Abstract
The capsular antigen detection (CAD) kit is widely used in clinics to detect Streptococcus pneumoniae infection from urine, because it is rapid, convenient, and effective. However, there are several disadvantages, including false-positive results in children colonized with S. pneumoniae and prolonged positive readings even after the bacteria have been cleared. RP-L7/L12 is a component of the 50S ribosome that is abundant in all bacteria and is specific for each bacterial species. We investigated whether RP-L7/L12 could be used to accurately diagnose pneumococcal pneumonia infection in mouse models of pneumonia and colonization generated by infecting CBA/JN or CBA/N mice, respectively, with S. pneumoniae strain 741. RP-L7/L12 detection by enzyme-linked immunosorbent assay accurately assessed active lung infection, as RP-L7/L12 levels decreased simultaneously with the bacterial lung burden after imipenem administration in the pneumonia mouse model. Based on the data, antibodies detecting RP-L7/L12 were applied to rapid immunochromatographic strips (ICS) for urine sample testing. When we compared the ICS test with the CAD kit in the pneumonia model, the results correlated well. Interestingly, however, when the lung bacterial burden became undetectable after antibiotic treatment, the ICS test was correspondingly negative, even though the same samples tested by the CAD kit remained positive. Similarly, while the ICS test exhibited negative results in the nasal colonization model, the CAD kit demonstrated positive results. Bacterial RP-L7/L12 may be a promising target for the development of new methods to diagnose infectious disease. Further studies are warranted to determine whether such a test could be useful in children.
INTRODUCTION
Streptococcus pneumoniae is the common pathogen associated with meningitis, otitis media, sepsis, and community-acquired pneumonia (CAP) (1–5). Mortality from pneumococcal pneumonia is particularly high in infants and the elderly (6, 7). Despite its importance for CAP pathogenicity, current diagnostic methods for S. pneumoniae infection are frequently problematic. The current standard diagnostic method determining the presence of S. pneumoniae in blood cultures (8, 9) has low sensitivity and requires a waiting period of at least 2 days (10, 11). In addition, expectorated-sputum cultures provide only a probable—but not definitive—diagnosis, since pneumococcal organisms are often carried in the nasopharynx (12). Thirty-five percent of children aged 3 to 6 years have nasopharyngeal colonization, even in patients effectively immunized with the PCV7 vaccine (13). Therefore, children are more likely to be asymptomatic carriers of pneumococci than adults (14–16).
Antigen detection assays are an alternative to the standard culture-based methods for pneumococcal pneumonia diagnosis. A rapid urinary pneumococcal antigen test (e.g., Binax NOW) that detects the capsular C-polysaccharide antigen present in S. pneumoniae is commercially available for rapid diagnosis and has been widely used in clinical practice (11, 17). Although the method is highly specific and moderately sensitive for adults (18, 19), it is not as effective in accurately diagnosing infection in children, due in part to the following problems: false-positive results occurring because of S. pneumoniae colonization in children (15, 20), an inability to detect infection immediately after onset, and sustained antigen-positive results regardless of treatment (21). Another antigen detection method, ODK0501, has been developed to detect the C-polysaccharide moiety in sputum samples, although whether this test can effectively discriminate between children with and without pneumococcal infection is questionable (22, 23). Therefore, a more effective target for diagnosing pneumococcal infections, especially in children, is greatly desired.
L7/L12 ribosomal protein (RP-L7/L12) is among the most investigated components of prokaryotic ribosomes, and it interacts with translation factors during protein biosynthesis in bacteria (24). RP-L7/L12 is present at approximately a 4-fold higher level than other ribosomal proteins, and it increases in proportion to the bacterial growth rate (25). Similar proteins are found in the large ribosomal subunits of archaebacteria, eukaryotes, and all eubacteria. Although archaebacterial and eukaryotic proteins are homologous to each other, they show little homology to eubacterial proteins, as assessed by various physical and functional criteria (24). Alignments of the complete RP-L7/L12 amino acid sequences available from 16 different bacterial species show that the C-terminus region is highly conserved; however, one of the monoclonal antibodies (MAbs) cross-reacted only with streptococci in Western blotting (26). In contrast, a specific epitope on L7/L12 of Neisseria gonorrhoeae was used for a laboratory-based evaluation system (27). Therefore, bacterial RP-L7/L12 may be a promising target for the development of new methods to diagnose infectious disease.
In the present study, we generated an anti-RP-L7/L12 antibody to detect S. pneumoniae and assessed RP-L7/L12 antigen production in a pneumococcal pneumonia mouse model. In addition, we determined the ability of anti-RP-L7/L12 antibody-coated immunochromatographic strips (ICS) to rapidly detect S. pneumoniae from urine samples taken from S. pneumoniae-induced pneumonia and colonization mouse models and compared its performance with the currently used capsular antigen detection kit.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free 5- to 6-week-old CBA/JN mice (Charles River Japan, Tokyo, Japan) were used for the pneumonia model (28), and CBA/N mice (Sankyo Labo Service, Hamamatsu, Japan) (7) were used for the colonization model. The mice were maintained under specific-pathogen-free conditions within the animal care facility in the Laboratory Animal Research Center of Toho University of Medicine until the day of sacrifice. Experiments were conducted according to our institution's ethical guidelines for animal experiments. Animal protocols were approved by the institutional animal care and use committee (approval number 10-52-54).
Bacteria.
A clinical isolate of S. pneumoniae strain 741 (serotype 19F) stocked in the Department of Microbiology and Infectious Diseases, Toho University School of Medicine, Tokyo, Japan, was used in this study. The bacteria were incubated on Mueller-Hinton agar (Difco Laboratories, Detroit, MI) plates supplemented with 5% defibrinated horse blood at 35°C for 14 h. Bacterial scrapes from these plates were suspended in brain heart infusion broth (Becton, Dickinson and Company) supplemented with 0.5% yeast extract and cultured at 35°C for 5 h at the mid-log phase. CBA/JN or CBA/N mice were challenged with approximately 106 CFU of S. pneumoniae.
Experimental animal models.
Mice were anesthetized with an intramuscular injection of a mixture of 60 mg/kg of body weight ketamine (Sankyo Pharmaceutical, Tokyo, Japan) and 10 mg/kg xylazine (Bayer Japan, Tokyo, Japan). A 30-μl bacterial suspension was intranasally administered to CBA/JN mice for the experimental pneumonia model. For the colonization model, a 10-μl bacterial suspension was intranasally administered to anesthetized CBA/N mice by slow delivery over several minutes (29). After bacterial inoculation of CBA/JN mice, 50 mg/kg of imipenem hydrate/cilastatin sodium (IPM/CS) (MSD, Tokyo, Japan) was intraperitoneally administered twice a day.
Sample collection and microbiological investigation.
At designated time points (as shown in the figure legends), the mice were sacrificed by CO2 asphyxiation. Whole lungs were homogenized in 1.0 ml of saline using a tissue homogenizer (IKA, Osaka, Japan). Nasal passages were washed with 500 μl of saline. Serial dilutions of the lung and nasal washes were plated on 5% horse blood agar plates containing 4 μg/ml gentamicin (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Colonies were counted after the plates were incubated for 24 h at 35°C. The resulting lung homogenate, nasal wash, serum, and urine samples were collected and stored at −20°C for further analysis.
Monoclonal antibodies.
Anti-RP-L7/L12 monoclonal antibodies were obtained by conventional methods. Numerous monoclonal antibodies were prepared by Asahi Kasei Corporation, Japan. Then, we selected clones highly specific for S. pneumoniae by sandwich enzyme-linked immunosorbent assay (ELISA) with several microbes. Briefly, S. pneumoniae (ATCC 27336) RPL7/L12 cDNA was cloned into a glutathione S-transferase (GST) fusion expression vector (pGEX-6P-1; GE Healthcare). The recombinant RP-L7/L12 was expressed in Escherichia coli, purified on an affinity column, and cleaved into final protein form by enzyme removal of the GST tag (PreScission Protease; GE Healthcare). The purified protein was used as an antigen for mouse immunization. Monoclonal antibodies were obtained and purified using conventional mouse hybridoma techniques.
ELISA.
Two anti-RP-L7/L12 monoclonal antibodies were used for sandwich ELISA. The detection antibody was chemically conjugated to peroxidase (enzyme immunoassay [EIA] grade; Roche Ltd., Switzerland) by conventional methods. Microtiter plates (Nunc-Immuno Plate; Thermo Fisher Scientific Inc., Waltham, MA) were coated with 100 μl of 10 μg/ml capture anti-RP-L7/L12 monoclonal antibody. After overnight incubation at 4°C, the wells were washed 2 times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T). Then, 200 μl of 1% bovine serum albumin blocking solution (in PBS) was added to each well, and the plate was blocked for 1 h at room temperature. Each sample was diluted with diluent (buffer solution containing fetal bovine serum [FBS], dextran, casein, and 1% Triton X-100 surfactant) to obtain measurable signal within the standard curve. The recombinant RP-L7/L12 protein was used as a control. Serially diluted samples were prepared using a standard curve. The wells were washed once with PBS-T, 25 μl of prediluted sample was added to duplicate wells, and the plate was incubated for 1 h at room temperature. After repeated washing with PBS-T, 50 μl of 1-μg/ml peroxidase-labeled detection antibody was added, and the plate was incubated for 1 h at room temperature. After another wash, 100 μl of TMB (3,3′,5,5′-tetramethylbenzidine) substrate (KPL, Gaithersburg, MD) was added for quantitative color development, and 100 μl of 1 M hydrochloric acid was added to stop the reaction after optimal color development was obtained. The results were recorded by measuring absorbance at 450 nm (Spectra Max 190; Molecular Devices, Downingtown, PA). The detection limits of ELISA measurement were 0.06 ng/ml in lung homogenates and 0.15 ng/ml in urine and serum samples, respectively.
Immunochromatography format assay.
The RP-L7/L12 ICS test was developed as follows. Similar to ELISA, the capture anti-RP-L7/L12 monoclonal antibody was immobilized on a nitrocellulose strip, while a colloidal gold-conjugated anti-RP-L7/L12 monoclonal antibody was used in a mobile phase for detection. A sample containing a urine specimen in dilution buffer was dropped onto the L7/L12 ICS specimen window to allow the S. pneumoniae antigen (RP-L7/L12) and the anti-RP-L7/L12 gold-labeled detection antibody to react. The resulting immunocomplexes traveled via capillary action to the membrane and were trapped by the captured anti-RP-L7/L12 solid-phase monoclonal antibody, thereby forming sandwich complexes on the strip. The presence of RP-L7/L12 was indicated by the test line turning red. In the absence of RP-L7/L12, no sandwich complex formed, and the test line did not turn red. Rabbit anti-mouse IgG antibody immobilized on the control line captured excess gold passing through the test line, causing red color to develop on the control line, indicating that the liquid sample successfully flowed up the membrane as a positive control. Cross-reactivity toward various pathogenic bacteria (the 27 microbes listed in Table 1) was assessed by in vitro testing of surfactant-extracted RP-L7/L12 antigen from the bacteria (106 CFU/ml).
Table 1.
Microorganisms used in this study
| Microorganisma | Strain |
|---|---|
| Bacillus subtilis | NBRP GTC01672 |
| Chlamydia pneumoniaeb | CWL-029 |
| Enterococcus faecalis | ATCC 19433 |
| Escherichia coli | ATCC 25922 |
| Haemophilus influenzae | ATCC 10211 |
| Haemophilus parahaemolyticus | NBRP GTC1529 |
| Haemophilus parainfluenzae | NBRP GTC2091 |
| Klebsiella pneumoniae | ATCC 13883 |
| Moraxella catarrhalis | ATCC 25240 |
| Mycoplasma pneumoniae | ATCC 15531 |
| Neisseria gonorrhoeae | ATCC 43070 |
| Neisseria lactamica | ATCC 23970 |
| Neisseria meningitidis | ATCC 13090 |
| Propionibacterium acnes | NBRP GTC00154 |
| Proteus mirabilis | NBRP GTC01262 |
| Pseudomonas aeruginosa | ATCC 27853 |
| Sarratia marcescens | NBRP GTC00135 |
| Staphylococcus aureus | ATCC 25923 |
| Staphylococcus epidermidis | NBRP GTCDY0064 |
| Streptococcus agalactiae | ATCC 12386 |
| Streptococcus mitior | NBRP GTC1140 |
| Streptococcus mitis | NBRP GTC0495 |
| Streptococcus mutans | NBRP GTC0218 |
| Streptococcus pneumoniae | ATCC 27336 |
| Streptococcus pyogenes | ATCC 19615 |
| Streptococcus salivarius | NBRP GTC0215 |
| Streptococcus sanguis | NBRP IID1633 |
Obtained from ATCC or NBRP (National Bioresource Project, Japan).
Cultured in HL cells. Chlamydia pneumoniae cell lysate was purchased from Microbix Biosystems Inc., Canada.
IC procedure with urine specimen and signal interpretation.
Mouse urine specimens were diluted between 2 and 20 times with PBS for analysis and used for S. pneumoniae antigen detection by the RP-L7/L12 ICS test. Commercially available diagnostics (Binax Now S. pneumoniae; Binax, Inc., Waltham, MA) were simultaneously used to measure the same samples in accordance with the manufacturer's instructions. The assays were read after a 15-min incubation and interpreted by detecting the presence or absence of a line, as follows: 3 independent judgments classified each line as positive or negative by light reflection intensity. In cases of discrepancy among the 3 independent evaluations for low-intensity lines that might be weakly positive (a faint line in comparison with the control line) or negative, the lines were labeled plus-minus.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism 5 software (GraphPad, Inc., CA). All values are expressed as means ± standard deviations (SD). Data were analyzed with Student's t test. A P value of <0.05 was regarded as statistically significant.
RESULTS
RP-L7/L12 protein can be detected in an S. pneumoniae pneumonia mouse model.
To determine whether RP-L7/L12 can be detected in a mouse model of S. pneumoniae pneumonia, we measured RP-L7/L12 production in the lungs, sera, and urine of infected mice. First, we evaluated the bacterial burden in the lung after intranasal administration of S. pneumoniae in CBA/JN mice. As shown in Fig. 1A, approximately 104 CFU of bacteria was found in the lung on day 2 postinfection, which gradually increased to 107 CFU in the lung at day 7. Of 10 mice, 5 died by day 7. During this infection, we examined RP-L7/L12 production in samples harvested from infected mice by ELISA. No RP-L7/L12 was detected in mice before infection (data not shown). At day 2 postinfection, RP-L7/L12 was detected in lung homogenates and urine, but it was below the detection limit in serum (Fig. 1B to D). At day 7, approximately 800, 650, and 40 ng/ml of RP-L7/L12 was detected in lung, urine, and serum samples, respectively. We next examined whether RP-L7/L12 levels depended on bacterial numbers in the lung by collecting samples the day after inoculating CBA/JN mice with 103, 104, 105, or 106 CFU/ml of S. pneumoniae. The lung bacterial burden correlated with the inoculum dose 1 day after infection (Fig. 1E). RP-L7/L12 was detected in lung homogenates from mice inoculated with more than 105 CFU/ml of S. pneumoniae, whereas an inoculum dose of 106 CFU/ml was necessary to detect RP-L7/L12 in urine (Fig. 1F and G). RP-L7/L12 in serum remained below detection limits at all inoculation doses (data not shown). These results indicated that RP-L7/L12 could be detected in S. pneumoniae-infected mice in an inoculum dose- and time-dependent manner.
Fig 1.
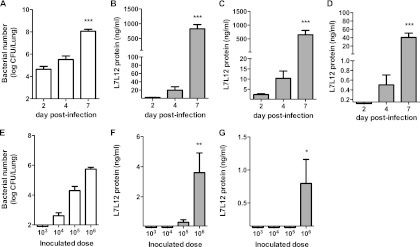
Effect of bacterial burden on RP-L7/L12 production in the pneumococcal pneumonia mouse model. (A to D) Bacterial numbers in the lungs were determined (A), and RP-L7/L12 production was measured in lung homogenates (B), urine (C), and serum (D) at days 2 (n = 10), 4 (n = 8), and 7 (n = 5) after infection of CBA/JN mice with S. pneumoniae at 106 CFU/ml. (E, F, and G) After 24 h of infection (n = 5) with various doses of S. pneumoniae in CBA/JN mice, bacterial numbers in lungs were determined (E), and RP-L7/L12 production was measured in lung homogenates (F) and urine (G). The results are expressed as means and SD. The limits of detection for ELISA measurement were 0.06 ng/ml in lung homogenates and 0.15 ng/ml in urine and serum. The asterisks denote significant differences (*, P < 0.05; **, P < 0.001; ***, P < 0.0001) compared to day 2 or 103 CFU/ml.
RP-L7/L12 production decreases upon antibiotic treatment in a mouse model of pneumonia.
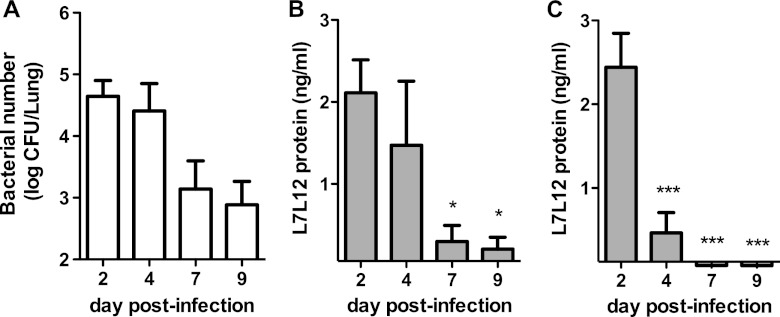
To further evaluate whether RP-L7/L12 is a good readout for the levels of S. pneumoniae present in the lungs of an infected host, we determined whether RP-L7/L12 levels correlated with decreasing bacterial burden after antibiotic treatment in the mouse model of S. pneumoniae-induced pneumonia. CBA/JN mice were treated with IPM/CS 2 times a day starting at 48 h postinfection and continuing for 7 days. As the bacterial burden in the lungs of antibiotic-treated mice gradually decreased (Fig. 2A), RP-L7/L12 production detected in lung homogenates and urine correspondingly decreased (Fig. 2B to C). No RP-L7/L12 was detected at any time point in serum (data not shown). These data suggest that there is a correlation between the lung bacterial burden in infected mice and RP-L7/L12 production detected in the lungs or urine throughout antibiotic treatment.
Fig 2.
Effect of antibiotic treatment on RP-L7/L12 production in a mouse model of pneumonia. CBA/JN mice (n = 10) infected with S. pneumoniae at 106 CFU/ml were treated with IPM/CS 2 times a day starting at day 2 postinfection and continuing for 7 days. (A) The bacterial burdens in lungs at days 4, 7, and 9 after infection were determined. (B and C) RP-L7/L12 production in lung homogenates (B) and urine (C) were measured by ELISA. The results are expressed as means and standard errors of the mean (SEM). The limit of detection for ELISA measurement was 0.06 ng/ml in lung homogenates and 0.15 ng/ml in urine and serum. The asterisks denote significant differences (*, P < 0.01; ***, P < 0.0001) compared to the onset of treatment.
RP-L7/L12 protein can be detected in nasal wash, but not urine, after S. pneumoniae colonization of the mouse nasal cavity.
To examine whether RP-L7/L12 could be detected in urine following S. pneumoniae colonization, CBA/N mice received a dose of 5.5 × 105 CFU in the nasal cavity. Nasally infected mice consistently harbored 104 to 105 CFU of bacteria within the nasal cavity throughout day 11, whereas the bacterial burden in the lung decreased till day 11 (day 5, 103 CFU/lung; day 11, 102 CFU/lung). Under these conditions, RP-L7/L12 production was detected in nasal cavity washes, but not in urine or lungs, at days 5 and 11 after colonization (data not shown).
Development of the immunochromatographic strip assay for RP-L7/L12.
We developed a rapid antigen detection method by generating an ICS test that detected S. pneumoniae-derived RP-L7/L12 in urine samples within 15 min, as evaluated by our mouse model of pneumonia. Cross-reactivity toward various other pathogenic bacteria (27 microbes) was assessed by in vitro testing of surfactant-extracted RP-L7/L12 antigen from these bacteria (Table 1). No cross-reactivity was detected in 25 tested microbes; however, cross-reactivity was observed against Streptococcus mitis and Streptococcus mitior at 106 CFU/ml. Among 5 strains (serotypes 19F, 21, 29, 34, and 35) of clinical isolates of S. pneumoniae, positive results were found in all strains tested by ICS testing.
The ICS test rapidly detects RP-L7/L12 production in a mouse model of pneumonia.
We used CBA/JN mice for our mouse model of pneumonia and evaluated RP-L7/L12 production in urine samples from each mouse using the ICS test. In the mouse pneumonia model, 104 to 108 CFU of S. pneumoniae was present in the lungs of infected mice; during the course of infection, 3/8 mice with pneumonia died between days 4 and 7 (Fig. 1A). The ICS test exhibited high detection sensitivity by RP-L7/L12 recognition in urine from 80% (8/10) of infected mice at day 2 and 100% (8/8) at day 4 after infection (Table 2). The capsular antigen detection (CAD) kit also detected infection with high sensitivity in serum samples (Table 2). To confirm that the ICS test could accurately distinguish pneumonia infection in the lung from bacterial colonization in nasal passages, we evaluated RP-L7/L12 production in urine samples from CBA/N mice colonized with S. pneumoniae in their nasal cavities (Table 3). In this colonization model, colonized CBA/N mice harbored approximately 104 CFU of bacteria in the nasal cavity but demonstrated no bacterial burden in the lungs at day 6 or 13 after infection. While urine samples from these mice remained negative on ICS testing throughout the testing period, the CAD kit exhibited positive readings in 80% (4/5) of colonized mice at day 3 and 100% (5/5) at days 6 and 13. These results suggest that RP-L7/L12 ICS testing correlates well with the presence of S. pneumoniae only in the context of lung infection that indicates pneumococcal pneumonia.
Table 2.
Comparison of ICS test and CAD kit results in the mouse pneumonia model
| Test resulta | No. of results on day: |
|||||
|---|---|---|---|---|---|---|
| ICS test |
CAD kit |
|||||
| 2 (n = 10)b | 4 (n = 8) | 7 (n = 3) | 2 (n = 10) | 4 (n = 8) | 7 (n = 3) | |
| − | 2 | 0 | 0 | 1 | 0 | 0 |
| ± | 5 | 2 | 0 | 1 | 0 | 0 |
| + | 3 | 6 | 3 | 8 | 8 | 3 |
The performances of the ICS test and CAD kit in urine were evaluated at days 2, 4, and 7 after infection of CBA/JN mice with S. pneumoniae at 106 CFU/ml. +, positive; −, negative; ±, low-intensity lines that might be weakly positive (a faint line in comparison with the control line) or negative.
n, number of mice tested.
Table 3.
Comparison of ICS test and CAD kit results in the mouse colonization model
| Test resulta | No. of results on day: |
|||||
|---|---|---|---|---|---|---|
| ICS test |
CAD kit |
|||||
| 3 (n = 5)b | 6 (n = 5) | 13 (n = 5) | 3 (n = 5) | 6 (n = 5) | 13 (n = 5) | |
| − | 5 | 5 | 5 | 1 | 0 | 0 |
| ± | 0 | 0 | 0 | 0 | 1 | 0 |
| + | 0 | 0 | 0 | 4 | 4 | 5 |
The performances of the ICS test and CAD kit in urine were evaluated at days 3, 6, and 13 after infection of CBA/N mice with S. pneumoniae at 106 CFU/ml. +, positive; −, negative; ±, low-intensity lines that might be weakly positive (a faint line in comparison with the control line) or negative.
n, number of mice tested.
ICS test results from urine samples correlate with a decrease in the lung bacterial load after antibiotic therapy in a mouse model of pneumonia.
Testing for the presence of pneumococcal pneumonia with the CAD kit has been reported to exhibit long-term antigen-positive results regardless of antibiotic treatment (21, 30). We therefore evaluated the effect of antibiotic treatment on RP-L7/L12 production in the mouse pneumonia model to determine whether this was a more sensitive and accurate readout of infection. Both the ICS test and the CAD kit showed positive readouts from urine samples in 80% (8/10) and 90% (9/10) of infected mice, respectively, before the initiation of antibiotic treatment. After antibiotic treatment, we first assessed the pulmonary bacterial burden in the mouse and found that it gradually decreased after IPM/CS treatment at day 2 postinfection (Fig. 2A). Correspondingly, the ICS test was negative for the presence of RP-L7/L12 in 100% (0/8) of antibiotic-treated mice at days 7 and 9 after inoculation (Table 4). In contrast, the CAD kit exhibited positive results in 25% (2/8) and 37% (3/8) of antibiotic-treated mice at days 7 and 9 after inoculation, respectively, even though these mice demonstrated lower bacterial counts in the lung (Table 4). Although these results suggest that the positive readouts from both tests largely correlated with the bacterial burden in the lung, the ICS test may more accurately reflect the diminishing bacterial load in the lung in the mouse model of pneumonia after antibiotic treatment.
Table 4.
Comparison of ICS test and CAD kit results during antibiotic treatment in the mouse pneumonia model
| Test resulta | No. of results on day: |
|||||||
|---|---|---|---|---|---|---|---|---|
| ICS test |
CAD kit |
|||||||
| 2 (n = 10)b | 4 (n = 10) | 7 (n = 8) | 9 (n = 8) | 2 (n = 10) | 4 (n = 10) | 7 (n = 8) | 9 (n = 8) | |
| − | 2 | 9 | 8 | 8 | 1 | 5 | 6 | 5 |
| ± | 5 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| + | 3 | 0 | 0 | 0 | 8 | 4 | 1 | 3 |
The performances of the ICS test and CAD kit in urine were evaluated at days 2, 4, 7, and 9 after infection of CBA/JN mice with S. pneumoniae at 106 CFU/ml. CBA/JN mice infected with S. pneumoniae were treated with IPM/CS 2 times a day starting at day 3 postinfection and continuing for 7 days. +, positive; −, negative; ±, low-intensity lines that might be weakly positive (a faint line in comparison with the control line) or negative.
n, number of mice tested.
DISCUSSION
There is a need to develop improved methods for rapid and accurate determination of pneumococcal pneumonia, especially in children and the elderly. This study revealed that the presence of RP-L7/L12 in urine correlated well with the lung bacterial burden in a mouse model of pneumococcal pneumonia. In addition, the ICS test measuring S. pneumoniae-derived RP-L7/L12, which we developed as a new detection method, exhibited efficient and accurate assessment of pneumococcal pneumonia in infected mice.
The bacterial capsular polysaccharide antigen has been widely used as a target for rapid detection of the presence of pneumococcal pneumonia, including the CAD kit (13, 18, 22). Dominguez et al. reported that the sensitivity and specificity of this CAD test using urine samples from patients with a definite diagnosis of pneumococcal pneumonia were 80.4% and 97.2%, respectively (18). Other studies testing CAD kit sensitivity and specificity in blood cultures reported a range of 79 to 87% for sensitivity and a range of 83 to 97% for specificity (17, 28, 31). While these detection rates are high, the CAD kit presents some disadvantages, including false-positive results due to pneumococcal colonization, particularly among children, and long-lasting positive results after antibody treatment (32, 33). Therefore, other detection methods for rapidly identifying pneumococcal pneumonia have been developed by several laboratories. Recently, new targets for rapid detection testing, such as putative proteinase maturation protein A (PpmA) (22) and the C-polysaccharide moiety of S. pneumoniae (ODK0501) (23), have been exploited. Although these targets have been useful for diagnosing pneumococcal pneumonia, the same limitations exhibited by the CAD kit mentioned above apply to these new methods and have not been resolved. In this study, we focused on testing the S. pneumoniae-derived RP-L7/L12 protein, which we argue is a more appropriate target for detecting S. pneumoniae in the following ways. First, RP-L7/L12 is a component of the 50S ribosome, which is abundant in all bacteria and is specific for each bacterial species (26, 34). Second, the RP-L7/L12 levels increase in proportion to the bacterial growth rate, since the protein is essential for protein synthesis in bacteria (25, 35). In this study, our newly developed assay detected RP-L7/L12 in lung homogenates, urine, and sera harvested from the mouse model of pneumococcal pneumonia in a dose-and time-dependent manner. In addition, the decreased RP-L7/L12 protein levels detected in urine by our assay accurately reflected the reduced bacterial numbers in the lungs due to antibiotic treatment of infected mice. Therefore, further studies are needed to determine whether this protein can be used to accurately diagnose pneumonia in children.
In this study, we applied the antibodies that we generated to detect the target protein RP-L7/L12 to ICS testing technology and determined that the ICS test exhibited positive correlation with the bacterial burden in the lungs of mice in the pneumonia model. We further compared the detection capability of the RP-L7/L12-targeted ICS test developed here with that of the widely used CAD kit for the presence of S. pneumoniae lung infection in both the pneumonia and colonization mouse models. The ICS test and CAD kit test have similar minimal detection levels for S. pneumoniae lysate. In the S. pneumoniae colonization model that contained bacteria only in the nasal cavity, we found that the CAD kit exhibited false-positive results, whereas the ICS test accurately gave negative results for all sample types. This was consistent with RP-L7/L12 protein levels in these mice as measured by ELISA, where antigen was present in the nasal wash but not in lung homogenate, serum, or urine samples. These results indicated that the RP-L7/L12-targeted ICS test might be a useful diagnostic method in children who harbor S. pneumoniae colonization and do not have pneumonia. When mice with pneumococcal pneumonia were treated with antibiotics, ICS tests were negative for the presence of infection, which corresponded to low or absent bacterial burden in the lung. Under these conditions, however, the CAD kit results indicated that 4 of 5 mice remained positive. While the reason for this difference is still unclear, we speculate that differences in how long these detected antigens remain in the host after bacterial death may be an underlying factor. While the capsular antigen remained present in clinical samples such as urine even in the absence of viable S. pneumoniae (21), RP-L7/L12 decreased simultaneously with bacterial growth suppression in our mouse model. Consequently, RP-L7/L12-targeted ICS tests may address the problem of long-lasting positive readings by the CAD kit even after bacteria have been cleared by antibiotic treatment. In addition, although specimen dilution might affect the test result slightly in each experiment, it might still be applicable in human clinical or animal models.
In conclusion, RP-L7/L12 detection in murine urine samples reflects the real-time infection status due to pneumococcal pneumonia. In addition, our study indicated that RP-L7/L12-targeted ICS testing is more accurate than current detection methods by providing fewer false-positive results in cases of colonization and lower incidence of lasting positivity after antibiotic treatment in a mouse model of pneumonia. The results of our study suggest that the RP-L7/L12-targeted ICS test may be used as a complementary tool to accurately detect pneumococcal pneumonia in a clinical setting, especially in young children, although clinical trials need to be performed to support the data shown here and confirm the applicability of the ICS test to the clinic.
ACKNOWLEDGMENTS
This work was partially supported by Asahi Kasei Corporation.
We thank Shigeki Misawa from Juntendo University Hospital for kindly providing S. pneumoniae strains.
Y. Sugiyama, Y. Harada, T. Sohka, and K. Matsuyama are employees of Asahi Kasei Corporation but do not have stock or options. We have no other possible conflicts of interest to report.
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Bartlett JG, Breiman RF, Mandell LA, File TM., Jr 1998. Community-acquired pneumonia in adults: guidelines for management. The Infectious Diseases Society of America. Clin. Infect. Dis. 26: 811–838 [DOI] [PubMed] [Google Scholar]
- 2. File TM. 2003. Community-acquired pneumonia. Lancet 362: 1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M. 2004. A 3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: utility and clinical impact on the reported etiology. J. Infect. Chemother. 10: 359–363 [DOI] [PubMed] [Google Scholar]
- 4. Juven T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, Eskola J, Saikku P, Ruuskanen O. 2000. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 19: 293–298 [DOI] [PubMed] [Google Scholar]
- 5. Saito A, Kohno S, Matsushima T, Watanabe A, Oizumi K, Yamaguchi K, Oda H. 2006. Prospective multicenter study of the causative organisms of community-acquired pneumonia in adults in Japan. J. Infect. Chemother. 12: 63–69 [DOI] [PubMed] [Google Scholar]
- 6. Feldman C, Anderson R. 2011. Bacteraemic pneumococcal pneumonia: current therapeutic options. Drugs 71: 131–153 [DOI] [PubMed] [Google Scholar]
- 7. Varon E, Mainardi JL, Gutmann L. 2010. Streptococcus pneumoniae: still a major pathogen. Clin. Microbiol. Infect. 16: 401. [DOI] [PubMed] [Google Scholar]
- 8. Marston BJ, Plouffe JF, File TM, Jr, Hackman BA, Salstrom SJ, Lipman HB, Kolczak MS, Breiman RF. 1997. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch. Intern. Med. 157: 1709–1718 [PubMed] [Google Scholar]
- 9. Wubbel L, Muniz L, Ahmed A, Trujillo M, Carubelli C, McCoig C, Abramo T, Leinonen M, McCracken GH., Jr 1999. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr. Infect. Dis. J. 18: 98–104 [DOI] [PubMed] [Google Scholar]
- 10. Burman LA, Trollfors B, Andersson B, Henrichsen J, Juto P, Kallings I, Lagergard T, Mollby R, Norrby R. 1991. Diagnosis of pneumonia by cultures, bacterial and viral antigen detection tests, and serology with special reference to antibodies against pneumococcal antigens. J. Infect. Dis. 163: 1087–1093 [DOI] [PubMed] [Google Scholar]
- 11. Reller LB, Weinstein MP, Werno AM, Murdoch DR. 2008. Laboratory diagnosis of invasive pneumococcal disease. Clin. Infect. Dis. 46: 926–932 [DOI] [PubMed] [Google Scholar]
- 12. Lentino JR, Lucks DA. 1987. Nonvalue of sputum culture in the management of lower respiratory tract infections. J. Clin. Microbiol. 25: 758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. 2009. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124: e1–e11 doi:10.1542/peds.2008-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adegbola RA, Obaro SK, Biney E, Greenwood BM. 2001. Evaluation of Binax now Streptococcus pneumoniae urinary antigen test in children in a community with a high carriage rate of pneumococcus. Pediatr. Infect. Dis. J. 20: 718–719 [DOI] [PubMed] [Google Scholar]
- 15. Dowell SF, Garman RL, Liu G, Levine OS, Yang Y-H. 2001. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, preformed among pediatric patients. Clin. Infect. Dis. 32: 824–825 [DOI] [PubMed] [Google Scholar]
- 16. Faden H, Heimerl M, Varma C, Goodman G, Winkelstein P. 2002. Urinary excretion of pneumococcal cell wall polysaccharide in children. Pediatr. Infect. Dis. J. 21: 791–793 [DOI] [PubMed] [Google Scholar]
- 17. Bartlett JG. 2011. Diagnostic tests for agents of community-acquired pneumonia. Clin. Infect. Dis. 52: S296–S304 [DOI] [PubMed] [Google Scholar]
- 18. Dominguez J. 2001. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest 119: 243–249 [DOI] [PubMed] [Google Scholar]
- 19. Gutierrez F, Masia M, Rodriguez JC, Ayelo A, Soldan B, Cebrian L, Mirete C, Royo G, Hidalgo AM. 2003. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin. Infect. Dis. 36: 286–292 [DOI] [PubMed] [Google Scholar]
- 20. Hamer DH, Egas J, Estrella B, Macleod WB, Griffiths JK, Sempertegui F. 2002. Assessment of the Binax NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage. Clin. Infect. Dis. 34: 1025–1028 [DOI] [PubMed] [Google Scholar]
- 21. Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, Gonzalez J, Martinez E, Garcia E, Mensa J, de Roux Torres AA. 2003. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur. Respir. J. 21: 209–214 [DOI] [PubMed] [Google Scholar]
- 22. Ehara N, Fukushima K, Kakeya H, Mukae H, Akamatsu S, Kageyama A, Saito A, Kohno S. 2008. A novel method for rapid detection of Streptococcus pneumoniae antigen in sputum and its application in adult respiratory tract infections. J. Med. Microbiol. 57: 820–826 [DOI] [PubMed] [Google Scholar]
- 23. Izumikawa K, Akamatsu S, Kageyama A, Okada K, Kazuyama Y, Takayanagi N, Nakamura S, Inoue Y, Higashiyama Y, Fukushima K, Ishida T, Sawai T, Yoshimura K, Nakahama C, Ohmichi M, Kakugawa T, Nishioka Y, Aoki N, Seki M, Kakeya H, Yamamoto Y, Yanagihara K, Kohno S. 2009. Evaluation of a rapid immunochromatographic ODK0501 assay for detecting Streptococcus pneumoniae antigen in sputum samples from patients with lower respiratory tract infection. Clin. Vaccine Immunol. 16: 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gudkov AT. 1997. The L7/L12 ribosomal domain of the ribosome: structural and functional studies. FEBS Lett. 407: 253–256 [DOI] [PubMed] [Google Scholar]
- 25. Howe JG, Hershey JW. 1983. Initiation factor and ribosome levels are coordinately controlled in Escherichia coli growing at different rates. J. Biol. Chem. 258: 1954–1959 [PubMed] [Google Scholar]
- 26. Kolberg J, Hoiby EA, Lopez R, Sletten K. 1997. Monoclonal antibodies against Streptococcus pneumoniae detect epitopes on eubacterial ribosomal proteins L7/L12 and on streptococcal elongation factor Ts. Microbiology 143: 55–61 [DOI] [PubMed] [Google Scholar]
- 27. Samarawickrama A, Alexander S, Ison C. 2011. A laboratory-based evaluation of the BioStar Optical ImmunoAssay point-of-care test for diagnosing Neisseria gonorrhoeae infection. J. Med. Microbiol. 60: 1779–1781 [DOI] [PubMed] [Google Scholar]
- 28. Smith MD, Derrington P, Evans R, Creek M, Morris R, Dance DA, Cartwright K. 2003. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J. Clin. Microbiol. 41: 2810–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu H-Y, Virolainen A, Mathews B, King J, Russell MW, Briles DE. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23: 127–137 [DOI] [PubMed] [Google Scholar]
- 30. Murdoch DR, Reller LB, Faden H. 2003. Immunochromatographic test for rapid detection of Streptococcus pneumoniae in the nasopharynx. J. Clin. Microbiol. 41: 2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murdoch DR, Laing RT, Mills GD, Karalus NC, Town GI, Mirrett S, Reller LB. 2001. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J. Clin. Microbiol. 39: 3495–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Navarro D, Garcia-Maset L, Gimeno C, Escribano A, Garcia-de-Lomas J. 2004. Performance of the Binax NOW Streptococcus pneumoniae urinary antigen assay for diagnosis of pneumonia in children with underlying pulmonary diseases in the absence of acute pneumococcal infection. J. Clin. Microbiol. 42: 4853–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tateda K, Kusano E, Matsumoto T, Kimura K, Uchida K, Nakata K, Yamaguchi K. 2006. Semi-quantitative analysis of Streptococcus pneumoniae urinary antigen: kinetics of antigen titers and severity of diseases. Scand. J. Infect. Dis. 38: 166–171 [DOI] [PubMed] [Google Scholar]
- 34. Jomaa M, Kyd JM, Cripps AW. 2005. Mucosal immunisation with novel Streptococcus pneumoniae eprotein antigens enhances bacterial clearance in an acute mouse lung infection model. FEMS Immunol. Med. Microbiol. 44: 59–67 [DOI] [PubMed] [Google Scholar]
- 35. Liljas A. 1991. Comparative biochemistry and biophysics of ribosomal proteins. Int. Rev. Cytol. 124: 103–136 [DOI] [PubMed] [Google Scholar]