O-ring tubes (Sarstedt) with no headspace. Methylene blue (1.5 μg/ml) was added to three control tubes as an oxygen indicator. Tubes were sealed with parafilm and incubated at 37°C. CFU from triplicate tubes were enumerated at 0, 30, 60, 100, and 200 days by plating serially diluted culture aliquots on 7H10 agar.
Transcriptional profiling.
Bacteria grown at 37°C in 7H9 broth were maintained in early exponential phase for 4 days by daily dilution. Bacteria were harvested at an OD600 of 0.2, and RNA was extracted and converted to labeled cDNA as described previously (36). Cells were collected by centrifugation (3,320 × g for 4 min) and snap-frozen on dry ice. RNA was harvested by dissolving frozen pellets in 1 ml TRIzol reagent (Gibco BRL) and bead beating for 30 s (3 times) with 0.5 ml of 0.1-mm diameter zirconia/silica beads (BioSpec Products). Cell debris was pelleted by centrifugation (3,320 × g for 1 min), and the supernatant containing the RNA was transferred to a new tube containing 2 ml Phase Lock Gel I heavy (Eppendorf) and 300 μl chloroform. After rapid inversion for 2 min, samples were centrifuged (3,320 × g for 5 to 7 min) to separate phases and the top aqueous layer was removed and combined with 270 μl isopropanol and 270 μl of high-salt solution (0.8 M sodium citrate and 1.2 M NaCl). After 12 to 24 h at 4°C, RNA was pelleted, washed with 75% ethanol, and air dried briefly. DNA was removed by treatment with 6 units of DNase I (Ambion) for 30 min at 37°C. Final purification of RNA was performed using an RNeasy column (Qiagen).
To convert RNA to labeled cDNA for arrays, 0.5 to 2 μg total RNA was combined with 4.4 μg of random oligonucleotide hexamers and incubated for 2 min at 98°C. Samples were cooled on ice and combined with StrataScript reverse transcriptase buffer, 0.5 mM dATP/dGTP/dCTP, 0.02 mM dTTP, 1.5 nmol Cy3 or Cy5-dUTP (Amersham Biosciences), and 1.8 μl StrataScript reverse transcriptase (Stratagene) in a total volume of 25 μl. Samples were incubated for 10 min at 25°C and 90 min at 42°C. cDNA was purified using Microcon-30 (Amicon).
Oligonucleotides (Operon) were printed in microarrays at the University of Colorado Denver microarray core. For hybridization, oligonucleotide microarrays were prehybridized for 1 h in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1% bovine serum albumin (BSA), and 0.1% SDS and washed with H2O and isopropanol. Then, the microarray was spotted with 10 μl of hybridization solution (labeled cDNA, 5 μg tRNA, 2× SSC, 25% formamide, and 0.1% SDS), covered with a glass coverslip, and hybridized at 54°C overnight. Microarrays were processed by washing briefly in 1× SSC-0.05% SDS and then in 0.06× SSC. Arrays were scanned with a GenePix 4000b scanner (Molecular Devices), and spot intensities were obtained by using GenePix Pro 6.0 (Molecular Devices). Data were analyzed with ArrayStar software using the Robust Multichip Averaging (RMA) with Quantile parameter for normalization. Differentially expressed genes were selected using a 2.0-fold-change cutoff. Differential gene expression was considered significant for P values of <0.05 by Student's t test. Complete microarray data will be made available on the Gene Expression Omnibus (GEO) database.
Quantitative reverse transcription-PCR (qRT-PCR).
For confirmation of the transcriptional profiling results, bacteria were grown to mid-exponential phase (OD600 of 0.5) in 7H9 broth. To assess the response to Pi starvation, bacteria were grown to mid-exponential phase in 7H9 broth and then washed twice and resuspended to an OD600 of 0.05 in Pi-free 7H9 broth. Cultures were incubated at 37°C, and bacteria were collected for RNA extraction at 0, 8, 24, 48, 72, and 96 h. Cells were collected by centrifugation (4,700 × g for 10 min at 4°C), and RNA was extracted in 500 μl of TRIzol (Invitrogen) plus 0.1% polyacryl carrier (Molecular Research Center) by bead beating with 0.1-mm zirconia beads (BioSpec). RNA was treated with Turbo DNase (Ambion) and reverse transcribed to cDNA using the SuperScript II first-strand synthesis kit and random hexamers for priming (Invitrogen). The reverse transcription reaction used the following cycling conditions: 10 min at 25°C for annealing, 50 min at 42°C for extension, and 15 min at 72°C for heat inactivation of SuperScript II enzyme. cDNA was stored at −20°C until real-time quantitative PCRs were performed.
Primers for real-time quantitative reverse transcription-PCR (qRT-PCR) specific for the 16S rRNA, sigA, pstS3, senX3, regX3, pitB, pstS1, pstS2, udgA, mgtA, rv0784, rv3026, pimC, and rv1387 genes were designed using Primer Express software (Applied Biosystems) with similar melting temperatures (58 to 60°C) and are listed in Table S3 in the supplemental material. Primers were tested in PCRs using 100 M. tuberculosis genome equivalents as the template, and products were analyzed by gel electrophoresis. Real-time quantitative PCRs were prepared with 2× Sybr master mix (Applied Biosystems), 2 μl cDNA, and 0.3 μM primers and were run in absolute quantification mode on an ABI7900 real-time PCR machine (Applied Biosystems) using the following cycling conditions: 50°C for 2 min; 95°C for 10 min; 40 cycles of 95°C for 15 s and 60°C for 1 min with data collection during each cycle; and 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s with 2% ramp rate during the final denaturation to generate melting curves for confirmation of product specificity. Mock reactions (no RT) were performed on each sample to confirm the absence of genomic DNA contamination. Cycle threshold CT values were converted to copy numbers using standard curves for each gene. Target cDNA was internally normalized to sigA cDNA (Pi-rich cultures) or 16S cDNA (Pi-starved cultures).
Statistical analysis.
Student's unpaired t test (one-tailed) was used for pairwise comparisons between wild-type and mutant strains of M. tuberculosis. The Mantel-Cox log-rank test was used for comparison of Kaplan-Meier plots of mouse survival. P values were calculated using GraphPad Prism 5.0 software (GraphPad Software, Inc.). P values of <0.05 were considered significant.
RESULTS
PstA1 is an M. tuberculosis counterimmune factor.
In a previous report, we used a differential signature-tagged mutagenesis strategy to identify M. tuberculosis genes involved in counteracting IFN-γ-dependent immune mechanisms other than NOS2 (17, 25). These “counterimmune” mutants replicated normally in IFN-γ−/− mice but were attenuated for growth and virulence in NOS2−/− mice. Three mutants were described previously (17, 25). A fourth mutant harbored a transposon insertion in the gene pstA1 (rv0930), which encodes a membrane-spanning component of a putative Pst Pi uptake system (20). The pstA1::Tn mutant was severely attenuated for replication and virulence in NOS2−/− mice but exhibited little or no attenuation in IFN-γ−/− mice infected by either the intravenous or aerosol route (data not shown). These observations suggest that PstA1 is required to counteract an IFN-γ-dependent, NOS2-independent immune mechanism(s).
PstA1 counters an IFN-γ-dependent immune mechanism independent of NOS2, Irgm1, and Phox.
To confirm that the pstA1::Tn mutant phenotypes were due to disruption of pstA1, we generated an unmarked, in-frame deletion of pstA1 in M. tuberculosis Erdman that removes all but the first 3 and last 6 codons of pstA1. The deletion leaves intact the upstream pstC2 gene and the downstream pknD gene. The ΔpstA1 deletion was confirmed by PCR and Southern blot (Fig. 1A and B). During routine culture, spontaneous mutations can arise that cause deficiency in the production of phthiocerol dimycocerosate (PDIM) (25), a complex lipid that is required for full virulence of M. tuberculosis (30). Therefore, we confirmed that the ΔpstA1 mutant retained the ability to produce PDIM in vitro (Fig. 1C). We also complemented the ΔpstA1 mutation by introducing an episomal vector harboring pstA1 under the control of the pstS3C2A1 operon promoter (pMV-pstA1).
Fig 1.

Confirmation of the ΔpstA1 chromosomal deletion by Southern blotting and analysis of phthiocerol dimycocerosate production. (A) Genetic organization of the pstS3C2A1 locus in M. tuberculosis. Genes are indicated by arrows. The probe used for Southern blotting is indicated by the black rectangle 3′ to the pstA1 gene. (B) Southern blot of M. tuberculosis wild-type and ΔpstA1 mutant genomic DNA digested with the indicated restriction enzymes. Positions of molecular weight standards are indicated at the right. The ΔpstA1 deletion removes 0.89 kbp. (C) Phthiocerol dimycocerosate (PDIM) was detected in 14C-labeled apolar lipid fractions extracted from M. tuberculosis wild-type and ΔpstA1 mutant by thin-layer chromatography. Two spots corresponding to phthiocerol (methoxy) and phthiodiolone (keto) forms of PDIM are indicated.
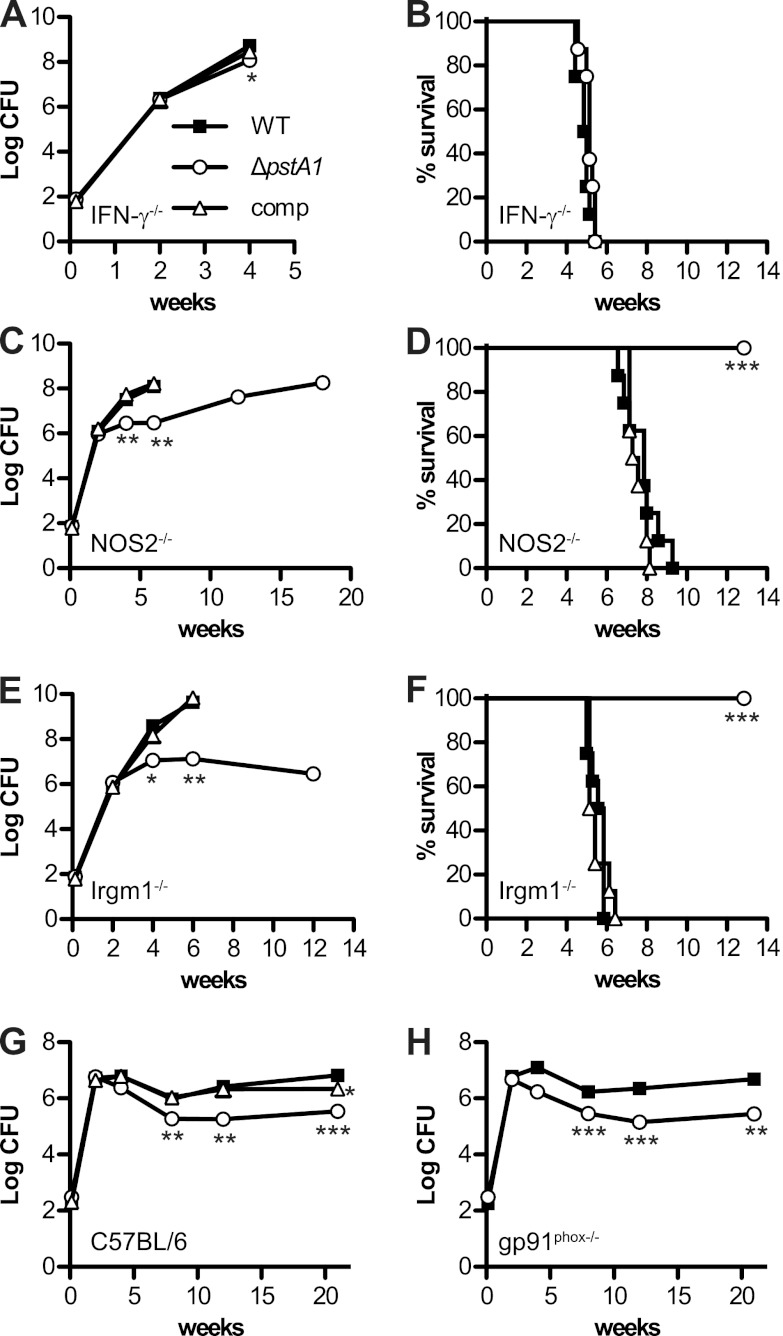
IFN-γ−/−, NOS2−/−, and Irgm1−/− mice were infected by the aerosol route with ∼100 CFU of M. tuberculosis wild type (WT), ΔpstA1, or ΔpstA1 pMV-pstA1 strains. Irgm1−/− mice were included to test whether PstA1 is required to resist phagosome maturation and acidification mediated by this GTPase. In the lungs of IFN-γ−/− mice, ΔpstA1 bacteria replicated with kinetics similar to those of the WT (Fig. 2A). We observed a small but reproducible (P = 0.03) difference in CFU of WT and ΔpstA1 strains at 4 weeks postinfection that was reversed by complementation. This modest replication defect had no discernible impact on virulence, since IFN-γ−/− mice infected with WT or ΔpstA1 bacteria succumbed to infection with similar kinetics (Fig. 2B). These results indicate that the in vivo replication and virulence defects of ΔpstA1 bacteria are dependent on activation of immune responses by IFN-γ.
Fig 2.
Growth kinetics and virulence of M. tuberculosis ΔpstA1 in mice. Mice were aerosol infected with ∼100 CFU of M. tuberculosis WT, ΔpstA1, or ΔpstA1 pMV-pstA1 (comp). Mouse strains were as follows: IFN-γ−/− (A, B), NOS2−/− (C, D), Irgm1−/− (E, F), C57BL/6 (G), and gp91phox−/− (H). All mouse strains were on the C57BL/6 background. (A, C, E, G, H) Growth kinetics. Groups of infected mice (n = 4) were sacrificed at the indicated time points. Bacterial CFU were enumerated by plating lung homogenates on 7H10 agar and incubating for 3 to 4 weeks at 37°C. Symbols represent means; error bars indicate standard errors of the means. Asterisks indicate statistically significant differences from WT (*, P < 0.05; **, P < 0.01; ***, P < 0.0005). (B, D, F) Virulence. Groups of infected mice (n = 8) were monitored for signs of illness. Moribund mice were euthanized and scored as dead. Asterisks indicate statistically significant differences from WT (P < 0.0005).
Like the pstA1::Tn mutant, the ΔpstA1 mutant exhibited marked replication and virulence defects in NOS2−/− mice. During the first 2 weeks of infection, ΔpstA1 bacteria replicated in the lungs of NOS2−/− mice with kinetics indistinguishable from those of WT bacteria. Between 2 and 6 weeks postinfection, ΔpstA1 CFU showed little change, whereas WT CFU increased by more than 100-fold (Fig. 2C). Beyond 6 weeks, ΔpstA1 CFU slowly increased. The in vivo replication defect of the ΔpstA1 mutant was accompanied by significantly reduced virulence (Fig. 2D). The mean survival time of NOS2−/− mice infected with WT bacteria was 54 days, whereas all NOS2−/− mice infected with ΔpstA1 bacteria survived to 13 weeks postinfection, when the experiment was terminated (P < 0.0001). Complementation reversed the replication and virulence defects of ΔpstA1 bacteria in NOS2−/− mice (Fig. 2C and D). These results confirm that PstA1 is required to counteract an immune mechanism that is IFN-γ dependent but NOS2 independent.
The ΔpstA1 mutant was also attenuated for replication and virulence in Irgm1−/− mice. As in NOS2−/− mice, ΔpstA1 bacteria replicated in the lungs of Irgm1−/− mice with kinetics identical to those of WT bacteria during the first 2 weeks of infection, but replication was significantly impaired thereafter (Fig. 2E). Pulmonary CFU of the ΔpstA1 mutant in Irgm1−/− mice showed little change between 4 and 12 weeks postinfection. The ΔpstA1 mutant was also significantly less virulent (Fig. 2F). The mean survival time of Irgm1−/− mice infected with WT bacteria was 38.5 days, whereas all Irgm1−/− mice infected with ΔpstA1 bacteria survived to 13 weeks postinfection, when the experiment was terminated (P < 0.0001). Complementation reversed the replication and virulence defects of ΔpstA1 bacteria in Irgm1−/− mice (Fig. 2E and F). These data suggest that PstA1 counteracts an IFN-γ-dependent immune mechanism that is independent of phagosome maturation mediated by Irgm1.
The IFN-γ-dependent attenuation of the ΔpstA1 mutant might be explained by sensitivity of the mutant to the oxidative burst generated by phagocyte oxidase (phox). This hypothesis is consistent with in vitro experiments demonstrating hypersensitivity of the ΔpstA1 mutant to reactive oxygen species (ROS) (see below). To examine this possibility, we monitored the replication and persistence of ΔpstA1 bacteria in aerosol-infected gp91phox−/− mice lacking the enzymatic gp91 subunit of phox. The ΔpstA1 mutant exhibited no replication defect during the first 2 weeks postinfection, but CFU began to decline at the transition between the acute and chronic phases of infection (Fig. 2H). By 8 weeks postinfection, there was a statistically significant difference in the number of CFU recovered from the lungs of gp91phox−/− mice infected with WT or ΔpstA1 bacteria that was maintained throughout the chronic phase of infection (Fig. 2H). These results suggest that the ΔpstA1 mutant is sensitive to an IFN-γ-dependent immune response that is independent of ROS produced by phox.
PstA1 is required for persistence in wild-type mice.
The results of infections in immune-deficient mice suggested that PstA1 is important for bacterial resistance to host immune responses. Therefore, we investigated whether PstA1 is required for normal replication and persistence in aerosol-infected wild-type C57BL/6 mice. These mice are relatively resistant to M. tuberculosis and maintain a long-term chronic persistent infection in the lungs. Although ΔpstA1 bacteria replicated in the lungs like WT bacteria during the acute phase of infection, between 2 and 4 weeks postinfection, ΔpstA1 CFU began to decline (Fig. 2G). By 8 weeks postinfection, ΔpstA1 CFU were significantly reduced compared to WT CFU, and this difference was maintained throughout the chronic phase of infection. The persistence defect of the ΔpstA1 mutant is consistent with a specific role for PstA1 in counteracting an IFN-γ-dependent adaptive immune response, since IFN-γ-producing T cells are not recruited to the lungs of aerosol-infected mice until 2 to 3 weeks postinfection (37). Complementation of the ΔpstA1 mutation restored persistence in C57BL/6 mice (Fig. 2G). CFU of the ΔpstA1 pMV-pstA1 strain were slightly reduced compared to WT CFU at 21 weeks postinfection but were significantly higher than CFU of the noncomplemented ΔpstA1 mutant (P = 0.006). These results demonstrate that PstA1 is required for M. tuberculosis persistence in wild-type mice, presumably because it contributes to bacterial resistance to IFN-γ-dependent immunity.
PstA1 is required for surviving phosphate starvation in vitro.
To identify factors that might contribute to the IFN-γ-dependent attenuation of the ΔpstA1 mutant, we tested the sensitivity of the mutant to various stress conditions in vitro. Because the M. tuberculosis Pst systems have previously been implicated in Pi uptake under Pi-limiting growth conditions (22), we compared replication of the ΔpstA1 mutant in Pi-rich and Pi-limiting 7H9 broth cultures. The ΔpstA1 mutant replicated with kinetics identical to those of WT bacteria in Pi-rich medium (Fig. 3). Under Pi-limiting conditions, the ΔpstA1 mutant exhibited a survival defect. CFU of the ΔpstA1 mutant began to decrease between days 3 and 5, continued to fall until day 10, and were significantly different from the WT CFU (Fig. 4A). The complemented strain survived during Pi limitation similar to WT bacteria (Fig. 4A), confirming that the M. tuberculosis Pst system is required for survival during Pi starvation.
Fig 3.
Growth of ΔpstA1 bacteria in Pi-rich medium. M. tuberculosis wild-type and ΔpstA1 were inoculated in 7H9 broth at an OD600 of 0.02 and incubated at 37°C with aeration. Growth was monitored by measuring the optical density (OD600) of the cultures (A) and by enumerating CFU (B) at the indicated times. Plates were incubated at 37°C for 3 to 4 weeks prior to CFU enumeration. Results are representative of three independent experiments.
Fig 4.
Stress sensitivity of the ΔpstA1 mutant. M. tuberculosis WT, ΔpstA1, and ΔpstA1 pMV-pstA1 (comp) were grown to mid-exponential phase in Pi-rich 7H9 medium and then subjected to the indicated stresses. Bacterial CFU were enumerated by plating serially diluted cultures on 7H10 agar and incubating for 3 to 4 weeks at 37°C. Percent survival was calculated as (CFU poststress)/(CFU prestress) × 100. Data shown are the means ± standard deviations for three independent cultures from one representative experiment. Data in panels A to D are representative of at least three independent experiments. Data in panel E are representative of two independent experiments. Asterisks indicate statistically significant differences from WT (*, P < 0.05; **, P < 0.005; ***, P < 0.0001). (A) Pi starvation. Bacteria were shifted to Pi-free 7H9 medium, and CFU were enumerated at t = 0, 3, 5, 7, and 10 days. (B) Reactive oxygen species. Hydrogen peroxide (H2O2) was added to the cultures at a final concentration of 0, 0.75, 1.5, 3, or 5 mM. CFU were enumerated at t = 0 and 24 h. (C) Reactive nitrogen species. The nitric oxide donor DETA-NO was added to the cultures at a final concentration of 0.2 mM at t = 0, 24, and 48 h. CFU were enumerated at t = 0 and 72 h. (D) Cell wall stress. Sodium dodecyl sulfate (SDS) was added to the cultures at a final concentration of 0, 0.03, 0.06, 0.125, 0.25, or 0.5% (wt/vol). CFU were enumerated at t = 0 and 24 h. (E) Hypoxic stress. Oxygen was depleted using a standard sealed culture tube model. CFU were enumerated at 0, 30, 60, 100, and 200 days after tubes were sealed.
PstA1 is required for resistance to stress conditions in vitro.
Because the results of experiments in immune-deficient mice suggested that the ΔpstA1 mutant might be sensitive to an as-yet unidentified IFN-γ-dependent immune mechanism, we tested responses of the mutant to relevant stress conditions in vitro. Bacteria in exponential-phase Pi-rich 7H9 broth cultures were exposed to hydrogen peroxide (H2O2) or to the nitric oxide (NO) donor DETA-NO, and survival was monitored by enumerating CFU. The ΔpstA1 mutant was more sensitive than WT bacteria to H2O2 (Fig. 4B) and NO (Fig. 4C), and hypersensitivity to these stresses was reversed by complementation.
We also tested bacterial sensitivity to the detergent SDS, which causes perturbations to the mycobacterial cell wall (38). The ΔpstA1 mutant was hypersensitive to SDS over a range of concentrations (Fig. 4D). Complementation restored WT levels of SDS resistance to the ΔpstA1 mutant, confirming that SDS sensitivity was due to deletion of pstA1 (Fig. 4D).
Lastly, we examined the survival of ΔpstA1 bacteria in a model of hypoxia-induced nonreplicating persistence that recapitulates the reduced oxygen tension encountered by M. tuberculosis during growth within granulomas (39). The ΔpstA1 mutant exhibited a significant survival defect in this model, which was partially reversed by complementation (Fig. 4E).
Although pstA1 deficiency conferred pleiotropic stress sensitivity phenotypes, it did not cause hypersensitivity to all stress conditions. For example, deletion of pstA1 did not confer increased sensitivity to high osmolarity or to several different classes of antibiotics (data not shown). We also note that the stress sensitivity assays reported above were performed in Pi-rich 7H9 medium that is phosphate-buffered and contains 25 mM Pi, a concentration 100-fold higher than is required for optimal growth of M. tuberculosis. The ΔpstA1 mutant exhibited no growth defect in Pi-rich 7H9 medium (Fig. 3), suggesting that ΔpstA1 bacteria are capable of acquiring sufficient Pi for replication by alternative Pi uptake mechanisms under these growth conditions. Indeed, the Pst high-affinity Pi transporter is predicted to be dispensable for Pi uptake when Pi is abundant, since the M. tuberculosis genome encodes two alternative low-affinity Pi transporters, PitA and PitB, that function when the external Pi concentration is relatively high (18). These observations suggested that PstA1 might have an important function separate from its role in Pi uptake.
Transcriptome profiling identifies dysregulated genes in ΔpstA1 bacteria.
In other species, inactivation of the Pst system leads to constitutive expression of the Pi-responsive regulon that is normally induced only during Pi starvation. To test whether the M. tuberculosis Pst system performs a similar negative regulatory function, we performed transcriptome profiling experiments on WT and ΔpstA1 bacteria grown to early exponential phase in Pi-rich 7H9 broth. Compared to the WT, the ΔpstA1 mutant reproducibly exhibited more than 2-fold overexpression of 41 genes and underexpression of 25 genes (see Table S4 in the supplemental material). Among the overexpressed genes were several related to Pi uptake and metabolism, including pstS3, pstC2, pitB, and rv2577, which encodes a putative acid phosphatase (see Table S4). Genes encoding the two-component signal transduction system SenX3-RegX3 were also overexpressed by 2.6-fold and 1.8-fold, respectively, in the ΔpstA1 mutant (see Table S4).
To confirm the transcriptome profiling results, we examined the levels of select dysregulated transcripts by real-time quantitative RT-PCR (qRT-PCR). The qRT-PCR results supported the microarray results. The senX3 and regX3 genes were overexpressed 4.5-fold and 2.9-fold, respectively, in the ΔpstA1 mutant compared to the WT control (Fig. 5A). Likewise, qRT-PCR confirmed that pstS3 and pitB were overexpressed 10-fold and 15-fold, respectively, in the ΔpstA1 mutant (Fig. 5B). M. tuberculosis encodes two alternative PstS substrate-binding proteins that were not identified as dysregulated in the microarray analysis; the levels of the pstS1 and pstS2 transcripts were unaffected by the ΔpstA1 deletion (Fig. 5B). Finally, we verified increased transcript levels for a set of genes that were highly overexpressed in the ΔpstA1 mutant. We found that each of these genes was expressed at an increased level in the ΔpstA1 mutant compared to the WT control, with fold changes in expression ranging from 8.6-fold for the mgtA transcript to 125-fold for the udgA transcript (Fig. 5C). These observations suggest that pstA1 deficiency causes marked changes to the bacterial transcriptome that could affect cellular physiology.
Fig 5.
Quantitative RT-PCR analysis of genes dysregulated in the ΔpstA1 mutant. RNA was extracted from cultures of M. tuberculosis WT, ΔpstA1, ΔregX3, ΔpstA1 ΔregX3, ΔregX3 pND-regX3, and ΔpstA1 ΔregX3 pND-regX3 grown to mid-exponential phase (OD600 of 0.5) in Pi-rich 7H9 medium. Transcript abundance relative to abundance of sigA was determined by real-time quantitative RT-PCR. Data shown are the means ± standard deviations of two independent experiments. (A) senX3 and regX3 transcripts. (B) pstS3, pstS1, pstS2, and pitB transcripts. (C) rv3026, udgA, mgtA, rv0784, and pimC transcripts.
SenX3-RegX3 is responsible for aberrant gene expression in ΔpstA1 bacteria.
Because the senX3 and regX3 genes are overexpressed in ΔpstA1 bacteria and this system has previously been implicated in Pi starvation-responsive gene regulation (23, 24), we hypothesized that constitutive activation of SenX3-RegX3 causes aberrant gene expression in the ΔpstA1 mutant. To test this possibility, we deleted regX3, encoding the DNA-binding response regulator, and performed transcriptional profiling experiments comparing ΔregX3 and ΔpstA1 ΔregX3 mutants to WT bacteria. In the ΔpstA1 mutant background, deletion of regX3 restored the expression of most dysregulated transcripts to WT levels (see Table S4 in the supplemental material), supporting our hypothesis. Deletion of regX3 alone likewise had little impact on transcript levels for this set of genes (see Table S4). We confirmed these transcriptional profiling results by qRT-PCR. For each transcript tested, deletion of regX3 alone had no impact on transcript levels whereas deletion of regX3 in the ΔpstA1 mutant reversed the aberrant pattern of expression (Fig. 5). Complementation of the ΔpstA1 ΔregX3 mutant by expressing regX3 from a plasmid (pND-regX3) restored the aberrant gene expression pattern characteristic of the ΔpstA1 mutant (Fig. 5).
Our transcriptional profiling analysis also revealed significant changes in gene expression compared to the WT control for both the ΔregX3 and ΔpstA1 ΔregX3 mutants under the Pi-rich growth conditions that we used (see Table S5 and S6, respectively, in the supplemental material). Most of the differentially expressed genes were underexpressed in the ΔregX3 mutants compared to WT bacteria (82 genes in the ΔregX3 mutant and 69 genes in ΔpstA1 ΔregX3 mutant). These data suggest that RegX3 is required for maximal expression of certain transcripts, even when Pi is abundant.
RegX3 is required for Pi-responsive gene regulation.
RegX3 has previously been implicated in the M. tuberculosis transcriptional response to Pi starvation (23). We hypothesized that genes whose expression is dysregulated in the ΔpstA1 mutant might require RegX3 for their activation or repression during Pi limitation. We examined the levels of several putative RegX3-dependent transcripts over time during growth in Pi-limiting medium. We found that ugdA and mgtA were constitutively expressed in the ΔpstA1 mutant and were induced in WT bacteria within 24 h of Pi starvation in a RegX3-dependent manner (Fig. 6A and B). Similar patterns of RegX3-dependent induction in response to Pi starvation were observed for the pstS3 and pitB transcripts (data not shown). In contrast, the rv1387 transcript was constitutively repressed in the ΔpstA1 mutant and repressed in a RegX3-dependent manner within 24 h of Pi starvation in WT bacteria (Fig. 6C). These data suggest that RegX3 can be activated either by Pi limitation or by Pst deficiency.
Fig 6.
RegX3 is required for regulation of gene expression in response to phosphate limitation. RNA was extracted from cultures of M. tuberculosis WT, ΔpstA1, ΔregX3, and ΔpstA1 ΔregX3 at t = 0, 8, 24, 48, 72, and 96 h after shifting to Pi-free 7H9 medium. Transcript abundance relative to 16S rRNA was determined by real-time qRT-PCR. Data shown are the means ± standard deviations of two independent experiments. (A) mgtA transcript. (B) udgA transcript. (C) rv1387 transcript.
Aberrant activation of RegX3 causes stress sensitivity in ΔpstA1 bacteria.
We hypothesized that sensitivity of the ΔpstA1 mutant to stresses (Fig. 4) might be caused by constitutive activation of SenX3-RegX3. Deletion of regX3 had no impact on stress resistance in the WT background but suppressed the hypersensitivity of the ΔpstA1 mutant to SDS (Fig. 7A), H2O2 (Fig. 7B), and DETA-NO (Fig. 7C). Stress hypersensitivity was restored by complementation of the ΔpstA1 ΔregX3 mutant with pND-regX3 (Fig. 7). These results suggest that ΔpstA1 bacteria are hypersensitive to stresses due to dysregulated gene expression rather than impaired Pi uptake. To test whether phosphorylation-mediated activation of RegX3 is required for stress hypersensitivity, we introduced a point mutation in regX3 that converts the phosphorylated Asp at position 52 (40) to a nonphosphorylatable Ala (regX3D52A). The ΔpstA1 regX3D52A double mutant was as resistant as WT bacteria to SDS and H2O2 (Fig. 7A and B). These results suggest that the stress hypersensitivity of the ΔpstA1 mutant under Pi-rich conditions is due to dysregulated gene expression mediated by phosphorylation-activated RegX3.
Fig 7.
Deletion of regX3 suppresses stress hypersensitivity of the ΔpstA1 mutant. M. tuberculosis WT, ΔpstA1, ΔregX3, ΔpstA1 ΔregX3, ΔpstA1 ΔregX3 pND-regX3, regX3D52A, and ΔpstA1 regX3D52A were grown to mid-exponential phase in Pi-rich 7H9 medium and then subjected to the indicated stresses. Bacterial CFU were enumerated by plating serially diluted cultures on 7H10 agar and incubating for 3 to 4 weeks at 37°C. Percent survival was calculated as (CFU poststress)/(CFU prestress) × 100. Data shown are means ± standard deviations of at least three independent experiments. Asterisks indicate statistically significant differences from WT (*, P < 0.05 by Student's unpaired t test). (A) Cell wall stress. Sodium dodecyl sulfate (SDS) was added to the cultures at a final concentration of 0.125% (wt/vol). CFU were enumerated at t = 0 and 24 h. (B) Reactive oxygen species. Hydrogen peroxide (H2O2) was added to the cultures at a final concentration of 3 mM. CFU were enumerated at t = 0 and 24 h. (C) Reactive nitrogen species. The nitric oxide donor DETA-NO was added to the cultures at a final concentration of 0.2 mM at t = 0, 24, and 48 h. CFU were enumerated at t = 0 and 72 h.
RegX3-dependent gene expression mediates attenuation of ΔpstA1 bacteria in NOS2−/− mice.
We hypothesized that the ΔpstA1 mutant is hypersensitive to an IFN-γ-dependent immune mechanism because it is fully virulent in IFN-γ−/− mice but attenuated in C57BL/6, NOS2−/−, Irgm1−/−, and gp91phox−/− mice (Fig. 2). To examine whether aberrant gene expression mediated by RegX3 sensitizes the bacteria to host immune responses, we tested whether deletion of regX3 would restore replication competence and virulence to the ΔpstA1 mutant in aerosol-infected mice. In contrast to the situation in vitro, in which deletion of regX3 alone had no impact on M. tuberculosis stress resistance, ΔregX3 bacteria were impaired for acute-phase growth and chronic-phase persistence in the lungs of wild-type C57BL/6 mice (Fig. 8A). The growth defects during the acute phase of infection were independent of IFN-γ, since the ΔregX3 and ΔpstA1 ΔregX3 mutants were attenuated for virulence in IFN-γ−/− mice compared to WT bacteria (P < 0.0001) (Fig. 8B). The ΔregX3 mutant was likewise impaired for growth in the lungs of Irgm1−/− mice (Fig. 8C), and Irgm1−/− mice infected with the ΔregX3 or ΔpstA1 ΔregX3 mutant survived at least 15 weeks longer than mice infected with WT bacteria (P < 0.0001) (Fig. 8D). Although these results precluded a determination of whether or not aberrant gene expression mediated by RegX3 causes attenuation of the ΔpstA1 mutant in C57BL/6 and Irgm1−/− mice, nevertheless they confirm that signal transduction by the SenX3-RegX3 system is important for M. tuberculosis pathogenesis.
Fig 8.
Deletion of regX3 partially suppresses attenuation of the ΔpstA1 mutant in NOS2−/− mice. Mice were aerosol infected with ∼100 CFU of M. tuberculosis WT, ΔregX3, ΔpstA1, or ΔpstA1 ΔregX3. Mouse strains were as follows: C57BL/6 (A), IFN-γ−/− (B), Irgm1−/− (C and D), and NOS2−/− (E and F). All mouse strains were on the C57BL/6 background. (A, C, E) Growth kinetics. Groups of infected mice (n = 4) were sacrificed at the indicated time points. Bacterial CFU were enumerated by plating lung homogenates on 7H10 agar and incubating for 3 to 4 weeks at 37°C. Symbols represent means; error bars indicate standard errors of the means. (B, D, F) Virulence. Groups of infected mice (n = 4 to 8) were monitored for signs of illness. Moribund mice were euthanized and scored as dead.
In contrast, deletion of regX3 had little impact on M. tuberculosis replication in the lungs of NOS2−/− mice (Fig. 8E). Although the ΔregX3 mutant exhibited a significant replication defect during the first 2 weeks of infection in NOS2−/− mice (P = 0.0002), by 6 weeks there was no significant difference in CFU recovered from the lungs of mice infected with WT or ΔregX3 bacteria (P = 0.76). Remarkably, deletion of regX3 partially restored the ability of the ΔpstA1 mutant to replicate in the lungs of NOS2−/− mice (Fig. 8E). Bacterial loads were higher in the lungs of ΔpstA1 ΔregX3-infected mice than in ΔpstA1-infected mice at 4 weeks (P = 0.0006) and 6 weeks (P = 0.0037) postinfection. The ΔpstA1 ΔregX3 double mutant also exhibited increased virulence in NOS2−/− mice compared to the ΔpstA1 mutant (P = 0.0003) (Fig. 8F). All NOS2−/− mice infected with ΔpstA1 ΔregX3 bacteria succumbed to infection within 10 weeks, whereas all mice infected with ΔpstA1 bacteria survived for at least 13 weeks, when the experiment was terminated (Fig. 8F). These results demonstrate that regX3 deficiency is epistatic to pstA1 deficiency and suggest that the ΔpstA1 mutant is attenuated in NOS2−/− mice due to RegX3-mediated dysregulation of an as-yet-unidentified factor(s).
DISCUSSION
Immunocompetent individuals harboring persistent M. tuberculosis constitute a global reservoir of potentially transmissible bacteria, but the mechanisms that enable M. tuberculosis to survive host immune defenses remain poorly understood. We used a mouse infection model in combination with bacterial and host genetics to identify M. tuberculosis factors that counteract IFN-γ-dependent immunity (17). We demonstrate that the M. tuberculosis Pi uptake system component PstA1 is essential to counteract an IFN-γ-dependent immune response that is independent of RNS produced by NOS2, ROS produced by phox, or phagosome maturation driven by Irgm1. We further show that attenuation of the ΔpstA1 mutant is caused, in part, by constitutive activation of the Pi starvation-responsive signal transduction system SenX3-RegX3.
Previous studies suggested that the Pst system is required for M. tuberculosis replication in both macrophages and mice because its Pi uptake function counteracts Pi limitation experienced in host tissues (21, 22). Our data challenge this interpretation. We demonstrate that the Pst system also participates in transcriptional control by negatively regulating the SenX3-RegX3 signal transduction system under Pi-rich growth conditions. We provide evidence that this regulatory function of the Pst system is critical to survive host immune responses in the mouse infection model. The replication and virulence defects of ΔpstA1 bacteria in NOS2−/− mice are reversed by deletion of regX3, which also restores a wild-type gene expression pattern in Pi-rich medium. Our results therefore support an alternative interpretation: that Pi is readily available in host tissues and that the Pst system is required to sense this nutritional signal and prevent inappropriate activation of the RegX3 response regulator.
Our transcriptome profiling experiments revealed a set of 66 genes that were aberrantly expressed in the ΔpstA1 mutant in a RegX3-dependent manner. We have confirmed by qRT-PCR that at least five of these genes require RegX3 for their induction or repression during Pi limitation. In addition, half of the genes we identified are regulated at the transcriptional level in response to Pi starvation (23). We predict that these genes represent the RegX3-dependent Pi starvation-responsive regulon of M. tuberculosis.
Our results contrast with those of two previous reports that claimed to identify the M. tuberculosis RegX3 regulon. One study that analyzed the transcriptional response to Pi starvation identified over 600 genes that were differentially expressed during Pi limitation and suggested that these genes are controlled by SenX3-RegX3 (23). Comparing the transcriptional profiles of bacteria grown in Pi-rich versus Pi-limiting conditions, as was done in this study, is problematic because many transcriptional responses to Pi limitation may actually be secondary effects due to changes in metabolic activity or growth rate, instead of a specific response to the nutritional stress (41). The relatively broad transcriptional response to Pi starvation may therefore reflect global alterations in the activity of many transcription factors, rather than activation of RegX3 specifically. Our results also differ substantially from the previously reported RegX3 regulon that was identified by transcriptome profiling of a senX3-regX3 mutant grown in Pi-rich medium (42). Of the nearly 100 genes identified by this study, only two overlap either the putative RegX3-dependent genes that were dysregulated in the ΔpstA1 mutant or the 80 genes that were differentially expressed by our ΔregX3 mutant. Differences in the growth state of the cultures might account for these different results.
Our observations indicate that dysregulated gene expression mediated by RegX3 is responsible for hypersensitivity of the ΔpstA1 mutant to diverse stress conditions in vitro and IFN-γ-dependent immune responses in vivo. This implies that a factor or factors controlled by RegX3 directly influences sensitivity to stress. However, most of the RegX3-dependent genes that we identified have not previously been implicated in stress responses or virulence, and they encode proteins of unknown function. Notable exceptions are the esxA, esxB, espA, and espE genes that encode secreted substrates of the virulence-associated ESX-1 secretion system (43). Because these genes were underexpressed in the ΔpstA1 mutant, it is possible that decreased ESX-1 secretion system activity attenuates ΔpstA1 bacteria. Alternatively, one or more RegX3-dependent genes may influence the permeability or integrity of the mycobacterial cell wall, since the pleiotropic stress sensitivity of the ΔpstA1 mutant resembles that of M. tuberculosis with cell wall defects (34). Future studies will focus on identifying the specific RegX3-dependent factor(s) responsible for sensitivity to stress and to host immunity.
The IFN-γ-dependent immune mechanism(s) responsible for controlling the replication of ΔpstA1 bacteria also remains undefined. The ΔpstA1 mutant might be hypersensitive to a combination of IFN-γ-activated macrophage functions, for example, RNS produced by NOS2 plus phagosome acidification mediated by Irgm1. In this scenario, the ΔpstA1 mutant would be attenuated in mice lacking either NOS2 or Irgm1 because the other can effectively control bacterial replication. This hypothesis is consistent with the hypersensitivity of the ΔpstA1 mutant to multiple stress conditions in vitro. Alternatively, the ΔpstA1 mutant might be sensitive to a known IFN-γ-dependent immune mechanism other than the effector mechanisms that we have tested, such as the ubiquitin-derived or hepcidin antimicrobial peptides (44, 45). Finally, the ΔpstA1 mutant could be sensitive to an as-yet-unidentified immune mechanism that is dependent on IFN-γ activation. Because the phenotypes of the ΔpstA1 mutant point to the potential existence of previously unrecognized IFN-γ-dependent immune system functions, we speculate that the ΔpstA1 mutant could be used in genetic screens to discover these novel host factors. A host gene knockdown that reverses the in vivo growth and virulence defects of ΔpstA1 bacteria would suggest that the ablated host factor is normally responsible for limiting replication of the ΔpstA1 mutant.
We demonstrate that RegX3 is also essential for normal growth in wild-type mice and virulence in IFN-γ−/− mice, consistent with a previous report that regX3-deficient bacteria are attenuated in immune-deficient SCID mice (42). Because RegX3 mediates transcriptional responses to Pi limitation, M. tuberculosis may experience Pi starvation during replication in host tissues and require RegX3 to respond to this condition. This conclusion contrasts with our interpretation that Pi is readily available in host tissues, since constitutive activation of RegX3 sensitizes ΔpstA1 bacteria to host immune responses. There are two ways these conflicting interpretations could be reconciled. One possibility is that M. tuberculosis does experience Pi limitation, but only at specific times postinfection or places within host tissue. Alternatively, one or more of the 80 genes that require RegX3 for maximal expression during growth in Pi-rich conditions might be essential for replication in host tissue. It may be possible to address these hypotheses by monitoring the expression of RegX3-dependent genes during infection.
In summary, we have identified a signal transduction system that plays a critical role in M. tuberculosis immune evasion and persistence by controlling gene expression in response to Pi availability. However, the molecular mechanisms by which the Pst system senses Pi concentration and controls SenX3-RegX3 activity remain mysterious. In Escherichia coli, a similar collaboration between the Pst Pi uptake system and the orthologous PhoBR two-component system was discovered over 2 decades ago. Despite intensive investigation, the molecular nature of the E. coli Pst-PhoBR interaction is still unclear (46). Because communication between the Pst system and its cognate two-component signal transduction system is critical for virulence of M. tuberculosis and many other pathogens, characterization of this interaction may reveal novel targets for the development of antimicrobials with broad-spectrum activity.
Supplementary Material
ACKNOWLEDGMENTS
We thank Neeraj Dhar for plasmid pND200. Jonathan Plenn, Sonia Garcia, and Laetitia Martin provided expert technical assistance with animal experiments.
A.D.T. was supported by an Irvington Institute Postdoctoral Fellowship of the Cancer Research Institute. M.A.K. was supported by a Robert D. Watkins Graduate Fellowship of the American Society of Microbiology and NIH MSTP grant GM07739 to the M.D./Ph.D. program of Weill-Cornell Medical School. R.L.L. was supported by NIH training grant T32 AI052066. This work was supported by National Institutes of Health grants AI061505 (M.I.V.) and HL88906 (J.D.M.) and the intramural research program of the School of Life Sciences, EPFL (J.D.M.).
Footnotes
Published ahead of print 6 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01136-12.
REFERENCES
- 1. Lin PL, Flynn JL. 2010. Understanding latent tuberculosis: a moving target. J. Immunol. 185:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van de Vosse E, Hoeve MA, Ottenhoff TH. 2004. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect. Dis. 4:739–749 [DOI] [PubMed] [Google Scholar]
- 3. Cooper AM, Dalton DK, Stewart TA, Griffen JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J. Exp. Med. 178:2243–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart T, Bloom BR. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf AJ, Linas B, Trevejo-Nuñez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. 2007. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179:2509–2519 [DOI] [PubMed] [Google Scholar]
- 6. Flannagan RS, Cosîo G, Grinstein S. 2009. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7:355–366 [DOI] [PubMed] [Google Scholar]
- 7. Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. U. S. A. 97:8841–8848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung Y-J, LaCourse R, Ryan L, North RJ. 2002. Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J. Exp. Med. 196:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. 2004. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol. Microbiol. 52:1291–1302 [DOI] [PubMed] [Google Scholar]
- 10. Miller JL, Velmurugan K, Cowan MJ, Briken V. 2010. The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-α-mediated host cell apoptosis. PLoS Pathog. 6:e1000864 doi:10.1371/journal.ppat.1000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 94:5243–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shenoy AR, Kim B-H, Choi H-P, Matsuzawa T, Tiwari S, MacMicking JD. 2007. Emerging themes in IFN-γ-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiol. 212:771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tiwari S, Choi H-P, Matsuzawa T, Pypaert M, MacMicking JD. 2009. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat. Immunol. 8:907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacMicking JD, Taylor GA, McKinney JD. 2003. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302:654–659 [DOI] [PubMed] [Google Scholar]
- 15. Ehrt S, Schnappinger D. 2009. Mycobacterial survival strategies in the phagosome: defense against host stresses. Cell. Microbiol. 11:1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vandal OH, Nathan CF, Ehrt S. 2009. Acid resistance in Mycobacterium tuberculosis. J. Bacteriol. 191:4714–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hisert KB, Kirksey MA, Gomez JE, Sousa AO, Cox JS, Jacobs WR, Jr, Nathan CF, McKinney JD. 2004. Identification of Mycobacterium tuberculosis counterimmune (cim) mutants in immunodeficient mice by differential screening. Infect. Immun. 72:5315–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon, p 1357–1381 In Neidhardt FC. (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 19. Lamarche MG, Wanner BL, Crépin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 32:461–473 [DOI] [PubMed] [Google Scholar]
- 20. Braibant M, Gilot P, Content J. 2000. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 24:449–467 [DOI] [PubMed] [Google Scholar]
- 21. Rengarajan J, Bloom BR, Rubin EJ. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 102:8327–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peirs P, Lefevre P, Boarbi S, Wang XM, Denis O, Braibant M, Pethe K, Locht C, Huygen K, Content J. 2005. Mycobacterium tuberculosis with disruption in genes encoding the phosphate binding proteins PstS1 and PstS2 is deficient in phosphate uptake and demonstrates reduced in vivo virulence. Infect. Immun. 73:1898–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rifat D, Bishai WR, Karakousis PC. 2009. Phosphate depletion: a novel trigger for Mycobacterium tuberculosis persistence. J. Infect. Dis. 200:1126–1135 [DOI] [PubMed] [Google Scholar]
- 24. Glover RT, Kriakov J, Garforth SJ, Baughn AD, Jacobs WR., Jr 2007. The two-component regulatory system senX3-regX3 regulates phosphate-dependent gene expression in Mycobacterium smegmatis. J. Bacteriol. 189:5495–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirksey MA, Tischler AD, Siméone R, Hisert KB, Uplekar S, Guilhot C, McKinney JD. 2011. Spontaneous phthiocerol dimycocerosate-deficient variants of Mycobacterium tuberculosis are susceptible to gamma interferon-mediated immunity. Infect. Immun. 79:2829–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muñoz-Elías EJ, McKinney JD. 2005. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senanayake SD, Brian DA. 1995. Precise large deletions by the PCR-based overlap extension method. Mol. Biotechnol. 4:13–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pavelka MS, Jr, Jacobs WR., Jr 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis Bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parish T, Stoker NG. 2000. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiol. 146:1969–1975 [DOI] [PubMed] [Google Scholar]
- 30. Cox JS, Chen B, McNeil M, Jacobs WR., Jr 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79–83 [DOI] [PubMed] [Google Scholar]
- 31. Slayden RA, Barry CE., III 2001. Analysis of the lipids of Mycobacterium tuberculosis, p 229–245 In Parish T, Stoker NG. (ed), Mycobacterium tuberculosis protocols. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 32. Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Vande Woude GF, Sher A, Taylor GA. 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon γ-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 194:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wiegeshaus EH, McMurray DN, Grover AA, Harding GE, Smith DW. 1970. Host-parasite relationships in experimental airborne tuberculosis. III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 102:422–429 [DOI] [PubMed] [Google Scholar]
- 34. Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. 2009. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J. Bacteriol. 191:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wayne LG, Lin K-Y. 1982. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect. Immun. 37:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377 [DOI] [PubMed] [Google Scholar]
- 38. Manganelli R, Voskuil MI, Schoolnik GK, Smith I. 2001. The Mycobacterium tuberculosis ECF sigma factor σE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423–437 [DOI] [PubMed] [Google Scholar]
- 39. Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha E, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., III 2008. Tuberculosis granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Himpens S, Locht C, Supply P. 2000. Molecular characterization of the mycobacterial SenX3-RegX3 two-component system: evidence for autoregulation. Microbiol. 146:3091–3098 [DOI] [PubMed] [Google Scholar]
- 41. Brauer MJ, Huttenhower C, Airoldi EM, Rosenstein R, Matese JC, Gresham D, Boer VM, Troyanskaya OG, Botstein D. 2008. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol. Biol. Cell 19:352–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parish T, Smith DA, Roberts G, Betts J, Stoker NG. 2003. The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiol. 149:1423–1435 [DOI] [PubMed] [Google Scholar]
- 43. Bitter W, Houben ENG, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire KM, Fortune SM, Gao Liu L-YJ, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DS, Cole ST, Brosch R. 2009. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 5:e1000507 doi:10.1371/journal.ppat.1000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alonso S, Pethe K, Russell DG, Purdy GE. 2007. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. U. S. A. 104:6031–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. 2007. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J. Leukoc. Biol. 82:934–945 [DOI] [PubMed] [Google Scholar]
- 46. Hsieh Y-J, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 13:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.