Abstract
The filamentous cyanobacterium Anabaena fixes nitrogen in specialized cells called heterocysts. The immediate product of fixation, ammonia, is known to be assimilated by addition to glutamate to make glutamine. How fixed nitrogen is transported along the filament to the 10 to 20 vegetative cells that separate heterocysts is unknown. N-fixing heterocysts accumulate an insoluble polymer containing aspartate and arginine at the cell poles. Lockau's group has proposed that the polymer is degraded at the poles to provide a mobile carrier, arginine, to the vegetative cells (R. Richter, M. Hejazi, R. Kraft, K. Ziegler, and W. Lockau, Eur. J. Biochem. 263:163–169, 1999). We wished to use the Sakaguchi reaction for arginine to determine the relative cellular concentration of arginine along the filament. At present, the methods for measuring absorption of the Sakaguchi reaction product at 520 nm are insufficiently sensitive for that purpose. However, that product quenches the fluorescence of phycobiliproteins, which we have adapted to a determination of arginine. Our results are consistent with the proposal that arginine is a principal nitrogen carrier from heterocysts to vegetative cells in Anabaena.
INTRODUCTION
The filamentous cyanobacterium Anabaena sp. strain PCC 7120 fixes nitrogen in specialized cells called heterocysts, which differentiate from oxygenic vegetative cells at regular intervals along the filaments under conditions of nitrogen deprivation. The heterocyst is essentially a factory for reduction of atmospheric nitrogen (1–3). To do this, the cell synthesizes large amounts of the nitrogenase complex in addition to electron carriers that feed the enzyme with low-potential electrons and protons. The normal pathways of carbon fixation are inactivated in these specialized cells. Instead, carbon stores such as glycogen are consumed first, and then sucrose is brought in from neighboring vegetative cells where photosynthesis continues. The sucrose is converted to hexoses by invertase (4), and these are metabolized to provide reduced pyridine nucleotides for several purposes: the synthesis of glycolipids and polysaccharides to make the protective outer envelope and the reduction of the electron carriers to nitrogenase. Carbon is also needed for the carriers that shuttle newly fixed nitrogen to the vegetative cells. These carriers have been suggested to be 2-oxo-glutarate, glutamate, glutamine, aspartate, and arginine. The product of nitrogen fixation, ammonia, is added to glutamate to make glutamine; that much has been established by studies of the incorporation of radioactive N2 (5, 6). How the heterocyst acquires enough glutamate is still a mystery (2). Some of the glutamine may be transported directly to the neighboring vegetative cells. Most of it, particularly at high light intensity when nitrogen fixation is rapid, is converted to arginine, which is then polymerized into the storage polymer cyanophycin. The latter insoluble material has a backbone of polyaspartate; the arginine is bound to the polymer by isopeptide linkage to the carboxyl group of each aspartate side chain (7). The cyanophycin accumulates at the poles of the heterocyst where, in both phase-contrast micrographs and electron micrographs, it appears to form polar plugs. However, these plugs appear to penetrate the membranes separating the two cell types (J. Austin, Z. Ye, and R. Haselkorn, unpublished data). Enzymes exist that specifically break down cyanophycin to yield dipeptides and degrade the dipeptides to aspartate and arginine, so it is possible that cyanophycin provides the major mechanism for transport of fixed nitrogen from the heterocyst to the vegetative cell (8, 9).
The Sakaguchi reaction is a colorimetric reaction for identification and quantitation of guanidino groups, involving reaction with naphthol and sodium hypochlorite, providing a qualitative test for arginine that is free or in protein (10). This reaction was used by Fogg to show that heterocysts contain arginine (11). The absorption spectrum of the Sakaguchi reaction product ranges from 400 to 600 nm with a maximum at 520 nm. Based on this absorption, Simon developed an assay to determine the amount of cyanophycin granule polypeptide in Anabaena cylindrica Lemm (7). His in vitro data support the view that the cyanophycin granule is a cellular nitrogen reserve.
In this work, we focus on the intercellular transport of fixed nitrogen between each pair of neighboring cells, especially between heterocyst and vegetative cell, by treating Anabaena sp. strain PCC 7120 filaments with Sakaguchi reagents and measuring the resulting fluorescence emission spectra at the cellular interfaces. Anabaena PCC 7120 filaments were excited at 488 nm, and emission was measured from 500 to 580 nm. At this wavelength range, the fluorescence emission is due to phycobiliproteins assembled for the most part in phycobilisomes (12). Absorbance is extremely difficult to measure at the single-cell level (13), but we reasoned that the absorbance of the Sakaguchi reaction product would quench the fluorescence at 520 nm, so the concentration of arginine might be estimated based on the degree of quenching at that wavelength.
MATERIALS AND METHODS
Anabaena sp. strain PCC 7120 cultures were grown in BG11 medium under room light (∼9 microeinsteins/m2/s) and temperature (∼24°C). Filaments of Anabaena sp. strain PCC 7120 were centrifuged to remove BG11 medium and immediately treated with the Sakaguchi reagents for 10 min. The 1-ml Sakaguchi reaction solution was prepared by mixing 268 μl 8% Ba(OH)2, 29 μl 5% NaClO, 143 μl 1% dichloronaphthol, and 560 μl H2O. After treatment, the filament was placed on a slide with a coverslip, and emission spectra were acquired using a Leica SP5 Tandem Scanner Spectral 2-Photon confocal microscope with a pinhole value of 1.3 arbitrary units (AU). The focus thickness along the z axis is about 4.5 μm. The samples were excited at 488 nm, and emission was measured from 500 to 580 nm.
RESULTS AND DISCUSSION
We show first the consequences for the fluorescence emission spectra of Anabaena filaments after treating them with the Sakaguchi reagents. We then apply this method for assessing the concentration of arginine along a differentiated filament. The filaments with only vegetative cells were also monitored for comparison. Finally, we discuss possible interpretations of the observed gradient of arginine whose concentration decreases with distance from the heterocyst.
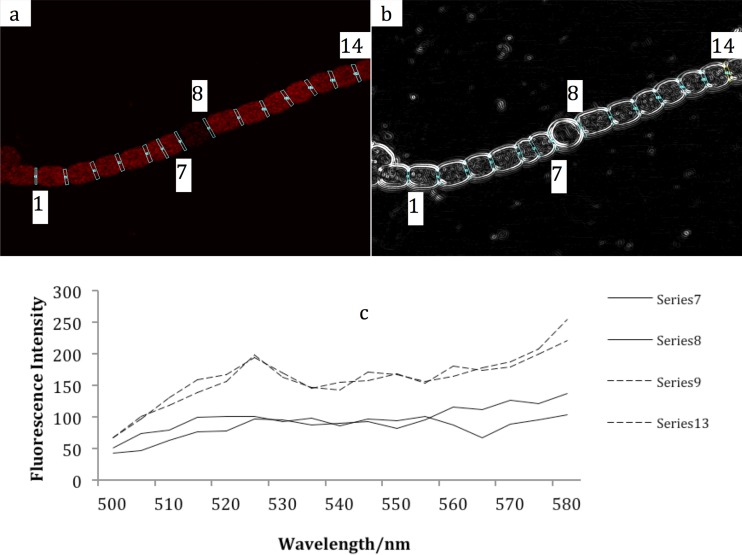
Figures 1a and b show the differential interference contrast (DIC) images and fluorescence of an Anabaena sp. strain PCC 7120 filament with a heterocyst. Sampling areas for fluorescence spectroscopy were selected at the interface between two cells, shown by the blue outlines in each figure. The emission spectra shown in Fig. 1c as spectra 7 and 8 are from interfaces between a heterocyst and two vegetative cells, while spectra 9 and 13 are from the interfaces between two vegetative cells. Other spectra from vegetative cell pairs were similar to the ones shown. The fluorescence intensity from the heterocyst is less than that from the vegetative cells, which is expected because of the degradation of phycobilisomes in the heterocyst. Each spectrum is the mean of 32 scans.
Fig 1.
Fluorescence of an Anabaena sp. strain PCC 7120 filament without Sakaguchi treatment. The sampling positions were chosen to show the intercellular connection of each pair of neighboring cells. (a) Fluorescence image; (b) DIC image. (c) The fluorescence spectra were obtained by excitation at 488 nm and scanning from 500 to 580 nm with 5 nm/step. Each series is the mean from 32 interfaces at the same position relative to a heterocyst.
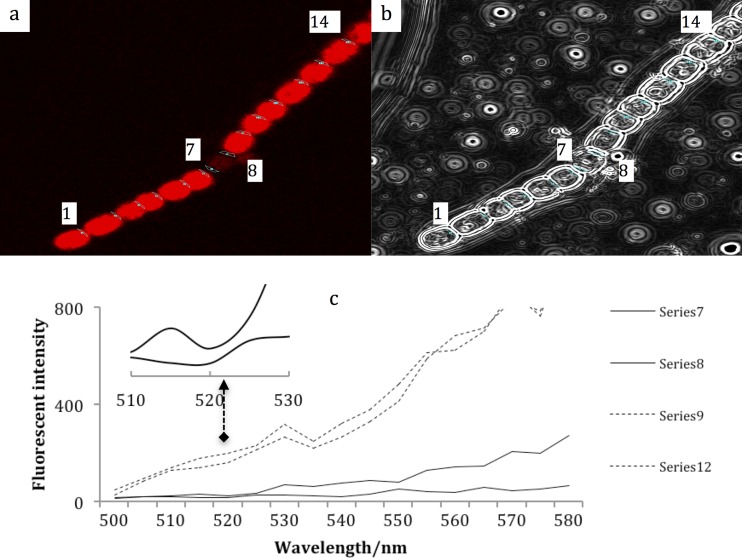
Figure 2 shows the DIC images and fluorescence of an Anabaena sp. strain PCC 7120 filament following Sakaguchi treatment. The spectra shown in Fig. 2c as 7 and 8 are from the connections between the heterocyst and vegetative cell, while 9 and 12 are from the connections between two vegetative cells. Compared to Fig. 1, the fluorescence intensity from the heterocyst is much lower than that from vegetative cells. The fluorescence intensity of the vegetative cells following Sakaguchi treatment is higher than that before the Sakaguchi treatment, possibly because the Sakaguchi reagents damage the cell membrane. However, an intensity decrease was clearly observed in the fluorescence spectra from 515 to 525 nm in series 7 and 8, that is, the connections between heterocysts and vegetative cells.
Fig 2.
Fluorescence of Anabaena sp. strain PCC 7120 filament following Sakaguchi treatment. The sampling positions were chosen as described for Fig. 1, as were the variables for measuring fluorescence. Note that spectra 6 and 7 measure the same interface between vegetative cell and heterocyst. The inset shows the enlarged fluorescence emission spectra around 520 nm.
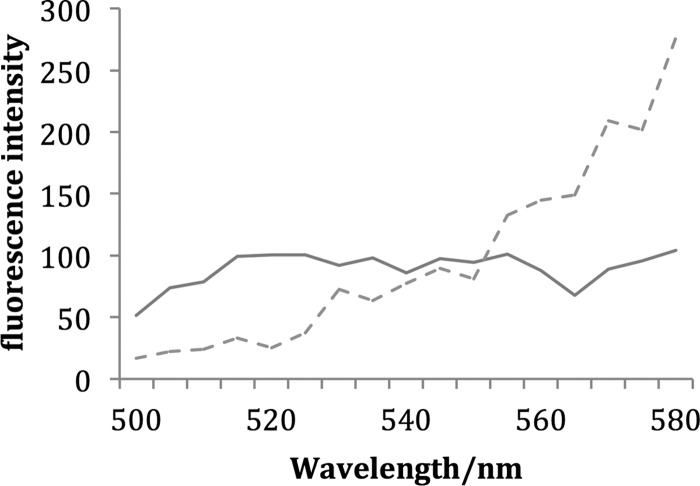
Comparison of the fluorescence spectra obtained from heterocysts with and without Sakaguchi treatment is shown in Fig. 3. Except for the region around the Sakaguchi product absorption, the fluorescence intensity from the heterocyst following Sakaguchi treatment is greater than that prior to treatment. We interpret these results to mean that arginine is abundant at the interface between the heterocyst and the vegetative cell.
Fig 3.
Fluorescence emission from the interface between the heterocyst and vegetative cell following (dashed line) and without (solid line) Sakaguchi treatment.
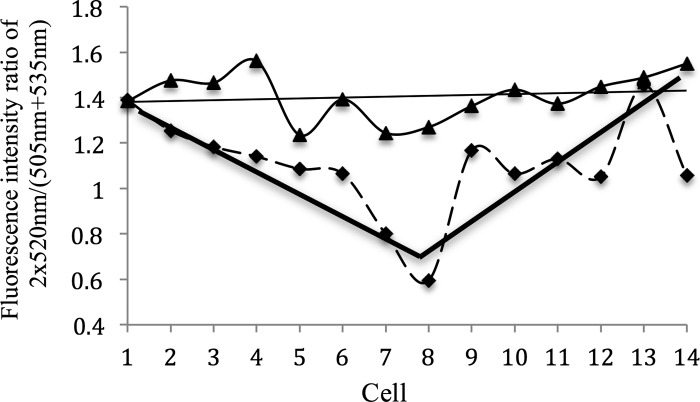
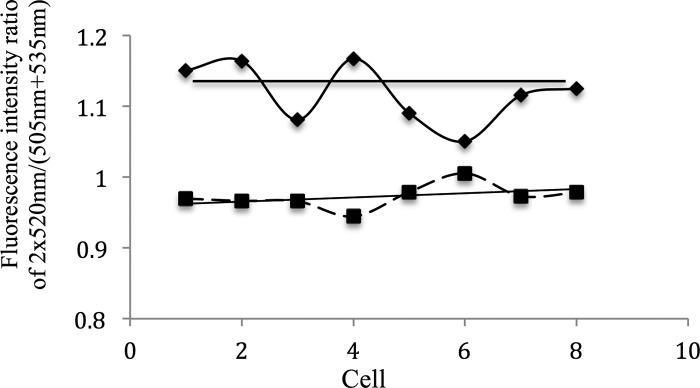
During nitrogen deprivation, the products of nitrogen fixation, such as arginine (as well as regulatory peptides and perhaps even proteins), are transported from the heterocysts to the neighboring vegetative cells and continue to other vegetative cells along the filament. Based on the discussion above, if a filament is treated with Sakaguchi reagents, the fluorescence intensity around 520 nm might increase as a function of the distance from the heterocyst. To reduce errors from the background, the ratio of the fluorescence intensity at 520 nm to the average fluorescence intensity from 505 and 535 nm was used as a parameter to monitor the fluorescence intensity. This ratio is shown in Fig. 4. Cells 7 and 8 in the x axis represent the two ends of the heterocyst. Cells 6 and 9 are the vegetative cells closest to the heterocyst along the filament, while cells 1 and 14 were most distant from the heterocyst.
Fig 4.
Fluorescence intensity ratio of the fluorescence (520 nm) to the wavelength (505 to +535 nm) along a differentiated filament. Each cell along the filament was monitored with (dashed line) and without (solid line) Sakaguchi treatment.
In the filament without Sakaguchi treatment, no trend is identified from the fluorescence along the filament. On the contrary, in the treated filament, the heterocyst has the lowest ratio, which we attribute to arginine absorption. Furthermore, a trend exists, namely, the ratio increases as a function of distance of cells away from the heterocyst. This result is consistent with a concentration gradient of arginine decreasing with distance from the heterocyst along the filament.
This arginine concentration trend requires differentiation. Anabaena sp. strain PCC 7120 filaments grown on complete medium (BG11) were also monitored following Sakaguchi treatment. The decrease of fluorescence emission at 520 nm following Sakaguchi treatment was identified again as shown in Fig. 5. The Anabaena sp. strain PCC 7120 filaments without differentiation and Sakaguchi treatment have higher fluorescence emission intensities at 520 nm than do filaments following Sakaguchi treatment, but no positional trend is observed in either case.
Fig 5.
Fluorescence intensity ratio of the fluorescence (520 nm) to the wavelength (505 to +535 nm) along an undifferentiated filament. Each vegetative cell along the filament was monitored with (dashed line) and without (solid line) Sakaguchi treatment.
Even though single-cell absorption might measure the concentration of arginine directly through the Sakaguchi reaction, the indirect method, depending on the ability of the Sakaguchi product to quench fluorescence from phycobiliproteins, provides higher resolution and sensitivity. The fluorescence emission intensity is about 1,000 times higher than that of absorption, with a corresponding higher signal-to-noise ratio.
We consider three possibilities for the gradient observed. One is the polymer cyanophycin, which is made up of aspartate and arginine and has been located at the poles of heterocysts by both phase-contrast and electron microscopy (14). To date the evidence from electron microscopy locates the polymer solely in the heterocysts; there is none or very little in vegetative cells in cultures growing on N2 as the nitrogen source. If the quenching is due to the polymer alone, then the fluorescence intensity at vegetative cell interfaces should be constant, but they are not, as shown in Fig. 4. Instead, the fluorescence intensity increases with distance from the heterocyst-vegetative cell interface. That increase is not likely to be due to cyanophycin.
A second possibility is a gradient of the arginine-containing peptide RGSGR, the product of the patS gene that regulates the activity of HetR. The peptide is synthesized in heterocysts as part of the PatS protein, and its gradient along the filament has been shown by Risser and Callahan (15). The third possibility is arginine itself, which is stored in cyanophycin and released where the heterocyst and vegetative cell meet by cyanophycinase, according to the model proposed by Lockau's group (8, 9).
All of the enzymes needed for the conversion of glutamine to arginine except one were demonstrated by in vitro assay to be present in heterocysts of Anabaena by Gupta and Carr (16). The missing enzyme was subsequently found, and its coding sequence exists in the genome sequence of Anabaena sp. strain 7120 (17). It makes ultimate good sense to have the immediate product of nitrogen fixation converted to arginine and polymerized, thereby removing it from solution, where it might participate in feedback or repressive actions to limit further fixation and assimilation of nitrogen. Following Lockau, we believe that the polymer is solubilized and the dipeptide product is split into aspartate and arginine, the former returned to the heterocyst to provide the polymer backbone while the arginine is transported along the filament from one vegetative cell to another. Arginine, with its four nitrogens, is an efficient nitrogen carrier.
ACKNOWLEDGMENTS
This research was supported in part by the University of Chicago and the Ellison Medical Foundation.
We thank V. Bindokas for assistance with the fluorescence measurements.
Footnotes
Published ahead of print 19 October 2012
REFERENCES
- 1. Flores E, Herrero A. 2010. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 8: 39–50 [DOI] [PubMed] [Google Scholar]
- 2. Haselkorn R. 2007. Heterocyst differentiation and nitrogen fixation in cyanobacteria, p 233–255 In Elmerich C, Newton WE. (ed), Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Springer, Dordrecht, Netherlands [Google Scholar]
- 3. Kumar K, Mella-Herrera R, Golden J. 2010. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2: a000315 doi:10.1101/cshperspect.a000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vargas W, Cumino A, Salerno GL. 2003. Cyanobacterial alkaline/neutral invertases. Origin of sucrose hydrolysis in the plant cytosol? Planta 216: 951–960 [DOI] [PubMed] [Google Scholar]
- 5. Thomas J, Meeks JC, Wolk Shaffer CPPW, Austin SM, Chien WS. 1977. Formation of glutamine from [13N]ammonia, [13N]dinitrogen and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J. Bacteriol. 129: 1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas J, Wolk CP, Shaffer PW, Austin SM, Galonsky A. 1975. The initial organic products of fixation of 13N-labelled nitrogen gas by the blue-green alga Anabaena cylindrica. Biochem. Biophys. Res. Commun. 67: 501–507 [DOI] [PubMed] [Google Scholar]
- 7. Simon RD. 1973. Measurement of the cyanophycin granule polypeptide contained in the blue-green alga Anabaena cylindrica. J. Bacteriol. 114: 1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richter R, Hejazi M, Kraft R, Ziegler K, Lockau W. 1999. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartic acid (cyanophycin): molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur. J. Biochem. 263: 163–169 [DOI] [PubMed] [Google Scholar]
- 9. Ziegler K, Diener A, Herpin C, Richter R, Deutzmann R, Lockau W. 1998. Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartate (cyanophycin). Euro. J. Biochem. 254: 154–159 [DOI] [PubMed] [Google Scholar]
- 10. Deitch AD. 1961. An improved Sakaguchi reaction for microspectrophotometric use. J. Histochem. Cytochem. 9: 477–483 [DOI] [PubMed] [Google Scholar]
- 11. Fogg GE. 1949. Growth and heterocyst production in Anabaena cylindrica. II. In relation to carbon and nitrogen metabolism. Ann. Botany 13: 241–259 [Google Scholar]
- 12. Gray B, Gantt E. 1975. Spectral properties of phycobilisomes and phycobiliproteins from the blue-green alga Nostoc. Photochem. Photobiol. 21: 121–128 [DOI] [PubMed] [Google Scholar]
- 13. Sugiura K, Itoh S. 2012. Single-cell confocal spectrometry of a filamentous cyanobacterium nostoc at room and cryogenic temperature. Diversity and differentiation of pigment systems in 311 cells. Plant Cell Physiol. 53: 1492–1506 [DOI] [PubMed] [Google Scholar]
- 14. Black K, Buikema W, Haselkorn R. 1995. The hglK gene is required for localization of heterocyst glycolipids in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 177: 6440–6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Risser D, Callahan S. 2009. Genetic and cytological evidence that heterocyst patterning is regulated by inhibitor gradients that promote activator decay. Proc. Natl. Acad. Sci. U. S. A. 106: 19884–19888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta M, Carr NG. 1981. Enzyme activities related to cyanophycin metabolism in heterocysts and vegetative cells of Anabaena spp. J. Gen. Microbiol. 125: 17–23 [Google Scholar]
- 17. Kaneko T, Nakamural Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A, Iriguchi Ishikawa MA, Kawashima K, Kimura T, Kishida Y, Kohara M, Matsumoto M, Matsuno A, Muraki A, Nakazaki N, Shimpo S, Sugimoto M, Takazawa M, Yamada M, Yasuda M, Tabata S. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8: 227–253 [DOI] [PubMed] [Google Scholar]