Abstract
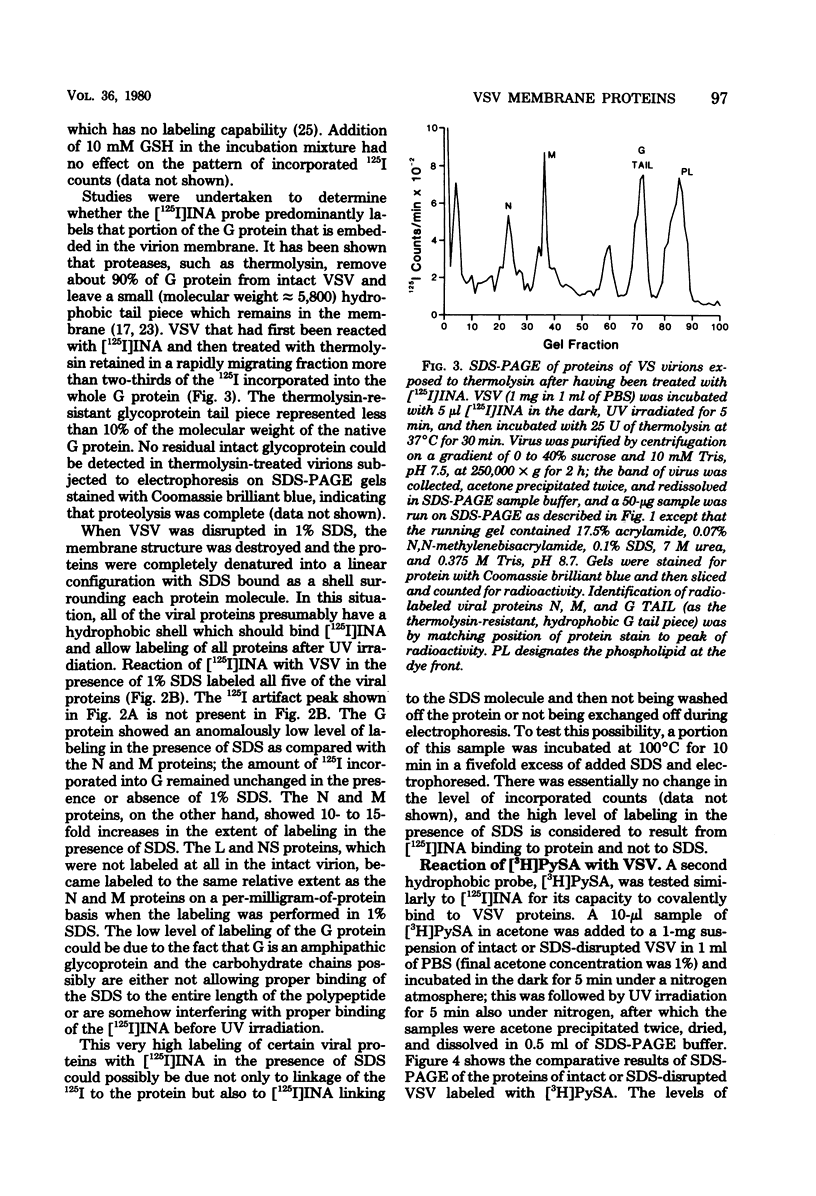
The location of membrane-associated proteins of vesicular stomatitis virus was investigated by using two monofunctional and three bifunctional probes that differ in the degree to which they partition into membranes and in their specific group reactivity. Two hydrophobic aryl azide probes, [125I]5-iodonaphthyl-1-azide and [3H]pyrenesulfonylazide, readily partitioned into virion membrane and, when activated to nitrenes by UV irradiation, formed stable covalent adducts to membrane constituents. Both of these monofunctional probes labeled the glyco-protein G and matrix M proteins, but [125I]5-iodonaphthyl-1-azide also labeled the nucleocapsid N protein and an unidentified low-molecular-weight component. Protein labeling of intact virions was unaffected by the presence of cytochrome c or glutathione, but disruption of membrane by sodium dodecyl sulfate greatly enhanced the labeling of all viral proteins except G. Labeling of G protein was essentially restricted to the membrane-embedded, thermolysin-resistant tail fragment. Three bifunctional reagents, tartryl diazide, dimethylsuberimidate, and 4,4′-dithiobisphenylazide, were tested for their capacity to cross-link proteins to membrane phospholipids of virions grown in the presence of [3H]palmitate. Only G and M proteins of intact virions were labeled with 3H-phospholipid by these cross-linkers; the reactions were not affected by cytochrome c but were abolished by disruption of virus with sodium dodecyl sulfate. Dimethylsuberimidate, which reacts with free amino groups, cross-linked 3H-phospholipid to both G and M protein. In contrast, the hydrophilic tartryl diazide cross-linked phospholipid primarily to the M protein, whereas the hydrophobic 4,4′-dithiobisphenylazide cross-linked phospholipid primarily to the intrinsic G protein. These data support the hypothesis that the G protein traverses the virion membrane and that the M protein is membrane associated but does not penetrate very deeply, if at all.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley H., Knowles J. R. Photogenerated reagents for membrane labeling. 1. Phenylnitrene formed within the lipid bilayer. Biochemistry. 1978 Jun 13;17(12):2414–2419. doi: 10.1021/bi00605a025. [DOI] [PubMed] [Google Scholar]
- Bercovici T., Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978 Apr 18;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- Bisson R., Montecucco C., Gutweniger H., Azzi A. Cytochrome c oxidase subunits in contact with phospholipids. Hydrophobic photolabeling with azidophospholipids. J Biol Chem. 1979 Oct 25;254(20):9962–9965. [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Inhibition of transcription by immunoglobulins directed against the ribonucleoprotein of homotypic and heterotypic vesicular stomatitis viruses. J Virol. 1978 Feb;25(2):675–684. doi: 10.1128/jvi.25.2.675-684.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerletti N., Schatz G. Cytochrome c oxidase from bakers' yeast. Photolabeling of subunits exposed to the lipid bilayer. J Biol Chem. 1979 Aug 25;254(16):7746–7751. [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U. Vesicular stomatitis virus: structure and function of virion components. Curr Top Microbiol Immunol. 1976;73:1–34. doi: 10.1007/978-3-642-66306-2_1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Specific incorporation of host cell surface proteins into budding vesicular stomatitis virus particles. Cell. 1980 Jan;19(1):161–169. doi: 10.1016/0092-8674(80)90397-9. [DOI] [PubMed] [Google Scholar]
- Lutter L. C., Ortanderl F., Fasold H. The use of a new series of cleavable protein-crosslinkers on the Escherichia coli ribosome. FEBS Lett. 1974 Nov 15;48(2):288–292. doi: 10.1016/0014-5793(74)80488-6. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. doi: 10.1128/jvi.7.1.59-70.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F. Photoactivated cross-linking of proteins within the erythrocyte membrane core. J Biol Chem. 1976 Dec 10;251(23):7413–7416. [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Patzer E. J., Wagner R. R., Dubovi E. J. Viral membranes: model systems for studying biological membranes. CRC Crit Rev Biochem. 1979;6(2):165–217. doi: 10.3109/10409237909102563. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979 Jun 10;254(11):4313–4316. [PubMed] [Google Scholar]
- Sator V., Gonzalez-Ros J. M., Calvo-Fernandez P., Martinez-Carrion M. Pyrenesulfonyl azide: a marker of acetylcholine receptor subunits in contact with membrane hydrophobic environment. Biochemistry. 1979 Apr 3;18(7):1200–1206. doi: 10.1021/bi00574a013. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Staros J. V., Bayley H., Standring D. N., Knowles J. R. Reduction of aryl azides by thiols: implications for the use of photoaffinity reagents. Biochem Biophys Res Commun. 1978 Feb 14;80(3):568–572. doi: 10.1016/0006-291x(78)91606-6. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Schreiber C., Scheefers H. Lipids with photosensitive groups as chemical probes for the structural analysis of biological membranes. On the localization of the G- and M-protein of vesicular stomatitis virus. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):923–931. doi: 10.1515/bchm2.1978.359.2.923. [DOI] [PubMed] [Google Scholar]



