Abstract
Naturally occurring human immunity to both schistosomiasis and hookworm infection has been associated with IgE responses against parasite allergen-like proteins. Since the two helminths frequently coinfect the same individuals, there is growing advocacy for their concurrent treatment. However, both helminths are known to exert strong immunomodulatory effects; therefore, coinfected individuals could have immune responses different from those characteristically seen in monoinfected individuals. In this study, we measured changes in IgE, IgG1, and IgG4 responses to schistosome and hookworm antigens, including the allergen-like proteins Schistosoma mansoni tegumental-allergen-like 1 protein (SmTAL1), SmTAL2, and Necator americanus Ancylostoma-secreted protein-2 (Na-ASP-2), following concurrent treatment of schoolchildren coinfected with Schistosoma mansoni and hookworm. Antibody responses to schistosome egg (soluble egg antigen and SmTAL2) or somatic adult hookworm (AHW) antigens either decreased after treatment or were unchanged, whereas those to schistosome worm antigens (soluble worm antigen and SmTAL1) increased. The observed different effects of treatment likely reflect the different modes of drug action and sites of infection for these two helminths. Importantly, there was no evidence that the simultaneous treatment of coinfected children with praziquantel and albendazole affected schistosome- and hookworm-specific humoral responses differently from those characteristic of populations in which only one organism is endemic; schistosome- and hookworm-specific responses were not associated, and there was no evidence for cross-regulation. Posttreatment increases in the levels of IgE to schistosome worm antigens were associated with lower Schistosoma mansoni reinfection intensity, while no associations between humoral responses to AHW antigen and protection from hookworm reinfection were observed in this sample of school-aged children.
INTRODUCTION
Schistosomiasis and hookworms are estimated to affect about 207 million and 740 million people worldwide, respectively (1, 2). Although there is effective chemotherapy for both infections, treatment is insufficient to halt transmission and individuals remain susceptible to reinfection after treatment. However, age-infection profiles observed among populations in which the diseases are endemic provide epidemiological evidence that a natural age-dependent partial immunity can develop to both helminth infections (3, 4).
For both helminth infections, this immunity has been associated with specific IgE responses to crude and defined parasite antigens. In schistosomiasis, IgE to adult worm antigens has been associated with resistance to reinfection (5–8); these responses tend to increase with age and after chemotherapeutic treatment in populations in which the disease is endemic (9, 10). In hookworm infection, negative relationships between both total and specific IgE and worm weight, fecundity, and heavy hookworm infection have been described (11, 12). In contrast to schistosomiasis, there is little evidence for treatment boosting of antibody responses; instead, responses tend to decrease posttreatment (13, 14).
In both helminth infections, IgE responses to a number of defined antigens are strongly associated with immunity (5, 15, 16), and many of these antigens share structural homology with allergens (17). For example, IgE to Schistosoma mansoni tegumental-allergen-like 1 protein (SmTAL1-IgE) is a marker for human immunity in schistosomiasis mansoni (5, 16); SmTAL1 is a member of the TAL family, a group of proteins sharing structural homology with the EF hand allergens, one of the most common groups of clinical allergens (9). In necatoriasis americanus, negative associations between IgE to the recombinant larval protein Necator americanus Ancylostoma-secreted protein-2 (Na-ASP-2) and heavy infection intensity have been described (11); Na-ASP-2 shares structural homology with the pathogenesis-related 1 (PR-1) allergens, the majority of which are derived from insect venoms (18).
While in allergy similar IgE responses are associated with severe pathology, in helminth infection, strong immunomodulatory effects, including regulatory cells and cytokines, and also IgG4, which is capable of blocking IgE-allergen complex formation (19; reviewed in references20, 21, and22), limit IgE-mediated morbidity. Although this immunoregulation reduces pathology during chronic infection and may also offer protection against allergy and autoimmune disease (reviewed in references20, 22, and23), it can also influence immune responses to vaccines (24, 25), coinfecting microparasites (26), and also coinfecting helminths (27, 28). Despite helminth-helminth coinfection often being the norm in tropical regions (29–31) and the potential implications that this may have for vaccine development and control programs, relatively few studies have investigated the immunology of helminth coinfections. Animal studies generally suggest that infection with a Schistosoma sp. may have protective effects against other helminth coinfections (32–34), while human studies point toward downmodulation of specific immune responses among coinfected individuals (27, 28). For example, Geiger et al. observed further downmodulation of hookworm-specific Th1 cytokine responses among individuals coinfected with S. mansoni or Ascaris lumbricoides, and although coinfected individuals also had higher levels of both hookworm-specific IgE and IgG4, correlations between hookworm infection intensity and hookworm-specific IgG4 were also stronger among these individuals (28). A number of studies have also indicated an increased risk of concurrent schistosome-hookworm infection in populations in which both organisms are endemic (35–37) and a propensity toward heavier infection among coinfected individuals (38).
In the current study, we examined posttreatment parasite-specific immunological changes and whether helminth coinfection influences these changes and subsequent reinfection immunity. The study was conducted among school-aged children living in the same community where both diseases are endemic and receiving treatment for schistosomiasis and hookworm as part of the Ugandan national control program. In these children, we compared the effects of concurrent praziquantel and albendazole treatment on IgE, IgG1, and IgG4 responses to defined schistosomal and hookworm antigens derived from different stages of the life cycle, including the allergen-like proteins SmTAL1, SmTAL2, and Na-ASP-2. Findings will help establish the nature of the immune response to these infections after treatment in a setting in which both diseases are endemic and whether there is evidence for cross-regulation and influences on reinfection immunity.
MATERIALS AND METHODS
Study area and population.
This study was conducted among children aged 7 to 16 years attending Bwondha Primary School in Bwondha Village, located along the shoreline of Lake Victoria in Mayuge District, eastern Uganda. As part of the Ugandan national control program, praziquantel and albendazole are administered to all schoolchildren by teachers annually and biannually, respectively (39).
The principal water sources in Bwondha are the lake and shallow wells; boreholes are used by a small minority. Sanitation is poor: ≤33% of households have access to a pit latrine.
Parasitological surveys.
A register of all children (n = 795) attending Bwondha Primary School was drawn up; from this, a simple random sample of 350 children aged 7 to 16 years was selected. A socioeconomic questionnaire was translated to Luganda (the principle language) and administered to the most relevant parent/guardian.
From the end of July 2010, children were treated with albendazole (400 mg) and twice with praziquantel (40 mg/kg of body weight), 1 week apart; in line with national guidelines, children were treated again for hookworm 6 months after the initial treatment. Venous blood samples (5 ml) were collected from children before the initial treatment and 8 weeks after treatment, and stool samples were collected for parasitology prior to treatment and 5 weeks, 6 months, and 12 months after the second dose of initial treatment. Stool samples collected at 5 weeks were used to detect treatment failure or noncompliance and determine treatment efficacy. All stool samples were collected on three consecutive days, and two 50-mg Kato-Katz (40) slides were prepared from each day's sample and examined microscopically (within 30 min for hookworm quantification).
Parasite antigens.
A Puerto Rican strain of S. mansoni, maintained in outbred mice and Biomphalaria glabrata, was used for production of native schistosome antigens. Adult worms were recovered from mice by portal perfusion 6 weeks after infection, and parasite eggs were isolated from liver tissue. Soluble worm antigen (SWA) was prepared from frozen parasites, and saline-soluble egg antigen (SEA) was prepared from frozen eggs, all as previously described (23, 41). The recombinant schistosome antigens SmTAL1 (Sm22.6) and SmTAL2 (Sm21.7) were prepared as previously described (5, 16). Somatic adult hookworm (AHW) crude antigen extract was derived from Ancylostoma caninum, taken from canines. Na-ASP-2, an excretory-secretory (ES) product released by third-stage Necator americanus larvae, was expressed in Pichia pastoris as previously described (11, 42).
Antibody assays.
Plasma was removed from venous blood samples and stored at −80°C until required. Levels of IgE, IgG1, and IgG4 to SEA, SWA, SmTAL1, SmTAL2, AHW, and Na-ASP-2 antigens were measured by enzyme-linked immunosorbent assay (ELISA) as described elsewhere (9). Briefly, 384-well plates were coated with 15 μl/well of antigen at saturation coating concentrations of 1.2 μg/ml (SEA), 8 μg/ml (SWA), 2.75 μg/ml (SmTAL1), 3.8 μg/ml (SmTAL2), 5 μg/ml (AHW), and 5 μg/ml (Na-ASP-2), as determined by titration. Fifteen microliters of sample plasma and plasma from noninfected controls was assayed in duplicate at dilutions of 1/20 for IgE and 1/200 for IgG1 and IgG4. A 3-fold serial dilution of purified human IgG1 or IgG4 (Sigma-Aldrich) or IgE myeloma (Calbiochem) was added directly to each plate to form a 14-point standard curve, starting at 30 μg/ml.
For schistosome antigen assays, detection was as described previously (9). For AHW and Na-ASP-2 assays, horseradish peroxidase (HRP)-conjugated mouse anti-human IgG1, mouse anti-human IgG4-HRP (Invitrogen), or polyclonal goat anti-human IgE-HRP (ABD Serotec) was used for detection. Plates were read at dual wavelengths of 490 and 630 nm on a Powerwave HT microplate reader (BioTek Instruments Inc.). Results were interpolated from the standard curves with a 5-parameter curve fit using Gen5 analysis software (BioTek Instruments Inc.).
Statistical analysis.
Infection intensity was expressed as the mean egg count per gram (epg). Due to overdispersion, epg values were transformed to the logarithm, ln(epg + 1), and geometric means (GMs) were calculated. Details relating to household economic characteristics (house construction, sources of water, sanitation, and asset ownership) were used to construct a proxy measure of economic status, using principle component analysis, as described previously (43, 44). Scores were ranked and divided into tertiles, allowing households to be classified as low, middle, or high economic status.
Detection thresholds for ELISA readings for each antigen and isotype assayed were calculated as the mean plus 3 standard deviations of noninfected European control plasma samples. Readings were analyzed as the logarithm, with the detection threshold added to remove nonpositive numbers. Changes to isotype responses after treatment were calculated as the difference in log pre- and posttreatment antibody levels. These were analyzed in three ways: (i) significance in changes was tested using paired t tests, (ii) differences in changes by age group (≤10 years versus >10 years) were determined using two-sample t tests, and (iii) associations between antibody changes and pretreatment infection intensity [ln(epg + 1)] and how this varied by age group were determined using multiple-regression analysis, adjusting for age and sex, with an interaction term between ln(epg + 1) and age.
Multiple regressions were used to investigate associations between antibody changes and reinfection [ln(epg + 1)] among children infected at baseline, adjusting for age, sex, and treatment efficacy [5-week ln(epg + 1)] as a priori confounders; other variables were assessed by forward selection; no other significant risk factors were found. For hookworm, since the rate of reinfection at 6 months was relatively low (<50%), zero-inflated negative-binomial models were used. For all analyses, age was classified into two binary groups of roughly equal size: 7 to 10 years and 11 to 16 years.
All analyses were conducted using the Stata (version 10.1) program (StataCorp).
Ethical clearance and informed consent.
Ethical clearance was obtained from the Uganda National Council of Science and Technology. Written informed consent was obtained from all parents/guardians of the selected children.
RESULTS
Consent was obtained from the parents or guardian of 277 (79%) of the 350 selected children; 4 of them either failed to comply with or respond to treatment. Of the remaining 273 children, 240 (96%) donated blood at baseline and 8 weeks posttreatment; 179 (75% of 240) and 155 (65%) were present, respectively, for the 6- and 12-month parasitology surveys. There was some evidence that consenting children were slightly older than nonconsenting children (10.4 versus 9.8 years of age); otherwise, children lost to follow-up were not significantly different (results not shown).
The overall pretreatment rates of prevalence of S. mansoni and hookworm infection were high: 93.8% (95% confidence interval [CI], 89.9 to 96.5) and 80.4% (95% CI, 74.8 to 85.2), respectively. The prevalence of S. mansoni and hookworm coinfection was 74.6% (95% CI, 68.6 to 80.0). Both S. mansoni and hookworm infection intensities were lower among monoinfected children (Table 1), but not to the extent of significance [age-, sex-, and socioeconomic status-adjusted S. mansoni GM (epg + 1) ratio = 0.80 (95% CI, 0.44 to 1.46; P = 0.46) and adjusted hookworm GM ratio = 0.85 (95% CI, 0.29 to 2.51) for monoinfected versus coinfected groups].
Table 1.
Distribution of S. mansoni infection and hookworm infection by age and sex among children bled both before and 8 weeks after treatment
| Infection and characteristic | Pretreatment |
12 mo posttreatment |
||||
|---|---|---|---|---|---|---|
| No.a | % prevalence (95% CI) | GMb (epg + 1) (95% CI) | No.c | % prevalence (95% CI) | GM (epg + 1) (95% CI) | |
| S. mansoni | ||||||
| Overall | 240 | 93.8 (89.9, 96.5) | 172.73 (131.61, 226.72) | 155 | 92.9 (87.7, 96.4) | 103.04 (76.54, 138.71) |
| Sex | ||||||
| Male | 117 | 94.9 (89.2, 98.1) | 217.94 (146.89, 323.35) | 79 | 94.9 (87.5, 98.6) | 133.38 (88.55, 200.90) |
| Female | 123 | 92.7 (86.6, 96.6) | 138.47 (95.04, 201.74) | 76 | 90.8 (81.9, 96.2) | 78.79 (51.11, 121.46) |
| P value | 0.48 | 0.10 | 0.31 | 0.08 | ||
| Age (yr) | ||||||
| 7–10 | 118 | 95.8 (90.4, 98.6) | 213.69 (149.66, 305.12) | 73 | 98.6 (92.6, 100.0) | 157.70 (113.01, 220.05) |
| 11–16 | 122 | 91.8 (85.4, 96.0) | 140.60 (93.22, 212.07) | 82 | 87.8 (78.7, 94.0) | 70.54 (44.14, 112.73) |
| P value | 0.20 | 0.13 | 0.01 | 0.01 | ||
| Socioeconomic backgroundd | ||||||
| Low | 64 | 96.9 (89.2, 99.6) | 245.49 (157.79, 381.94) | 41 | 100.0 (91.4, 1e) | 125.02 (79.46, 196.70) |
| Medium | 65 | 96.9 (89.3, 99.6) | 249.37 (154.76, 401.80) | 39 | 92.3 (79.1, 98.4) | 119.99 (66.50, 216.53) |
| High | 61 | 86.9 (75.8, 94.2) | 86.30 (46.49, 160.22) | 39 | 89.7 (75.8, 97.1) | 77.68 (38.51, 156.68) |
| P value | 0.04 | 0.01 | 0.01 | 0.43f | ||
| Coinfection | ||||||
| Monoinfected | 46 | 195.17 (117.67, 323.70) | 30 | 93.3 (77.9, 99.2) | 98.97 (48.95, 200.13) | |
| Coinfected | 179 | 257.78 (199.61, 332.89) | 115 | 94.8 (89.0, 98.1) | 125.21 (90.60, 173.05) | |
| P value | 0.33 | 0.76 | 0.52 | |||
| Hookworm | ||||||
| Overall | 80.4 (74.8, 85.2) | 71.75 (51.52, 99.90) | 179 | 44.7 (37.3, 52.3) | 8.00 (5.43, 11.76) | |
| Sex | ||||||
| Male | 87.2 (79.7, 92.6) | 114.67 (72.76, 180.72) | 85 | 49.4 (38.4, 60.5) | 9.94 (5.55, 17.80) | |
| Female | 74.0 (65.3, 81.5) | 45.93 (28.64, 73.65) | 94 | 40.4 (30.4, 51.0) | 6.57 (3.91, 11.04) | |
| P value | 0.01 | 0.01 | 0.23 | 0.29 | ||
| Age (yr) | ||||||
| 7–10 | 84.7 (77.0, 90.7) | 92.22 (59.22, 143.61) | 88 | 43.2 (32.7, 54.2) | 7.84 (4.42, 13.90) | |
| 11–16 | 76.2 (67.7, 83.5) | 56.28 (34.38, 92.12) | 91 | 46.2 (35.6, 56.9) | 8.15 (4.79, 13.86) | |
| P value | 0.10 | 0.14 | 0.69 | 0.92 | ||
| Socioeconomic backgroundd | ||||||
| Low | 78.1 (66.0, 87.5) | 59.47 (31.24, 113.23) | 47 | 40.4 (26.4, 55.7) | 6.67 (3.06, 14.52) | |
| Medium | 86.2 (75.3, 93.5) | 111.84 (59.31, 210.89) | 50 | 48.0 (33.7, 62.6) | 10.47 (4.91, 22.34) | |
| High | 73.8 (60.9, 84.2) | 48.50 (24.11, 97.57) | 41 | 48.8 (32.9, 64.9) | 7.56 (3.33, 17.13) | |
| P value | 0.21 | 0.17 | 0.67 | 0.68 | ||
| Coinfection | ||||||
| Monoinfected | 158.86 (54.79, 460.62) | 10 | 50.0 (18.7, 81.3) | 16.13 (1.59, 163.45) | ||
| Coinfected | 207.05 (161.39, 265.61) | 129 | 50.4 (41.5, 59.3) | 10.60 (6.66, 16.85) | ||
| P value | 0.57 | 0.98 | 0.63 | |||
Among children who donated at least one stool sample at baseline.
GM, geometric mean.
Among children who donated at least one stool sample at 12 months posttreatment for S. mansoni and among children who donated at least one stool sample at 6 months posttreatment for hookworm.
Based on household characteristics (number of people sharing a sleeping room, materials used for construction, overall condition, main source of fuel for lighting and cooking, toilet facilities, and sources of water) and asset ownership (radio, mobile phone, lantern, boat, sheep or goat, cattle, and poultry).
One-sided, 97.5% confidence interval.
The quadratic term was significant (P = 0.02).
The overall cure rate for S. mansoni infection in Bwondha was relatively low (45.5%), but among those with detectable infection at 5 weeks posttreatment, infection intensity was markedly reduced (GM epg = 16.43 [95% CI, 12.66, 21.33] compared with GM epg = 363.49 [95% CI, 271.22, 487.14] before treatment). S. mansoni cure rates increased significantly with age (likelihood ratio [LR] test for trend, P < 0.01), from 29.6% at 7 to 8 years to 58.3% at 13 to 16 years. The overall cure rate for hookworm infection was 79.4%, with no significant age association (P = 0.64).
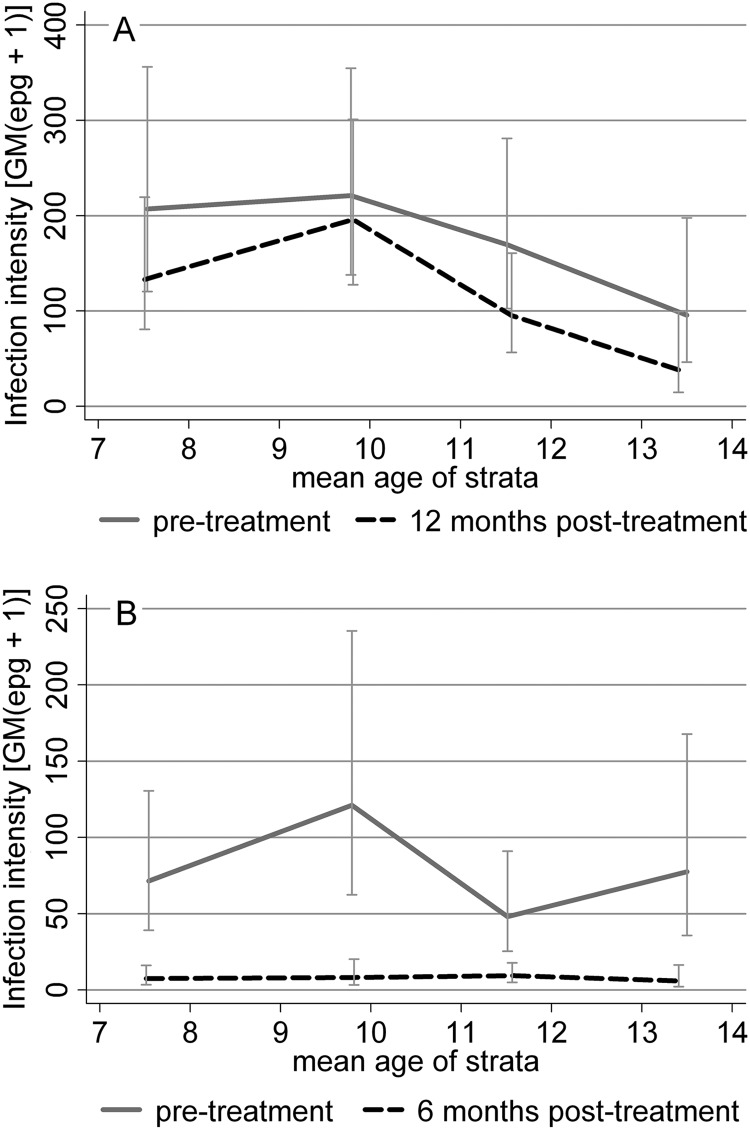
The posttreatment S. mansoni infection prevalence and intensity were significantly lower among older children (P < 0.01; Table 1); both before and after treatment, infection intensities followed a convex relationship with age, peaking at about age 10 years (Fig. 1A). The prevalence and intensity of hookworm infection showed no clear association with age (P ≥ 0.10; Table 1 and Fig. 1B). Hookworm infection also showed no association with socioeconomic background, whereas the S. mansoni infection prevalence and intensity decreased with increasing socioeconomic status in a quadratic relationship (Table 1).
Fig 1.
Geometric mean egg counts (epg + 1) for S. mansoni (A) and hookworm (B) before and after treatment.
Parasite-specific antibody profiles over age.
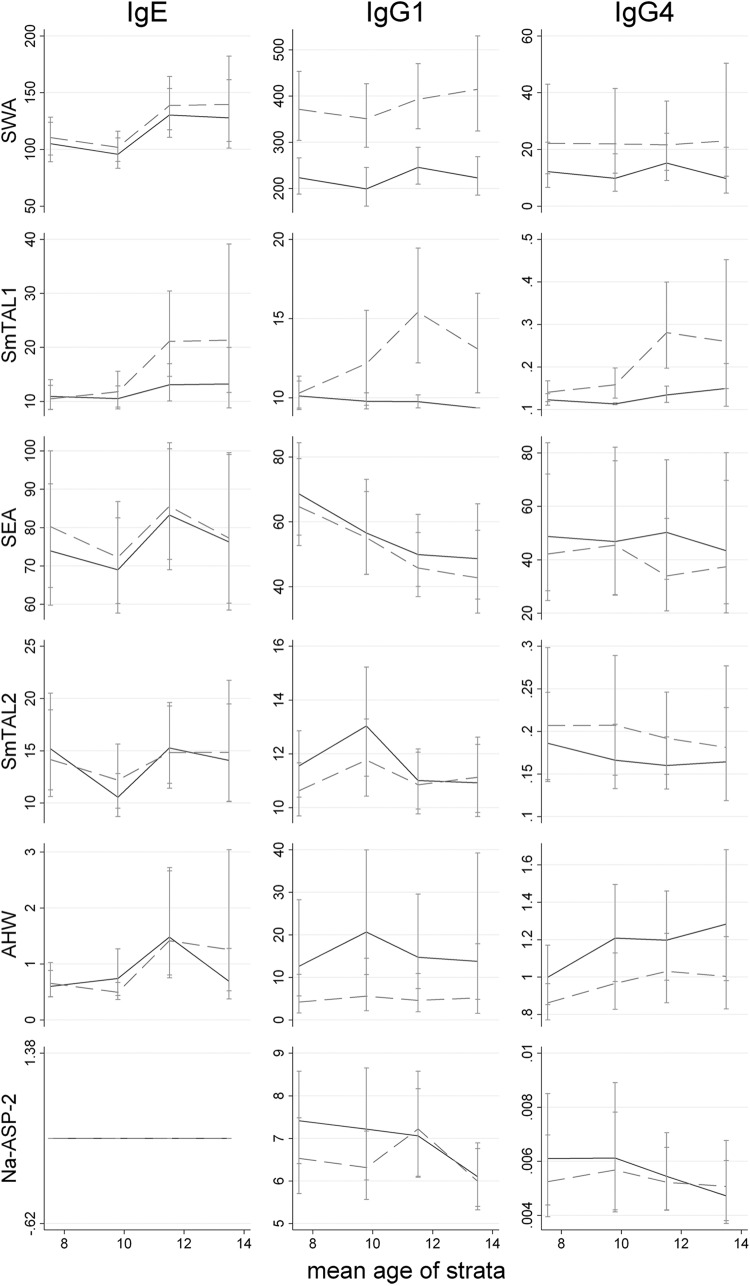
Pretreatment mean levels of the antibody isotype to each of the antigens measured are displayed by age in Fig. 2. There was some evidence that mean anti-SWA IgE and anti-AHW IgG4 responses increased with age and that mean anti-SEA IgG1 responses declined with age, but generally, no significant trends were observed. Of note was the lack of a detectable anti-Na-ASP-2 IgE response among children.
Fig 2.
Geometric mean isotype responses to SWA, SmTAL1, SEA, SmTAL2, AHW, and Na-ASP-2 over age before (solid line) and 8 weeks after (dashed line) treatment. Detection thresholds were added to values before calculating GMs to remove nonpositive values. IgE levels are expressed in ng/ml, and IgG1 and IgG4 levels are expressed in μg/ml.
Also displayed in Fig. 2 are the 8-week posttreatment mean antigen-specific isotype levels, with the changes following treatment quantified in Table 2. Treatment had the effect of significantly increasing mean levels of IgE, IgG1, and IgG4 to SWA; responses to TAL1, a protein mainly expressed in the adult worm, increased predominately in older children (age, >10 years; P < 0.001). In contrast, responses to AHW crude antigen extract either were not significantly affected by treatment (anti-AHW IgE) or were markedly reduced posttreatment (anti-AHW IgG1 and anti-AHW IgG4; P ≤ 0.001). Similarly, levels of IgE to S. mansoni egg antigens were not significantly affected by treatment, while anti-SEA IgG1 and IgG4 levels significantly decreased following treatment (Table 2).
Table 2.
Increase in isotype responses to SWA, SmTAL1, SEA, SmTAL2, AHW, and ASP2 following treatment among all children by age group
| Antigen and antibody | All children |
Children ≤10 yr of age |
Children >10 yr of age |
|||
|---|---|---|---|---|---|---|
| % boosta (95% CI) | P valueb | % boost (95% CI) | P value | % boost (95% CI) | P value | |
| SWA | ||||||
| IgE | 7 (1, 13) | 0.03 | 6 (−2, 14) | 0.14 | 7 (−2, 17) | 0.12 |
| IgG1 | 69 (59, 81) | <0.0001 | 71 (56, 88) | <0.0001 | 68 (53, 84) | <0.0001 |
| IgG4 | 83 (54, 118) | <0.0001 | 101 (57, 157) | <0.0001 | 68 (30, 116) | <0.0001 |
| TAL1 | ||||||
| IgE | 30 (11, 51) | 0.001 | 3 (−13, 23) | 0.70 | 61 (26, 107) | 0.0002 |
| IgG1 | 31 (17, 46) | <0.0001 | 12 (−1, 28) | 0.09 | 52 (28, 80) | <0.0001 |
| IgG4 | 58 (37, 81) | <0.0001 | 26 (11, 42) | 0.0003 | 96 (54, 150) | <0.0001 |
| SEA | ||||||
| IgE | 4 (−3, 12) | 0.23 | 7 (−3, 17) | 0.19 | 2 (−8, 13) | 0.67 |
| IgG1 | −7 (−11, −2) | 0.004 | −4 (−11, 3) | 0.23 | −10 (−16, −3) | 0.01 |
| IgG4 | −18 (−28, −8) | 0.001 | −8 (−22, 7) | 0.27 | −27 (−39, −13) | 0.001 |
| TAL2 | ||||||
| IgE | 2 (−14, 20) | 0.86 | 3 (−18, 30) | 0.77 | 0 (−22, 28) | 0.99 |
| IgG1 | −5 (−11, 2) | 0.17 | −9 (−18, 1) | 0.09 | 0 (−8, 8) | 0.94 |
| IgG4 | 17 (7, 28) | 0.001 | 17 (1, 36) | 0.04 | 17 (4, 31) | 0.01 |
| AHW | ||||||
| IgE | 1 (−30, 44) | 0.97 | −14 (−42, 27) | 0.44 | 18 (−35, 115) | 0.59 |
| IgG1 | −68 (−79, −51) | <0.0001 | −70 (−84, −42) | 0.001 | −67 (−81, −41) | 0.0003 |
| IgG4 | −17 (−22, −11) | <0.0001 | −17 (−24, −9) | 0.0001 | −17 (−24, −9) | 0.0001 |
| ASP2 | ||||||
| IgE | ||||||
| IgG1 | −6 (−15, 4) | 0.24 | −12 (−24, 1) | 0.08 | 1 (−12, 16) | 0.89 |
| IgG4 | −6 (−22, 15) | 0.56 | −11 (−33, 19) | 0.44 | 0 (−23, 30) | 0.97 |
Average percent increase in antibody responses, calculated as follows: 100 × (exp{mean[ln(posttreatment level + assay cutoff) − ln(pretreatment level + assay cutoff)]} − 1).
Paired t test comparing ln(posttreatment level + assay cutoff) with ln(pretreatment level + assay cutoff).
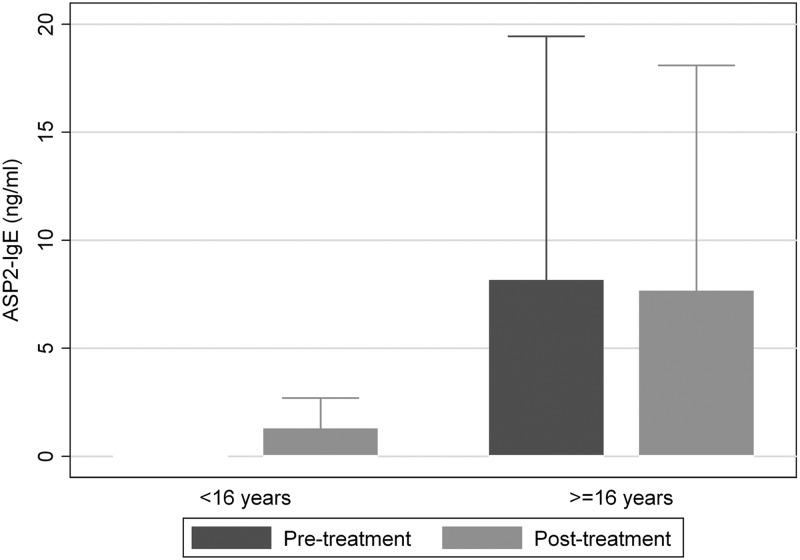
Mean levels of IgG4 to SmTAL2, a protein expressed throughout the S. mansoni life cycle, rose after treatment (P ≤ 0.04), while anti-SmTAL2 IgE and IgG1 levels were not significantly affected by treatment. Responses to Na-ASP-2 were also unaffected by treatment: anti-Na-ASP-2 IgG1 and anti Na-ASP-2 IgG4 levels were not significantly different posttreatment, and as was the case before treatment, none of the children mounted an anti-Na-ASP-2 IgE response. To ensure that the absence of a detectable anti-Na-ASP-2 IgE response among children was not due to an assay failure, we repeated the assay using sera from a previous study of children and adults (9) conducted in a similar Lake Victoria village where the two infections are coendemic. Few children had detectable anti-Na-ASP-2 IgE levels (<25%), but IgE responses were clearly detected among adults (>38%) (Fig. 3).
Fig 3.
Box plot displaying IgE responses to Na-ASP-2 among individuals (age range, 7 to 76 years) in a community where schistosomiasis and hookworm are coendemic; the figure excludes outside values.
Table 3 displays the influence of pretreatment infection intensity on observed posttreatment antigen-specific antibody boosts. Posttreatment increases in anti-SWA IgG1, anti-TAL1 IgG1 and IgG4, and anti-TAL2 IgG4 titers were associated with pretreatment S. mansoni infection intensity, with greater increases observed among more heavily infected children (P ≤ 0.01). Boosts in anti-TAL1 IgE levels were likewise associated with heavier pretreatment infection intensities, but only among older children (significant interaction, according to a χ2 value [1 degree of freedom {df}] by the LR test of 5.08; P = 0.02).
Table 3.
Associationsa between percent change in isotype responses to SWA, SmTAL1, SEA, SmTAL2, AHW, and ASP2 and pretreatment S. mansoni or hookworm infection intensity
| Antigen and antibody |
S. mansoni |
Hookworm |
||
|---|---|---|---|---|
| Adjusted % boost (95% CI) | P valueb | Adjusted % boost (95% CI) | P value | |
| SWA | ||||
| IgE | −0.1 (−2.8, 2.7) | 0.95 | 1.8 (−0.5, 4.1) | 0.13 |
| IgG1 | 4.3 (1.2, 7.5) | 0.01 | 1.8 (−0.7, 4.4) | 0.16 |
| IgG4 | −1.0 (−8.9, 7.6) | 0.81 | 3.7 (−3.2, 11.1) | 0.30 |
| TAL1 | ||||
| IgE | 3.8 (−3.26, 11.43) | 0.29c | 0.3 (−5.4, 6.4) | 0.91 |
| IgG1 | 11.2 (5.8, 16.8) | <0.001 | −1.1 (−5.2, 3.1) | 0.59 |
| IgG4 | 14.7 (7.8, 21.9) | <0.001 | 0.3 (−4.8, 5.8) | 0.90 |
| SEA | ||||
| IgE | 2.1 (−1.2, 5.5) | 0.21 | 1.9 (−0.9, 4.7) | 0.18 |
| IgG1 | 1.2 (−1.2, 3.6) | 0.34 | 1.6 (−0.4, 3.6) | 0.11 |
| IgG4 | −0.6 (−6.0, 5.2) | 0.84d | −0.9 (−5.4, 3.8) | 0.70 |
| TAL2 | ||||
| IgE | 4.6 (−3.4, 13.3) | 0.26 | 1.9 (−4.6, 8.9) | 0.57 |
| IgG1 | 2.7 (−0.5, 6.0) | 0.10 | 1.0 (−1.7, 3.7) | 0.47 |
| IgG4 | 5.6 (1.1, 10.3) | 0.01 | −0.7 (−4.3, 3.0) | 0.71 |
| HW | ||||
| IgE | 11.1 (−6.3, 31.8) | 0.22 | −3.5 (−16.2, 11.3) | 0.62 |
| IgG1 | 1.3 (−17.6, 24.6) | 0.90e | −21.2 (−33.5, −6.7) | 0.01f |
| IgG4 | 2.9 (−0.0, 6.0) | 0.13 | −4.5 (−6.8, −2.2) | 0.0001 |
| ASP2 | ||||
| IgE | ||||
| IgG1 | 2.3 (−2.4, 7.2) | 0.33 | −0.6 (−4.4, 3.3) | 0.75 |
| IgG4 | −2.0 (−10.8, 7.5) | 0.66g | −1.5 (−8.9, 6.4) | 0.69 |
Percent change in isotype responses with each unit increase in ln(epg), or equivalently, an increase in epg by a multiple of e (roughly 2.71828), adjusting for age and sex using multiple regression analysis.
Likelihood ratio (LR) test.
Significant age-pretreatment infection intensity interaction (χ2 [df = 1] by LR test = 5.08, P = 0.02): adjusted percent boost for the group aged ≤10 years = −5.6 (95% CI, −15.3, 5.3); adjusted percent boost for the group aged >10 yrs = 11.1 (95% CI, 1.4, 21.9).
Significant age-pretreatment infection intensity interaction (χ2 [df = 1] by LR test = 4.02, P = 0.04): adjusted percent boost for the group aged ≤10 years = −7.0 (95% CI, −14.7, 1.4); adjusted percent boost for the group aged >10 years = 4.3 (95% CI, −3.0, 12.3).
Significant age-pretreatment infection intensity interaction (χ2 [df = 1] by LR test = 4.17, P = 0.04): adjusted percent boost for the group aged ≤10 years = −21.3 (95% CI, −42.8, 8.3); adjusted percent boost for the group aged >10 years = 21.4 (95% CI, −7.3, 59.1).
Significant age-pretreatment infection intensity interaction (χ2 [df = 1] by LR test = 4.55, P = 0.03): adjusted percent boost for the group aged ≤10 years = −36.1 (95% CI, −50.6, −17.5); adjusted percent boost for the group aged >10 years = −8.2 (95% CI, −26.3, 14.4).
Significant age-pretreatment infection intensity interaction (χ2 [df = 1] by LR test = 8.42, P = 0.004): adjusted percent boost for the group aged ≤10 years = −16.6 (95% CI, −27.6, −3.8); adjusted percent boost for the group aged >10 years = 9.9 (95% CI, −2.5, 24.0).
Posttreatment changes in the levels of IgG1 and IgG4 against AHW were similarly associated with pretreatment hookworm infection intensity but differed in that greater decreases were observed among children with heavier pretreatment hookworm infections (P < 0.01). For anti-AHW IgG1, this relationship was much stronger among younger children (significant interaction, according to a χ2 value [1 df] by the LR test of 4.55, P = 0.03).
No associations were observed between pretreatment hookworm infection and posttreatment changes in schistosome-specific antibody levels, or vice versa, for pretreatment S. mansoni infection and posttreatment changes in anti-AHW IgG1 and IgG4 levels (Table 3).
Associations between posttreatment changes in antibody levels and reinfection.
Further analysis used multiple-regression analysis to explore associations between antibody boosts and reinfection among children infected at baseline, controlling for age, sex, and treatment efficacy. Stronger anti-SWA IgE boosts were associated with significantly lower S. mansoni infection intensity at 12 months posttreatment (GM ratio, 0.54 [95% CI, 0.29, 0.99]; P = 0.04; Table 4). Stronger anti-SmTAL1 IgE boosts were borderline significantly associated with lower rates of reinfection, but only after controlling for posttreatment levels of anti-SmTAL1 IgG4 (GM ratio, 0.73 [95% CI, 0.53 to 1.00]; P = 0.05). No other isotype changes (including for hookworm antigens) were associated with S. mansoni reinfection (P > 0.5; results not shown).
Table 4.
Associations between posttreatment increases in titer of IgE to SWA and that at 12 months reinfectiona
| Characteristic | GM ratiob (95% CI) | P valuec |
|---|---|---|
| Sex (male) | 0.63 (0.35, 1.14) | 0.53 |
| Age (yr) | ||
| 7–10 | ||
| 11–16 | 0.84 (0.47, 1.49) | 0.12 |
| Posttreatment SWA IgE boostd | 0.54 (0.29, 0.99) | 0.04 |
| ln(epg + 1)e at 5 wk | 1.38 (1.16, 1.64) | <0.001 |
Restricted to children with detectable S. mansoni eggs before treatment.
Geometric mean (GM) ratio determined using multiple regression analysis.
By the likelihood ratio (LR) test.
ln(posttreatment level + assay cutoff) − ln(pretreatment level + assay cutoff).
Used as an indicator of treatment efficacy.
Due to the low levels of hookworm reinfection observed, zero-inflated negative-binomial models were used to investigate associations between hookworm reinfection at 6 months and posttreatment changes in antibody levels. Reinfection intensities were significantly greater among children with sustained (i.e., less reduced) anti-AHW IgG1 levels (adjusted β = 0.21 [95% CI, 0.09 to 0.32]; P < 0.001). Conversely, the risk of reinfection was reduced among children with a sustained anti-Na-ASP-2 IgG1 response (adjusted β = 0.68 [95% CI, 0.07 to 1.30]; P = 0.03). Otherwise, no associations (including with schistosome-specific antibody boosts) were observed (P > 0.1; results not shown).
DISCUSSION
Helminth infections are known to exert strong immunomodulatory effects on their mammalian hosts (19; reviewed in references20, 21, and22); therefore, individuals living in regions where both infections occur may have altered immune responses to those characteristically seen in populations of monoendemicity. The current study investigated changes in parasite-specific IgG1, IgG4, and IgE responses to crude parasite extracts and defined allergen-like antigens following praziquantel and albendazole treatment of schoolchildren coinfected with schistosomes and hookworm and receiving treatment as part of a national control program. Specifically, we measured antibody responses to schistosome egg (SEA and SmTAL2) and worm (SWA and SmTAL1) antigens, hookworm somatic adult worm crude antigen extract, and the recombinant hookworm larval protein Na-ASP-2. Responses to schistosome antigens predominantly expressed in the adult worm tended to increase after treatment, while those to egg antigens generally either decreased or were unchanged by treatment. In contrast to responses to schistosomal adult worm antigens, antibody responses to hookworm adult somatic crude antigen extract remained either unchanged or decreased following treatment, while those to Na-ASP-2 were seemingly unaffected. There was evidence for an association between posttreatment increases in IgE responses to schistosomal worm antigens and reduced susceptibility to S. mansoni reinfection 12 months after treatment. There were no observable interactions between the two infections.
Posttreatment increases in antibody responses to adult worm antigens have been observed in other communities where schistosomiasis is endemic (10, 45–47). Grogan et al. (46) suggested that these posttreatment increases in antischistosomal worm responses could be due to either the boosting of antischistosomal worm responses by praziquantel-induced exposure of specific worm antigens or the removal of parasite-driven suppressive mechanisms. The contrasting increase in schistosome worm antibody responses and decrease in hookworm responses when coinfected children are simultaneously treated strongly suggest that these increases are exposure driven rather than due to the removal of immunosuppressive factors. This could explain the observed associations between pretreatment infection intensity and posttreatment antibody boosts: a greater worm burden before treatment is likely to result in greater antigenic exposure after treatment. The stronger boosts to the tegumental antigen SmTAL1 observed among older children (11 to 16 years) and the significant interaction between pretreatment infection intensity and age for anti-TAL1 IgE boosts suggest that these boosts are age related. We have observed age-related posttreatment anti-TAL1 and anti-SWA boosts in other S. mansoni-infected communities (10). This age relatedness is likely linked to the extent of previous sensitization by exposure to normally sequestered antigens following natural or treatment-induced worm death. Age or exposure dependency could explain the relatively small posttreatment anti-SWA IgE boosts observed here among schoolchildren compared with those that we have observed in older individuals (10).
It has been suggested that posttreatment increases in schistosome-specific antibody responses indicate an individual's potential to mount a protective response when required (10). Indeed, associations between posttreatment anti- worm IgE boosts and resistance to reinfection have been described (45), and here, posttreatment anti-SWA IgE increases were associated with resistance to S. mansoni reinfection at 12 months posttreatment, independently of age. There is evidence that increased resistance to reinfection can be induced by repeated rounds of praziquantel treatment (41, 48), most likely due to treatment-associated exposure to specific antigens. Hence, resistance to infection may develop sooner among populations receiving regular treatment for schistosomiasis. Since we know that the Bwondha community has received previous treatment for schistosomiasis, the immunizing effect of previous praziquantel treatments may explain the relatively early peak in infection intensity seen in our study sample (in those ages 9 to 10 years versus a more typical peak in those ages 13 to 16 years) (49).
In contrast to the increase in responses directed at antigens predominantly expressed in the schistosome adult worm, anti-SEA IgG1 and IgG4 responses fell after treatment. Unlike the potentially immunizing doses of schistosome adult worm antigen associated with treatment, levels of egg-associated antigens, to which the immune system will be continuously exposed during chronic infection (50), are likely to fall rather than increase after treatment, due to the removal of adult worms.
Similarly, adult hookworm-specific antibody levels were either unaffected by treatment (IgE) or fell after treatment (IgG1 and IgG2). Geiger et al. observed similar reductions in anti-adult hookworm IgG1 and IgG4 responses but similarly unaffected anti-adult hookworm IgE levels among Necator americanus-infected schoolchildren 6 months after curative treatment (51). The observed reductions in anti-AHW responses, which contrast with those to adult schistosomes, may reflect the different sites of infection for these two helminths: the small intestine versus the mesenteric veins. They could also reflect the different modes of action of praziquantel and albendazole. Whereas praziquantel causes disruption of the tegument and exposure to antigen intravenously, albendazole causes metabolic disruption, resulting in the paralysis and death of worms, which are then expelled intact from the gut; hence, albendazole-associated exposure to antigen is unlikely. The sustained anti-AHW IgE levels observed after treatment may reflect different dose effects, as postulated by Pritchard et al., who observed analogously sustained IgE responses to adult ES antigen at 1 year posttreatment but significant decreases in anti-adult ES IgM and IgG responses (14). In experimental Nippostrongylus brasiliensis infection in rats, low-level infection induced a more sustained specific IgE response, whereas specific IgG levels were much more strongly associated with the dose of infection (52).
Pritchard et al. also failed to observe any marked change in IgG levels to larval somatic antigen (14), as similarly observed here for anti-Na-ASP-2 IgG1 and IgG4. This is not unexpected: responses to larval antigens are likely to reflect exposure to infection as opposed to chronic patent infection. Higher anti-Na-ASP-2 IgE levels have been positively associated and higher anti-Na-ASP-2 IgG4 levels have been negatively associated with heavy hookworm infection in individuals from areas with high rates of transmission in Brazil and China (11). None of the children included in our study produced a detectable anti-Na-ASP-2 IgE response. However, in our parallel study of children and adults, similarly few children had detectable anti-Na-ASP-2 IgE responses, whereas significant anti-Na-ASP-2 IgE responses were detected among adults. From experimental human infections, it is known that antihookworm IgE responses tend to develop very slowly and over repeated exposures (53, 54). Hence, our school-aged children may not have had a sufficient history of exposure to have developed an anti-Na-ASP-2 IgE response. Provided that anti-Na-ASP-2 IgE responses are protective for hookworm infection, this would be consistent with age-hookworm infection profiles, which point toward the slow development of partial immunity (4).
In summary, we investigated changes in parasite-specific IgG1, IgG4, and IgE antibody responses following praziquantel and albendazole treatment of school-aged children living in a community where schistosomiasis mansoni and hookworm are coendemic. Antibody responses to adult hookworm or S. mansoni egg antigens either decreased after treatment or were unchanged, whereas those to S. mansoni adult worm antigens increased. Posttreatment increases in anti-adult worm IgE were associated with reduced susceptibility to S. mansoni reinfection, indicating that treatment-associated boosting of immune responses potentially induces schistosome reinfection immunity. In contrast, there was little evidence for treatment-induced IgE-mediated immunity to hookworm infection in this cohort of children; however, given the contrasting development of antischistosome and antihookworm IgE responses, a wider age range may be required to demonstrate hookworm immunity. Importantly, there was no evidence for the simultaneous treatment of coinfected children with praziquantel and albendazole affecting posttreatment changes in specific antibody responses and subsequent reinfection immunity. Our findings have importance for control programs conducted in countries where the two infections are coendemic, where coadministration of praziquantel and albendazole is increasingly called for.
ACKNOWLEDGMENTS
We are grateful for the help and cooperation of the students and staff at Bwondha Primary School and the parents/guardians of the children involved in this study. We also thank the field assistants, Rashid Ssalongo, Hakim Irumba, Musa Mubiru, and Musitwa, for their crucial involvement in this study. Additional gratitude extends to David Ogutu, Benjamin Tinkitina, and Moses Adriko for their invaluable assistance in the field.
This study was supported by the Wellcome Trust (programme grant WT 083931/Z/07/Z).
Footnotes
Published ahead of print 15 October 2012
REFERENCES
- 1. de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 19:547–551 [DOI] [PubMed] [Google Scholar]
- 2. Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411–425 [DOI] [PubMed] [Google Scholar]
- 3. Fulford AJ, Webster M, Ouma JH, Kimani G, Dunne DW. 1998. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol. Today 14:23–26 [DOI] [PubMed] [Google Scholar]
- 4. Woolhouse ME. 1992. A theoretical framework for the immunoepidemiology of helminth infection. Parasite Immunol. 14:563–578 [DOI] [PubMed] [Google Scholar]
- 5. Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. 1992. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22:1483–1494 [DOI] [PubMed] [Google Scholar]
- 6. Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243–245 [DOI] [PubMed] [Google Scholar]
- 7. Pinot de Moira A, Fulford AJ, Kabatereine NB, Ouma JH, Booth M, Dunne DW. 2010. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: the influence of age, sex, ethnicity and IgE. PLoS Negl. Trop. Dis. 4:e820 doi:10.1371/journal.pntd.0000820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Satti MZ, Lind P, Vennervald BJ, Sulaiman SM, Daffalla AA, Ghalib HW. 1996. Specific immunoglobulin measurements related to exposure and resistance to Schistosoma mansoni infection in Sudanese canal cleaners. Clin. Exp. Immunol. 106:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitzsimmons CM, Jones FM, Stearn A, Chalmers IW, Hoffmann KF, Wawrzyniak J, Wilson S, Kabatereine NB, Dunne DW. 2012. The Schistosoma mansoni tegumental-allergen-like (TAL) protein family: influence of developmental expression on human IgE responses. PLoS Negl. Trop. Dis. 6:e1593 doi:10.1371/journal.pntd.0001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walter K, Fulford AJ, McBeath R, Joseph S, Jones FM, Kariuki HC, Mwatha JK, Kimani G, Kabatereine NB, Vennervald BJ, Ouma JH, Dunne DW. 2006. Increased human IgE induced by killing Schistosoma mansoni in vivo is associated with pretreatment Th2 cytokine responsiveness to worm antigens. J. Immunol. 177:5490–5498 [DOI] [PubMed] [Google Scholar]
- 11. Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, Goud G, Bottazzi ME, Zhan B, Wang Y, Williamson A, Lustigman S, Correa-Oliveira R, Xiao S, Hotez PJ. 2005. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J. 19:1743–1745 [DOI] [PubMed] [Google Scholar]
- 12. Pritchard DI, Quinnell RJ, Walsh EA. 1995. Immunity in humans to Necator americanus: IgE, parasite weight and fecundity. Parasite Immunol. 17:71–75 [DOI] [PubMed] [Google Scholar]
- 13. Ganguly NK, Mahajan RC, Sehgal R, Shetty P, Dilawari JB. 1988. Role of specific immunoglobulin E to excretory-secretory antigen in diagnosis and prognosis of hookworm infection. J. Clin. Microbiol. 26:739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pritchard DI, Walsh EA, Quinell RJ, Raiko A, Edmonds P, Keymer AE. 1992. Isotypic variation in antibody responses in a community in Papua New Guinea to larval and adult antigens during infection, and following reinfection, with the hookworm Necator americanus. Parasite Immunol. 14:617–631 [DOI] [PubMed] [Google Scholar]
- 15. Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, Pond-Tor S, Wu HW, Manalo D, Olveda R, Acosta L, Kurtis JD. 2009. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect. Immun. 77:2051–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Webster M, Fulford AJ, Braun G, Ouma JH, Kariuki HC, Havercroft JC, Gachuhi K, Sturrock RF, Butterworth AE, Dunne DW. 1996. Human immunoglobulin E responses to a recombinant 22.6-kilodalton antigen from Schistosoma mansoni adult worms are associated with low intensities of reinfection after treatment. Infect. Immun. 64:4042–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzsimmons CM, Dunne DW. 2009. Survival of the fittest: allergology or parasitology? Trends Parasitol. 25:447–451 [DOI] [PubMed] [Google Scholar]
- 18. Asojo OA, Goud G, Dhar K, Loukas A, Zhan B, Deumic V, Liu S, Borgstahl GE, Hotez PJ. 2005. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J. Mol. Biol. 346:801–814 [DOI] [PubMed] [Google Scholar]
- 19. Turner JD, Jackson JA, Faulkner H, Behnke J, Else KJ, Kamgno J, Boussinesq M, Bradley JE. 2008. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis. 197:1204–1212 [DOI] [PubMed] [Google Scholar]
- 20. Danilowicz-Luebert E, O'Regan NL, Steinfelder S, Hartmann S. 2011. Modulation of specific and allergy-related immune responses by helminths. J. Biomed. Biotechnol. 2011:821578 doi:10.1155/2011/821578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maizels RM, Yazdanbakhsh M. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733–744 [DOI] [PubMed] [Google Scholar]
- 22. van Riet E, Hartgers FC, Yazdanbakhsh M. 2007. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212:475–490 [DOI] [PubMed] [Google Scholar]
- 23. Capron M. 2011. Effect of parasite infection on allergic disease. Allergy 66(Suppl 95):16–18 [DOI] [PubMed] [Google Scholar]
- 24. Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123:219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 173:269–272 [DOI] [PubMed] [Google Scholar]
- 26. Borkow G, Bentwich Z. 2006. HIV and helminth co-infection: is deworming necessary? Parasite Immunol. 28:605–612 [DOI] [PubMed] [Google Scholar]
- 27. Correa-Oliveira R, Golgher DB, Oliveira GC, Carvalho OS, Massara CL, Caldas IR, Colley DG, Gazzinelli G. 2002. Infection with Schistosoma mansoni correlates with altered immune responses to Ascaris lumbricoides and hookworm. Acta Trop. 83:123–132 [DOI] [PubMed] [Google Scholar]
- 28. Geiger SM, Alexander ND, Fujiwara RT, Brooker S, Cundill B, Diemert DJ, Correa-Oliveira R, Bethony JM. 2011. Necator americanus and helminth co-infections: further down-modulation of hookworm-specific type 1 immune responses. PLoS Negl. Trop. Dis. 5:e1280 doi:10.1371/journal.pntd.0001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brooker S, Clements AC. 2009. Spatial heterogeneity of parasite co-infection: determinants and geostatistical prediction at regional scales. Int. J. Parasitol. 39:591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clements AC, Deville MA, Ndayishimiye O, Brooker S, Fenwick A. 2010. Spatial co-distribution of neglected tropical diseases in the east African great lakes region: revisiting the justification for integrated control. Trop. Med. Int. Health 15:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raso G, Vounatsou P, McManus DP, Utzinger J. 2007. Bayesian risk maps for Schistosoma mansoni and hookworm mono-infections in a setting where both parasites co-exist. Geospat. Health 2:85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chernin J, McLaren DJ, Morinan A, Jamieson BN. 1988. Mesocestoides corti: parameters of infection in CBA/Ca mice and the effect of introducing a concomitant trematode infection. Parasitology 97(Pt 3):393–402 [DOI] [PubMed] [Google Scholar]
- 33. Curry AJ, Else KJ, Jones F, Bancroft A, Grencis RK, Dunne DW. 1995. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J. Exp. Med. 181:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshida A, Maruyama H, Yabu Y, Amano T, Kobayakawa T, Ohta N. 1999. Immune response against protozoal and nematodal infection in mice with underlying Schistosoma mansoni infection. Parasitol. Int. 48:73–79 [DOI] [PubMed] [Google Scholar]
- 35. Hamm DM, Agossou A, Gantin RG, Kocherscheidt L, Banla M, Dietz K, Soboslay PT. 2009. Coinfections with Schistosoma haematobium, Necator americanus, and Entamoeba histolytica/Entamoeba dispar in children: chemokine and cytokine responses and changes after antiparasite treatment. J. Infect. Dis. 199:1583–1591 [DOI] [PubMed] [Google Scholar]
- 36. Keiser J, N′Goran EK, Singer BH, Lengeler C, Tanner M, Utzinger J. 2002. Association between Schistosoma mansoni and hookworm infections among schoolchildren in Cote d'Ivoire. Acta Trop. 84:31–41 [DOI] [PubMed] [Google Scholar]
- 37. Webster M, Correa-Oliveira R, Gazzinelli G, Viana IR, Fraga LA, Silveira AM, Dunne DW. 1997. Factors affecting high and low human IgE responses to schistosome worm antigens in an area of Brazil endemic for Schistosoma mansoni and hookworm. Am. J. Trop. Med. Hyg. 57:487–494 [DOI] [PubMed] [Google Scholar]
- 38. Fleming FM, Brooker S, Geiger SM, Caldas IR, Correa-Oliveira R, Hotez PJ, Bethony JM. 2006. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health 11:56–64 [DOI] [PubMed] [Google Scholar]
- 39. Kabatereine NB, Brooker S, Koukounari A, Kazibwe F, Tukahebwa EM, Fleming FM, Zhang Y, Webster JP, Stothard JR, Fenwick A. 2007. Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bull. World Health Organ. 85:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katz N, Chaves A, Pellegrino J. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397–400 [PubMed] [Google Scholar]
- 41. Black CL, Mwinzi PN, Muok EM, Abudho B, Fitzsimmons CM, Dunne DW, Karanja DM, Secor WE, Colley DG. 2010. Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 4:e637 doi:10.1371/journal.pntd.0000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hawdon JM, Jones BF, Hoffman DR, Hotez PJ. 1996. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J. Biol. Chem. 271:6672–6678 [DOI] [PubMed] [Google Scholar]
- 43. Filmer D, Pritchett L. 1999. The effect of household wealth on educational attainment: evidence from 35 countries. Popul. Dev. Rev. 25:85–120 [Google Scholar]
- 44. Filmer D, Pritchett LH. 2001. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography 38:115–132 [DOI] [PubMed] [Google Scholar]
- 45. Caldas IR, Correa-Oliveira R, Colosimo E, Carvalho OS, Massara CL, Colley DG, Gazzinelli G. 2000. Susceptibility and resistance to Schistosoma mansoni reinfection: parallel cellular and isotypic immunologic assessment. Am. J. Trop. Med. Hyg. 62:57–64 [DOI] [PubMed] [Google Scholar]
- 46. Grogan JL, Kremsner PG, van Dam GJ, Metzger W, Mordmuller B, Deelder AM, Yazdanbakhsh M. 1996. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J. Infect. Dis. 173:1242–1247 [DOI] [PubMed] [Google Scholar]
- 47. Webster M, Fallon PG, Fulford AJ, Butterworth AE, Ouma JH, Kimani G, Dunne DW. 1997. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 19:333–335 [DOI] [PubMed] [Google Scholar]
- 48. Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, Secor WE. 2002. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360:592–596 [DOI] [PubMed] [Google Scholar]
- 49. Fulford AJ, Butterworth AE, Sturrock RF, Ouma JH. 1992. On the use of age-intensity data to detect immunity to parasitic infections, with special reference to Schistosoma mansoni in Kenya. Parasitology 105(Pt 2):219–227 [DOI] [PubMed] [Google Scholar]
- 50. Fitzsimmons CM, McBeath R, Joseph S, Jones FM, Walter K, Hoffmann KF, Kariuki HC, Mwatha JK, Kimani G, Kabatereine NB, Vennervald BJ, Ouma JH, Dunne DW. 2007. Factors affecting human IgE and IgG responses to allergen-like Schistosoma mansoni antigens: molecular structure and patterns of in vivo exposure. Int. Arch. Allergy Immunol. 142:40–50 [DOI] [PubMed] [Google Scholar]
- 51. Geiger SM, Massara CL, Bethony J, Soboslay PT, Correa-Oliveira R. 2004. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clin. Exp. Immunol. 136:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamada M, Nakazawa M, Kamata I, Arizono N. 1992. Low-level infection with the nematode Nippostrongylus brasiliensis induces significant and sustained specific and non-specific IgE antibody responses in rats. Immunology 75:36–40 [PMC free article] [PubMed] [Google Scholar]
- 53. Maxwell C, Hussain R, Nutman TB, Poindexter RW, Little MD, Schad GA, Ottesen EA. 1987. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am. J. Trop. Med. Hyg. 37:126–134 [DOI] [PubMed] [Google Scholar]
- 54. Ogilvie BM, Bartlett A, Godfrey RC, Turton JA, Worms MJ, Yeates RA. 1978. Antibody responses in self-infections with Necator americanus. Trans. R. Soc. Trop. Med. Hyg. 72:66–71 [DOI] [PubMed] [Google Scholar]