Abstract
Iron is an indispensable nutrient for most organisms. Ferric iron (Fe3+) predominates under aerobic conditions, while during oxygen limitation ferrous (Fe2+) iron is usually present. The Feo system is a bacterial ferrous iron transport system first discovered in Escherichia coli K-12. It consists of three genes, feoA, feoB, and feoC (yhgG). FeoB is thought to be the main transmembrane transporter while FeoC is considered to be a transcriptional regulator. Using multidimensional nuclear magnetic resonance (NMR) spectroscopy, we have determined the solution structure of E. coli FeoA. The structure of FeoA reveals a Src-homology 3 (SH3)-like fold. The structure is composed of a β-barrel with two α-helices where one helix is positioned over the barrel. In comparison to the standard eukaryotic SH3 fold, FeoA has two additional α-helices. FeoA was further characterized by heteronuclear NMR dynamics measurements, which suggest that it is a monomeric, stable globular protein. Model-free analysis of the NMR relaxation results indicates that a slow conformational dynamic process is occurring in β-strand 4 that may be important for function. 31P NMR-based GTPase activity measurements with the N-terminal domain of FeoB (NFeoB) indicate a higher GTP hydrolysis rate in the presence of potassium than with sodium. Further enzymatic assays with NFeoB suggest that FeoA may not act as a GTPase-activating protein as previously proposed. These findings, together with bioinformatics and structural analyses, suggest that FeoA may have a different role, possibly interacting with the cytoplasmic domain of the highly conserved core portion of the FeoB transmembrane region.
INTRODUCTION
Iron is an essential nutrient for most organisms (1). Its importance arises from its role as a protein cofactor in processes such as DNA precursor synthesis, respiration, and oxygen transport or as an electron carrier (2–4). Two forms of iron exist naturally, ferric (Fe3+) and ferrous (Fe2+), where ferric iron is the form that is dominant under oxidizing conditions. In Gram-negative bacteria, Fe3+ is transported into the periplasm through dedicated outer membrane receptors that are part of the TonB-ExbB-ExbD-dependent transport systems (3, 5–7). Anaerobiosis and acidification lead to the transition of ferric to ferrous iron (3, 8). Different bacterial ferrous iron transport pathways exist: the metal-ABC transporters YfeABCD and FutABC, MntH, ZupT, and EfeUOB as well as the ferrous iron transport system, Feo (9–18). YfeABCD is part of the SitABCD family of ATP-binding cassette (ABC) transporters that regulate manganese and iron levels within the cell (15, 17). FutABC is also an ABC transporter that selectively transports ferrous iron (13, 14). MntH and ZupT are part of the NRAMP (natural resistance-associated macrophage proteins) and the Zip transporter family, respectively, and both have broad-spectrum specificity for various divalent metal ions (10, 12, 16). The EfeUOB transport system is specific for ferrous iron transport; however, it is present only in specific pathogenic species (9, 11, 12). The Feo system is the only transport pathway that is widely distributed and that is dedicated to the transport of ferrous iron (19, 20).
The Feo system was first discovered in Escherichia coli K-12 (E. coli), and it consists of the feoA, feoB, and feoC (yhgG) genes (19–21) (Fig. 1A). Residing upstream of feoA, feoB, and feoC are the fnr and fur regulatory elements (Fig. 1A) (5, 20). Fnr is an anaerobically induced transcriptional activator (22), which acts as an oxygen sensor for the feo operon and, in the absence of oxygen, activates transcription of the feo genes. Fur is an iron-dependent regulator of iron transport genes. In the presence of iron, the Fe2+-Fur complex binds to the fur box to inhibit transcription of the feo genes (5, 23). Iron deprivation results in the dissociation of Fur from the fur box to allow for transcription of iron transport genes. Fur and Fnr play a crucial role in maintaining cellular iron homeostasis and preventing the formation of damaging free radicals. The presence of another regulatory element, the rstA box, has also been found in the Salmonella enterica feo operon, which is absent in its E. coli counterpart, to precede the fur binding sequence (24). RstA was shown to regulate the expression of the feoB gene in S. enterica (24). The Feo system is important for the virulence of various pathogenic bacterial strains such as Helicobacter pylori, Legionella pneumophila, and Campylobacter jejuni (25–27). As such, it is important to gain a better insight into the mode of action of the Feo proteins.
Fig 1.
(A) The Feo operon in E. coli K-12. (B) Different feo operon organizations in all sequenced bacterial genomes to date (October 2011). Frequency indicates prevalence of each organization in the NCBI Gene database. NfeoB represents the gene encoding the soluble N-terminal cytoplasmic G protein and GDI domains of FeoB. CfeoB represents the gene encoding the C-terminal transmembrane region of FeoB.
Within the Feo system, the FeoB protein is thought to be the main ferrous iron transporter in the cytoplasmic membrane. E. coli FeoB is a 773-residue transmembrane protein comprised of two main regions: an N-terminal cytoplasmic domain and a C-terminal polytopic transmembrane domain. Within the N-terminal domain (NFeoB) reside the G-protein and the guanine nucleotide dissociation inhibitor (GDI) domains (28). The G domain of FeoB is essential for ferrous iron uptake (29). It is thought that the G domain provides energy for the transport process or that it senses the energy state of the cell and relays this information to the transmembrane domain to regulate transport. The GDI domain is an internal regulator that specifically stabilizes the binding of GDP to the G domain (28). The polytopic transmembrane region of FeoB is proposed to act as a permease for the diffusion of Fe2+ into the cell (21, 29).
E. coli FeoC is a small 78-residue protein with a winged-helix fold (5, 30). This fold belongs to the helix-turn-helix family that is usually involved in DNA binding (5, 31). It has been suggested that FeoC may act as a transcriptional regulator (5). In comparison to FeoA and FeoB, FeoC is not well conserved between species, and it is thought to be present only in gammaproteobacteria (5) (Fig. 1B).
The role of FeoA is not well understood within the Feo system. E. coli FeoA is a 75-residue cytoplasmic protein with an unknown function. It was proposed that FeoA may interact with FeoB to take part in ferrous iron transport (32). Surveying the available sequenced bacterial genomes to date (NCBI database, accessed October 2011), we find that ∼45% possess one or more Feo systems (Fig. 1B). In some Feo systems, multiple FeoA proteins exist that are covalently attached to each other (Fig. 1B). Sequence analysis of the double and triple FeoA proteins reveals that they have low amino acid sequence homology; typically, there is only ∼30% identity or less between the individual domains. Further analysis of the operons reveals that FeoA occurs together with FeoB 89% of the time (Fig. 1B). In prokaryotes, interacting proteins are often located adjacent to one another within one operon (33). Consequently, the fact that the FeoA and FeoB genes are being expressed together suggests intertwined functional roles. Here, we present the solution structure of E. coli FeoA along with a dynamics analysis of the behavior of the protein in aqueous solution. Moreover, we also performed 31P nuclear magnetic resonance (NMR) studies to examine the role of FeoA within ferrous iron transport. Our results show that FeoA may not act as a GTPase-activating protein (GAP) as was earlier suggested (5); instead, we propose that it may function by interacting with the highly conserved core region in the transmembrane domain of FeoB.
MATERIALS AND METHODS
Cloning of E. coli FeoA and NFeoB.
FeoA and NFeoB (residues 1 to 276) were amplified from the E. coli K-12 genome through PCR with oligonucleotides engineered to contain 5′ NdeI (CATATG) and 3′ XhoI (CTCGAG) (New England BioLabs) restriction sites. Amplified FeoA and NFeoB were cloned into pET30a and pSUMO expression vectors, respectively. The pET30a vector (Novagen) attaches a hexahistidine tag to the C terminus of the expressed protein while the pSUMO (small ubiquitin-like modifier) system (LifeSensors) attaches a hexahistidine SUMO tag on the N terminus (34). The original pSUMO vector was modified to contain an NdeI restriction site in the multiple-cloning site. Constructs were verified through nucleotide sequencing at the University of Calgary Genetic Analysis laboratory. Vectors with constructs are maintained in E. coli DH5α cells. Plasmids were transformed to E. coli BL21(DE3) cells for protein expression.
Site-directed mutagenesis of FeoA.
The C-terminal cysteine residue of wild-type FeoA was mutated to a serine (C75S) to prevent any undesired spontaneous disulfide formation and covalent dimerization of the protein during the lengthy NMR experiments. The cytoplasm of E. coli is a reducing environment; thus, Cys75 would be in its reduced form in vivo. The C75S mutation ensures that FeoA is always present in its intracellular form. Moreover, Cys75 is the terminal residue, and it does not appear to be conserved among FeoA proteins from different species; thus, C75S can be considered to be equivalent to FeoA. Preliminary structural data on wild-type FeoA were identical to those for C75S; therefore, we will refer to the mutant protein as FeoA henceforth. The pET30a vector with wild-type FeoA was used as a template for site-directed mutagenesis. Mutagenesis was performed using a protocol outlined in the QuikChange site-directed mutagenesis system (Stratagene).
Overexpression and purification of FeoA and NFeoB.
For overexpression of FeoA, E. coli cells were grown in 1 liter of Luria Bertani (LB) liquid medium in the presence of 30 μg/ml of kanamycin. Cells were expressed to an optical density at 600 nm (OD600) of ∼0.4 at 37°C and induced with 0.8 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at room temperature for 5 h. Cells were harvested and resuspended in 20 mM Tris, 0.5 M NaCl, and 40 mM imidazole (pH 8) and lysed by a French pressure cell. The cell lysate was centrifuged at 18,500 × g for 45 min at 8°C. All histidine-tagged proteins were purified with a chelating Sepharose column (GE Healthcare) through nickel affinity chromatography. Bound proteins were washed with 20 mM Tris, 0.5 M NaCl, and 100 mM imidazole and eluted off the column with 20 mM Tris, 0.15 M NaCl, and 300 mM imidazole (pH 8). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to visualize protein homogeneity. The FeoA protein concentration was determined by measuring the A280 using an extinction coefficient calculated through the ExPaSy ProtParam program. The C-terminal hexahistidine tag of the FeoA construct was not removed for these studies as it enhanced the solubility for the in vitro studies.
For the production of 13C, 15N isotope-enriched FeoA, cells were grown under the conditions described above with the exception that M9 minimal medium supplemented with 15NH4Cl (1g/liter) and [13C]glucose (3g/liter) (Sigma-Aldrich) were used instead of the LB medium. Isotope-labeled protein was purified as per the protocol described above.
NFeoB was expressed in 1 liter of LB medium with 30 μg/ml of kanamycin and induced at an OD600 of ∼0.6 with 0.25 mM IPTG for 4 h. Cells were harvested and resuspended in 20 mM Tris, 0.5 M NaCl, and 20 mM imidazole, pH 8, and lysed by a French pressure cell. The lysed cells were centrifuged at 18,500 × g for 45 min at 8°C and applied to a nickel-chelating Sepharose column. Bound proteins were washed with resuspension buffer, and SUMO-NFeoB fusion proteins were eluted with 20 mM Tris, 0.15 M NaCl, and 300 mM imidazole at pH 8. The SUMO-NFeoB fusion protein was cleaved with SUMO protease (100 μg of fusion protein/unit of protease) (LifeSensors). Undigested fusion proteins along with the cut SUMO proteins were removed from NFeoB through reapplication to the nickel-chelating Sepharose column, and NFeoB was eluted using resuspension buffer. SDS-PAGE was used to visualize protein homogeneity; proteins were >95% pure. The protein concentration of NFeoB was also determined through A280 measurements.
NMR measurements of FeoA.
NMR samples contained approximately 0.5 mM uniformly 15N, 13C-labeled FeoA, 1 mM 2,2-dimethyl-2-silapentane-5-sulfonate (DSS), and 10% D2O buffered in 20 mM HEPES, 100 mM NaCl at pH 7. All NMR experiments were performed at 298 K on a Bruker Avance 500 MHz NMR spectrometer equipped with a triple-resonance inverse Cryoprobe with a single axis z-gradient. The sequential backbone resonance assignment of FeoA was obtained through HNCACB, CBCA(CO)NH, HNCO, and HN(CA)CO experiments. Aliphatic side chain assignments were obtained through three-dimensional experiments including C(CCO)NH-total correlation spectroscopy (TOCSY), H(CCO)NH-TOCSY, HBHA(CBCACO)NH, CCH-TOCSY, and HCCH-TOCSY. Aromatic side chain assignments were determined through two-dimensional (HB)CB(CGCD)HD and (HB)CB(CGCDCE)HE (35) and double-quantum-filtered correlation spectroscopy (DQF-COSY) experiments. All nuclear Overhauser effect spectroscopy (NOESY) experiments including three-dimensional 13C-edited NOESY-heteronuclear single-quantum coherence (HSQC), three-dimensional 15N-edited NOESY, and two-dimensional NOESY were acquired with a mixing time of 120 ms.
Backbone dynamics studies of 15N-labeled FeoA were acquired at 50.68 MHz for the 15N frequency. Longitudinal relaxation (T1) experiments were performed using sensitivity-enhanced inversion-recovery pulse sequences (36). Each spectrum was acquired with 1,024 points in the 1H dimension and 64 points in the 15N dimension. Sweep widths of 8,012 Hz and 912 Hz were employed for the 1H and 15N dimensions, respectively, with 24 scans per t1 point. Delay times of 14 (twice), 98, 350 (twice), 490, 700, 896, and 1,190 ms were used. 15N spin-spin relaxation time (T2) data were acquired using sensitivity-enhanced Carr-Purcell-Meiboom-Gill (CPMG) pulse sequences with pulsed-field gradients (36). All T2 spectra were acquired with the same parameters as above except with 32 scans per t1 point. Delay times of 6.55 (twice), 13.09, 26.17 (twice), 39.25, 52.33, 65.41, 78.49, 91.57, 117.737 ms were used. {1H}-15N heteronuclear NOE data were acquired with a 5-s presaturation period with the reference spectrum acquired with a 5-s delay time. NMR spectra were processed with NMRPipe/NMRDraw (37) and analyzed with NMRView software (38).
Structure calculation.
The initial structure of FeoA was calculated with CYANA (version 2.1) (39) using distance restraints generated from the automated NOE assignment protocol implemented in CYANA and dihedral angle restraints predicted by TALOS+ (40). Further structural refinement with the addition of hydrogen bond restraints based on secondary structure from the chemical shift index was performed with XPLOR-NIH (version 2.26) (41). The 30 lowest-energy structures from a total of 200 calculated were used for analysis. Structures were validated with the Protein Structure Validation Software (PSVS) suite (42). All molecular graphics used in the manuscript were generated with MOLMOL (43) or PyMOL (version 1.3r1; Schrodinger, LLC) software.
Relaxation data analysis.
T1 and T2 data were fitted with the program CurveFIT (A. G. Palmer, Columbia University). The uncertainties for {1H}-15N NOE values were evaluated from spectra with and without proton saturation. Residues that show NOE values of <0.65 were eliminated from analysis due to possible fast internal motions (44, 45). Residues involved in chemical exchange processes affect T2 relaxation time and, thus, were removed as described by Tjandra et al. (46). Residue-specific correlation times were determined from the ratio of the spin-spin relaxation rate constant to the spin-lattice relaxation rate constant (R2/R1) using the program R2R1_τm (A. G. Palmer, Columbia University), and a single isotropic total correlation time (τc) of the protein was calculated. Estimates of axial and fully anisotropic diffusion tensors were obtained using the program QUADRIC_DIFFUSION (A. G. Palmer, Columbia University). Relaxation data were fit to five models according to the model-free formalism (47, 48), where each N-H vector is assigned a specific model from which relevant motional parameters (the squared order parameter, S2, and the chemical exchange rate, Rex) were extracted. Model selection was performed through the FAST-ModelFree program (49) that uses ModelFree, version 4.20 (50).
31P NMR.
31P NMR spectroscopy was used to monitor the GTPase activity of NFeoB. The 31P NMR samples contained 60 μM NFeoB in 20 mM HEPES, 100 mM KCl or NaCl, 3 mM GTP, 4 mM MgSO4, and 10% D2O at pH 7. One-dimensional NMR experiments were acquired every hour for 24 h to monitor GTP hydrolysis. All experiments were acquired on a Bruker Avance 400 MHz spectrometer equipped with a 10-mm Broadband probe at 310 K in a 10-mm NMR tube. GTP self-hydrolysis was monitored by performing the same experiment as described above except that no NFeoB was added.
To examine the role of FeoA, it was added to an identical sample to observe its effect on the GTPase activity of NFeoB. FeoA and NFeoB were mixed together in 20 mM HEPES and 100 mM KCl at pH 7 and concentrated down to 60 μM each. GTP (3 mM) and MgSO4 (4 mM) and D2O (10%) were added to the concentrated solution, and hydrolysis activity was immediately recorded. The control sample contained the same reagents with the exception of FeoA. These experiments were acquired at 298 K.
Protein structure accession numbers.
The atomic coordinates of the 30 lowest-energy structures have been deposited in the RCSB Protein Data Bank (PDB) under accession number 2lx9. The NMR chemical shifts were deposited in the BioMagResBank (BMRB) under accession number 18668.
RESULTS
Protein purification.
The His-tagged FeoA construct could be expressed with a yield of ∼10 mg of purified protein per liter of cell culture in LB medium; the expression levels in minimal medium were around ∼7 mg of protein per liter of culture. FeoA was usually expressed in the cytoplasm of E. coli BL21(DE3) and was not detectable in inclusion bodies. NFeoB, with a SUMO tag, was expressed in LB medium with a yield of ∼50 mg of tag-free purified NFeoB protein per liter of culture. Both proteins could be purified to homogeneity as determined by SDS-PAGE (see Fig. S1 in the supplemental material). Molecular weights were confirmed with mass spectrometry (data not shown).
Solution structure of FeoA.
The backbone amide resonances of FeoA could be assigned unambiguously with the exception of a few residues that experienced severe broadening through chemical exchange (e.g., Gln2 and Ser75). The NMR solution structure of FeoA was calculated based on 1,197 NOE-derived distance restraints (Table 1). Superposition of the 30 lowest-energy structures reveals a well-defined structure that converges to a backbone root mean square deviation (RMSD) of 0.40 ± 0.09 Å for the folded region (Fig. 2A). FeoA possesses two antiparallel β-sheets with β-strands 1 and 5 being orthogonal to β-strand 2, 3, and 4 (Fig. 2B). There are two α-helices in FeoA; the first α-helix sequentially follows the first β-strand and is situated above the β-barrel structure resembling a lid (Fig. 2B; see also Fig. S2A in the supplemental material). The second α-helix is slightly shorter than the first helix, consisting of only two turns, and it is located between β-strands 4 and 5. Ramachandran analysis shows that 88.2% of the residues are in the favored regions, while 11.8% of the residues are in the additionally allowed regions (Table 1). No residues are present in the disallowed regions of the Ramachandran plot.
Table 1.
Experimental restraints and structural statistics for the 30 lowest-energy structures
| Parameter | Value for the parameter |
|---|---|
| No. of NOE cross-peaks assigneda | 1,924 |
| Experimental restraints | |
| No. of distance restraints from NOEsb | |
| Total | 1,197 |
| Intraresidue | 297 |
| Sequential | 351 |
| Medium-range (2–4) | 192 |
| Long-range (5+) | 357 |
| No. of dihedral angle restraintsc | 130 |
| No. of hydrogen bond distance restraints | 40 |
| Avg RMSD from experimental restraints | |
| Distance restraint violation (Å) | 0.039 ± 0.002 |
| Dihedral angle restraint violation (°) | 0.574 ± 0.090 |
| Avg RMSD from ideal stereochemistry | |
| Bond length (Å) | 0.003 ± 0.000 |
| Bond angle (°) | 0.471 ± 0.010 |
| Improper bond (°) | 0.318 ± 0.010 |
| PROCHECK Ramachandran analysis (%)d | |
| Residues in favored regions | 88.2 |
| Residues in additional allowed regions | 11.8 |
| Residues in generously allowed regions | 0.0 |
| Residues in disallowed regions | 0.0 |
| Coordinate precision of folded regions (Å)e | |
| Backbone | 0.40 ± 0.09 |
| All heavy atoms | 1.02 ± 0.11 |
Determined from CYANA, version 2.1 (39).
Numbers of restraints per residue were calculated using FeoA C75S residues 5 to 70 from Xplor-NIH, version 2.26 (41).
Predicted from TALOS+ (40).
Validated through the Protein Structure Validation Suite (42), excluding glycine and proline residues.
Residues 5 to 70.
Fig 2.

(A) The 30 lowest-energy structures of FeoA C75S superimposed for the well-folded region (residues 5 to 70). (B) Ribbon representation of the lowest-energy structure. β-Sheet and α-helices are represented in navy and yellow, respectively. (C) Comparison of solution NMR structures of E. coli FeoA (navy) (PDB ID 2lx9) and K. pneumonia FeoA (green) (PDB ID 2gcx). (D) Superposition of NMR structure of E. coli FeoA (navy) (PDB ID 2lx9) and crystal structure of S. maltophilia FeoA (yellow) (PDB ID 3mhx).
Figure 2C shows an overlay of the PDB-deposited solution NMR structure of Klebsiella pneumonia FeoA (PDB identification number [ID] 2gcx) and the E. coli protein. The sequence identity between these two proteins is 90%. The residues that differ are all conservative substitutions with the exception of Asn-38 in E. coli, which is a His in K. pneumonia. This residue is solvent exposed and does not alter the structure significantly. All secondary structural elements are the same for both proteins, with the overall structures agreeing to a backbone RMSD of 2.22 Å.
The crystal structure of Stenotrophomonas maltophilia FeoA complexed with zinc was recently reported (PDB ID 3mhx) (32). The amino acid sequence of S. maltophilia FeoA is significantly different from the sequences of E. coli and K. pneumonia FeoA (data not shown). Superposition of this structure on that of E. coli FeoA shows the typical β-barrel structure being conserved with a backbone RMSD of 2.61 Å (Fig. 2D). In contrast to the two helices and five β-strands of E. coli FeoA, S. maltophilia FeoA contains three helices and five β-strands (Fig. 2D; see also Fig. S2B in the supplemental material). Despite these differences, the core β-barrel fold is conserved.
Dynamic properties of FeoA.
Backbone dynamics experiments including {1H}-15N heteronuclear NOE, T1, and T2 measurements were carried out at 500 MHz with 68 residues selected for final analysis (Fig. 3). Of note, analysis of the dynamics data excluded the hexahistidine tag region and all five proline residues which do not have an amide proton. NOE and R2 results reveal a relatively ordered conformation for FeoA, with the exception of the loop region between β-strands 3 and 4, which undergoes chemical exchange. The S2 order parameter (average S2 [S2ave] of ∼0.87) derived from model-free analysis agrees well with the R1/R2/NOE data, suggesting that FeoA is a rigid protein (Fig. 3). Lower S2 values near the C terminus of FeoA indicate a more flexible region (Fig. 3). Four residues with conformational exchange occurring in the milli- to microsecond timescale are observed in the region of β-strands 3 and 4 (Fig. 3). These residues also have a higher effective R2 [R2eff] relaxation rate. The higher R2eff of these residues are a result of the higher Rex contribution. Of specific interest is Val56, which has the highest Rex value of all the residues. The total correlation time of FeoA was calculated to be 6.2 ns. Quadratic diffusion (D) analysis reveals an isotropic globular protein with a Dparallel/Dperpendicular of 0.87 ± 0.03. All data are in accordance with secondary structural elements as illustrated in Fig. 3. Moreover, they indicate that E. coli FeoA behaves as a monomeric, almost globular, protein in aqueous solution.
Fig 3.
15N relaxation data and FAST-ModelFree analysis for FeoA. R1, R2, NOE values, S2 order parameters, and chemical exchange rates (Rex) are plotted as a function of the residue number. S2 order parameters reflect motions on the pico- to nanosecond timescale, and Rex values reflect motions on the micro- to millisecond timescale. Positions of the secondary structural elements are shown.
31P NMR of NFeoB.
To monitor the GTPase activity of NFeoB, GTP hydrolysis was followed over time by 31P NMR. This assay simultaneously monitors the levels of GTP, GDP, and Pi in the sample. Each phosphorus atom in the GTP/GDP moieties is identified through its characteristic chemical shift. The first experiment after 1 h shows nonhydrolyzed GTP with three phosphate peaks: γ-, α-, and β-phosphate (Fig. 4A). As time progresses, the Pi peak becomes increasingly visible around 2 ppm. After 21 h, the majority of GTP has been hydrolyzed, with small residual amounts observed through the small β-phosphate GTP peak (Fig. 4A). A corresponding control experiment showed no GTP autohydrolysis under the same conditions (data not shown); thus, the hydrolysis observed is due to the addition of NFeoB.
Fig 4.
GTPase activity assay of NFeoB. 31P NMR spectra of NFeoB in the presence of NaCl (A) and KCl (B).
Previously, Streptococcus thermophilus NFeoB was shown to accelerate GTP hydrolysis by 16-fold in the presence of potassium (51). Our initial 31P NMR experiments were performed in NaCl. To investigate whether E. coli NFeoB acts through a similar mechanism as S. thermophilus NFeoB, the same 31P NMR assay was performed in the presence of KCl. Figure 4B illustrates the complete hydrolysis of GTP after 4 h. The only visible peaks after 4 h represent the Pi and the β- and α-GDP peaks. A comparison of the NaCl and KCl spectra shows that the hydrolysis activity is five times faster in KCl than in NaCl (Fig. 4).
To determine whether FeoA can act as a GAP of NFeoB, the same assay was performed in the presence of FeoA. The addition of an equimolar amount of FeoA had no effect on NFeoB's hydrolytic activity; the amount of time required to hydrolyze GTP was the same as without FeoA (data not shown).
DISCUSSION
Bacterial ferrous iron transport systems are currently much less understood in comparison with ferric iron transport pathways. For example, it is not yet known whether ferrous iron enters the cell through active transport or through a passive diffusion mechanism. In vivo models have shown that FeoA and FeoB are closely linked and that deletion of FeoA reduces ferrous iron uptake while deletion of FeoB completely abolishes ferrous iron transport (20). FeoA has previously been classified as an Src-homology 3 (SH3)-like domain based on its low amino acid sequence similarity to the C-terminal domain of DtxR (5), while FeoB is thought to provide energy and regulate transport (5, 20, 28, 29, 51). Our NMR studies of E. coli FeoA contribute to the understanding of bacterial ferrous iron transport as we have been able to investigate both the solution structure and the dynamic properties of the protein and the role that it may play in ferrous iron transport.
The structure of E. coli FeoA is composed of a β-barrel and two α-helices. The FeoA fold resembles that of a eukaryotic SH3 domain. SH3 domains are a group of interacting modules that are prevalent in eukaryotic organisms (52). They have been noted to interact with small G proteins and may bind GAPs or guanine exchange factors (GEFs) (53). In order to highlight the similarity, the FeoA structure was overlaid with the prototypical SH3 domain of Abl tyrosine kinase (ATK) (PDB ID 1ju5) (Fig. 5A). Superposition of FeoA and ATK reveals an RMSD of 2.64 Å, with the core β-barrel structurally conserved with several distinct differences (54). Specifically, the first α-helix that resides above the barrel and the second α-helix that completes the barrel are absent in the prototypical ATK SH3 domain (Fig. 2B and 5A). Normally, SH3 domains possess three conserved loops that are important for protein-protein interactions: the RT-Src, N-Src, and distal loop (Fig. 5A E) (55). The RT-Src loop of ATK is replaced by an α-helix in FeoA (Fig. 5A and E). While the N-Src loop of ATK is present in FeoA, its position and length are quite different (Fig. 5A and E). The distal loop is present and appears to be in the same orientation in both FeoA and ATK (Fig. 5A and E). Hydrophobic pockets filled with aromatic residues that are typical of SH3 domains (52) are also absent in FeoA. The lack of standard SH3 domain features is consistent with the notion that SH3 domains are less prevalent in prokaryotes than eukaryotes, which suggests that perhaps the SH3-like fold of FeoA could fulfill a distinct role (52, 55, 56).
Fig 5.
Structural comparison of FeoA with prototypical SH3 domain and Dali-determined structural homolog, IdeR. (A) Prototypical SH3 domain Abl tyrosine kinase (PDB ID 1ju5 chain C) superposed on E. coli FeoA (PDB ID 2lx9) in green and black, respectively. The RT-Src, N-Src, and distal loops of SH3 domains are indicated. Val56 of FeoA is highlighted in pink. (B) Structural superposition of E. coli FeoA (PDB ID 2lx9) (black) and IdeR (PDB ID 1u8r chain C) (gray). Ancillary site and primary site of IdeR are shown in blue and pink, respectively. Hydrogen bond contacts are illustrated in yellow. (C) Ancillary site (blue) and primary site (pink) of IdeR (PDB ID 1u8r chain C). E172 and Q175 of the SH3-like domain donate two metal ligands for the ancillary site. Hydrogen bond contacts are highlighted in yellow. (D) Potential metal ligands of E. coli FeoA. The SH3-like domain of IdeR is replaced by FeoA in this structure. Highlighted in green are residues of FeoA close to the binding sites of IdeR. Metal binding sites and contacts of IdeR are colored as in panel C. FeoA is depicted in black for all structures. (E) Three-dimensional structural alignment of E. coli FeoA with Abl tyrosine kinase and the C-terminal SH3-like domains of the three Dali structural homologs. Positions of β-sheets and α-helices are shown in dashed boxes. The location of the RT-Src, N-Src, and distal loops with respect to the secondary structure of Abl tyrosine kinase is indicated. Highlighted in yellow is one of the metal binding ligands in the SH3-like domain of the homologs.
Currently, seven structures for FeoA from different bacterial species have been deposited in the PDB: five NMR solution and two crystal structures. In comparison with E. coli FeoA, the deposited but as yet unpublished NMR structure of K. pneumonia FeoA (90% sequence identity) shows that both structures agree well with each other, with an RMSD of 2.2 Å (Fig. 2C). Of the seven structures, only the S. maltophilia FeoA crystal structure has been published with a suggested function as a bacterial ferrous iron transport-activating factor (32). Compared to E. coli FeoA, S. maltophilia FeoA possesses an additional helix (Fig. 2D). Despite their different sequences, the β-barrel structure is conserved, and thus this feature may be important for the function of this protein.
In eukaryotes, some SH3 domains are known to bind to G proteins through proline-rich sequences (PXXP), where they can regulate the enzymatic activity or interactions further down the signaling cascade (57–59). G proteins, depending on their nucleotide-bound state, can interact with different effectors to propagate or terminate signals. Intriguingly, the FeoB protein also possesses a cytoplasmic G protein in its N-terminal domain, which has led to the suggestion that the cytoplasmic FeoA protein can interact with the cytoplasmic G domain of FeoB (5). Furthermore, a bioinformatics analysis reveals that FeoA and FeoB occur together in one operon for 89% of all the Feo systems (Fig. 1B). In fact, in a limited number of cases FeoA also appears to be directly fused to FeoB, indicating that these proteins act in a spatially proximal manner (Fig. 1B). As suggested by Lee et al., the presence of two genes within the same operon is a strong indicator of intertwined biological roles (33). We first examined the potential for FeoA-FeoB interactions on the assumption that FeoA may act as a GAP for FeoB. Using 31P NMR, we established that the E. coli NFeoB (residues 1 to 276) construct possesses GTPase activity (Fig. 4); the observed GTP hydrolysis activity was extremely slow in the presence of sodium, as noted previously (Fig. 4A) (28, 29, 51). Interestingly, replacing sodium with potassium drastically increased the rate of GTP hydrolysis by NFeoB, similar to what was observed for S. thermophilus FeoB (Fig. 4) (51). It appears that E. coli NFeoB has a preference for potassium like its counterparts in other organisms. Potassium has a larger ionic radius than sodium and thus provides a more ideal geometry in the active site of the G protein for the hydrolysis of guanine nucleotides (51). Unexpectedly, our 31P NMR data indicate that no change is observed in the rate of GTP hydrolysis by NFeoB in vitro in the presence of FeoA. This result suggests that FeoA probably does not act as a GAP, as had been suggested (5), or that additional cofactors are required to induce an increased rate of hydrolysis. As previously noted, the affinity of NFeoB and the complete FeoB protein toward their guanine nucleotide substrates may vary depending on the construct being investigated (28). In particular, the transmembrane portion of FeoB could have a pronounced effect in regulating the affinity of the G domain toward its substrates, and, thus, its potential role in regulating protein-protein interactions with FeoA or other interacting species should not be disregarded. To this end, performing future in vitro experiments with full-length FeoB could provide additional insight toward the potential role of FeoA as a GAP. Be that as it may, E. coli NFeoB contains only one PXXP recognition site for typical SH3 domains, but this site is buried inside the protein. Moreover, the regular interactions would be disrupted by the insertion of the extra helix in FeoA compared to eukaryotic SH3 domains. Hence, FeoA and NFeoB binding, if any, could not occur in the same way as described for the eukaryotic SH3 domains.
Our NMR relaxation results indicate that some regions of the E. coli FeoA protein experience flexibility and chemical exchange (Fig. 3). In particular, slow conformational exchange is observed for several residues in β-strand 4 (Fig. 3). Val56 has the highest Rex value and is situated at the end of the distal loop which is sandwiched between β-strands 3 and 4 (Fig. 5A). The distal loop, as previously mentioned, is a defining feature of eukaryotic SH3 domains that is important for protein-protein interactions (55). With the high flexibility of this region observed in the NMR relaxation data, this suggests that this loop may be important for protein-protein interactions involving FeoA. Apart from V56 experiencing higher conformational exchange, it is also situated in one of the surface-exposed hydrophobic patches on FeoA (Fig. 6A), suggesting that it could partake in protein-protein interactions.
Fig 6.

Electrostatic surface potential plots of FeoA (PDB ID 2lx9) (A) and ScaR (PDB ID 3hru chain B) (B). Val56 of FeoA is highlighted in pink on the cartoon representation in panel A. The SH3-like domain of ScaR is highlighted in black in panel B. Positively charged regions are indicated in blue, while negatively charged regions are shown in red. Hydrophobic areas are represented in white. The orientation of the surface potential plot is represented by its corresponding cartoon depiction.
In an attempt to identify potential alternative functions for E. coli FeoA from its structure, we submitted the FeoA structure to the Dali server (60). The Dali server compares newly determined structures against the structures deposited in the PDB to identify structural homologues, which can lead to the establishment of potential evolutionary relationships (60). These results revealed similarity to streptococcal coaggregation regulator (ScaR) (PDB ID 3hru) (Z-score, 7.1), iron-dependent regulator (IdeR) (PDB ID 1u8r) (Z-score, 6.7), and diphtheria toxin repressor protein (DtxR) (PDB ID 1c0w) (Z-score, 5.6). These proteins are all part of the metal and DNA-binding protein families that function as transcriptional regulators (61–68). In addition, these proteins all have three domains: a DNA-binding, a dimerization, and an SH3-like domain (64, 69, 70), where the structure of FeoA resembles the last domain (see Fig. S3 in the supplemental material). Interestingly, primary sequence alignment of FeoA with these proteins reveals a low overall sequence identity (71, 72) despite the structural similarity. Figure S3 shows the superposed structures of FeoA with the structural homologues. FeoA aligns well with the SH3-like C-terminal domains of all three homologs, with overall RMSDs of 2.73 Å, 2.42 Å, and 2.83 Å for ScaR, IdeR, and DtxR, respectively (see Fig. S3). Structural alignment shows that most secondary structural elements and the global fold are conserved (Fig. 5B and E; see also Fig. S3). Minor differences exist, however; the core β-barrel structure that is reminiscent of SH3 domains is conserved.
All three of these structural homologues possess two metal binding sites with one residing in between the dimerization and SH3-like domains (Fig. 5B). The presence of metal ions signals these proteins to transition between the DNA-binding and nonbinding forms (61–70, 73). The SH3-like domains of IdeR and DtxR also play an important role in this conversion apart from sole metal binding. They interact with the other domains of the protein either on an intermolecular or intramolecular level that results in global conformational changes, allowing the binding or release of DNA (62, 63, 65–68, 74). Examining our solution NMR studies, done at high protein concentrations, has shown that FeoA is a monomeric protein, making it unlikely that it dimerizes noncovalently in a similar fashion to IdeR for function. Of the three homologues, the SH3-like domain of ScaR is only known to bind metal, with no specific function like the other two (64, 68). FeoA shares greater structural similarity between all three C-terminal domains of these proteins than with the prototypical SH3 domains (Fig. 5A and B; see also Fig. S3 in the supplemental material). Therefore, it is possible that it would bind metal ions and interact with other proteins to function as a metal-sensing transcriptional regulator.
To investigate this, we examined the metal binding sites of all three proteins and compared them to FeoA for similarity. The contributing ligands from the SH3-like domain of IdeR for the ancillary site are Glu172 and Gln175 (Fig. 5B and C). Gln175 is not present in the structural alignment between the two, while Glu172 aligns with Arg16 of FeoA, an acidic-to-basic substitution with an extended side chain (Fig. 5D and E). This is similar to the SH3-like domain of ScaR, which donates Asp160 for metal coordination in the secondary site, which corresponds to Arg16 of FeoA (Fig. 5E). Therefore, E. coli FeoA does not appear to have the appropriate ligands for metal ion coordination, in contrast to IdeR/DtxR/ScaR. The evidence for FeoA being a transcriptional regulator has not been established, and thus the reason for its structural similarity to these C-terminal SH3-like domains remain unclear. A surface potential plot of ScaR reveals that the only positively charged region is the DNA-binding domain, with the dimerization and SH3-like domains being acidic (Fig. 6B). In contrast, the surface of FeoA is mainly basic (Fig. 6). FeoA could perhaps interact with other proteins, similar to the C-terminal domain of ScaR/IdeR/DtxR, to function in a noncovalent transcriptional regulator complex.
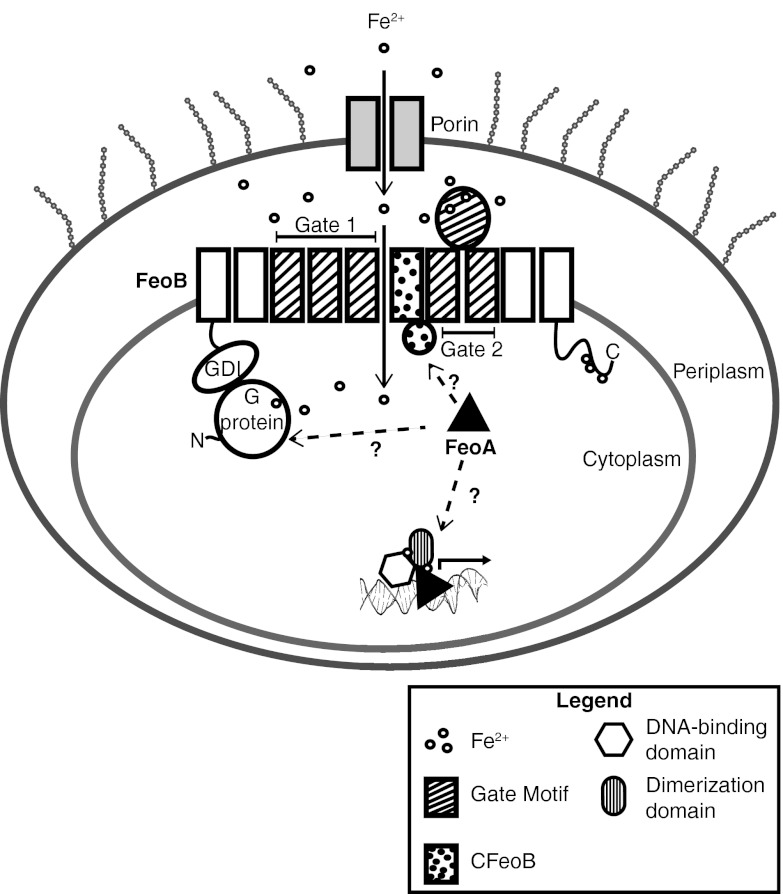
It is thought that ferrous iron diffuses into the periplasm through porins to be transported into the cell through the transmembrane domain of FeoB (Fig. 7) (5). The exact mechanism of uptake is not understood; however, two Gate motifs reminiscent of the Saccharomyces cerevisiae iron permease can be identified within the transmembrane domain (5, 75). It is generally assumed that ferrous iron is transported into the cell through the Gate motifs while the G domain relays information about the intracellular environment to the membrane. Although FeoA was first thought to function as a GAP, this has not been observed in our in vitro studies. Perhaps its function as a GAP in vivo could be dependent on other cofactor(s) which are absent under the conditions tested. However, it is equally possible that FeoA interacts with other highly conserved parts of FeoB. To explore other possible interacting regions of FeoB, its primary sequence was submitted to Pfam, a curated database of protein domain organizations (76). A Pfam analysis of FeoB indicates that the protein is composed of four domains: the N-terminal domain (NFeoB), the two Gate motifs, and another domain in between the two Gate motifs annotated as the C domain of FeoB (CFeoB; residues 457 to 507) (Fig. 7), which we have termed the core domain. Interestingly, part of the core CFeoB region and the carboxy-terminal region (residues 743 to 773) are predicted to form cytoplasmic domains, and these could both be potential alternative FeoA interaction partners (Fig. 7). The conserved core CFeoB region, which is present in all FeoB proteins, may play a role in bringing iron into the cytoplasm. The CFeoB core possesses His, Met, and Glu residues that could act as potential iron-coordinating ligands, and it is possible that FeoA interacts with this region to regulate the rate at which iron enters the cell (Fig. 7). The carboxy-terminal region of FeoB (residues 743 to 773) also has some characteristic features that could be important for iron binding, such as multiple cysteines that could form an iron sulfur cluster to move the iron away from the Gate regions (Fig. 7). However, this region, which is prominent in E. coli FeoB, is found only in a small number of FeoB proteins and therefore seems less likely to play a role in interacting with FeoA. Consequently, we propose that the conserved cytoplasmic core CFeoB (residues 457 to 507) region is another potential interaction partner for FeoA. The importance of the conserved core region to the regulation of the transport of Fe2+ through FeoB has not been emphasized before and merits further analysis.
Fig 7.
Proposed function for FeoA in E. coli ferrous iron transport. The striped transmembrane regions represent the Gate motifs while the dotted transmembrane regions represent the core CFeoB domain as represented in the Pfam database. FeoB is thought to take up iron through the Gate motifs while the G protein and the GDI domains signal to the membrane regions about the state of the cell or potentially provide energy for the membrane region for active transport. FeoA is thought to fulfill a dual role: (i) to take part in iron transport through interacting with FeoB and (ii) to form a regulatory complex with a transcriptional regulator.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a grant from the Novel Alternatives for Antibiotics program of the Canadian Institutes for Health Research. H.J.V. is the holder of an AHFMR-Scientist award from Alberta Innovates Health Solutions.
We express our gratitude to the late Deane McIntyre for his invaluable help with the 31P NMR experiments and his maintenance of the NMR instruments at the Bio-NMR Center. We also thank Byron Chu for critical reading and discussion of the manuscript.
Footnotes
Published ahead of print 26 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01121-12.
REFERENCES
- 1.Andrews SC, Robinson AK, Rodriguez-Quinones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 2.Braun V, Hantke K. 2011. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 15:328–334 [DOI] [PubMed] [Google Scholar]
- 3.Chu BC, Garcia-Herrero A, Johanson TH, Krewulak KD, Lau CK, Peacock RS, Slavinskaya Z, Vogel HJ. 2010. Siderophore uptake in bacteria and the battle for iron with the host: a bird's eye view. Biometals 23:601–611 [DOI] [PubMed] [Google Scholar]
- 4.Crichton RR. 2009. Iron metabolism: from molecular mechanisms to clinical consequences, 3rd ed. John Wiley & Sons Ltd., Chichester, United Kingdom [Google Scholar]
- 5.Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. 2006. Feo—transport of ferrous iron into bacteria. Biometals 19:143–157 [DOI] [PubMed] [Google Scholar]
- 6.Chu BC, Vogel HJ. 2011. A structural and functional analysis of type III periplasmic and substrate binding proteins: their role in bacterial siderophore and heme transport. Biol. Chem. 392:39–52 [DOI] [PubMed] [Google Scholar]
- 7.Krewulak KD, Vogel HJ. 2008. Structural biology of bacterial iron uptake. Biochim. Biophys. Acta 1778:1781–1804 [DOI] [PubMed] [Google Scholar]
- 8.Rong C, Huang Y, Zhang W, Jiang W, Li Y, Li J. 2008. Ferrous iron transport protein B gene (feoB1) plays an accessory role in magnetosome formation in Magnetospirillum gryphiswaldense strain MSR-1. Res. Microbiol. 159:530–536 [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Woodhall MR, Alvarez J, Cartron ML, Andrews SC. 2007. EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157:H7. Mol. Microbiol. 65:857–875 [DOI] [PubMed] [Google Scholar]
- 10.Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. 2005. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J. Bacteriol. 187:1604–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosse C, Scherer J, Koch D, Otto M, Taudte N, Grass G. 2006. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol. Microbiol. 62:120–131 [DOI] [PubMed] [Google Scholar]
- 12.Haemig HA, Moen PJ, Brooker RJ. 2010. Evidence that highly conserved residues of transmembrane segment 6 of Escherichia coli MntH are important for transport activity. Biochemistry 49:4662–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katoh H, Hagino N, Grossman AR, Ogawa T. 2001. Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 183:2779–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katoh H, Hagino N, Ogawa T. 2001. Iron-binding activity of FutA1 subunit of an ABC-type iron transporter in the cyanobacterium Synechocystis sp. Strain PCC 6803. Plant Cell Physiol. 42:823–827 [DOI] [PubMed] [Google Scholar]
- 15.Kehres DG, Janakiraman A, Slauch JM, Maguire ME. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065–1078 [DOI] [PubMed] [Google Scholar]
- 17.Perry RD, Mier I, Jr, Fetherston JD. 2007. Roles of the Yfe and Feo transporters of Yersinia pestis in iron uptake and intracellular growth. Biometals 20:699–703 [DOI] [PubMed] [Google Scholar]
- 18.Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. 2004. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect. Immun. 72:5522–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hantke K. 1987. Ferrous iron transport mutants in Escherichia coli K-12. FEMS Microbiol. Lett. 44:53–57 [DOI] [PubMed] [Google Scholar]
- 20.Kammler M, Schon C, Hantke K. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212–6219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hantke K. 2003. Is the bacterial ferrous iron transporter FeoB a living fossil? Trends Microbiol. 11:192–195 [DOI] [PubMed] [Google Scholar]
- 22.Spiro S, Guest JR. 1990. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol. Rev. 6:399–428 [DOI] [PubMed] [Google Scholar]
- 23.Bagg A, Neilands JB. 1987. Ferric uptake regulation protein acts as a repressor, employing iron(Ii) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471–5477 [DOI] [PubMed] [Google Scholar]
- 24.Jeon J, Kim H, Yun J, Ryu S, Groisman EA, Shin D. 2008. RstA-promoted expression of the ferrous iron transporter FeoB under iron-replete conditions enhances Fur activity in Salmonella enterica. J. Bacteriol. 190:7326–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A. 2006. Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect. Immun. 74:5433–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robey M, Cianciotto NP. 2002. Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect. Immun. 70:5659–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velayudhan J, Hughes NJ, McColm AA, Bagshaw J, Clayton CL, Andrews SC, Kelly DJ. 2000. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 37:274–286 [DOI] [PubMed] [Google Scholar]
- 28.Eng ET, Jalilian AR, Spasov KA, Unger VM. 2008. Characterization of a novel prokaryotic GDP dissociation inhibitor domain from the G protein coupled membrane protein FeoB. J. Mol. Biol. 375:1086–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marlovits TC, Haase W, Herrmann C, Aller SG, Unger VM. 2002. The membrane protein FeoB contains an intramolecular G protein essential for Fe(II) uptake in bacteria. Proc. Natl. Acad. Sci. U. S. A. 99:16243–16248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung KW, Juan TH, Hsu YL, Huang TH. 2012. NMR structure note: the ferrous iron transport protein C (FeoC) from Klebsiella pneumoniae. J. Biomol. NMR 53:161–165 [DOI] [PubMed] [Google Scholar]
- 31.Gajiwala KS, Burley SK. 2000. Winged helix proteins. Curr. Opin. Struct. Biol. 10:110–116 [DOI] [PubMed] [Google Scholar]
- 32.Su YC, Chin KH, Hung HC, Shen GH, Wang AH, Chou SH. 2010. Structure of Stenotrophomonas maltophilia FeoA complexed with zinc: a unique prokaryotic SH3-domain protein that possibly acts as a bacterial ferrous iron-transport activating factor. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66:636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee D, Redfern O, Orengo C. 2007. Predicting protein function from sequence and structure. Nat. Rev. Mol. Cell Biol. 8:995–1005 [DOI] [PubMed] [Google Scholar]
- 34.Butt TR, Edavettal SC, Hall JP, Mattern MR. 2005. SUMO fusion technology for difficult-to-express proteins. Protein Expr. Purif 43:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazaki T, Forman-Kay JD, Kay LE. 1993. Two-dimensional NMR experiments for correlating 13Cβ and 1Hδ/ε chemical shifts of aromatic residues in 13C-labeled proteins via scalar couplings. J. Am. Chem. Soc. 115:11054–11055 [Google Scholar]
- 36.Farrow NA, Muhandiram R, Singer AU, Pascal SM, Kay CM, Gish G, Shoelson SE, Pawson T, Forman-Kay JD, Kay LE. 1994. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry 33:5984–6003 [DOI] [PubMed] [Google Scholar]
- 37.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6:277–293 [DOI] [PubMed] [Google Scholar]
- 38.Johnson BA, Blevins RA. 1994. NMR view: a computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4:603–614 [DOI] [PubMed] [Google Scholar]
- 39.Guntert P. 2004. Automated NMR structure calculation with CYANA. Methods Mol. Biol. 278:353–378 [DOI] [PubMed] [Google Scholar]
- 40.Shen Y, Delaglio F, Cornilescu G, Bax A. 2009. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. 2003. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 160:65–73 [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharya A, Tejero R, Montelione GT. 2007. Evaluating protein structures determined by structural genomics consortia. Proteins 66:778–795 [DOI] [PubMed] [Google Scholar]
- 43.Koradi R, Billeter M, Wuthrich K. 1996. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14:51–55, 29–32 [DOI] [PubMed] [Google Scholar]
- 44.Dutta K, Cox CJ, Basavappa R, Pascal SM. 2008. 15N relaxation studies of Apo-Mts1: a dynamic S100 protein. Biochemistry 47:7637–7647 [DOI] [PubMed] [Google Scholar]
- 45.Inman KG, Baldisseri DM, Miller KE, Weber DJ. 2001. Backbone dynamics of the calcium-signaling protein apo-S100B as determined by 15N NMR relaxation. Biochemistry 40:3439–3448 [DOI] [PubMed] [Google Scholar]
- 46.Tjandra N, Wingfield P, Stahl S, Bax A. 1996. Anisotropic rotational diffusion of perdeuterated HIV protease from 15N NMR relaxation measurements at two magnetic fields. J. Biomol. NMR 8:273–284 [DOI] [PubMed] [Google Scholar]
- 47.Lipari G, Szabo A. 1982. Model-free approach to the interpretation of nuclear magnetic-resonance relaxation in macromolecules. 1. Theory and range of validity. J. Am. Chem. Soc. 104:4546–4559 [Google Scholar]
- 48.Lipari G, Szabo A. 1982. Model-free approach to the interpretation of nuclear magnetic-resonance relaxation in macromolecules. 2. Analysis of experimental results. J. Am. Chem. Soc. 104:4559–4570 [Google Scholar]
- 49.Cole R, Loria JP. 2003. FAST-Modelfree: a program for rapid automated analysis of solution NMR spin-relaxation data. J. Biomol. NMR 26:203–213 [DOI] [PubMed] [Google Scholar]
- 50.Palmer AG, Rance M, Wright PE. 1991. Intramolecular motions of a zinc finger DNA-binding domain from Xfin characterized by proton-detected natural abundance C-12 heteronuclear NMR-spectroscopy. J. Am. Chem. Soc. 113:4371–4380 [Google Scholar]
- 51.Ash MR, Guilfoyle A, Clarke RJ, Guss JM, Maher MJ, Jormakka M. 2010. Potassium-activated GTPase reaction in the G Protein-coupled ferrous iron transporter B. J. Biol. Chem. 285:14594–14602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li SS. 2005. Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction. Biochem. J. 390:641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu H, Rosen MK, Shin TB, Seidel-Dugan C, Brugge JS, Schreiber SL. 1992. Solution structure of the SH3 domain of Src and identification of its ligand-binding site. Science 258:1665–1668 [DOI] [PubMed] [Google Scholar]
- 54.Krissinel E, Henrick K. 2004. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60:2256–2268 [DOI] [PubMed] [Google Scholar]
- 55.D'Aquino JA, Ringe D. 2003. Determinants of the SRC homology domain 3-like fold. J. Bacteriol. 185:4081–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuriyan J, Cowburn D. 1997. Modular peptide recognition domains in eukaryotic signaling. Annu. Rev. Biophys. Biomol. Struct. 26:259–288 [DOI] [PubMed] [Google Scholar]
- 57.Booker GW, Gout I, Downing AK, Driscoll PC, Boyd J, Waterfield MD, Campbell ID. 1993. Solution structure and ligand-binding site of the SH3 domain of the p85 alpha subunit of phosphatidylinositol 3-kinase. Cell 73:813–822 [DOI] [PubMed] [Google Scholar]
- 58.Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, et al. 1993. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell 75:25–36 [PubMed] [Google Scholar]
- 59.Scaife RM, Margolis RL. 1997. The role of the PH domain and SH3 binding domains in dynamin function. Cell Signal. 9:395–401 [DOI] [PubMed] [Google Scholar]
- 60.Holm L, Rosenstrom P. 2010. Dali server: conservation mapping in 3D. Nucleic Acids Res. 38:W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakubovics NS, Smith AW, Jenkinson HF. 2000. Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140–153 [DOI] [PubMed] [Google Scholar]
- 62.Schmitt MP, Holmes RK. 1991. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect. Immun. 59:1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmitt MP, Twiddy EM, Holmes RK. 1992. Purification and characterization of the diphtheria toxin repressor. Proc. Natl. Acad. Sci. U. S. A. 89:7576–7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoll KE, Draper WE, Kliegman JI, Golynskiy MV, Brew-Appiah RA, Phillips RK, Brown HK, Breyer WA, Jakubovics NS, Jenkinson HF, Brennan RG, Cohen SM, Glasfeld A. 2009. Characterization and structure of the manganese-responsive transcriptional regulator ScaR. Biochemistry 48:10308–10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tai SP, Krafft AE, Nootheti P, Holmes RK. 1990. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb. Pathog. 9:267–273 [DOI] [PubMed] [Google Scholar]
- 66.Tao X, Boyd J, Murphy JR. 1992. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc. Natl. Acad. Sci. U. S. A. 89:5897–5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang G, Wylie GP, Twigg PD, Caspar DL, Murphy JR, Logan TM. 1999. Solution structure and peptide binding studies of the C-terminal src homology 3-like domain of the diphtheria toxin repressor protein. Proc. Natl. Acad. Sci. U. S. A. 96:6119–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wylie GP, Rangachari V, Bienkiewicz EA, Marin V, Bhattacharya N, Love JF, Murphy JR, Logan TM. 2005. Prolylpeptide binding by the prokaryotic SH3-like domain of the diphtheria toxin repressor: a regulatory switch. Biochemistry 44:40–51 [DOI] [PubMed] [Google Scholar]
- 69.Feese MD, Ingason BP, Goranson-Siekierke J, Holmes RK, Hol WG. 2001. Crystal structure of the iron-dependent regulator from Mycobacterium tuberculosis at 2.0-Å resolution reveals the Src homology domain 3-like fold and metal binding function of the third domain. J. Biol. Chem. 276:5959–5966 [DOI] [PubMed] [Google Scholar]
- 70.Qiu X, Pohl E, Holmes RK, Hol WG. 1996. High-resolution structure of the diphtheria toxin repressor complexed with cobalt and manganese reveals an SH3-like third domain and suggests a possible role of phosphate as co-corepressor. Biochemistry 35:12292–12302 [DOI] [PubMed] [Google Scholar]
- 71.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. 2010. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38:W695–W699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 73.Pohl E, Holmes RK, Hol WG. 1999. Crystal structure of a cobalt-activated diphtheria toxin repressor-DNA complex reveals a metal-binding SH3-like domain. J. Mol. Biol. 292:653–667 [DOI] [PubMed] [Google Scholar]
- 74.Liu C, Mao K, Zhang M, Sun Z, Hong W, Li C, Peng B, Chang Z. 2008. The SH3-like domain switches its interaction partners to modulate the repression activity of mycobacterial iron-dependent transcription regulator in response to metal ion fluctuations. J. Biol. Chem. 283:2439–2453 [DOI] [PubMed] [Google Scholar]
- 75.Severance S, Chakraborty S, Kosman DJ. 2004. The Ftr1p iron permease in the yeast plasma membrane: orientation, topology and structure-function relationships. Biochem. J. 380:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD. 2012. The Pfam protein families database. Nucleic Acids Res. 40:D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.