Abstract
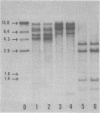
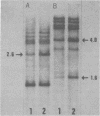
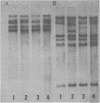
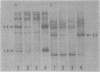
DNAs extracted from the mammary tumors of GR mice were analyzed for mouse mammary tumor virus proviral sequences by the restriction enzyme-Southern blot procedure. The tumor DNAs contain more proviral copies of mouse mammary tumor virus than DNA from a nonmalignant tissue. The degree of proviral amplification is small (ca. one to five additional copies) and appears to be variable from tumor to tumor. The restriction patterns of the amplified proviral sequences suggest a clonal origin for the tumor mass. In addition, the restriction patterns observed after digestion with the enzymes BglII and SacI indicate that only one of the proviruses endogenous to GR mice is amplified. The amplified provirus found in GR mammary tumors is identical to the provirus that is missing in GR-Mtv-2- mice, a congenic line exhibiting a low mammary tumor incidence.
Full text
PDF
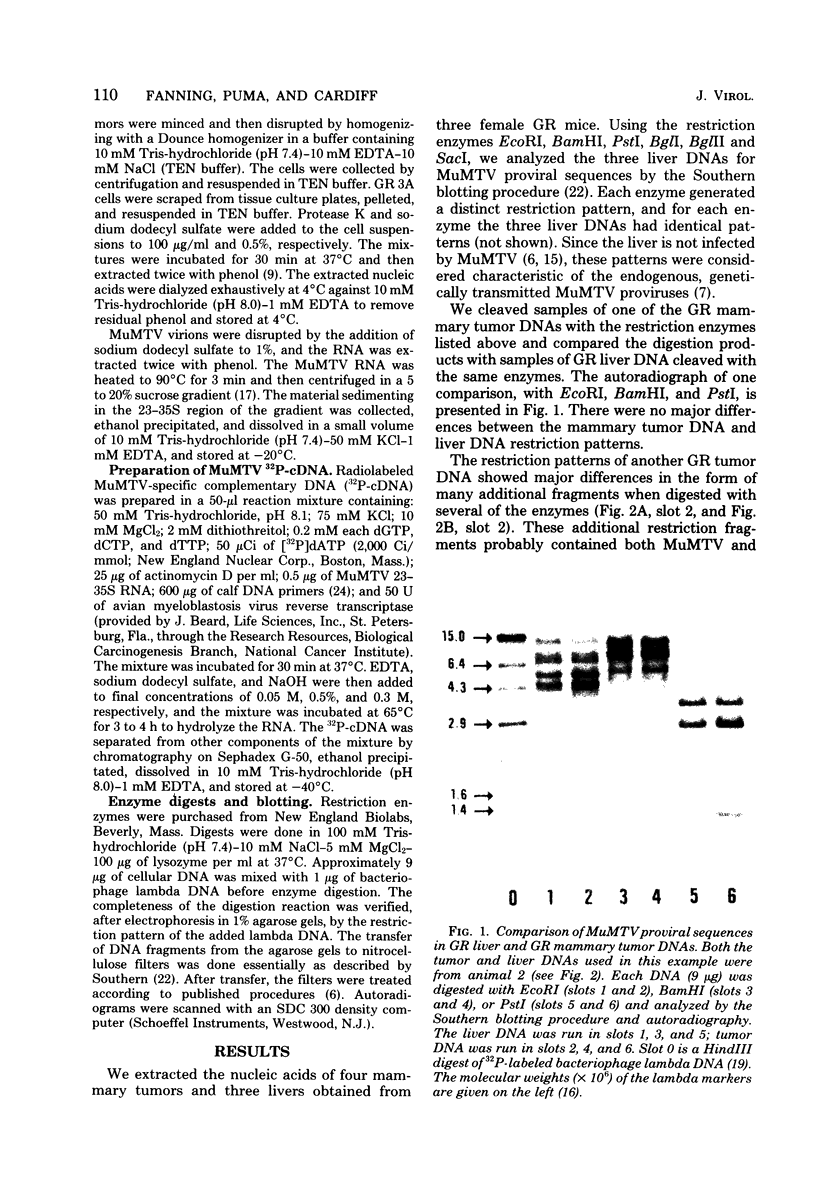



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P., Daams J. H., Hageman P., Calafat J. Genetic transmission of viruses that incite mammary tumor in mice. Proc Natl Acad Sci U S A. 1970 Sep;67(1):377–384. doi: 10.1073/pnas.67.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Bentvelzen P. Resistance to small amounts of Bittner mammary tumor virus in offspring of C57BL female mice with the virus. J Natl Cancer Inst. 1968 Sep;41(3):757–765. [PubMed] [Google Scholar]
- Berns A., Jaenisch R. Increase of AKR-specific sequences in tumor tissues of leukemic AKR mice. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2448–2452. doi: 10.1073/pnas.73.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Heine U. I., Pomenti A. A., Korb J., Weber G. H. Electrophoretic analysis of the molecular weight of murine mammary tumor virus RNA. J Virol. 1977 Jun;22(3):822–825. doi: 10.1128/jvi.22.3.822-825.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Vlahakis G., Schlom J. A biochemical approach to the study of the transmission of mouse mammary tumor viruses in mouse strains RIII and C3H. Int J Cancer. 1976 Jul 15;18(1):105–115. doi: 10.1002/ijc.2910180114. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nuss R. R., van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Sluyser M., Nouwen T., Hilgers J., Calafat J. Levels of mammary tumor virus in hormone-dependent and -independent mouse mammary tumor cells. Cancer Res. 1977 Jul;37(7 Pt 1):1986–1990. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Nowinski R. C., Sarker N. H. Mammary tumour virus specific nucleotide sequences in mouse DNA. Nat New Biol. 1972 Aug 9;238(84):189–191. doi: 10.1038/newbio238189a0. [DOI] [PubMed] [Google Scholar]
- van Nie R., de Moes J. Development of a congeneic line of the GR mouse strain without early mammary tumours. Int J Cancer. 1977 Oct 15;20(4):588–594. doi: 10.1002/ijc.2910200417. [DOI] [PubMed] [Google Scholar]