Abstract
Syphilis is a sexually transmitted disease caused by Treponema pallidum subsp. pallidum; it can be effectively treated with penicillin yet remains prevalent worldwide, due in part to the shortcomings of current diagnostic tests. Here we report the production of soluble recombinant versions of three novel diagnostic candidate proteins, Tp0326, Tp0453, and a Tp0453-Tp0326 chimera. The sensitivities of these recombinant proteins were assessed by screening characterized serum samples from primary, secondary, and latent stages of infection (n = 169). The specificities were assessed by screening false positives identified with the standard diagnostic testing algorithm (n = 21), samples from patients with potentially cross-reactive infections (Leptospira spp., Borrelia burgdorferi, Helicobacter pylori, Epstein-Barr virus, hepatitis B virus, hepatitis C virus, or cytomegalovirus) (n = 38), and samples from uninfected individuals (n = 11). The sensitivities of Tp0326, Tp0453, and the Tp0453-Tp0326 chimera were found to be 86%, 98%, and 98%, respectively, and the specificities were 99%, 100%, and 99%. In a direct comparison, the Captia syphilis (T. pallidum)-G enzyme immunoassay (Trinity Biotech) was used to screen the same serum samples and was found to have a sensitivity of 98% and a specificity of 90%. In particular, Tp0453 and the chimera exhibited superior accuracy in classifying analytical false-positive samples (100%, compared to 43% for the Captia assay). These findings identify Tp0453 and the Tp0453-Tp0326 chimera as novel syphilis-specific diagnostic candidates that surpass the performance of a currently available diagnostic enzyme immunoassay test for syphilis and that allow accurate detection of all stages of infection.
INTRODUCTION
Syphilis is a chronic infection caused by the spirochete Treponema pallidum subsp. pallidum and generally is transmitted either through sexual contact or from an infected mother to her fetus. Syphilis is a global health concern, with an estimated burden of 25 million affected people worldwide and an estimated annual incidence of 12 million cases (1). Further, infection with T. pallidum has been shown to increase the likelihood of contracting HIV, with risk estimates ranging from 2.3% to 8.6% (2). The incidence rates of syphilis among men who have sex with men have been increasing throughout North America and Europe (3, 4). In addition, congenital syphilis is recognized as the most significant disease affecting pregnancies and newborns throughout the world, with estimates of more than 2 million pregnant women per year being infected with syphilis (5).
The gold standard method for syphilis diagnosis relies on the use of a series of serological testing regimens. Traditional serological testing algorithms comprise detection of nontreponemal antibodies using the rapid plasma reagin (RPR) test or the Venereal Disease Research Laboratory (VDRL) test, followed by further screening of reactive samples for detection of treponeme-specific antibodies with the fluorescent treponemal antibody absorption (FTA-ABS) test, the microhemagglutination assay for T. pallidum (MHA-TP), or the T. pallidum particle agglutination (TP-PA) test. The RPR and VDRL tests show median sensitivities of 86% and 78%, respectively, during primary-stage infections and 73% and 71%, respectively, for late-stage infections (6, 7). Further, these nontreponemal tests exhibit cross-reactivities with a multitude of diseases and health conditions, including chickenpox, rheumatoid arthritis, pregnancy, and advanced age (6, 8). The confirmatory tests (MHA-TP, TP-PA, and FTA-ABS tests) are expensive and have major limitations due to their reliance on experienced technicians and adequate testing facilities, factors that are especially restricting in rural areas and developing countries (8). These confirmatory tests also have poor sensitivities for the detection of early infections, with values of 88% reported for the MHA-TP (7, 9) and TP-PA tests and 84% for the FTA-ABS test (7, 8).
In recent years, rapid point-of-care (POC) tests, automated enzyme immunoassays (EIAs), and chemiluminescence immunoassays (CIAs) have been developed for the diagnosis of syphilis. These assays, which use a panel of one or more of the recombinant treponemal proteins TpN15 (Tp0171), TpN17 (Tp0435), TpN47 (Tp0574), and TpN44 (Tp0768, TmpA) (alternative names are given in parentheses), have garnered significant interest in the syphilis diagnosis field due to their reproducibility, objectivity, and potential for automation (10). In the reverse testing algorithm, samples are first screened against the panel of recombinant treponemal proteins with EIAs/CIAs, positive samples are further screened with a nontreponemal test, and then discordant samples (e.g., EIA/CIA reactive and RPR/VDRL test nonreactive) are rescreened with a treponemal test (7, 10–12). This algorithm has shown higher false-reaction rates than the traditional screening algorithm in populations with a low prevalence of syphilis (13, 14); in three studies, 17% to 32% of discordant samples subsequently tested negative with a treponemal test (14–16). Further, a recent review of POC test usage in 15 studies undertaken at both sexually transmitted infection and prenatal clinics showed an average sensitivity of only 86% (17). These studies highlight the limitations associated with current versions of the automated EIAs/CIAs and POC tests.
Previous research identified two antigens, Tp0326 (Tp92) and Tp0453, which exhibited high sensitivities and specificities when insoluble preparations of these proteins were screened against a panel of serum samples from patients who tested positive for syphilis and negative controls, respectively (18). In order to ensure reliable results when recombinant proteins are incorporated into commercial diagnostic tests, it is essential that the proteins be expressed in a soluble form. The purpose of this study was to further investigate the diagnostic potential of these proteins by producing soluble recombinant versions of Tp0326 and Tp0453 and a Tp0453-Tp0326 chimera, subsequently screening these soluble diagnostic candidates against a more extensive serum bank, and directly comparing the performances of these proteins with that of a commercially available EIA. These investigations showed that Tp0453 and the Tp0453-Tp0326 chimera exhibited equivalent overall sensitivities and higher specificities than the Captia syphilis (T. pallidum)-G enzyme immunoassay from Trinity Biotech. Thus, Tp0453 and the Tp0453-Tp0326 chimera are promising new candidates for incorporation into automated and POC diagnostic tests for syphilis.
MATERIALS AND METHODS
Preparation of Treponema pallidum subsp. pallidum (Nichols strain) genomic DNA.
T. pallidum subsp. pallidum (Nichols strain) was propagated in New Zealand White rabbits as described elsewhere (19). All animal studies were approved by the University of Victoria institutional review board and were conducted in strict accordance with standard accepted principles, as set forth by the Canadian Council on Animal Care, the National Institutes of Health, and the U.S. Department of Agriculture, in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and the Canadian Council on Animal Care.
Synthesis of the tp0326 and tp0453 constructs and the tp0453-tp0326 chimera construct.
The tp0326 construct used in this study encodes amino acid residues Q22 to N434 (GenBank accession number NP_218766; note correction of the start site) and was described previously by Cox et al. (tp0326 POTRA1-5/pET28a, referred to here as tp0326) (20). The tp0453 construct (encoding residues A32 to S287) was amplified from T. pallidum subsp. pallidum genomic DNA using the primer pair 5′-CTAGACCATATGGCATCAGTAGATCCGTTGG-3′ (sense) (the NdeI site is underlined) and 5′-GTCAGCTCGAGTCACGAACTTCCCTTTTTGGAG-3′ (antisense) (the XhoI site is underlined). The resulting amplicon was ligated into the cloning vector pJET1 (Fermentas, Burlington, ON, Canada), digested with the restriction enzymes NdeI and XhoI, and ligated into a similarly digested pET28a expression vector (EMD Inc., Mississauga, ON, Canada). The tp0453-tp0326 chimera construct includes the regions in the tp0326 and tp0453 constructs that encode amino acids Q22 to N434 for Tp0326 and A32 to S287 for Tp0453. The tp0453 sequence was placed 5′ of the tp0326 sequence, with a linker sequence (5′-GGTGGAGGTGGCAGTGGTGGAGGAGGAAGCGGCGGGGGTGGGTCA) positioned between the two constructs. This intervening sequence encodes a glycine-serine linker [(GGGGS)3] to provide a flexible spacer between the regions, as described previously by Trinh et al. (21). The chimeric DNA sequence was manually codon harmonized, as described by Angov et al. (22), in order to optimize soluble expression in Escherichia coli. The NdeI and XhoI restriction site sequences were added to the 5′ and 3′ ends, respectively, of the chimera construct. The final sequence (see Fig. S1 in the supplemental material) was created through DNA synthesis by Integrated DNA Technologies (Coralville, IA). The synthesized sequence, which was received in the pIDTSMART-Kan vector, was digested with NdeI and XhoI and ligated into a similarly digested pET28a vector (EMD Inc.). The sequences of the tp0326 and tp0453 constructs, as well as the tp0453-tp0326 chimera construct, within the pET28a vector were verified through DNA sequencing.
Recombinant protein expression and purification.
Expression constructs tp0326 and tp0453 and the tp0453-tp0326 chimera were transformed into the E. coli expression strain BL21 Star(DE3) (Invitrogen) according to the manufacturer's instructions. Recombinant protein expressions of Tp0326 and Tp0453 and the Tp0453-Tp0326 chimera were performed with 6 liters (Tp0453 and Tp0326) or 18 liters (Tp0453-Tp0326 chimera) of culture material following overnight induction of protein expression through the addition of 0.4 mM isopropyl-d-thiogalactopyranoside (Invitrogen). Bacteria were harvested through centrifugation, resuspended in 20 mM Tris (pH 7.5), 500 mM NaCl, 20 mM imidazole (Tp0326 and the Tp0453-Tp0326 chimera) or 25 mM HEPES (pH 7.5), 500 mM NaCl, 20 mM imidazole (Tp0453), and lysed either through sonication (Tp0326 and the Tp0453-Tp0326 chimera) or with a French press (Tp0453). The resulting lysates were centrifuged for 45 min at 20,000 × g at 4°C, and the supernatants containing the soluble recombinant proteins were removed and subjected to immobilized metal ion affinity chromatography and size exclusion chromatography as described previously (23).
Serum panel.
Ethics approval for this study was obtained from the University of British Columbia and University of Victoria human ethics boards. The serum samples for this study were provided by the British Columbia Centre for Disease Control Public Health Microbiology and Reference Laboratory. Patients with signs and symptoms consistent with syphilis were evaluated clinically by physicians at the British Columbia Centre for Disease Control Sexually Transmitted Infection Clinic, and serum samples from those patients were collected and used in this study. The positive serum samples included 169 characterized samples from patients with confirmed primary (n = 70), secondary (n = 47), or early latent (n = 52) syphilis infections. Positive samples were defined on the basis of positive results for both RPR and TP-PA assays or, in cases where only the RPR or the TP-PA test was positive, positive FTA-ABS test results. The serum bank also contained 70 samples from patients with negative test results for syphilis infection, including nonreactive (n = 11), analytical false-positive (positive TP-PA test results but negative RPR and FTA-ABS test results; n = 7), biological false-positive (positive RPR test results but negative TP-PA and FTA-ABS test results; n = 14), and potentially cross-reactive (n = 38) samples. The latter samples included serum samples from patients infected with Borrelia burgdorferi (n = 4), Leptospira spp. (n = 4), Helicobacter pylori (n = 5), cytomegalovirus (n = 5), Epstein-Barr virus (n = 5), hepatitis B virus (n = 10), or hepatitis C virus (n = 5). The diagnostic tests used to confirm each cross-reactive sample are listed in Table 1. All cross-reactive samples were screened with the RPR and TP-PA tests and found to be negative. All serum samples were stored at −20°C and underwent only 2 freeze-thaw cycles. All serum samples were screened in a blinded manner.
Table 1.
Serological diagnostic tests used to characterize the serum sample set (n = 239)
| Bacterium/virus | Diagnostic testa | Manufacturer |
|---|---|---|
| T. pallidum | Rapid plasma reagin test | Becton-Dickinson, Mississauga, ON, Canada |
| T. pallidum | T. pallidum particle agglutination assay | Fujirebio Inc., Malvern, PA, USA |
| T. pallidum | Fluorescent treponemal antibody absorption assay | Zeus Scientific, Branchburg, NJ, USA |
| B. burgdorferi | IgG Western blotting | MarDx Diagnostics, Inc., Carlsbad, CA, USA |
| Leptospira spp. | Panbio Leptospira IgM assay | Panbio, Brisbane, Queensland, Australia |
| H. pylori | H. pylori IgG assay | Siemens Healthcare, Mississauga, ON, Canada |
| Cytomegalovirus | VIDAS cytomegalovirus IgG assay | bioMérieux, St Laurent, QC, Canada |
| Epstein-Barr virus | Enzygnost anti-EBV IgG/IgM assay | Dade Behring/Siemens Healthcare, Mississauga, ON, Canada |
| Hepatitis B virus | Total anti-HBs or anti-HBc assay | Siemens Healthcare |
| Hepatitis C virus | HCV assay | Siemens Healthcare |
CMV, cytomegalovirus; EBV, Epstein-Barr virus; HBs, hepatitis B surface antigen; HBc, hepatitis B core antigen; HCV, hepatitis C virus.
Enzyme-linked immunosorbent assays.
Ninety-six-well plates (Nunc-Immuno MaxiSorp; Sigma-Aldrich) were incubated overnight at 4°C with 50 μl of 6.0 μg/ml recombinant protein solution in phosphate-buffered saline (PBS) (pH 7.4). Plates were blocked for 2 h at room temperature with 1× PBS, 4% milk powder. Wells were washed 3 times with wash buffer (1× PBS, 0.05% Tween 20). Human serum was diluted 1:400 in dilution buffer (1× PBS [pH 7.4], 4% milk powder, 0.2% Triton X-100), and 50 μl of diluted serum was added to plates in triplicate and incubated for 1 h at room temperature. Plates were washed with 3 quick washes and 3 10-min washes. Goat anti-human IgG (Fab-specific) peroxidase (Sigma-Aldrich) was diluted 1:3,000 in dilution buffer, 50 μl was added to each well, and the mixture was incubated for 1 h at room temperature. Plates were washed as described above and developed by adding 100 μl of tetramethylbenzidine-H2O2 substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) to each well for 30 min at room temperature. Absorbance was read at 630 nm. All wash steps and the primary and secondary antibody incubations were done on a rotator operating at 80 rpm.
Statistical analyses.
Immunoassay cutoff value determination was done using serum samples from 24 patients who tested negative for syphilis infection. The samples used for this part of the study were obtained from patients with negative RPR and TP-PA test results and differed from the samples used for the final immunological screening. Each sample was run in triplicate in three independent assays. The average absorbance for the negative serum samples in three assay runs was calculated using the following equation:
where n is the number of replicates for each protein and y. represents the average absorbance for each run (for the 24 samples). The empirical standard error was estimated using the following equation:
Cutoff values with 98% confidence intervals were calculated using the following equation:
where 6.965 is the critical value of the T distribution for a 98% confidence interval (with 2 degrees of freedom). The upper limit of the 98% confidence interval defined the cutoff values for Tp0326, Tp0453, and the Tp0453-Tp0326 chimera, giving values of 0.138, 0.116, and 0.148, respectively. Equivocal range values of ±10% were calculated, giving ranges of 0.124 to 0.151, 0.104 to 0.127, and 0.133 to 0.163 for Tp0326, Tp0453, and the Tp0453-Tp0326 chimera, respectively (24). Positive samples were defined as those with values above the equivocal ranges, and negative samples were defined as those with values below the equivocal ranges. Values within the equivocal ranges were not included in the final sensitivity and specificity calculations.
Captia syphilis (T. pallidum)-G enzyme immunoassay.
The serum panel described above, including positive (from all stages of infection), nonreactive, analytical false-positive, biological false-positive, and potentially cross-reactive samples, was screened in duplicate using the Captia syphilis (T. pallidum)-G EIA (Trinity Biotech, Bray, Ireland) and 25 μl serum in a total volume of 500 μl, according to the manufacturer's instructions. Antibody indices were calculated by dividing the mean absorbance of the patient samples by the mean absorbance of the low-titer reactive controls provided with the kit. Antibody indices within the range of 0.9 to 1.1 were considered equivocal, whereas indices of ≥1.1 were considered positive, and indices of ≤0.9 were considered negative.
RESULTS
Production of soluble recombinant proteins.
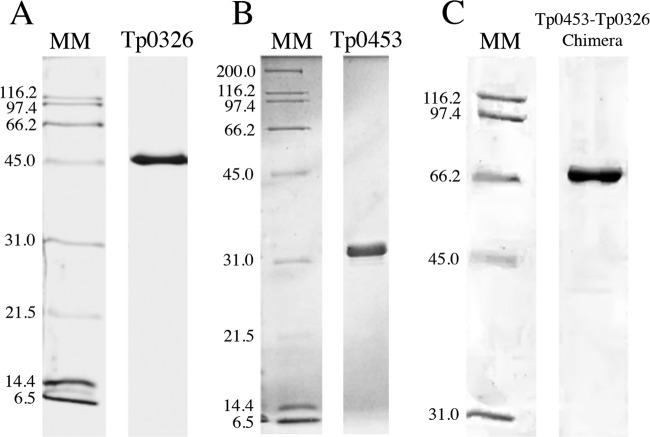
Previous investigations conducted using insoluble recombinant versions of the T. pallidum proteins Tp0326 (Tp92) and Tp0453 identified these proteins as being highly reactive with serum samples collected from syphilis patients (18). The insoluble nature of these recombinant proteins prevented further analyses of their potential for the serodiagnosis of syphilis, since commercial diagnostic tests require soluble proteins in order to facilitate standardization. To explore the diagnostic potential of the proteins, in this study we produced soluble versions of Tp0326 and Tp0453. The soluble Tp0326 recombinant protein, described previously by Cox et al. (20), encompasses amino acid residues Q22 to N434 of the full-length protein sequence and was produced as a single protein that was determined through SDS-PAGE analysis to have an estimated purity of >98% (Fig. 1A). The production of soluble recombinant Tp0453 was achieved through expression of amino acid residues A32 to S287 using the pET28a expression vector and the E. coli expression strain BL21 Star(DE3). This recombinant protein preparation was also judged to be >98% pure via SDS-PAGE analysis (Fig. 1B). Numerous attempts were made to produce a soluble chimeric construct of Tp0326 and Tp0453 (data not shown); only one construct (Tp0453-Tp0326 chimera) resulted in successful soluble expression in E. coli. This chimera included the amino acid residues in the individual Tp0326 and Tp0453 recombinant proteins (amino acids Q22 to N434 for Tp0326 and A32 to S287 for Tp0453), with the Tp0453 region being positioned N-terminally to the Tp0326 fragment (Fig. 2). Prior attempts to create a Tp0453-Tp0326 chimera consistently resulted in the expression of a truncated protein product, which was hypothesized to be due to the difference in codon usage between T. pallidum and the E. coli expression strain utilized in this study. Numerous studies have suggested that species-to-species differences in codon usage can account for the difficulties encountered in recombinant protein expression, including lack of expression, insoluble protein production, and premature translation termination (25–30). Codon harmonization is a technique that matches the codons used in the gene encoding the protein of interest with the codon preferences of the bacterial expression strain. Using codon frequency tables for T. pallidum subsp. pallidum and E. coli, the sequences of tp0326 and tp0453 were codon harmonized for expression in E. coli. A flexible linker consisting of three repeats of GGGGS, as described previously by Trinh et al. (21), was used to increase the distance between the tp0326 and tp0453 regions. Linker regions have been shown to promote proper folding and increased solubility by reducing the interactions between separate chimeric regions (21). Collectively, these optimization techniques resulted in the successful soluble expression and purification of a Tp0453-Tp0326 chimera that appeared as a single band in SDS-PAGE analyses and was judged to be >98% pure (Fig. 1C).
Fig 1.
SDS-PAGE analysis of the size exclusion chromatography elutions of soluble Tp0326 (49.4 kDa) (A), Tp0453 (30.8 kDa) (B), and the Tp0453-Tp0326 chimera (78.7 kDa) (C). MM, molecular mass markers.
Fig 2.
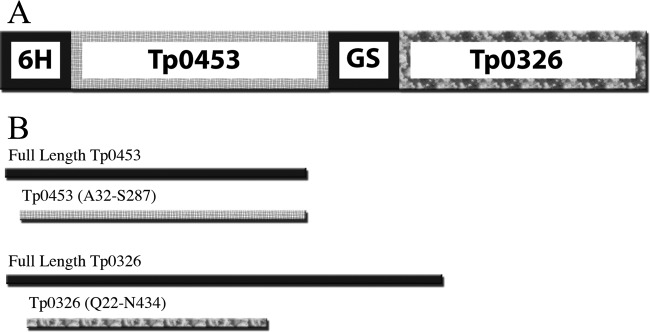
(A) Schematic of the Tp0453-Tp0326 chimera. 6H represents the 6× histidine tag, GS, incorporated glycine-serine linker region. (B) The portions of full-length Tp0453 (top) and Tp0326 (bottom) covered in the chimera.
Diagnostic performance of recombinant proteins.
The three soluble recombinant proteins (Tp0326, Tp0453, and the Tp0453-Tp0326 chimera) and, for comparison, the commercially available Captia syphilis (T. pallidum)-G EIA were tested against a well-characterized panel of serum samples from syphilis patients to determine their sensitivities for the diagnosis of syphilis infections (Table 2). Positive serum samples (n = 169) included those from patients with primary (n = 70), secondary (n = 47), or early latent (n = 52) syphilis infection. The overall sensitivity of Tp0326 was determined to be 86%, with sensitivities of 69%, 98%, and 94% for the detection of primary, secondary, and early latent infection, respectively. Tp0453 had an overall sensitivity of 98%, with sensitivities of 96%, 100%, and 100% for the detection of primary, secondary, and early latent infection, respectively. The Tp0453-Tp0326 chimera had an overall sensitivity of 98%, with sensitivities of 94%, 100%, and 100% for the detection of primary, secondary, and early latent infection, respectively. Finally, the Captia syphilis (T. pallidum)-G EIA exhibited an overall sensitivity of 98%, with sensitivities of 94%, 100%, and 100% for the detection of primary, secondary, and early latent infection, respectively.
Table 2.
Sensitivities of recombinant proteins Tp0326, Tp0453, and the Tp0453-Tp0326 chimera and Captia syphilis (T. pallidum)-G EIA, as determined through enzyme-linked immunosorbent assays of serum samples from patients who tested positive for primary, secondary, or early latent syphilis
| Protein screened and stage of syphilis infection | No. of sample results |
Sensitivity (%) | ||
|---|---|---|---|---|
| Positive | Negative | Equivocal | ||
| Tp0326 | ||||
| Primary | 41 | 18 | 11 | 69 |
| Secondary | 45 | 1 | 1 | 98 |
| Early latent | 46 | 3 | 3 | 94 |
| All | 132 | 22 | 15 | 86 |
| Tp0453 | ||||
| Primary | 64 | 3 | 3 | 96 |
| Secondary | 47 | 0 | 0 | 100 |
| Early latent | 52 | 0 | 0 | 100 |
| All | 163 | 3 | 3 | 98 |
| Tp0453-Tp0326 chimera | ||||
| Primary | 58 | 4 | 8 | 94 |
| Secondary | 47 | 0 | 0 | 100 |
| Early latent | 52 | 0 | 0 | 100 |
| All | 157 | 4 | 8 | 98 |
| Captia syphilis (T. pallidum)-G EIA | ||||
| Primary | 62 | 4 | 4 | 94 |
| Secondary | 47 | 0 | 0 | 100 |
| Early latent | 52 | 0 | 0 | 100 |
| All | 161 | 4 | 4 | 98 |
To determine the specificities of the recombinant proteins, Tp0326, Tp0453, and the Tp0453-Tp0326 chimera were tested against negative serum samples (n = 70), including nonreactive (n = 11), analytical false-positive (positive TP-PA test results but negative RPR and FTA-ABS test results) (n = 7), biological false-positive (positive RPR test results but negative TP-PA and FTA-ABS test results) (n = 14), and potentially cross-reactive (n = 38) samples (Table 3). The overall specificities for Tp0326, Tp0453, and the Tp0453-Tp0326 chimera were 99%, 100%, and 99%, respectively. All three proteins exhibited 100% specificity when tested against the nonreactive and false-positive serum samples. The potentially cross-reactive sera included samples from patients infected with B. burgdorferi (n = 4), Leptospira spp. (n = 4), H. pylori (n = 5), cytomegalovirus (n = 5), Epstein-Barr virus (n = 5), hepatitis B virus (n = 10), or hepatitis C virus (n = 5). Tp0326 exhibited a positive reaction to one serum sample from a patient infected with B. burgdorferi, while the Tp0453-Tp0326 chimera exhibited a similar positive reaction to one serum sample from a patient infected with the Epstein-Barr virus. Tp0453 showed no reactivity to any of the potentially cross-reactive serum samples. For comparison, the Captia syphilis (T. pallidum)-G EIA exhibited an overall specificity of 90% when screened with this sample set, with the commercially available EIA exhibiting positive reactions to serum samples from a patient infected with the Epstein-Barr virus, an uninfected individual, an individual who had a biological false-positive reaction, and four individuals who had analytical false-positive reactions.
Table 3.
Specificities of recombinant proteins Tp0326, Tp0453, and the Tp0453-Tp0326 chimera and Captia syphilis (T. pallidum)-G EIA, as determined through enzyme-linked immunosorbent assays of serum samples from individuals who tested negative for syphilis infection, including serum samples from patients with potentially cross-reactive infections
| Protein screened and serum used | No. of sample results |
Specificity (%) | ||
|---|---|---|---|---|
| Positive | Negative | Equivocal | ||
| Tp0326 | ||||
| Nonreactive | 0 | 11 | 0 | 100 |
| Analytical false positive | 0 | 7 | 0 | 100 |
| Biological false positive | 0 | 14 | 0 | 100 |
| Borrelia burgdorferi | 1 | 2 | 1 | 67 |
| Leptospira spp. | 0 | 4 | 0 | 100 |
| Helicobacter pylori | 0 | 5 | 0 | 100 |
| Cytomegalovirus | 0 | 4 | 1 | 100 |
| Epstein-Barr virus | 0 | 5 | 0 | 100 |
| Hepatitis B virus | 0 | 10 | 0 | 100 |
| Hepatitis C virus | 0 | 5 | 0 | 100 |
| All negative samples | 1 | 67 | 2 | 99 |
| Tp0453 | ||||
| Nonreactive | 0 | 11 | 0 | 100 |
| Analytical false positive | 0 | 6 | 1 | 100 |
| Biological false positive | 0 | 14 | 0 | 100 |
| Borrelia burgdorferi | 0 | 4 | 0 | 100 |
| Leptospira spp. | 0 | 4 | 0 | 100 |
| Helicobacter pylori | 0 | 5 | 0 | 100 |
| Cytomegalovirus | 0 | 5 | 0 | 100 |
| Epstein-Barr virus | 0 | 5 | 0 | 100 |
| Hepatitis B virus | 0 | 10 | 0 | 100 |
| Hepatitis C virus | 0 | 5 | 0 | 100 |
| All negative samples | 0 | 69 | 1 | 100 |
| Tp0453-Tp0326 chimera | ||||
| Nonreactive | 0 | 11 | 0 | 100 |
| Analytical false positive | 0 | 7 | 0 | 100 |
| Biological false positive | 0 | 14 | 0 | 100 |
| Borrelia burgdorferi | 0 | 4 | 0 | 100 |
| Leptospira spp. | 0 | 4 | 0 | 100 |
| Helicobacter pylori | 0 | 5 | 0 | 100 |
| Cytomegalovirus | 0 | 5 | 0 | 100 |
| Epstein-Barr virus | 1 | 3 | 1 | 75 |
| Hepatitis B virus | 0 | 10 | 0 | 100 |
| Hepatitis C virus | 0 | 5 | 0 | 100 |
| All negative samples | 1 | 68 | 1 | 99 |
| Captia syphilis (T. pallidum)-G EIA | ||||
| Nonreactive | 1 | 10 | 0 | 91 |
| Analytical false positive | 4 | 3 | 0 | 43 |
| Biological false positive | 1 | 13 | 0 | 93 |
| Borrelia burgdorferi | 0 | 3 | 1 | 100 |
| Leptospira spp. | 0 | 4 | 0 | 100 |
| Helicobacter pylori | 0 | 5 | 0 | 100 |
| Cytomegalovirus | 0 | 5 | 0 | 100 |
| Epstein-Barr virus | 1 | 4 | 0 | 80 |
| Hepatitis B virus | 0 | 10 | 0 | 100 |
| Hepatitis C virus | 0 | 5 | 0 | 100 |
| All negative samples | 7 | 62 | 1 | 90 |
DISCUSSION
Of the three diagnostic protein candidates analyzed in this study, Tp0326 was found to perform the least well, exhibiting a sensitivity of 86% and a specificity of 99%. Tp0326 is an ortholog of the BamA molecule (31, 32), and the polypeptide transport-associated (POTRA) region used in this study is partially conserved among Gram-negative bacteria (31, 33, 34). This may explain the reactivity seen in one serum sample from a patient who tested positive for infection with B. burgdorferi. Further, the moderate sensitivity observed for Tp0326 suggests that this protein by itself may not represent an effective diagnostic candidate.
Tp0453 was determined to be the best diagnostic candidate in this study, exhibiting a sensitivity of 98% and a specificity of 100%. These results match the results reported by Van Voorhis and coworkers, who showed that an insoluble preparation of Tp0453 exhibited 100% sensitivity and 100% specificity in the screening of 83 serum samples (18). No serum samples from patients who tested negative for syphilis infection showed reactivity against Tp0453, not even those from patients infected with the related spirochetes B. burgdorferi and Leptospira spp. The high specificities seen in this study may be explained by the unique nature of Tp0453, which has no homologs in other bacteria (35–37). The coding region used in the tp0453 construct is identical in all sequenced strains of T. pallidum, suggesting that patients infected with any strain of T. pallidum would likely have antibodies against this protein (36, 37).
The strong immunoreactivities exhibited by Tp0326 and Tp0453 suggested that creating a chimera of these two proteins that contains epitopes from both proteins might increase sensitivity. The soluble Tp0453-Tp0326 chimera created for this study showed high sensitivity and specificity (98% and 99%, respectively), making this construct another potential candidate for use in the diagnosis of syphilis. However, our hypothesis that the Tp0453-Tp0326 chimera would identify more syphilis infections has not yet been confirmed in the sample set we have analyzed to date, since the sensitivities of Tp0453 and the Tp0453-Tp0326 chimera were approximately equal. The difference in specificities was also not statistically significant, indicating that further screening with a larger sample set needs to be conducted to determine if the Tp0453-Tp0326 chimera indeed performs better in accurately detecting syphilis infections due to the incorporation of the two protein regions.
Direct comparison of the diagnostic performances of the soluble recombinant T. pallidum proteins that are the focus of this study and the Captia syphilis (T. pallidum)-G EIA showed that Tp0453 and the Tp0453-Tp0326 chimera exhibited an equal sensitivity (98%) and a higher specificity (99% to 100%) than the commercially available EIA (98% sensitivity and 90% specificity). In particular, both Tp0453 and the chimera exhibited 100% diagnostic accuracy with analytical false-positive samples, in comparison with the extremely low accuracy observed for these samples with the Captia assay (43%). The lower false-reaction rates exhibited by these novel T. pallidum antigens suggest that these antigens may relieve some of the limitations currently seen with implementation of the reverse algorithm in populations with low disease prevalence.
It should be emphasized that this study relied on the RPR, TP-PA, and FTA-ABS assays to define positive syphilis infections. Although the serum samples used in this study were clinically characterized, the inherent propensity of all three tests to generate false-positives allows for speculation that some of the false-negative results noted in these investigations might actually be true-negative results. Nontreponemal tests have been shown to exhibit cross-reactivity in a multitude of diseases and health conditions (8), and the false-positive results observed in traditional screening algorithms are thought to arise from cross-reactive antibodies derived from commensal bacteria (10, 38). The three samples identified in this study by Tp0326, Tp0453, and the Tp0453-Tp0326 chimera as potential false-negatives were defined as primary cases, with positive TP-PA test results but low titers in the RPR test (ranging from 1:1 to 1:4). These serum samples also tested negative with the Captia syphilis (T. pallidum)-G EIA in this study. These discordant results may result from the IgG-specific detection format used for our novel diagnostic candidates and the Captia EIA, in comparison with the broader IgG/IgM-specific detection capacity of the TP-PA test.
Using traditional screening algorithms, false-negative results for samples collected from patients with early primary infection may result from the frequent lack of nontreponemal antibodies in these patients (10). Our results show high sensitivities for primary-stage infection (96% and 94% for Tp0453 and the Tp0453-Tp0326 chimera, respectively), which were within the upper range of values for currently used EIAs and CIAs (77% to 100%) (7) and equaled (Tp0453-Tp0326 chimera) or surpassed (Tp0453) the 94% sensitivity observed for the Captia syphilis (T. pallidum)-G EIA with primary-stage samples in this study. A goal for future investigation might be to determine if Tp0453 and the Tp0453-Tp0326 chimera could accurately diagnose RPR test- or VDRL test-negative serum samples from patients determined through dark-field microscopy to have early primary syphilis infection. Demonstration of a positive result in such a study would serve to verify the results reported by Van Voorhis et al. (18), who found that four patients with dark-field microscopy-confirmed early primary syphilis infection and negative VDRL test results exhibited positive reactions with insoluble versions of Tp0453 and Tp0326, suggesting that these two diagnostic candidates may be particularly adept at identifying early cases of syphilis infection.
Since both Tp0453 and the Tp0453-Tp0326 chimera are expressed in soluble form, they are compatible with commercially available diagnostic test formats, including EIAs, CIAs, and POC tests. Although most automated and POC tests for syphilis currently use multiple recombinant treponemal proteins, which has the potential to confound results since patients may have different serological profiles for such antigens regardless of the presence of infection (39). Furthermore, the development of a test focusing on a single recombinant treponemal protein should reduce costs associated with the production and mixing of multiple antigens. Moreover, Tp0453 and/or the Tp0453-Tp0326 chimera show potential for exhibiting greater specificities than the currently used recombinant treponemal proteins, which may eliminate the need to perform multiple tests and may alleviate the high false-reaction rate observed in the screening of populations with low disease prevalence.
In summary, in this study we have identified the novel recombinant diagnostic candidates Tp0453 and the Tp0453-Tp0326 chimera, which exhibited high sensitivity (98%) for detection of all stages of infection and were extremely specific (100% and 99%, respectively) even when tested against potentially cross-reactive sera. In a direct comparison, the diagnostic performances of these proteins equaled or surpassed that of the commercially available Captia syphilis (T. pallidum)-G EIA with respect to sensitivity and specificity, respectively. Thus, the use of these proteins in a diagnostic test format could alleviate some of the limitations currently confronting the field of syphilis diagnosis. Further, the ability to produce these proteins in a soluble form should facilitate their incorporation into multiple diagnostic test formats, including automated assays and rapid POC tests.
Supplementary Material
ACKNOWLEDGMENTS
We thank Justin Radolf and Daniel Desrosiers, University of Connecticut Health Center, for their gift of the tp0326 POTRA1-5/pET28a construct, Quantine Wong, Raymond Wada, Ida Wang, Jonathan Laley, and all the staff at the Zoonotic Diseases and Emerging Pathogens Section of the British Columbia Centre for Disease Control Public Health Microbiology and Reference Laboratory for their assistance with screening, Sheila Lukehart and Wesley Van Voorhis, University of Washington, for helpful discussions related to this project, and Martin Boulanger, University of Victoria, for his assistance with the tp0453 construct design.
This work was funded by an operating grant from the Canadian Institutes of Health Research (grant HPR 85530) (to Caroline E. Cameron) and a Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarship (awarded to Brenden C. Smith).
Footnotes
Published ahead of print 24 October 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01390-12.
REFERENCES
- 1. Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl 1):S12–S16 [PubMed] [Google Scholar]
- 2. Fleming DT, Wasserheit JN. 1999. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex. Transm. Infect. 75: 3– 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciesielski CA. 2003. Sexually transmitted diseases in men who have sex with men: an epidemiologic review. Curr. Infect. Dis. Rep. 5: 145– 152 [DOI] [PubMed] [Google Scholar]
- 4. Simms I, Fenton KA, Ashton M, Turner KM, Crawley-Boevey EE, Gorton R, Thomas DR, Lynch A, Winter A, Fisher MJ, Lighton L, Maguire HC, Solomou M. 2005. The re-emergence of syphilis in the United Kingdom: the new epidemic phases. Sex. Transm. Dis. 32: 220– 226 [DOI] [PubMed] [Google Scholar]
- 5. Schmid GP, Stoner BP, Hawkes S, Broutet N. 2007. The need and plan for global elimination of congenital syphilis. Sex. Transm. Dis. 34(Suppl):S5–S10 [DOI] [PubMed] [Google Scholar]
- 6. Larsen SA, Steiner BM, Rudolph AH. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 8: 1– 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sena AC, White BL, Sparling PF. 2010. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin. Infect. Dis. 51: 700– 708 [DOI] [PubMed] [Google Scholar]
- 8. Ratnam S. 2005. The laboratory diagnosis of syphilis. Can. J. Infect. Dis. Med. Microbiol. 16: 45– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castro R, Prieto ES, Santo I, Azevedo J, Exposto FL. 2003. Evaluation of an enzyme immunoassay technique for detection of antibodies against Treponema pallidum. J. Clin. Microbiol. 41: 250– 253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoover KW, Radolf JD. 2011. Serodiagnosis of syphilis in the recombinant era: reversal of fortune. J. Infect. Dis. 204: 1295– 1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binnicker MJ, Jespersen DJ, Rollins LO. 2011. Treponema-specific tests for serodiagnosis of syphilis: comparative evaluation of seven assays. J. Clin. Microbiol. 49: 1313– 1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loeffelholz MJ, Binnicker MJ. 2012. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J. Clin. Microbiol. 50: 2– 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Binnicker MJ, Jespersen DJ, Rollins LO. 2012. Direct comparison of the traditional and reverse syphilis screening algorithms in a population with a low prevalence of syphilis. J. Clin. Microbiol. 50: 148– 150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention 2011. Discordant results from reverse sequence syphilis screening: five laboratories, United States, 2006-2010. MMWR Morb. Mortal. Wkly. Rep. 60: 133– 137 [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention 2008. Syphilis testing algorithms using treponemal tests for initial screening: four laboratories, New York City, 2005-2006. MMWR Morb. Mortal. Wkly. Rep. 57: 872– 875 [PubMed] [Google Scholar]
- 16.Park IU, Chow JM, Bolan G, Stanley M, Shieh J, Schapiro JM. 2011. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J. Infect. Dis. 204: 1297– 1304 [DOI] [PubMed] [Google Scholar]
- 17. Tucker JD, Bu J, Brown LB, Yin YP, Chen XS, Cohen MS. 2010. Accelerating worldwide syphilis screening through rapid testing: a systematic review. Lancet Infect. Dis. 10: 381– 386 [DOI] [PubMed] [Google Scholar]
- 18. Van Voorhis WC, Barrett LK, Lukehart SA, Schmidt B, Schriefer M, Cameron CE. 2003. Serodiagnosis of syphilis: antibodies to recombinant Tp0453, Tp92, and Gpd proteins are sensitive and specific indicators of infection by Treponema pallidum. J. Clin. Microbiol. 41: 3668– 3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lukehart SA, Marra CM. 2007. Isolation and laboratory maintenance of Treponema pallidum. Curr. Protoc. Microbiol. 12: Unit 12A.1. [DOI] [PubMed] [Google Scholar]
- 20. Cox DL, Luthra A, Dunham-Ems S, Desrosiers DC, Salazar JC, Caimano MJ, Radolf JD. 2010. Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect. Immun. 78:5178– 5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trinh R, Gurbaxani B, Morrison SL, Seyfzadeh M. 2004. Optimization of codon pair use within the (GGGGS)3 linker sequence results in enhanced protein expression. Mol. Immunol. 40: 717– 722 [DOI] [PubMed] [Google Scholar]
- 22. Angov E, Hillier CJ, Kincaid RL, Lyon JA. 2008. Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS One 3: e2189 doi:10.1371/journal.pone.0002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Houston S, Hof R, Francescutti T, Hawkes A, Boulanger MJ, Cameron CE. 2011. Bifunctional role of the Treponema pallidum extracellular matrix binding adhesin Tp0751. Infect. Immun. 79:1386– 1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burd EM. 2010. Validation of laboratory-developed molecular assays for infectious diseases. Clin. Microbiol. Rev. 23: 550– 576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adzhubei AA, Adzhubei IA, Krasheninnikov IA, Neidle S. 1996. Non-random usage of “degenerate” codons is related to protein three-dimensional structure. FEBS Lett. 399: 78– 82 [DOI] [PubMed] [Google Scholar]
- 26. Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. 2007. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315: 525– 528 [DOI] [PubMed] [Google Scholar]
- 27. Komar AA, Lesnik T, Reiss C. 1999. Synonymous codon substitutions affect ribosome traffic and protein folding during in vitro translation. FEBS Lett. 462: 387– 391 [DOI] [PubMed] [Google Scholar]
- 28. Kurland C, Gallant J. 1996. Errors of heterologous protein expression. Curr. Opin. Biotechnol. 7: 489– 493 [DOI] [PubMed] [Google Scholar]
- 29. Lindsley D, Gallant J, Guarneros G. 2003. Ribosome bypassing elicited by tRNA depletion. Mol. Microbiol. 48: 1267– 1274 [DOI] [PubMed] [Google Scholar]
- 30. Zhang G, Hubalewska M, Ignatova Z. 2009. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat. Struct. Mol. Biol. 16: 274– 280 [DOI] [PubMed] [Google Scholar]
- 31. Desrosiers DC, Anand A, Luthra A, Dunham-Ems SM, LeDoyt M, Cummings MA, Eshghi A, Cameron CE, Cruz AR, Salazar JC, Caimano MJ, Radolf JD. 2011. TP0326, a Treponema pallidum beta-barrel assembly machinery A (BamA) orthologue and rare outer membrane protein. Mol. Microbiol. 80: 1496– 1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gentle IE, Burri L, Lithgow T. 2005. Molecular architecture and function of the Omp85 family of proteins. Mol. Microbiol. 58: 1216– 1225 [DOI] [PubMed] [Google Scholar]
- 33. Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 181: 1401– 1413 [DOI] [PubMed] [Google Scholar]
- 34. Sanchez-Pulido L, Devos D, Genevrois S, Vicente M, Valencia A. 2003. POTRA: a conserved domain in the FtsQ family and a class of beta-barrel outer membrane proteins. Trends Biochem. Sci. 28: 523– 526 [DOI] [PubMed] [Google Scholar]
- 35. Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith HO, Venter JC. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281: 375– 388 [DOI] [PubMed] [Google Scholar]
- 36. Hazlett KR, Cox DL, Decaffmeyer M, Bennett MP, Desrosiers DC, La Vake CJ, La Vake ME, Bourell KW, Robinson EJ, Brasseur R, Radolf JD. 2005. Tp0453, a concealed outer membrane protein of Treponema pallidum, enhances membrane permeability. J. Bacteriol. 187: 6499– 6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matejková P, Strouhal M, Smajs D, Norris SJ, Palzkill T, Petrosino JF, Sodergren E, Norton JE, Singh J, Richmond TA, Molla MN, Albert TJ, Weinstock GM. 2008. Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 8: 76 doi:10.1186/1471-2180-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller JN. 1975. Value and limitations of nontreponemal and treponemal tests in the laboratory diagnosis of syphilis. Clin. Obstet. Gynecol. 18:191– 203 [DOI] [PubMed] [Google Scholar]
- 39. Martin IE, Lau A, Sawatzky P, Tsang RS, Cuff W, Lee C, Macpherson PA, Mazzulli T. 2008. Serological diagnosis of syphilis: enzyme-linked immunosorbent assay to measure antibodies to individual recombinant Treponema pallidum antigens. J. Immunoassay Immunochem. 29: 143– 151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.