Abstract
Mycobacterium bovis is responsible for a zoonosis originating in cattle. We report a case of a man with vertebral spondylodiscitis caused by Mycobacterium bovis. Diagnosis was complicated because of the lack of IS6110. These strains are rare, but microbiologists should be aware of their existence.
CASE REPORT
In May 2009, a 72-year-old man born in Belgium was seen in the orthopedic department because of back pain with right-sided radiation for a few weeks. This back pain progressively decreased his ability to perform daily activities. He had no pulmonary or cardiac complaints. He had already received antibiotics (first amoxicillin-clavulanic acid and then ciprofloxacin and oxacillin) from his general practitioner for a presumptive diagnosis of bacterial discitis.
His previous medical history was unremarkable. The patient was a diplomat and ambassador and had lived on almost every continent. His career started in Kinshasa in 1965 and ended in Canberra in 2002. He actively competed in running competitions. Relevant history included a 7-year stay in Asia, including Pakistan, Malaysia, and Thailand, and he returned 10 years prior to hospitalization. The patient had consumed dairy products and unpasteurized milk in Asia and also had close contact with wild boars.
During his hospitalization, a magnetic resonance imaging study was performed and showed the presence of spondylodiscitis involving the D9 and D10 vertebral bodies and a beginning paravertebral abscess. Biological testing showed a white blood cell count of 7,130/mm3, a C-reactive protein level of 3.90 mg/dl, and an erythrocyte sedimentation rate of 17 mm/h.
Culture of a biopsy specimen of the involved disc was negative. Blood cultures were taken but remained negative. Mycobacterial culture was not performed. The patient was treated with 1 g intravenous amoxicillin-clavulanic acid four times a day and 1 g intravenous vancomycin twice daily. The patient was discharged with 875 mg oral amoxicillin-clavulanic acid three times a day.
A few weeks later, the patient was rehospitalized for follow-up nuclear magnetic resonance (NMR) analysis. This showed spondylodiscitis of D8, D9, and D10 and a progression of the paravertebral abscess (Fig. 1A). At that time, a tuberculin skin test (TST) was performed and was positive, with an induration of 30 by 40 mm. Taking the diagnosis to be a Pott's abscess, another biopsy of the involved region was performed and an antituberculosis drug regimen consisting of 300 mg rifampin (RIF) twice daily, 300 mg isoniazid (INH) once daily, and 500 mg pyrazinamide (PZA) three times a day was initiated.
Fig 1.
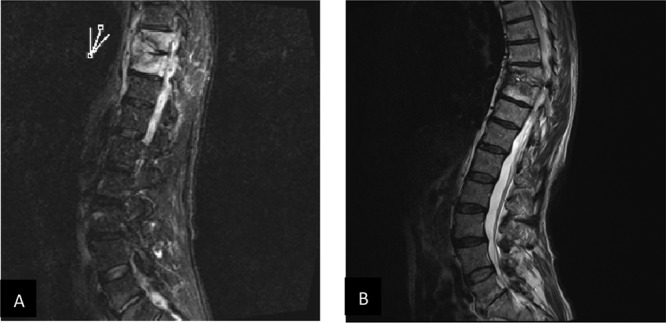
Magnetic resonance imaging scans of the lumbar spine. (A) Before treatment. The mark indicates the position of the paravertebral abscess around the involved discs. (B) Follow-up NMR scan 3 months after the end of treatment.
A Ziehl-Neelsen stain of the biopsy specimen was positive for acid-fast bacilli; however, PCR testing for the Mycobacterium tuberculosis complex based on IS6110 was negative. Subsequently, 500 mg clarithromycin twice daily was added to the triple therapy because atypical mycobacteria were not able to be excluded at that time.
After 5 weeks, acid-fast bacilli were grown on Lowenstein-Jensen medium (37°C). PCR for the M. tuberculosis complex based on IS6110 was again negative, but 16S rRNA gene sequencing showed 100% homology with the M. tuberculosis complex. The strain was niacin and nitrate reductase negative—which pointed toward Mycobacterium bovis—and was sent to the National Reference Laboratory for Mycobacteria for confirmation and subtyping: another PCR based on the IS6110 element (using other primers and probes) was also negative, and genotyping by IS6110-restriction fragment length polymorphism (RFLP) showed no bands. A multiplex PCR based on the deletion of a 12.7-kb DNA fragment in the genome of M. bovis compared to that of M. tuberculosis (1) identified the strain as M. bovis. A subsequent PCR revealed the presence of the RD1 region in the DNA (2), confirming that the isolated strain was M. bovis and not M. bovis BCG. Identification was also confirmed by GenoType MTBC (Hain). Drug susceptibility testing showed susceptibility to rifampin, ethambutol (EMB), and isoniazid but resistance to pyrazinamide with the specific C169G M. bovis mutation in the pncA gene (3). Spoligotyping revealed the octal code 616600000017600 and the mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) pattern 225322332353454243332512. A strain with a similar pattern had never been observed before in Belgium. Contact tracing found no incidence of tuberculosis (TB) among the patient's relatives or colleagues.
Approximately 1 month later, the patient developed a drug-induced hepatotoxicity, probably due to INH and/or RIF. Both drugs were discontinued, as was PZA after the confirmation of the etiologic agent as M. bovis (which is naturally resistant to PZA). A dose of 15 mg/kg ethambutol once daily was started, and 500 mg levofloxacin twice daily was added once the liver enzymes normalized. The patient temporarily developed an optic neuropathy caused by EMB.
Magnetic resonance imaging was performed 3 months after the end of treatment and showed no abscess but calcification around the involved discs and regression of the lesions (Fig. 1B). Symptoms and clinical findings showed excellent response, so the patient was allowed to participate again in running competitions, with equally good results.
A few cases of possible symptomatic primary M. bovis infection have been described in the literature (3–8). Our case is unusual because the strain does not contain a copy of IS6110. To our knowledge, this is the first isolation of an M. bovis strain lacking IS6110 that has been reported in Europe.
M. bovis, a bacterial species of the M. tuberculosis complex, is a pathogen that infects primarily cattle. However, humans can also become infected, most commonly through consumption of unpasteurized dairy products or close contact with infected animals. Therefore, these infections are most often suspected of being of zoonotic origin (9). A few studies have estimated the proportion of M. bovis infection in human tuberculosis cases to be in the range of 0.3 to 1.5% in developed countries (8, 10–13). Cases of interhuman transmission have only rarely been documented (7, 12, 14, 15). However, the exact source of infection remains undetermined in most cases. Although human diseases caused by M. bovis and other species of M. tuberculosis complex are similar, the anatomic site of M. bovis disease is more often extrapulmonary.
Genetically, all the members of M. tuberculosis complex are extremely similar, having identical 16S rRNA sequences and 99.9% genomic similarity at the nucleotide level (2). The list of its members (M. tuberculosis, M. bovis, M. bovis BCG, M. microti, M. canetti, M. africanum, M. caprae, M. pinnipedii, M. mungi, and M. orygis) is increasing.
The insertion sequence IS6110 is specific for the members of the M. tuberculosis complex, and the difference in the location and number of copies of this IS6110 in the genome is a source of polymorphism between isolates. The first technique used to observe such polymorphisms was the IS6110-based restriction fragment length polymorphism (16). In contrast to Mycobacterium tuberculosis, this technique provides only limited discrimination among M. bovis isolates, since M. bovis strains usually have only a single copy or a few copies of the IS6110 element. Alternative markers used for genetic typing were both the direct-repeat (DR) element and the GC-rich repetitive element (17, 18). The PCR-based genotyping techniques currently used, spoligotyping (19) and MIRU-VNTR (20), have both shown a good discriminatory power for M. bovis (9).
PCR has the potential to provide a more rapid, sensitive, and specific detection of M. tuberculosis complex in clinical specimens. Most (commercial) assays are based on IS6110 because of specificity for the M. tuberculosis complex and the multicopy number. However, in Asia, M. tuberculosis without IS6110 is described and, according to the literature, would occur more frequently in the group of Asian patients/travelers (9, 18, 21–23). Because of the much higher incidence of tuberculosis among recent immigrants than in the general population, it is likely that in the majority of these cases, the disease was contracted in the country of origin, thus increasing the likelihood of strains lacking IS6110. While the most immediate concern is the treatment of these patients, implementing the use of alternative genetic markers is important for identifying any spread of these strains within local communities and farther afield. This case report demonstrates that not all strains can be detected with primers directed toward IS6110. Because this can lead to false-negative results, multitargeted testing in molecular assays should be the rule.
In conclusion, although rare, primary M. bovis infection should be considered in travelers and immigrants returning from a zone where M. bovis is endemic, particularly in the case of a history of exposure to risk factors. The absence of IS6110 is rare but must be kept in mind and should give drive to multitargeted molecular testing.
Footnotes
Published ahead of print 7 November 2012
REFERENCES
- 1.Bakshi CS, Shah DH, Verma R, Singh RK, Malik M. 2005. Rapid differentiation of Mycobacterium bovis and Mycobacterium tuberculosis based on a 12.7-kb fragment by a single tube multiplex-PCR. Vet. Microbiol. 109:211–216 [DOI] [PubMed] [Google Scholar]
- 2.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. U. S. A. 99:3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allix-Béguec C, Fauville-Dufaux M, Stoffels K, Ommeslag D, Walravens K, Saegerman C, Supply P. 2010. Importance of identifying Mycobacterium bovis as a causative agent of human tuberculosis. Eur. Respir. J. 35(3):692–694 [DOI] [PubMed] [Google Scholar]
- 4.Hlavsa MC, Moonan PK, Cowan LS, Navin TR, Kammerer JS, Morlock GP, Crawford JT, LoBue PA. 2008. Human tuberculosis due to Mycobacterium bovis in the United States, 1995-2005. Clin. Infect. Dis. 47:168–175 [DOI] [PubMed] [Google Scholar]
- 5.de Kantor IN, Ambroggi M, Poggi S, Morcillo N, Da Silva Telles MA, Osorio Ribeiro M, Garzon Torres MC, Llerena Polo C, Ribon W, Garcıa V, Kuffo D, Asencios L, Vasquez Campos LM, Rivas C, de Waard JH. 2008. Human Mycobacterium bovis infection in ten Latin American countries. Tuberculosis 88:358–365 [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly LM, Daborn CJ. 1995. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber. Lung Dis. 76(Suppl 1):S1–S46 [DOI] [PubMed] [Google Scholar]
- 7.Evans JT, Smith EG, Banerjee A, Smith RM, Dale J, Innes JA, Hunt D, Tweddell A, Wood A, Anderson C, Hewinson RG, Smith NH, Hawkey PM, Sonnenberg P. 2007. Cluster of human tuberculosis caused by Mycobacterium bovis: evidence for person-to-person transmission in the UK. Lancet 369:1270–1276 [DOI] [PubMed] [Google Scholar]
- 8.Majoor CJ, Magis-Escurra C, van Ingen J, Boeree MJ, van Soolingen D. 2011. Epidemiology of Mycobacterium bovis disease in humans, The Netherlands, 1993-2007. Emerg. Infect. Dis. 17:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allix C, Walravens K, Saegerman C, Godfroid J, Supply P, Fauville-Dufaux M. 2006. Evaluation of the epidemiological relevance of variable-number tandem-repeat genotyping of Mycobacterium bovis and comparison of the method with IS6110 restriction fragment length polymorphism analysis and spoligotyping. J. Clin. Microbiol. 44:1951–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, de Kantor I, Meslin FX. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan LS, Crawford JT. 2002. Genotype analysis of Mycobacterium tuberculosis isolates from a sentinel surveillance population. Emerg. Infect. Dis. 8:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson AL, Hewinson G, Goodchild T, Watt B, Story A, Inwald J, Drobniewski FA. 2004. Molecular epidemiology of disease due to Mycobacterium bovis in humans in the United Kingdom. J. Clin. Microbiol. 42:431–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robert J, Boulahbal F, Trystram D, Truffot-Pernot C, de Benoist A- C, Vincent V, Jarlier V, Grosset J, Network of Microbiology Laboratories in France 1999. A national survey of human Mycobacterium bovis infection in France. Int. J. Tuberc. Lung Dis. 3:711–714 [PubMed] [Google Scholar]
- 14.Gutierrez MC, Galan JC, Blazquez J, Bouvet E, Vincent V. 1999. Molecular markers demonstrate that the first described multidrug-resistant Mycobacterium bovis outbreak was due to Mycobacterium tuberculosis. J. Clin. Microbiol. 37:971–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LoBue PA, Betancourt W, Cowan L, Seli L, Peter C, Moser KS. 2004. Identification of a familial cluster of pulmonary Mycobacterium bovis disease. Int. J. Tuberc. Lung Dis. 8:1142–1146 [PubMed] [Google Scholar]
- 16.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Soolingen D, de Haas PW, Hermans PW, Groenen PM, van Embden JD. 1993. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J. Clin. Microbiol. 31:1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahadevan R, Narayanan S, Paramasivan CN, Prabhakar R, Narayanan PR. 1995. Restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, India, by use of direct-repeat probe. J. Clin. Microbiol. 33:3037–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht C, van Soolingen D. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit–variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:4498–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agasino CB, Ponce de Leon A, Jasmer RM, Small PM. 1998. Epidemiology of Mycobacterium tuberculosis strains in San Francisco that do not contain IS6110. Int. J. Tuberc. Lung Dis. 2:518–520 [PubMed] [Google Scholar]
- 22.Das S, Paramasivan CN, Lowrie DB, Prabhakar R, Narayanan PR. 1995. IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, south India. Tuber. Lung Dis. 76:550–554 [DOI] [PubMed] [Google Scholar]
- 23.Howard ST, Oughton MT, Haddad A, Johnson WM. 1998. Absence of the genetic marker IS6110 from a strain of Mycobacterium tuberculosis isolated in Ontario. Can. J. Infect. Dis. 9(1):48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]


