Abstract
Urinary catheterization elicits major histological and immunological changes that render the bladder susceptible to microbial invasion, colonization, and dissemination. However, it is not understood how catheters induce these changes, how these changes act to promote infection, or whether they may have any protective benefit. In the present study, we examined how catheter-associated inflammation impacts infection by Enterococcus faecalis, a leading cause of catheter-associated urinary tract infection (CAUTI), a source of significant societal and clinical challenges. Using a recently optimized murine model of foreign body-associated UTI, we found that the implanted catheter itself was the primary inducer of inflammation. In the absence of the silicone tubing implant, E. faecalis induced only minimal inflammation and was rapidly cleared from the bladder. The catheter-induced inflammation was only minimally altered by subsequent enterococcal infection and was not suppressed by inhibitors of the neurogenic pathway and only partially by dexamethasone. Despite the robust inflammatory response induced by urinary implantation, E. faecalis produced biofilm and high bladder titers in these animals. Induction of inflammation in the absence of an implanted catheter failed to promote infection, suggesting that the presence of the catheter itself is essential for E. faecalis persistence in the bladder. Immunosuppression prior to urinary catheterization enhanced E. faecalis colonization, suggesting that implant-mediated inflammation contributes to the control of enterococcal infection. Thus, this study underscores the need for novel strategies against CAUTIs that seek to reduce the deleterious effects of implant-mediated inflammation on bladder homeostasis while maintaining an active immune response that effectively limits bacterial invaders.
INTRODUCTION
Urinary catheterization is directly associated with 80% of hospital-acquired urinary tract infections (UTIs) (1). The insertion and presence of indwelling urinary catheters disrupt the normal mechanical and host defenses of the urinary tract, allow extracellular microbes access to the sterile environment of the bladder by ascending through the catheter lumen or from the urethral meatus along the catheter, and provide an additional surface for biofilm formation and the establishment of antibiotic-recalcitrant chronic or recurrent infections (2–9). Even in the absence of microbial colonization, urinary catheterization was shown to be associated with histological and immunological alterations in the bladder, including urothelial damage and exfoliation, bladder wall edema, inflammatory cytokine production, immune cell infiltration, and mucosal lesions of the bladders and kidneys (7, 10–13) which can lead to bladder cancers (14, 15). However, there remains a need to uncover molecular details and the functional role of the catheter-induced host responses during bacterial colonization and catheter-associated UTIs (CAUTIs).
We recently optimized a murine model of foreign body-associated UTI to investigate the pathophysiology of enterococcal CAUTIs, which account for 15 to 30% of CAUTIs (16). We demonstrated that the transpeptidase enzymes sortase A and sortase C and the endocarditis- and biofilm-associated pilus (Ebp) contribute to Enterococcus faecalis biofilm formation on the surface of silicone implants in vivo, allowing for the establishment of persistent cystitis and pyelonephritis in this murine model (17, 18). Interestingly, this high and chronic enterococcal colonization occurs in the face of a robust inflammatory response primarily caused by the foreign body (18). However, from this study it was unclear whether E. faecalis takes advantage of the host inflammatory response for colonization and biofilm formation, as was previously reported for uropathogenic Escherichia coli (UPEC) (19) and other pathogens such as Salmonella enterica serovar Typhimurium and nontypeable Haemophilus influenzae (20–22), or if it employs other strategies to persist in the catheter-inflamed bladder.
In the present report, we sought first to characterize the immune response associated with urinary catheterization using genetic knockout mouse strains and flow cytometry-based assays and second to investigate the consequences of immune suppression and induction for the outcome of E. faecalis CAUTI. Our findings indicate that the inflammation ensuing from bladder implantation is primarily mediated by myeloid cells, in particular neutrophils, which serve to control and limit E. faecalis infection. This inflammatory response did not predispose the bladder to infection by E. faecalis, since the induction of inflammation in the absence of a foreign body did not promote infection. However, not only is E. faecalis able to withstand this foreign body-induced inflammatory response, but it depends on the catheter implant for persistence via an unknown mechanism that more than likely involves its ability to produce biofilms on the silicone tubing (18). This study thus provides an explanation for the clinical observations that E. faecalis is commonly recovered from patients with foreign body-associated infections or under immunosuppressive therapies and suggests that although immunosuppressive approaches for the management of CAUTIs may help limit the deleterious consequences of urinary catheterization for bladder biology, they may inadvertently predispose patients to increased bacterial colonization and dissemination leading to adverse side effects and more severe infections.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
E. faecalis strain OG1RF, resistant to rifampin and fusidic acid (23, 24), was used in this study. Unless otherwise specified, experiments were performed using an overnight bacterial culture grown in brain heart infusion broth (BHI) (Becton, Dickinson, Franklin Lakes, NJ) from a single colony of OG1RF grown on BHI agar plates supplemented with 25 μg/ml of rifampin and 25 μg/ml of fusidic acid (BHIRF25). Liquid cultures were grown statically at 37°C for 18 h.
Inhibitors and chemicals.
The drug treatments used in this study, their relevant modes of action, and references for dosage and effectiveness are described in Table 1. Vehicles were saline or dimethyl sulfoxide (DMSO) as indicated.
Table 1.
List of inhibitors used in this study
| Name | Dosage/routea | Mode of action | Company/references |
|---|---|---|---|
| Dexamethasone sodium phosphate | 10 mg/kg, i.p., 30 min prior to implantation | Glucocorticoid, anti–inflammatory, and immunosuppressant | American Regent, Inc., Shirley, NY (48) |
| CP-99,994 dihydrochloride | 5–10 mg/kg, i.p., i.v., s.c., 30 min and 3 h postimplantation (when indicated) | High-affinity neurokinin 1 receptor (NK1R) antagonist | Tocris Bioscience, Ellisville, MO (56, 57) |
| CP-96,345 | 5–10 mg/kg, i.p., i.v., s.c., 30 min and 3 h postimplantation (when indicated) | High-affinity NK1R antagonist | Tocris Bioscience (79) |
| Aminoguanidine hydrochloride | 200 mg/kg 1 h and 3 h postimplantation (when indicated) | Irreversible inducible nitric oxide synthase (iNOS) inhibitor | Tocris Bioscience (27) |
| Cyclophosphamide | 150 mg/kg at time of infection | Chemotherapeutic agent/prodrug | Tocris Bioscience (33, 67) |
i.v., intravenously; s.c., subcutaneously.
Mouse strains.
Six- to 7-week-old female wild-type C57BL/6Ncr mice purchased from the National Cancer Institute (NCI) were used in this study. Experiments were performed following a 1-week adaptation in the animal facility. All studies and procedures were approved by the Animal Studies Committee at Washington University School of Medicine.
Animal implantation and infection.
Animals were transurethrally implanted and inoculated as previously described (18). Briefly, 7- to 8-week-old female mice were anesthetized by inhalation of isoflurane and implanted with a 4- to 5-mm length of platinum-cured silicone tubing. Immediately following implantation, or at time of infection for nonimplanted animals, 50 μl of ∼1 × 107 to 2 × 107 CFU of bacteria in phosphate-buffered saline (PBS) was introduced in the bladder lumen by transurethral inoculation, when indicated.
Bladder weight determination and PPE.
When indicated, bladders were aseptically removed, bisected, and blotted dry. Bladders were placed in preweighed Eppendorf tubes and weighed. Bladder weight (in grams) was determined as the difference between bladder and Eppendorf tube weight and that of the empty Eppendorf tube. Plasma protein extravasation (PPE) in the bladder was determined using the Evans blue technique (25) in nonimplanted and implanted animals at 6 h after treatments with the indicated chemicals listed in Table 1. Mice were anesthetized by inhalation of isoflurane, and 30 mg/kg of body weight Evans blue (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was administered intravenously (i.v.). Animals were sacrificed 15 min later by cervical dislocation. The bladder was then excised, bisected, and blotted dry before weighing. Bladders were then placed in 1 ml of 100% formamide (Sigma-Aldrich) and incubated at 60°C for 24 h for Evans blue extraction. The extracted Evans blue from each bladder was quantified by colorimetric measurement at 620 nm with an automated microplate reader. The dye content extravasated in each bladder was determined from an Evans blue standard curve and expressed as μg/g of tissue.
Bacterial titer determination on implants and organs.
Animals were sacrificed at indicated time points by cervical dislocation after anesthesia inhalation, and the bladders and kidneys were aseptically harvested. Subsequently, the silicone implant was retrieved from the bladder when present, placed in PBS, sonicated for 10 min, and then vortexed at maximum speed for 3 min. The bladder and kidneys from each mouse were homogenized in PBS. Samples were serially diluted and plated onto BHI agar plates containing rifampin. CFU were enumerated after 24 h of incubation at 37°C. In all cases, experiments were performed at least twice with n = 5 to 10 mice/condition/experiment.
Cytokine profiling.
Bladder homogenates from nonimplanted and implanted animals with or without bacterial infections were microcentrifuged at 14,000 × g for 5 min, and supernatants were frozen at −80°C until the time of the assay. Assays were carried out according to the manufacturers' protocols using the Bio-Plex Pro mouse cytokine 23-plex assay kit from Bio-Rad Laboratories (Hercules, CA).
Histopathology.
For histological analyses, bladders were fixed in methacarn (60% methanol, 30% chloroform, and 10% glacial acetic acid) or formalin for 1 to 2 h at room temperature and dehydrated in 70% ethanol overnight at 4°C. They were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H/E) for light microscopy.
Flow cytometry.
Single-cell bladder suspensions were made from minced bladder tissues subjected to collagenase/DNase I digestion for 90 min at 37°C and then passed through a 40-μm filter, and cells were washed as described previously (26). Staining of surface markers was performed in FcR block with fluorochrome-conjugated monoclonal antibodies (MAbs). Cells were counterstained with propidium iodide (PI) prior to flow cytometry, and only live (PI-low) cells were included in the analysis (gating strategies are shown in Fig. S1 in the supplemental material). To specifically characterize the immune infiltrates, specific combinations of MAbs were chosen which distinguish granulocytes (CD11b+ Gr1hi Ly6Ghi Ly6Clo), monocytes/macrophages (F4/80+), dendritic cells (CD11c+), basophils (cKit− FcεR1+), eosinophils (SiglecF+), mast cells (cKit+ FcεR1+), NK cells (NK1.1+), T cells (CD3+), and B cells (CD19+). All antibodies were from BD Pharmagen, Ebioscience, or Southern Biotech. Activation status was determined using specific MAbs for major histocompatibility complex class II (MHCII). Samples were acquired on a FACSCalibur (BD Biosciences), and data were analyzed using FlowJo software (version 7.6.4). The relative proportion of cellular infiltrates in each bladder was calculated as a percentage of live cells.
Neutrophil depletion.
Mice were rendered neutropenic as previously described (26). Briefly, an anti-Ly6G MAb (1A8) from Bio X Cell (West Lebanon, NH) was administered intraperitoneally (i.p.) on days 3 and 1 prior to implantation and bacterial challenge. Control mice received IgG isotype control 2A3 (Bio X Cell) in a similar manner.
Statistical methods.
Comparisons among groups were conducted by the Mann-Whitney U test using GraphPad Prism (GraphPad software, version 5). Values below the limit of detection (LOD) (40 CFU/ml for organs and 20 CFU for implants) were assigned the appropriate LOD value for statistical analyses. All tests were two-tailed, and a P value less than 0.05 was considered significant. Colonization and infection were defined as organs/implants with bacterial titers above LOD at 24 h postinfection (hpi).
RESULTS
Urinary catheterization induces severe edema and release of proinflammatory cytokines.
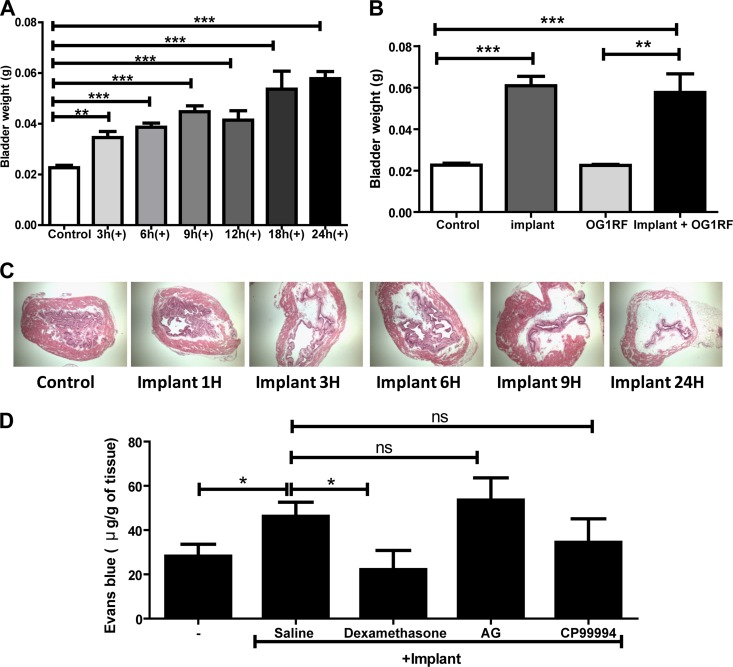
Examination of mouse bladders following implantation of silicone tubing reveals severe edema (18). However, the mechanisms of onset and progression of bladder wall edema following urinary catheterization are poorly defined. Thus, we investigated edema using bladder weight as a readout followed by histological verification. The bladder weights of uninfected mice were determined at 3, 6, 9, 12, 18, and 24 h postimplantation and compared to those of nonimplanted controls. The bladders of implanted mice significantly increased in weight as early as 3 h postimplantation (P = 0.0011 by the Mann-Whitney U test) compared to those of nonimplanted animals (normally 15 to 20 mg) and reached approximately 60 mg by 24 h (Fig. 1A). Histological analysis of bladder tissue from implanted animals at each time point depicts the gradual progression of bladder wall edema over time, corroborating the significant increase in bladder weight (Fig. 1C). The implant-induced edema correlates with plasma protein extravasation (PPE) in the tissue as assessed by the Evans blue extravasation assay performed 6 h postimplantation (Fig. 1D). Significantly higher quantities of Evans blue per gram of bladder tissue were extravasated and recovered from implanted bladders following intravenous injection in the murine tail vein (60 μg), compared to approximately 30 μg/g seen in nonimplanted controls (P = 0.0348). Urinary catheterization is also associated with the upregulation of several inflammatory cytokines (18). Interleukins 1β, 6, 12(p40), and 17 as well as granulocyte colony-stimulating factor (G-CSF) and keratinocyte-derived chemokine (KC) are upregulated at least 2-fold in saline-treated implanted animals over nonimplanted mock-infected controls following urinary implantation (Fig. 2A).
Fig 1.
Bladder foreign body implantation induces edema and plasma protein extravasation. (A) Bladder weights of nonimplanted (control) and implanted (+) animals at the indicated times. (B) Bladder weights at 24 hpi of nonimplanted and implanted mice in the presence or absence of the E. faecalis OG1RF strain. (C) H/E staining of bladder sections from nonimplanted and implanted animals at indicated time points observed under a light microscope at a magnification of ×10. (D) Plasma protein leakage in bladder tissue determined by Evans blue content at 6 hpi in nonimplanted animals treated with saline and implanted animals treated with saline, dexamethasone (10 mg/kg, i.p.), aminoguanidine (AG; 2 doses of 200 mg/kg, i.p.), or CP-99,994 (2 doses of 5 mg/kg, i.p.). All graphs represent the mean of each data set from at least two independent experiments. Error bars show standard errors of the means. *, P < 0.05; **, P < 0.005; ***, P < 0.001; and ns, P > 0.05, by the Mann-Whitney U test.
Fig 2.
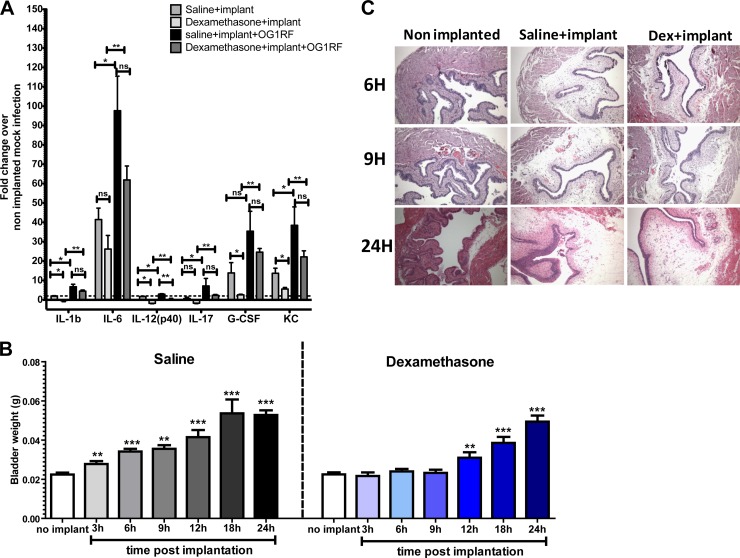
Dexamethasone treatment decreases cytokine production and delays onset of bladder edema. (A) Graph represents bladder cytokines with at least 2-fold differential expression relative to uninfected nonimplanted animals at 24 hpi for nonimplanted and saline- or dexamethasone-treated implanted animals with or without OG1RF from at least two independent experiments (n = 2 to 3 mice/condition/experiment). Error bars = standard errors of the means. *, P < 0.05, and **, P < 0.005, by the Mann-Whitney U test. ns, not significant (P > 0.05). (B) Bladder weights of female C57BL/6Ncr mice at the indicated time points treated with saline or dexamethasone (10 mg/kg, i.p.) 30 min prior to implantation. White bars from the same data set represent weights of nonimplanted animals' bladders. The experiment was done at least twice with n = 5 mice/experiment/time point, except at 12 and 18 h (experiment performed once). Error bar = standard error of the mean. **, P < 0.005, and ***, P < 0.001, by the Mann-Whitney U test. (C) H/E staining of bladder sections obtained at the indicated time points from nonimplanted and implanted animals treated with saline or dexamethasone (10 mg/kg, i.p.) observed under a light microscope at a magnification of ×40.
Glucocorticoid treatment partially inhibits implant-induced edema and inflammation.
We used anti-inflammatory and immunosuppressive agents in order to better understand the mechanism by which the severe edema occurs in the bladder as a result of urinary catheterization. We investigated the ability of (i) dexamethasone, a well-characterized glucocorticoid and potent anti-inflammatory agent; (ii) inhibitors of the neurogenic inflammatory pathway (NIP), including the neurokinin 1 receptor (NK1R) antagonists CP-99,994 and CP-96,345 (27–30); or (iii) aminoguanidine (AG), the irreversible inhibitor of inducible nitric oxide synthase (iNOS) (31–33), to immunosuppress catheter-induced edema and proinflammatory cytokines. Mice were intraperitoneally treated with each immunosuppressive agent 30 min prior to implantation. Immunosuppression was evaluated based on reduction in edema as determined by bladder weight, decreased plasma protein extravasation (PPE) by the Evans blue-based assay, and reduced production of proinflammatory cytokines.
Treatment with dexamethasone prevented the development of bladder wall edema at 6 hpi (Fig. 1D) and delayed the increase in bladder weight for up to 9 h postimplantation compared to saline-treated controls (Fig. 2B). However, by 12 h postimplantation, the bladders of dexamethasone-treated animals were as edematous and inflamed as were saline-treated implanted controls (Fig. 2C). Supplemental dosages of dexamethasone administered 30 min prior to implantation and at 9 h postimplantation did not prevent edema at 24 h postimplantation (data not shown). Dexamethasone also significantly decreased interleukins 1β and 12(p40) as well as G-CSF and KC in implanted animals compared to saline-treated implanted controls at 24 h postimplantation (Fig. 2A). In contrast, known inhibitors of the NIP (Fig. 1D), including the specific neurokinin 1 receptor (NK1R) antagonist CP-99,994 or aminoguanidine (AG), the irreversible inhibitor of inducible nitric oxide synthase (iNOS) (31–33), resulted in no reduction in bladder weights, and vascular permeability was observed in implanted animals. Similar findings were obtained with CP-96,345 (data not shown). Thus, NK1R-induced neurogenic inflammation does not appear to be a major contributor to the onset of bladder wall edema and vascular permeability following urinary implantation in mice. These data argue that activation of glucocorticoid-sensitive immune pathways contributes to the immediate inflammatory response following urinary implantation but that dexamethasone-insensitive pathways, not related to the NIP, dominate after 6 to 9 h postinfection.
Urinary implantation leads the specific recruitment of myeloid-derived cells in the murine bladder.
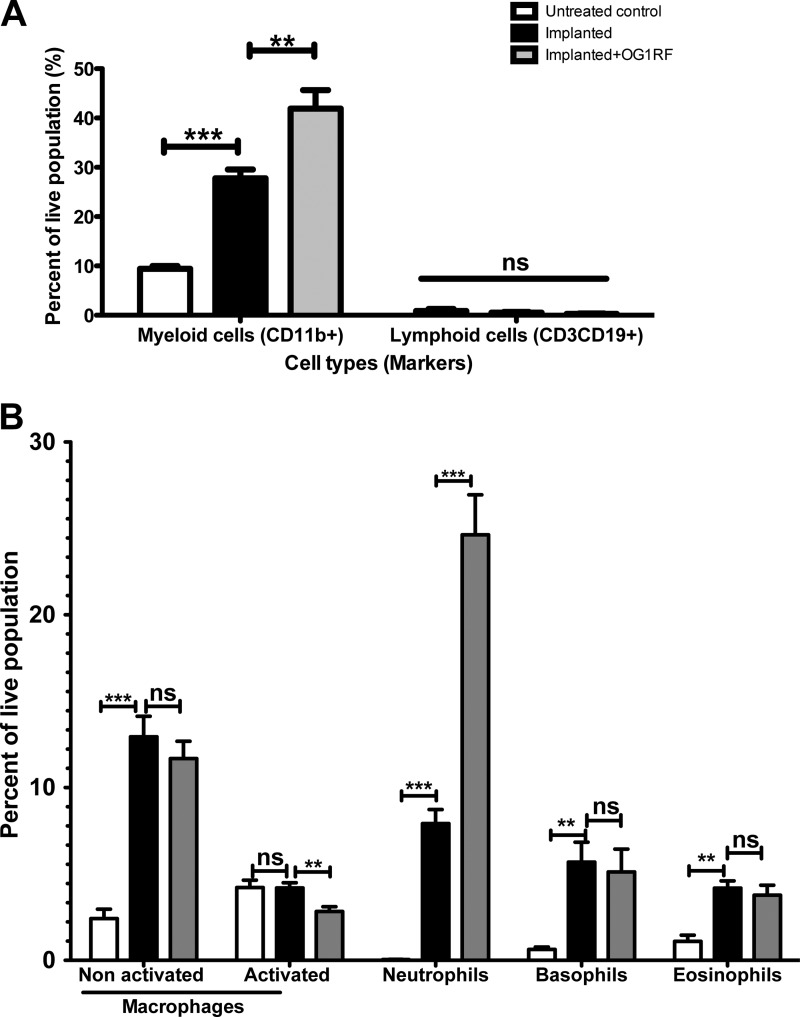
Very little is known about the cellular constituents of the immune response to urinary catheterization aside from histological analyses of bladder tissues (7, 13, 18). In order to establish the functional role of implant-mediated inflammation, we investigated the cellular nature of immune cells involved in the bladder immunological responses to the silicone implant. We performed flow cytometric analysis of bladder single cell suspensions in nonimplanted and implanted animals at 24 h postimplantation using antibodies raised against specific immune surface markers. These experiments reveal that CD11b+ myeloid cells account for approximately 30% of the live cell population in implanted animals compared to ∼10% in nonimplanted controls at 24 h postimplantation (P < 0.0001) (Fig. 3A). No significant difference was observed in lymphocyte (CD3+ CD19+) numbers in the presence or absence of implant at 24 hpi (Fig. 3A). Specific markers were used to further specify the myeloid cells. Nonactivated (CD11b+ F4/80+ MHCII−) and activated (CD11b+ F4/80+ MHCII+) macrophages are present with increases of approximately ∼12- and 2-fold, respectively, over nonimplanted controls (Fig. 3B). Neutrophils (CD11b+ Gr1hi Ly6Ghi Ly6Clo) are the most abundant immune cells recruited in response to urinary implantation, accounting for approximately 8% of live cells compared to less than 0.05% in nonimplanted controls (Fig. 3B). Further flow cytometric studies using an antibody raised against the neutrophil-specific receptor NB1 (also known as CD177) (34) corroborate the above findings that neutrophils are the major immune infiltrates in implanted murine bladders (data not shown). In addition, there was a significant increase in basophils (cKit− FcεR1+) and eosinophils (SiglecF+) in the bladder of implanted animals, representing 5.6 and 4.2%, respectively, of the live population (Fig. 3B). There was no statistically significant change in the number of dendritic cells (CD11b+ CD11c+ MHCII+/−) or mast cells (cKit+ FcεRI+) present in implanted animals compared to nonimplanted controls (data not shown). Dexamethasone treatment prior to implantation significantly reduced immune cell recruitment at 24 hpi in noninfected animals (data not shown).
Fig 3.
Neutrophils are important cellular infiltrates during enterococcal CAUTI. Cellular infiltrates from nonimplanted animals (white bars), implanted animals (black bars), or animals implanted and infected with E. faecalis OG1RF (gray bars) at 24 hpi derived from flow cytometry analysis. Nonactivated and activated macrophages (CD11b+ F4/80+ MHCII− and CD11b+ F4/80+ MHCII+, respectively), neutrophils (CD11b+ Gr1hi Ly6Ghi Ly6Clo), eosinophils (SiglecF+), and basophils (cKit− FcεR1+). Graphs represent the means derived from at least two independent experiments with n = 3 to 5/experiment/condition. Error bars represent standard errors of the means. *, P < 0.05; **, P < 0.005; and ***, P < 0.0005, by the Mann-Whitney U test. ns, not significant (P > 0.05).
Role of foreign body-induced bladder inflammation in E. faecalis CAUTI.
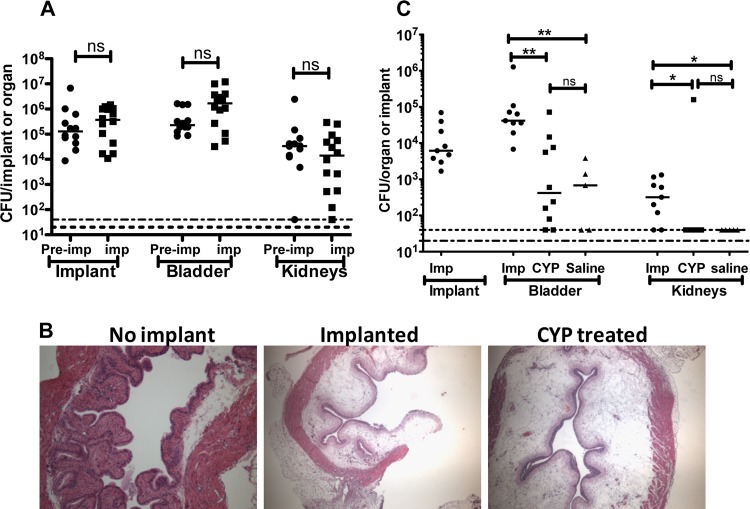
We investigated the role of the implant-mediated edema and inflammation in promoting or altering the outcome of enterococcal infections. In a murine model of cystitis, E. faecalis induces minimal inflammation and is rapidly eliminated from the bladder (35). However, with the introduction of silicone tubing implants into the bladder, E. faecalis becomes adept at causing CAUTI despite the edema and the robust inflammatory response induced by the catheter (36). Thus, we investigated whether E. faecalis virulence was altered in animals preimplanted 24 h before infection to determine the effect of a preexisting catheter-induced inflammatory response on the outcome of infection. Animals that were implanted and simultaneously infected served as a control. Bacterial titers from the implant and organs were then analyzed and compared. As shown in Fig. 4A, E. faecalis colonizes the implants and organs of the two groups to similar levels, indicating that preexisting implant-induced bladder inflammation does not enhance or prevent enterococcal colonization.
Fig 4.
Implant-induced inflammation is not sufficient for enterococcal urinary tract colonization. (A) CFU enumeration at 24 hpi of OG1RF from implants and homogenized bladders and kidneys of animals preimplanted (Pre-imp) 24 h before or implanted (imp) on the day of bacterial challenge. (B) H/E staining of bladder sections of 6-h cyclophosphamide (CYP)-treated or implanted mice observed under a light microscope (magnification, ×20). The experiment was performed in duplicate with n ≥ 3 to 5/group. (C) Graph represents OG1RF titers at 6 hpi from retrieved implants and homogenized bladders and kidneys from nonimplanted CYP- or saline-treated and implanted (Imp) animals. For panels A and C, the horizontal bars indicate the median of each data set. Two independent experiments performed with n = 5 to 10/condition/experiment. *, P < 0.05, and **, P < 0.005, by the Mann-Whitney U test. ns, not significant.
To assess whether any inflammation predisposes the bladder to E. faecalis infection in the absence of a foreign body, murine hemorrhagic cystitis was induced via treatment with cyclophosphamide (CYP) (150 kg/mg, intraperitoneally [i.p.]). CYP-induced cystitis is a well-characterized model of bladder inflammation. Even though CYP- and catheter-induced inflammatory responses are not equivalent, they share some important commonalities depicted in Fig. 4B, such as bladder wall edema, mucosal damage, and host immune cell infiltration (31, 32, 37, 38). When introduced in nonimplanted CYP-treated animals, E. faecalis is rapidly cleared from the urinary tract, similarly to saline-treated nonimplanted controls at 6 hpi (Fig. 4C). By 24 hpi, bacteria are recovered at very low levels (102 to 103 CFU/ml in organs) from both experimental groups compared to implanted animals, whose organs remain colonized at very high titers (105 to 106 CFU/ml; data not shown). These findings suggest that the inflammatory state of the bladder is not sufficient to promote E. faecalis infection of the urinary tract in the absence of a foreign body.
Similarly to uninfected implanted animals, CD11b+ myeloid cells comprise the predominant cellular infiltrates at 24 h in implanted animals with E. faecalis infection (Fig. 3A). However, CD11b+ myeloid cells account for approximately 40% of live cell populations in CAUTI animals, significantly higher than in implanted controls in the absence of E. faecalis (P < 0.005). This increase in myeloid cells in the presence of E. faecalis is mainly due to a 2-fold increase in the recruitment of neutrophils compared to mock-infected implanted controls (P < 0.001) (Fig. 3B). Similar numbers of macrophages, basophils, and eosinophils are present in the bladders of implanted animals whether or not E. faecalis is present (Fig. 3B), indicating that recruitment and activation of these immune cells occur specifically in response to the silicone implant. Together, these results indicate that while the silicone implant elicits the specific recruitment of myeloid infiltrates in the bladder, only neutrophil recruitment is enhanced during enterococcal CAUTI.
Neutrophil recruitment contributes to antienterococcal responses.
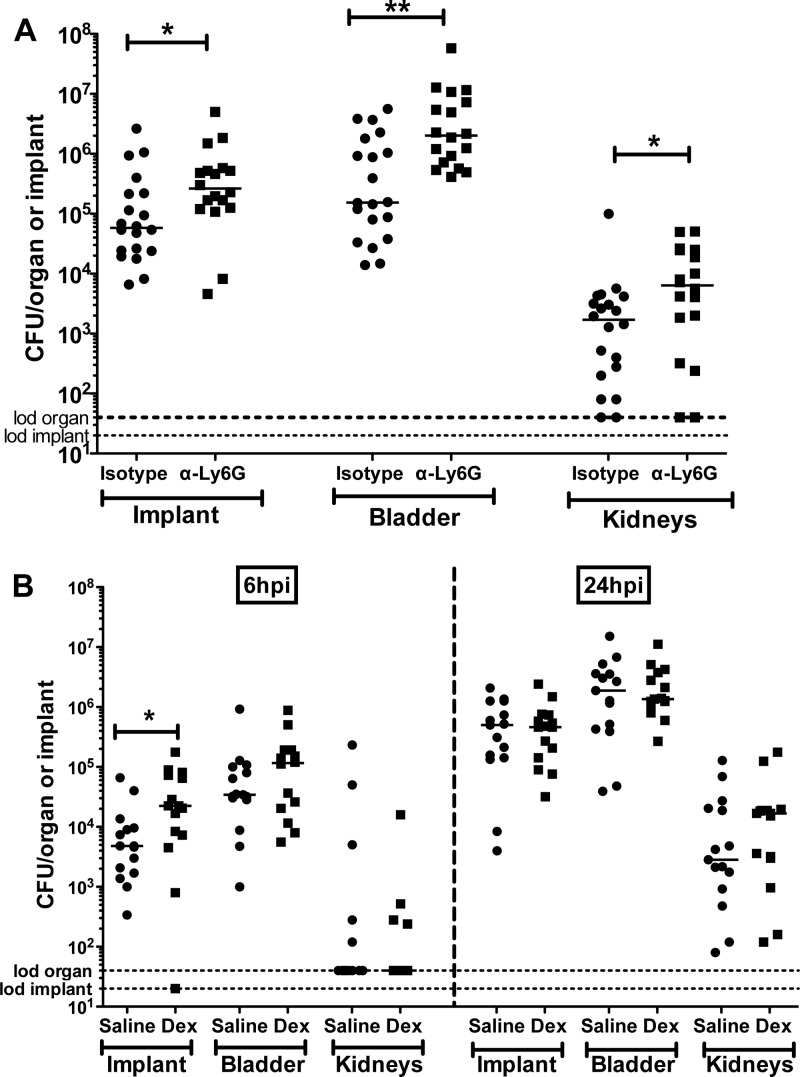
Having identified neutrophils as the major immune cells recruited in response to E. faecalis infection in the urinary tract of implanted animals (Fig. 3B), we assessed their contribution to the inflammatory response during E. faecalis infection. For neutrophil depletion, each mouse was rendered neutropenic following anti-Ly6G (MAb clone 1A8) treatment administered first i.p. 3 days and then 1 day prior to implantation and bacterial challenge (26). At 24 hpi, there were approximately 1- and 1.5-log increases in CFU recovered from implants and bladders of neutrophil-depleted animals, respectively, compared to isotype-control antibody-treated implanted animals (P < 0.05 in all cases) (Fig. 5A). There was also a statistically significant ∼0.5-log difference in the kidney titers between the two groups. Notably, the bladders from these neutropenic animals were as enlarged as were those from their littermate controls in the absence and presence of bacterial infections, indicating that neutrophils are not major contributors to the pathway leading to vascular permeability following urinary catheterization in mice. Together, these findings indicate that neutrophils are dispensable for implant-induced edema but are critical for controlling enterococcal colonization of the urinary tract during CAUTI.
Fig 5.
Neutrophil depletion and dexamethasone treatment enhances enterococcal CAUTI. (A) Graph represents OG1RF titers at 24 hpi on implants and homogenized bladders and kidneys of implanted isotype IgG control (Isotype)- and anti-Ly6G (α-Ly6G)-treated mice. (B) Graph represents OG1RF titers at 6 and 24 hpi from retrieved implants and homogenized bladders and kidneys from saline- or dexamethasone-treated implanted mice. The horizontal bars indicate the median of each data set from two independent experiments with n = 5 to 10/condition/experiment. The horizontal dashed lines represent the limit of detection (lod). *, P < 0.05, and **, P < 0.005, by the Mann-Whitney U test.
Glucocorticoid treatment significantly increases E. faecalis urovirulence at 6 hpi.
The effects of dexamethasone-induced immunosuppression on enterococcal virulence in implanted animals were assessed at 6 hpi and 24 hpi. As shown in Fig. 5B, enterococcal titers were 10-fold higher (P = 0.031) on implants recovered from dexamethasone-treated animals than on those from saline-treated implanted controls at 6 hpi. No significant difference in bacterial titers was observed in the bladder and kidneys between the two groups. By 24 hpi, E. faecalis recoveries from implants were similar in dexamethasone- and saline-treated animals (Fig. 5B). Enterococcal infection in dexamethasone-treated animals causes a significant increase in the production of proinflammatory cytokines, including interleukin-6 (IL-6), which was similar to that in saline-treated infected animals, as shown in Fig. 2A. These data suggest that although dexamethasone did not affect the ultimate production of many proinflammatory cytokines in the bladder consequent to CAUTI, other dexamethasone-sensitive pathways, such as immune cell activation and recruitment, contribute to bacterial clearance in this model.
DISCUSSION
E. faecalis is a major cause of nosocomial infections and an important etiological agent of CAUTI. During CAUTIs, E. faecalis takes advantage of the presence of the foreign body within the bladder to produce biofilms and establish persistent infections in the urinary tract. This infection occurs in the face of a robust immune response, which is induced in mice as well as in patients primarily in response to the presence of the foreign body within the bladder. The murine model of foreign body-associated UTI recently optimized for the study of enterococcal CAUTI and used in the present study helps unravel critical aspects of the interplay between inflammation and enterococcal colonization, providing new details of the molecular mechanisms leading to the foreign body-mediated inflammatory response and its role in the outcome of E. faecalis infection. We specifically showed that while induction of inflammation in the murine bladder either by implantation or chemically in the absence of implants is not sufficient to prevent or promote E. faecalis infection, partial immunosuppression with dexamethasone or neutrophil depletion prior to urinary implantation and bacterial challenge enhances enterococcal colonization of the implants and the urinary tract, suggesting that the immune response in the implanted bladder does not favor enterococcal infection but is simply ineffective at clearing the infection in our murine model of CAUTI.
The bladder responses to urinary catheterization are characterized by severe uroepithelial damage and exfoliation; the onset of bladder wall edema from increased vascular permeability; the production of proinflammatory cytokines IL-6, G-CSF, and KC, as previously reported (18); and the recruitment of myeloid-derived cells, particularly neutrophils. Neutrophils are the primary responders in implanted bladders followed by macrophages, basophils, and some eosinophils. As previously reported (18), infection with E. faecalis increases the above cytokines and induces the secretion of IL-1β and IL-12(p40). Here, we also report the induction of IL-17 following E. faecalis infection, which was significantly upregulated and showed at least a 2-fold increase over nonimplanted uninfected controls. This cytokine was not previously reported because it did not meet the 2-fold-change arbitrary requirement specified above, even though we observed the statistically significant increase in the bladder following infection of implanted animals with E. faecalis (data not shown). Furthermore, the neutrophil populations increased approximately 5-fold in the presence of E. faecalis in implanted animals compared to implanted animals without bacterial challenge.
All the above immune characteristics, from edema to neutrophilia, are associated with activation of the neurogenic inflammatory pathway in various experimental models of cystitis, including cyclophosphamide-induced hemorrhagic cystitis (39). This is an inflammatory response triggered by the release of proinflammatory neuropeptides and activation of surface receptors, including NK1R, on the surface of sensory neurons (40). However, our data indicate that the NK1R-mediated neurogenic inflammatory response is not a major contributor of implant-induced cystitis in mice since treatment with specific NK1R antagonists and iNOS inhibitors did not prevent plasma protein leakage and edema as was previously shown in cystitis or other experimental models involving activation of the neurogenic pathway (32, 41, 42). Although the contributions of other factors involved in the neurogenic inflammatory response, including mast cells, bradykinins, and NK2 receptors, need to be assessed, identifying the effects of urinary implantation on factors involved in vascular permeability, such as calcium channels, calveolin, RhoGTPases, sphingosine kinases (SPHK1), and protein tyrosine phosphatases (SHP2) (43), may shed light on the mechanisms underlying the onset of bladder wall edema following catheterization.
In contrast to studies with specific inhibitors of the neurogenic inflammatory response, glucocorticoid treatment delays the onset of implant-associated edema and vascular permeability and partially decreases cytokine production and cellular recruitment following urinary catheterization at 24 h posttreatment. Given that the effects of dexamethasone on cytokine production persist up to 24 h posttreatment, it is very likely that the cytokines reported here will also be suppressed at early stages (6 h posttreatment). Together, these findings implicate glucocorticoid-responsive inflammatory pathways in the immune response during the early stages of urinary implantation. While previous reports have shown that synthetic glucocorticoids, such as dexamethasone and prednicarbate, inhibit neurogenic vascular permeability in the respiratory tract of rodents and human forearm skin (44–52), the inhibitory effects of glucocorticoids on this pathway remain questionable, as other such compounds, including hydrocortisone and betamethasone, have been shown to have no effects or even to induce this response in airways of rats (53, 54). Synthetic glucocorticoids, including dexamethasone, are among the most effective anti-inflammatory agents used to date for the treatment of chronic inflammatory diseases (55). They are both anti-inflammatory and immunosuppressive molecules whose mechanisms of action involve in part transcriptional regulation via interaction and activation of glucocorticoid receptors (GR) in the host cytoplasm as well as posttranscriptional and -translational regulation of a myriad of genes encoding proteins for cellular and immune processes (55, 56). Glucocorticoids are known inhibitors of inflammatory processes mediated by interleukins (1β, 2, 6, and 8) and other proinflammatory cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor alpha (TNF-α), phospholipase A2, and iNOS. The unresponsiveness to dexamethasone observed in the later stages of urinary implantation has previously been reported in patients suffering from other inflammatory diseases (57), including asthma (58), inclusion body myositis (IBM) (59), and nephrotic syndrome (NS) in children (60). In the case of the implanted bladders, glucocorticoid refractoriness could be attributed to the ongoing exposure to the foreign body (61), the high levels of neutrophils in the bladder which have been previously associated with corticosteroid-resistant asthma (61), a significant reduction in glucocorticoid receptors (62) due to increased urothelial exfoliation, or other cellular and immunological pathways that can circumvent the effects of dexamethasone (61). These may include an increased expression of the dominant negative form of the glucocorticoid receptors (GRβ) on immune cells such as neutrophils and macrophages, rendering them insensitive to dexamethasone treatment (63, 64); upregulation of certain cytokines, including IL-2, IL-4, and IL-13; or that from activation of the mitogen-activated protein kinase (MAPK) signaling pathways (61). Notably, the suppression of the early phase of implant-mediated cystitis following glucocorticoid treatment or iNOS inhibition (see Fig. S3 in the supplemental material) led to a significant increase in enterococcal implant colonization, indicating that by reducing the inflammatory responses, these treatments favor E. faecalis survival in the implanted bladder. Remarkably, by 24 h postinfection, enterococcal infection in dexamethasone-treated animals induces an immune response similar to that elicited in implanted and infected saline-treated controls. At this later time point, no difference in bacterial colonization in glucocorticoid-treated animals relative to untreated controls is observed. Thus, the immune response, while detrimental to E. faecalis colonization, fails to completely clear enterococcal infection. Further, these findings suggest that E. faecalis possesses immune evasion mechanisms that allow its survival in the face of this glucocorticoid-resistant immune response and that implant-mediated bladder inflammation, as in the case of preimplanted animals, did not alter the outcome of infection. The acute inflammatory response induced by the implant may even alter or impair the host response to bacteria, as was demonstrated for Enterococcus faecium peritonitis following treatment with turpentine or casein prior to bacterial challenge (65).
E. faecalis colonization is also significantly increased in the bladders of neutropenic mice following urinary implantation, corroborating previous reports that neutrophils are important mediators of the antienterococcal host response in humans and other animal models of infections (66–69). Previous studies demonstrated that E. faecalis and E. faecium isolated from saliva and root canals are efficiently killed by neutrophils recruited to the site of infection (70) and that TLR-2 is involved in the immune response against E. faecium (71). However, the immune defense during E. faecalis infections of the urinary tract at 24 hpi occurs in a TLR-2- and IL-6-independent manner, as infection of implanted animals deficient in these immune modulators did not alter the outcome of infection (see Fig. S2 in the supplemental material). Further research is required to establish the molecular mechanisms underlying the role of neutrophils during E. faecalis infection. We have recently adapted an in vitro system to grow biofilms on silicone tubing in filtered human urine under fluid flow (72). However, unlike uropathogenic E. coli, E. faecalis failed to produce biofilms under these conditions, even with the addition of extra carbon sources, such as glucose (data not shown). Despite the absence of an in vitro system to study E. faecalis catheter colonization, our in vivo findings strongly support the critical role of neutrophils in delaying and controlling E. faecalis infection. Uncovering the contribution of macrophages and other immune cells, the Toll-like receptors, IL-8, and G-CSF signaling pathways in the host immune response to enterococcal CAUTI will provide more insights into the host response to these infections.
Despite the role of neutrophils in controlling E. faecalis infection, this bacterium is still able to colonize the urinary tract of implanted immunocompetent mice, implying the presence of potential mechanisms to help E. faecalis avoid and/or resist neutrophil killing. Recent studies have demonstrated that the cell wall-anchored pheromone-inducible aggregation substance (AS) and the enterococcal polysaccharide antigen (Epa) in E. faecalis are involved in resistance to neutrophil-mediated killing (73–75). However, the E. faecalis OG1RF strain used in the present study does not bear AS, arguing for alternative mechanisms of immune evasion, such as the downregulation of integrin 4 expression on the surface of neutrophils (76); alteration of the neutrophil properties rendering them nonresponsive to bacterial infections, as is the case during enterococcal sepsis in thermally injured patients and mice (66, 68, 69); and survival within immune cells such as macrophages and biofilm formation, which are well-characterized virulence attributes of enterococci (18, 77–89). Together, these findings indicate that the inflammatory response to the urinary implant is deleterious to E. faecalis but is inefficient at controlling bacterial proliferation and colonization over time. Furthermore, this report is in accord with epidemiological reports of severe enterococcal infections increasingly occurring in immunosuppressed and immunocompromised patients (90–93).
In addition to promoting persistent enterococcal cystitis, the presence of the silicone implants in the bladder allows E. faecalis to gradually and successfully ascend to the kidneys and establish renal colonization in implanted animals. The onset of acute pyelonephritis has been described in postmortem studies of the elderly with indwelling catheters at the time of death (94). In the clinical setting, the removal of infected indwelling urinary catheters is one of the most effective methods used to resolve bacteriuria and CAUTIs (3, 95). However, removing the indwelling medical device, even combined with a long course of antibiotic treatment (95), may not be sufficient for complete resolution of the infection, especially with the rise in antibiotic resistance observed in nosocomial settings (96). In addition, implant removal upon infection in other device-associated infections like prosthetic valve endocarditis is not in itself efficacious (97–99) and thus may not be a suitable therapeutic approach in all instances. Understanding the constituents of the pathophysiology of CAUTIs, namely, biofilm formation and host immune response to urinary catheters, may lead to the development of novel preventative and therapeutic strategies that limit the damage to the uroepithelium while enhancing effective immune responses to bacterial infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kimberly A. Kline at Nanyang Technological University in Singapore and Karen Dodson at Washington University in St. Louis for valuable comments on the manuscript, Marina Cella for technical assistance with flow cytometry, and Marco Colonna at Washington University in St. Louis for providing us with antibodies, reagents, and laboratory space for some of these experiments.
This work was funded by NIH grants DK64540, DK51406, and AI095542 (S.J.H.); AI46433 (M.G.C.); and AI083746 (T.J.H.) and an ASM Robert D. Watkins Graduate Research Fellowship Award (P.S.G.).
Footnotes
Published ahead of print 6 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00856-12.
REFERENCES
- 1. Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. 1981. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect. Control 2:380–386 [DOI] [PubMed] [Google Scholar]
- 2. Tambyah PA, Halvorson KT, Maki DG. 1999. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin. Proc. 74:131–136 [DOI] [PubMed] [Google Scholar]
- 3. Warren JW. 2001. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17:299–303 [DOI] [PubMed] [Google Scholar]
- 4. Norden CW. 1968. Study of urinary infections by catheterization. N. Engl. J. Med. 278:966–967 [DOI] [PubMed] [Google Scholar]
- 5. Garibaldi RA, Burke JP, Britt MR, Miller MA, Smith CB. 1980. Meatal colonization and catheter-associated bacteriuria. N. Engl. J. Med. 303:316–318 [DOI] [PubMed] [Google Scholar]
- 6. Parsons CL. 1986. Pathogenesis of urinary tract infections. Bacterial adherence, bladder defense mechanisms. Urol. Clin. North Am. 13:563–568 [PubMed] [Google Scholar]
- 7. Peychl L, Zalud R. 2008. Changes in the urinary bladder caused by short-term permanent catheter insertion. Cas. Lek. Cesk. 147:325–329 (In Czech.) [PubMed] [Google Scholar]
- 8. Saint S, Chenoweth CE. 2003. Biofilms and catheter-associated urinary tract infections. Infect. Dis. Clin. North Am. 17:411–432 [DOI] [PubMed] [Google Scholar]
- 9. Hashmi S, Kelly E, Rogers SO, Gates J. 2003. Urinary tract infection in surgical patients. Am. J. Surg. 186:53–56 [DOI] [PubMed] [Google Scholar]
- 10. Delnay KM, Stonehill WH, Goldman H, Jukkola AF, Dmochowski RR. 1999. Bladder histological changes associated with chronic indwelling urinary catheter. J. Urol. 161:1106–1109 [PubMed] [Google Scholar]
- 11. Vaidyanathan S, Mansour P, Ueno M, Yamazaki K, Wadhwa M, Soni BM, Singh G, Hughes PL, Watson ID, Sett P. 2002. Problems in early diagnosis of bladder cancer in a spinal cord injury patient: report of a case of simultaneous production of granulocyte colony stimulating factor and parathyroid hormone-related protein by squamous cell carcinoma of urinary bladder. BMC Urol. 2:8 doi:10.1186/1471-2490-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaidyanathan S, Soni BM, Bingley J, Brown E, Markey S. 2002. Prevention of pressure sore caused by indwelling urinary catheters. Spinal Cord 40:489. [DOI] [PubMed] [Google Scholar]
- 13. Kurosaka Y, Ishida Y, Yamamura E, Takase H, Otani T, Kumon H. 2001. A non-surgical rat model of foreign body-associated urinary tract infection with Pseudomonas aeruginosa. Microbiol. Immunol. 45:9–15 [DOI] [PubMed] [Google Scholar]
- 14. Kaufman JM, Fam B, Jacobs SC, Gabilondo F, Yalla S, Kane JP, Rossier AB. 1977. Bladder cancer and squamous metaplasia in spinal cord injury patients. J. Urol. 118:967–971 [DOI] [PubMed] [Google Scholar]
- 15. Jacobs SC, Kaufman JM. 1978. Complications of permanent bladder catheter drainage in spinal cord injury patients. J. Urol. 119:740–741 [DOI] [PubMed] [Google Scholar]
- 16. Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 17. Nielsen HV, Guiton PS, Kline KA, Port GC, Pinkner JS, Neiers F, Normark S, Henriques-Normark B, Caparon MG, Hultgren SJ. 2012. The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. mBio 3(4):e00177–00112 doi:10.1128/mBio.00177-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guiton PS, Hung CS, Hancock LE, Caparon MG, Hultgren SJ. 2010. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 78:4166–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ingersoll MA, Kline KA, Nielsen HV, Hultgren SJ. 2008. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell. Microbiol. 10:2568–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong W, Juneau RA, Pang B, Swords WE. 2009. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J. Innate Immun. 1:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. 2011. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect. Immun. 79:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Baumler AJ. 2011. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. U. S. A. 108:17480–17485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray BE, An FY, Clewell DB. 1988. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob. Agents Chemother. 32:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray BE, Singh KV, Ross RP, Heath JD, Dunny GM, Weinstock GM. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saria A, Lundberg JM. 1983. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J. Neurosci. Methods 8:41–49 [DOI] [PubMed] [Google Scholar]
- 26. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70 [DOI] [PubMed] [Google Scholar]
- 27. Snider RM, Constantine JW, Lowe JA, III, Longo KP, Lebel WS, Woody HA, Drozda SE, Desai MC, Vinick FJ, Spencer RW, Hess HJ. 1991. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science 251:435–437 [DOI] [PubMed] [Google Scholar]
- 28. Snider RM, Longo KP, Drozda SE, Lowe JA, III, Leeman SE. 1991. Effect of CP-96,345, a nonpeptide substance P receptor antagonist, on salivation in rats, Proc. Natl. Acad. Sci. U. S. A. 88:10042–10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McLean S, Ganong A, Seymour PA, Snider RM, Desai MC, Rosen T, Bryce DK, Longo KP, Reynolds LS, Robinson G, Schmidt AW, Siok C, Heym J. 1993. Pharmacology of CP-99,994; a nonpeptide antagonist of the tachykinin neurokinin-1 receptor. J. Pharmacol. Exp. Ther. 267:472–479 [PubMed] [Google Scholar]
- 30. McLean S, Snider RM, Desai MC, Rosen T, Bryce DK, Longo KP, Schmidt AW, Heym J. 1993. CP-99,994, a nonpeptide antagonist of the tachykinin NK1 receptor. Regul. Pept. 46:329–331 [DOI] [PubMed] [Google Scholar]
- 31. Abraham P, Rabi S, Kulothungan P. 2009. Aminoguanidine, selective nitric oxide synthase inhibitor, ameliorates cyclophosphamide-induced hemorrhagic cystitis by inhibiting protein nitration and PARS activation. Urology 73:1402–1406 [DOI] [PubMed] [Google Scholar]
- 32. Abraham P, Rabi S, Selvakumar D. 2009. Protective effect of aminoguanidine against oxidative stress and bladder injury in cyclophosphamide-induced hemorrhagic cystitis in rat. Cell Biochem. Funct. 27:56–62 [DOI] [PubMed] [Google Scholar]
- 33. Griffiths MJ, Messent M, MacAllister RJ, Evans TW. 1993. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br. J. Pharmacol. 110:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stroncek DF, Skubitz KM, McCullough JJ. 1990. Biochemical characterization of the neutrophil-specific antigen NB1. Blood 75:744–755 [PubMed] [Google Scholar]
- 35. Kau AL, Martin SM, Lyon W, Hayes E, Caparon MG, Hultgren SJ. 2005. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73:2461–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, Heuser J, Dodson KW, Caparon MG, Hultgren SJ. 2009. Contribution of autolysin and sortase A during Enterococcus faecalis DNA-dependent biofilm development. Infect. Immun. 77:3626–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishii H, Nomura M, Fujimoto N, Matsumoto T. 2006. Up-regulation of interleukin-6 gene expression in cyclophosphamide-induced cystitis in mice: an in situ hybridization histochemical study. Int. J. Urol. 13:1339–1343 [DOI] [PubMed] [Google Scholar]
- 38. Olivar T, Laird JM. 1999. Cyclophosphamide cystitis in mice: behavioural characterisation and correlation with bladder inflammation. Eur. J. Pain 3:141–149 [DOI] [PubMed] [Google Scholar]
- 39. Bjorling DE, Wang ZY, Bushman W. 2011. Models of inflammation of the lower urinary tract. Neurourol. Urodyn. 30:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geppetti P, Nassini R, Materazzi S, Benemei S. 2008. The concept of neurogenic inflammation. BJU Int. 101(Suppl 3):2–6 [DOI] [PubMed] [Google Scholar]
- 41. Eglezos A, Giuliani S, Viti G, Maggi CA. 1991. Direct evidence that capsaicin-induced plasma protein extravasation is mediated through tachykinin NK1 receptors. Eur. J. Pharmacol. 209:277–279 [DOI] [PubMed] [Google Scholar]
- 42. Trevisani M, Campi B, Gatti R, Andre E, Materazzi S, Nicoletti P, Gazzieri D, Geppetti P. 2007. The influence of alpha1-adrenoreceptors on neuropeptide release from primary sensory neurons of the lower urinary tract. Eur. Urol. 52:901–908 [DOI] [PubMed] [Google Scholar]
- 43. Chavez A, Smith M, Mehta D. 2011. New insights into the regulation of vascular permeability. Int. Rev. Cell Mol. Biol. 290:205–248 [DOI] [PubMed] [Google Scholar]
- 44. Andersson PT, Persson CG. 1988. Developments in anti-asthma glucocorticoids. Agents Actions Suppl. 23:239–260 [DOI] [PubMed] [Google Scholar]
- 45. Bowden JJ, Schoeb TR, Lindsey JR, McDonald DM. 1994. Dexamethasone and oxytetracycline reverse the potentiation of neurogenic inflammation in airways of rats with Mycoplasma pulmonis infection. Am. J. Respir. Crit. Care Med. 150:1391–1401 [DOI] [PubMed] [Google Scholar]
- 46. Brain SD, Newbold P, Kajekar R. 1995. Modulation of the release and activity of neuropeptides in the microcirculation. Can. J. Phys. Pharmacol. 73:995–998 [DOI] [PubMed] [Google Scholar]
- 47. Frode-Saleh TS, Calixto JB, Medeiros YS. 1999. Analysis of the inflammatory response induced by substance P in the mouse pleural cavity. Peptides 20:259–265 [DOI] [PubMed] [Google Scholar]
- 48. Huang HT, Haskell A, McDonald DM. 1989. Changes in epithelial secretory cells and potentiation of neurogenic inflammation in the trachea of rats with respiratory tract infections. Anat. Embryol. 180:325–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kingery WS, Castellote JM, Maze M. 1999. Methylprednisolone prevents the development of autotomy and neuropathic edema in rats, but has no effect on nociceptive thresholds. Pain 80:555–566 [DOI] [PubMed] [Google Scholar]
- 50. Piedimonte G, McDonald DM, Nadel JA. 1990. Glucocorticoids inhibit neurogenic plasma extravasation and prevent virus-potentiated extravasation in the rat trachea. J. Clin. Invest. 86:1409–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Piedimonte G, Pickles RJ, Lehmann JR, McCarty D, Costa DL, Boucher RC. 1997. Replication-deficient adenoviral vector for gene transfer potentiates airway neurogenic inflammation. Am. J. Respir. Cell Mol. Biol. 16:250–258 [DOI] [PubMed] [Google Scholar]
- 52. Tafler R, Herbert MK, Schmidt RF, Weis KH. 1993. Small reduction of capsaicin-induced neurogenic inflammation in human forearm skin by the glucocorticoid prednicarbate. Agents Actions 38(Spec No):C31–C34 [DOI] [PubMed] [Google Scholar]
- 53. Lundberg JM, Martling CR, Saria A, Folkers K, Rosell S. 1983. Cigarette smoke-induced airway oedema due to activation of capsaicin-sensitive vagal afferents and substance P release. Neuroscience 10:1361–1368 [DOI] [PubMed] [Google Scholar]
- 54. Martling CR, Lundberg JM. 1988. Capsaicin sensitive afferents contribute to acute airway edema following tracheal instillation of hydrochloric acid or gastric juice in the rat. Anesthesiology 68:350–356 [DOI] [PubMed] [Google Scholar]
- 55. Newton R. 2000. Molecular mechanisms of glucocorticoid action: what is important? Thorax 55:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lan NC, Karin M, Nguyen T, Weisz A, Birnbaum MJ, Eberhardt NL, Baxter JD. 1984. Mechanisms of glucocorticoid hormone action. J. Steroid Biochem. 20:77–88 [DOI] [PubMed] [Google Scholar]
- 57. Norman M, Hearing SD. 2002. Glucocorticoid resistance—what is known? Curr. Opin. Pharmacol. 2:723–729 [DOI] [PubMed] [Google Scholar]
- 58. Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G, Corrigan C, de Blic J, Fabbri L, Holgate ST, Ind P, Joos G, Kerstjens H, Leuenberger P, Lofdahl CG, McKenzie S, Magnussen H, Postma D, Saetta M, Salmeron S, Sterk P. 1999. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. Eur. Respir. J. 13:1198–1208 [DOI] [PubMed] [Google Scholar]
- 59. Dalakas MC. 2011. Inflammatory myopathies: management of steroid resistance. Curr. Opin. Neurol. 24:457–462 [DOI] [PubMed] [Google Scholar]
- 60. Park SJ, Shin JI. 2011. Complications of nephrotic syndrome. Korean J. Pediatr. 54:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ito K, Chung KF, Adcock IM. 2006. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 117:522–543 [DOI] [PubMed] [Google Scholar]
- 62. Lu YS, Lien HC, Yeh PY, Yeh KH, Kuo ML, Kuo SH, Cheng AL. 2005. Effects of glucocorticoids on the growth and chemosensitivity of carcinoma cells are heterogeneous and require high concentration of functional glucocorticoid receptors. World J. Gastroenterol. 11:6373–6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Strickland I, Kisich K, Hauk PJ, Vottero A, Chrousos GP, Klemm DJ, Leung DY. 2001. High constitutive glucocorticoid receptor beta in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J. Exp. Med. 193:585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goleva E, Li LB, Eves PT, Strand MJ, Martin RJ, Leung DY. 2006. Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am. J. Respir. Crit. Care Med. 173:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leendertse M, Willems RJ, Giebelen IA, van den Pangaart PS, Bonten MJ, van der Poll T. 2009. The acute-phase response impairs host defence against Enterococcus faecium peritonitis. Immunology 128:e335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kobayashi Y. 2006. Neutrophil infiltration and chemokines. Crit. Rev. Immunol. 26:307–316 [DOI] [PubMed] [Google Scholar]
- 67. Leendertse M, Willems RJ, Giebelen IA, Roelofs JJ, Bonten MJ, van der Poll T. 2009. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect. Immun. 77:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fazal N, Shelip A, Siddiqui E, Ali A, Azim AC, Al-Ghoul WM. 2012. Differential effector responses by circulating/blood and tissue/peritoneal neutrophils following burn combined with Enterococcus faecalis infection. FEMS Immunol. Med. Microbiol. 64:191–204 [DOI] [PubMed] [Google Scholar]
- 69. Tsuda Y, Shigematsu K, Kobayashi M, Herndon DN, Suzuki F. 2008. Role of polymorphonuclear neutrophils on infectious complications stemming from Enterococcus faecalis oral infection in thermally injured mice. J. Immunol. 180:4133–4138 [DOI] [PubMed] [Google Scholar]
- 70. Ma Z, Wang Y, Zhu X, Zhang C, Li S, Jin L, Shen Y, Haapasalo M. 2011. Role of polymorphonuclear neutrophils in the clearance of Enterococcus faecalis derived from saliva and infected root canals. J. Endod. 37:346–352 [DOI] [PubMed] [Google Scholar]
- 71. Leendertse M, Willems RJ, Giebelen IA, van den Pangaart PS, Wiersinga WJ, de Vos AF, Florquin S, Bonten MJ, van der Poll T. 2008. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J. Immunol. 180:4865–4874 [DOI] [PubMed] [Google Scholar]
- 72. Guiton PS, Cusumano CK, Kline KA, Dodson KW, Han Z, Janetka JW, Henderson JP, Caparon MG, Hultgren SJ. 2012. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob. Agents Chemother. 56:4738–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rakita RM, Vanek NN, Jacques-Palaz K, Mee M, Mariscalco MM, Dunny GM, Snuggs M, Van Winkle WB, Simon SI. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect. Immun. 67:6067–6075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vanek NN, Simon SI, Jacques-Palaz K, Mariscalco MM, Dunny GM, Rakita RM. 1999. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol. Med. Microbiol. 26:49–60 [DOI] [PubMed] [Google Scholar]
- 75. Teng F, Jacques-Palaz KD, Weinstock GM, Murray BE. 2002. Evidence that the enterococcal polysaccharide antigen gene (epa) cluster is widespread in Enterococcus faecalis and influences resistance to phagocytic killing of E. faecalis. Infect. Immun. 70:2010–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shon W, Lim S, Bae KS, Baek S, Lee W. 2005. The expression of alpha4 integrins by human polymorphonuclear neutrophils in response to sonicated extracts of Enterococcus faecalis. J. Endod. 31:369–372 [DOI] [PubMed] [Google Scholar]
- 77. Shigematsu K, Asai A, Kobayashi M, Herndon DN, Suzuki F. 2009. Enterococcus faecalis translocation in mice with severe burn injury: a pathogenic role of CCL2 and alternatively activated macrophages (M2aMphi and M2cMphi). J. Leukoc. Biol. 86:999–1005 [DOI] [PubMed] [Google Scholar]
- 78. La Carbona S, Sauvageot N, Giard JC, Benachour A, Posteraro B, Auffray Y, Sanguinetti M, Hartke A. 2007. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, alkyl hydroperoxide reductase and thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol. Microbiol. 66:1148–1163 [DOI] [PubMed] [Google Scholar]
- 79. Verneuil N, Maze A, Sanguinetti M, Laplace JM, Benachour A, Auffray Y, Giard JC, Hartke A. 2006. Implication of (Mn) superoxide dismutase of Enterococcus faecalis in oxidative stress responses and survival inside macrophages. Microbiology 152:2579–2589 [DOI] [PubMed] [Google Scholar]
- 80. Baldassarri L, Bertuccini L, Creti R, Filippini P, Ammendolia MG, Koch S, Huebner J, Orefici G. 2005. Glycosaminoglycans mediate invasion and survival of Enterococcus faecalis into macrophages. J. Infect. Dis. 191:1253–1262 [DOI] [PubMed] [Google Scholar]
- 81. Verneuil N, Sanguinetti M, Le Breton Y, Posteraro B, Fadda G, Auffray Y, Hartke A, Giard JC. 2004. Effects of the Enterococcus faecalis hypR gene encoding a new transcriptional regulator on oxidative stress response and intracellular survival within macrophages. Infect. Immun. 72:4424–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baldassarri L, Bertuccini L, Ammendolia MG, Cocconcelli P, Arciola CR, Montanaro L, Creti R, Orefici G. 2004. Receptor-mediated endocytosis of biofilm-forming Enterococcus faecalis by rat peritoneal macrophages. Indian J. Med. Res. 119(Suppl):131–135 [PubMed] [Google Scholar]
- 83. Baldassarri L, Cecchini R, Bertuccini L, Ammendolia MG, Iosi F, Arciola CR, Montanaro L, Di Rosa R, Gherardi G, Dicuonzo G, Orefici G, Creti R. 2001. Enterococcus spp. produces slime and survives in rat peritoneal macrophages. Med. Microbiol. Immunol. 190:113–120 [DOI] [PubMed] [Google Scholar]
- 84. Sussmuth SD, Muscholl-Silberhorn A, Wirth R, Susa M, Marre R, Rozdzinski E. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. 1999. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect. Immun. 67:2160–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hendrickx AP, van Luit-Asbroek M, Schapendonk CM, van Wamel WJ, Braat JC, Wijnands LM, Bonten MJ, Willems RJ. 2009. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect. Immun. 77:5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mohamed JA, Huang DB. 2007. Biofilm formation by enterococci. J. Med. Microbiol. 56:1581–1588 [DOI] [PubMed] [Google Scholar]
- 88. Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 116:2799–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kosmidis CI, Chandrasekar PH. 2012. Management of gram-positive bacterial infections in patients with cancer. Leuk. Lymphoma 53:8–18 [DOI] [PubMed] [Google Scholar]
- 91. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317 [DOI] [PubMed] [Google Scholar]
- 92. Sastry V, Brennan PJ, Levy MM, Fishman N, Friedman AL, Naji A, Barker CF, Brayman KL. 1995. Vancomycin-resistant enterococci: an emerging pathogen in immunosuppressed transplant recipients. Transplant. Proc. 27:954–955 [PubMed] [Google Scholar]
- 93. Bonten MJ, Willems R, Weinstein RA. 2001. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect. Dis. 1:314–325 [DOI] [PubMed] [Google Scholar]
- 94. Warren JW, Muncie HL, Jr, Hall-Craggs M. 1988. Acute pyelonephritis associated with bacteriuria during long-term catheterization: a prospective clinicopathological study. J. Infect. Dis. 158:1341–1346 [DOI] [PubMed] [Google Scholar]
- 95. Trautner BW, Darouiche RO. 2004. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 32:177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Donelli G, Guaglianone E. 2004. Emerging role of Enterococcus spp in catheter-related infections: biofilm formation and novel mechanisms of antibiotic resistance. J. Vasc. Access 5:3–9 [DOI] [PubMed] [Google Scholar]
- 97. Donlan RM. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dickinson GM, Bisno AL. 1989. Infections associated with indwelling devices: infections related to extravascular devices. Antimicrob. Agents Chemother. 33:602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dickinson GM, Bisno AL. 1989. Infections associated with indwelling devices: concepts of pathogenesis; infections associated with intravascular devices. Antimicrob. Agents Chemother. 33:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.