Abstract
The Bacillus cereus pathogenic spectrum ranges from strains used as probiotics to human-lethal strains. However, prediction of the pathogenic potential of a strain remains difficult. Here, we show that food poisoning and clinical strains can be differentiated from harmless strains on the basis of host colonization phenotypes.
TEXT
Bacillus cereus is an emerging human food-borne pathogen (1). B. cereus-induced gastroenteritis is generally mild, but bloody diarrhea and emetic poisoning leading to some fatal cases have been reported (2). B. cereus is also associated with severe local and systemic human infections, such as endophthalmitis, pneumonia, and meningitis, posing a public health problem (3, 4). The pathogenic spectrum of B. cereus ranges from strains that are used as probiotics to humans to lethal, highly toxic strains (5, 6). B. cereus is thus complex, harboring strains differing considerably in terms of their economic and sanitary importance. It is therefore important to determine the extent to which pathogenic strains can be distinguished from nonpathogenic strains. At the chromosomal level, the reasons for this strong heterogeneity are unknown and there are currently no specific markers for unambiguously differentiating pathogenic from harmless strains. Indeed, the genetic studies carried out to date have been inconclusive and, regardless of the diseases they cause, all strains seem to carry genes encoding at least one of the known diarrheal toxins (Nhe, Hbl, CytK) (7). Although the diarrheal toxin production seems on average higher in strains involved in diarrhea (7), highly toxic strains do not express these toxins (6, 7), and other factors are involved during the infection process. For example, HlyII, which causes macrophage death (8), is found in pathogenic strains, although its prevalence in such strains is only about 30% (9). Thus, although these markers provide indications as to the pathogenic potential of a given strain, they do not yet explain the pathogenicity of all strains and are not sufficient to differentiate them. In the absence of specific markers, our objective was to develop new criteria for the identification of pathogenic strains.
The pathogenicity of B. cereus is dependent on its ability to colonize and persist in the host and subsequently to invade tissues. This encompasses mechanisms for colonization and overcoming primary defense mechanisms (10–12). We therefore aimed to link different colonization/resistance phenotypes to virulence to try to identify traits that could be used to differentiate between pathogenic and harmless strains. For this purpose, we used a B. cereus strain collection representative of its pathological diversity in humans. We compared 25 strains with no history of involvement in food poisoning (NP) with 29 strains known to have caused food poisoning outbreaks of different origins (FP) or to be associated with other clinical independent nongastrointestinal diseases (C) (Table 1). All strains were grown in laboratory medium (14) at 30°C, under agitation, to mid-exponential growth phase, and for each strain, we assessed motility, adhesion to epithelial cells, cytotoxicity, biofilm formation, antimicrobial peptide (AMP) resistance, and virulence in an insect model of infection. For each group, the mean value of each phenotype is shown (Fig. 1A). In addition, a threshold value was defined (median value of all strains) for each phenotype and the percentage of strains with values above the threshold is given (Fig. 1B). Differences between the B. cereus groups were analyzed by the pairwise Student t test or by the Pearson chi-square analysis.
Table 1.
Strain collection, origin, and genetic group
| Pathogenic profile and genetic group | Strain | Sourcea | Reference or sourceb |
|---|---|---|---|
| Nonpathogenic (NP) | |||
| II | INRA BC′ | Vegetable | 7 |
| INRA BN | Vegetable | 13 | |
| III | INRA PF | Milk protein | 7, 13 |
| INRA PA | Milk protein | 7, 13 | |
| IV | INRA A3 | Starch | 13 |
| I23 | Cooked apple | 13 | |
| I13 | Cooked rice | 13 | |
| I2 | Dried fruit | 7, 13 | |
| V | SB′ | Vegetable field | 13 |
| I11 | Cooked food | 13 | |
| VI | INRA 1 | Pasteurized zucchini puree | 7, 13 |
| INRA 5 | Pasteurized zucchini puree | 7, 13 | |
| INRA BK | Vegetable | 7 | |
| INRA BL | Vegetable | 7 | |
| INRA C1 | Pasteurized vegetables | 7, 13 | |
| INRA C46 | Pasteurized vegetables | 7 | |
| INRA C64 | Pasteurized vegetables | 7 | |
| INRA C74 | Pasteurized vegetables | 7, 13 | |
| ADRIA I3 | Cooked foods | 7, 13 | |
| ADRIA I20 | Cooked foods | 7, 13 | |
| ADRIA I21 | Cooked foods | 7, 13 | |
| INRA SL′ | Soil | 7, 13 | |
| INRA SO | Soil | 7, 13 | |
| INRA SV | Soil | 7, 13 | |
| WSBC 10204 | Pasteurized milk | 13 | |
| Food poisoning (FP) | |||
| II | NVH 0861/00 | Diarrheal outbreak | 7, 13 |
| III | NVH 0500/00 | Diarrheal outbreak | 7, 13 |
| NVH 0075/95 | Diarrheal outbreak | 13 | |
| NVH 1519/00 | Diarrheal outbreak | 13 | |
| F3371/93 | Diarrheal outbreak | 7, 13 | |
| F4433/73 | Diarrheal outbreak | 7, 13 | |
| LMG 17615 (F289/78) | Diarrheal outbreak | 7 | |
| NVH 200 | Diarrheal outbreak | 13 | |
| IV | NVH 0230/00 | Diarrheal outbreak | 7, 13 |
| NVH 1230/88 | Diarrheal outbreak | 7, 13 | |
| 98HMPL63 | Diarrheal outbreak | 7, 13 | |
| F2081A/98 | Diarrheal outbreak | 7, 13 | |
| F352/90 | Diarrheal outbreak | 7, 13 | |
| F4430/73 | Diarrheal outbreak | 7, 13 | |
| VII | NVH 0391/98 | Diarrheal outbreak | 7, 13 |
| Clinical nongastrointestinal infection (C) | |||
| II | AH1125 | Human | c |
| III | DSM 4222 (F837/76) | Human, postoperative infection | 7, 13 |
| AH728 | Human, urine | c | |
| AH825 | Human, periodontitis | c | |
| AH1127 | Human, eye | c | |
| AH1131 | Human, eye | c | |
| B06_007 | Human, cutaneous | c | |
| B06_018 | Human, peritoneal | c | |
| IV | AH726 | Human, urine | c |
| AH1293 | Human, blood | c | |
| B06_019 | Human, wound | c | |
| B06_034 | Human | c | |
| B06_036 | Human, blood | c | |
| V | B06_015 | Human, drainage tube | c |
All diarrheal outbreaks were independent; all human strains were isolated from different patients.
c, University of Oslo Bacillus cereus group MultiLocus Sequence Typing website (http://mlstoslo.uio.no).
Fig 1.
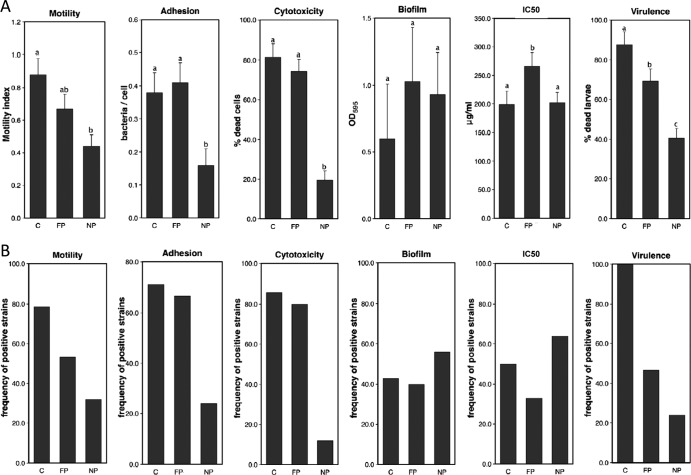
Phenotypic analyses of representative B. cereus strains. (A) Quantitative mean values ± standard deviations for motility, epithelial cell adhesion, cytotoxicity, biofilm formation, polymyxin B resistance, and virulence in the model insect G. mellonella for each group of strains. NP, nonpathogenic strains; FP, food poisoning strains; C, clinical strains. Bars with different letters (a, b, c) indicate significant differences in their mean values (P < 0.005). Results are the means of results of at least three independent experiments. (B) Frequencies of strains positive for each of the tested phenotypes in the various B. cereus groups. NP, nonpathogenic strains; FP, food poisoning strains; C, clinical strains. Frequencies were calculated relative to a threshold value defined for each phenotype as the median value for all strains.
Motility of the strains was assessed by measuring the zones of bacterial growth on soft agar plates (15). NP strains were generally less motile than FP and C strains (Fig. 1A). With respect to the threshold value (Fig. 1B), 30% of the NP strains were considered motile, whereas 53% and 80% of pathogenic strains (FP and C, respectively) were motile (P < 0.02).
Adhesion of the strains to epithelial cells was assessed as previously described (16). All NP strains displayed low levels of adhesion to epithelial cells (not shown). The mean adhesion level for this group was significantly lower (P < 0.004) than that for pathogenic strains (Fig. 1A). Only 20% of the NP strains adhered to epithelial cells, whereas more than 70% of the pathogenic strains adhered to epithelial cells (Fig. 1B).
Cytotoxicity of culture supernatants was evaluated by the trypan blue dye method (17, 18). The pathogenic strains (FP and C) were highly toxic to human cells (more than 80% of the strains), whereas cytotoxicity was detected for only 12% of the NP strains (P < 0.001; Fig. 1B). On average, NP strains were much less toxic (20% cell mortality) than FP or C strains (about 80% cell mortality; Fig. 1A). Adhesion, motility, and cytotoxicity may enable the bacteria to colonize their host, lyse specific tissues, disseminate, and reach deeper tissues. It is therefore not surprising that these properties confer on the strains a higher pathogenic potential.
Biofilm formation is a microbial survival strategy allowing cells to survive in hostile conditions and providing resistance to natural host defenses (19). Biofilm formation at the liquid-air interface was assessed for each strain, on polyvinyl chloride (PVC) microtiter plates, by crystal violet staining (20). On PVC plates, the pellicle is lost during the staining procedure and the biofilm quantified corresponds mainly to the ring formed in the microtiter wells. In all three groups, approximately 40 to 50% of the strains were able to form biofilms (Fig. 1B) of highly heterogeneous intensity (see standard errors in Fig. 1A), with no clear difference between groups. The ability to form biofilms might be a general fitness characteristic for B. cereus, required for the persistence of strains in various environments such as the host intestinal tract, food matrices, or hospital facilities. Thus, it is not possible to identify pathogenic strains on the basis of this characteristic. However, it is not excluded that biofilms might be involved in host colonization. In Staphylococcus aureus, biofilms are involved in infectious processes (21), and a probiotic strain of Bacillus subtilis requires biofilm-forming abilities to protect rodents from enteropathogenic Escherichia coli (22).
Host cells produce several bactericidal antimicrobial peptides, which are able to damage microbial membranes (23). Polymyxin B, a standard cyclic AMP (24), was used to allow a large-scale screening of the collection. Susceptibility to polymyxin B was evaluated by determining the inhibitory concentration of polymyxin B that reduces inoculum viability by 50% (IC50). Bacterial growth was scored after inoculation of the strains at an initial optical density at 600 nm (OD600) of 0.1 and incubation at 30°C for 6 h. The mean IC50 was slightly higher (P < 0.05) in FP strains than in NP strains (Fig. 1A). However, fewer FP strains than NP and C strains displayed polymyxin B resistance (Fig. 1B), indicating that only a small number of FP strains displayed a high IC50.
The virulence potential of the strains was assessed in vivo using the Galleria mellonella insect model as previously described (25). Defense mechanisms against bacterial infections are sufficiently similar in invertebrates and mammals to allow the use of invertebrates as infection models for mammalian pathogens (26). The FP and C strains were significantly more virulent in insects (70% and 87%, respectively) than the NP strains (Fig. 1A), and the C strains were more virulent than the FP strains. Furthermore, a much higher proportion of the C strains than of the NP strains were highly virulent (100% and 24%, respectively; P < 0.001) (Fig. 1B). Only 50% of the FP strains induced mortality levels of more than 65% (threshold value). However, 95% of FP and 100% of C strains had virulence values of more than 40%, versus only 48% of the NP strains (not shown).
We also used pairwise correlation analysis to investigate the correlations between the various phenotypes tested (Fig. 2). Correlation coefficients were calculated together with their 95% confidence intervals. Virulence in insects was found to be positively correlated with (i) motility (P < 0.0001), (ii) cytotoxicity (P = 0.0003), (iii) adhesion (P = 0.0014), and (iv) resistance to polymyxin B (P = 0.037). Thus, all these properties likely contribute to virulence. Adhesion was also correlated with cytotoxicity (P = 0.0006), suggesting that the adhesion of B. cereus to epithelial cells may lead to a local increase in the concentrations of extracellular membrane-active proteins potentially involved in B. cereus pathogenesis. No significant correlations were found between the other combinations of phenotypes (P > 0.05).
Fig 2.
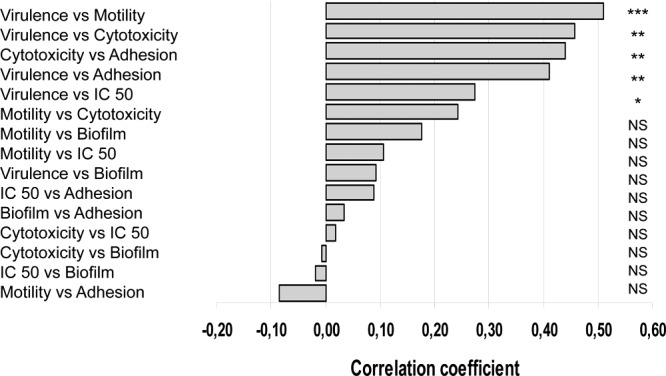
Pairwise correlation between the virulence phenotypes and statistical significance of the correlation coefficients. Each phenotype was compared to each of the others in pairwise tests, and correlation coefficients were determined for each pair of phenotypes. *, P < 0.05; **, P < 0.005; ***, P < 0.0001; NS, not significant.
A principal component analysis was performed to determine how strains cluster in a 2-dimensional scatterplot according to phenotype combinations. We plotted the strains according to their coordinates in the principal component 1 (PC1) and 2 (PC2) systems. PC1 corresponded to adhesion, motility, cytotoxicity, and virulence. PC2 corresponded to biofilm formation and motility (Fig. 3). Using this coordinate system, two groups of strains could be distinguished (green and red forms). Thus, taking the four phenotypes (adhesion to epithelial cells, motility, cytotoxicity, and virulence in insects) into account, all the FP and C strains (with the exception of one FP strain) were separated from the NP strains. The second group included only two NP strains. These strains (from soil and from milk) have pathogenic properties and may therefore have been wrongly classified as nonpathogenic. The soil strain might be pathogenic when in contact with a host. The strain isolated from milk did not induce food poisoning infections, and either the bacterial quantity in the food was not sufficient to induce pathology or the people who ingested the food were not sensitive to this strain.
Fig 3.
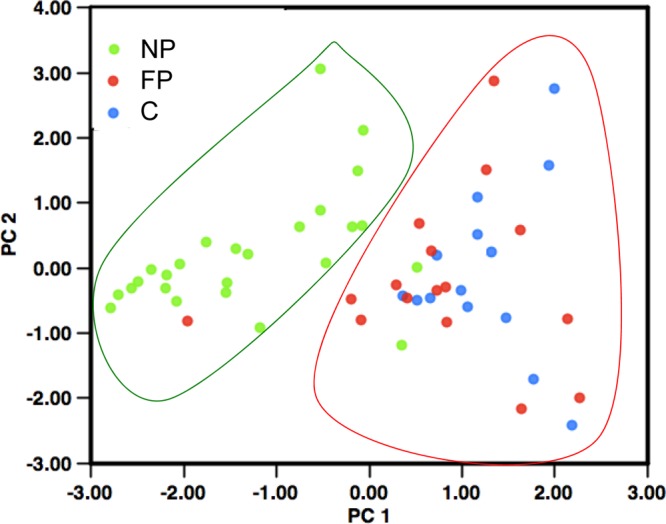
Principal component analysis. PC1 corresponds to a combination of adhesion, motility, cytotoxicity, and virulence in insects. PC2 corresponds to biofilm formation and motility. Each B. cereus strain was plotted according to its values in PC analysis. Green, red, and blue spots correspond to NP, FP, and C strains, respectively.
All together, our results demonstrate that disease-causing strains (gastrointestinal, local, or systemic diseases) can be differentiated from nonhazardous strains on the basis of several colonization phenotypes. This finding is new, because there has been a tendency to consider B. cereus an opportunistic pathogen, with all strains having virulence potential and disease severity mainly dependent on host immune system efficiency/deficiency. Host immune status probably plays a role in disease development, but we show here that there are significant differences in virulence capacity between the strains. These findings highlight the true pathogenic potential of this bacterial species.
Moreover, we have identified phenotypic characteristics of representative B. cereus strains that can be used to distinguish between pathogenic and innocuous strains. This finding is of great potential value for the food industry and hospitals. Indeed, the isolation of a B. cereus strain in these environments must be taken seriously, and an assessment of the virulence potential of strains is important for surveillance and outbreak investigation purposes. A molecular investigation should now be performed to identify the genetic determinants responsible for these phenotypes and to correlate toxin (e.g., Hbl, Nhe, etc.) or other colonization/virulence (FlhA, CwpFM, InhA, etc.) factor production (27–29) with strain toxicity.
We hope that our findings on the virulence traits of the various B. cereus strains will improve our understanding of the pathogenicity of B. cereus, thereby making it possible to improve both clinical diagnosis and food safety.
ACKNOWLEDGMENTS
We thank Alain Léréec for technical assistance. We also thank Marie Hélène Guinebretière for generously providing the strains.
Rita Kamar held a Mobilité Scientifique et Universitaire fellowship from the Agence Universitaire de la Francophonie (AUF), Lebanon.
Footnotes
Published ahead of print 7 November 2012
REFERENCES
- 1. Anonymous 2009. The community summary report on food-borne outbreaks in the European Union in 2007. EFSA J. doi:10.2903/j.efsa.2009.271r [Google Scholar]
- 2. Stenfors Arnesen L, Fagerlund A, Granum P. 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32:579–606 [DOI] [PubMed] [Google Scholar]
- 3. Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 23:382–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hilliard NJ, Schelonka RL, Waites KB. 2003. Bacillus cereus bacteremia in a preterm neonate. J. Clin. Microbiol. 41:3441–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong H, Duc Le H, Cutting S. 2005. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 29:813–835 [DOI] [PubMed] [Google Scholar]
- 6. Lund T, DeBuyser M-L, Granum PE. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254–261 [DOI] [PubMed] [Google Scholar]
- 7. Guinebretière MH, Broussolle V, Nguyen-The C. 2002. Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 40:3053–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran SL, Guillemet E, Ngo-Camus M, Clybouw C, Puhar A, Moris A, Gohar M, Lereclus D, Ramarao N. 2011. Hemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell. Microbiol. 13:92–108 [DOI] [PubMed] [Google Scholar]
- 9. Cadot C, Tran SL, Vignaud ML, De Buyser ML, Kolsto AB, Brisabois A, Nguyen-The C, Lereclus D, Guinebretiere MH, Ramarao N. 2010. InhA1, NprA, and HlyII as candidates to differentiate pathogenic from nonpathogenic Bacillus cereus strains. J. Clin. Microbiol. 48:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Houry A, Briandet R, Aymerich S, Gohar M. 2010. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology 156(Pt 4):1009–1018 [DOI] [PubMed] [Google Scholar]
- 11. Minnaard J, Lievin-Le Moal V, Coconnier MH, Servin AL, Pérez PF. 2004. Disassembly of F-actin cytoskeleton after interaction of Bacillus cereus with fully differentiated human intestinal Caco-2 cells. Infect. Immun. 72:3106–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wijman J, de Leeuw P, Moezelaar R, Zwietering M, Abee T. 2007. Air-liquid interface biofilms of Bacillus cereus: formation, sporulation, and dispersion. Appl. Environ. Microbiol. 73:1481–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guinebretière MH, Thompson FL, Sorokin A, Normand P, Dawyndt P, Ehling-Schulz M, Svensson B, Sanchis V, Nguyen-The C, Heyndrickx M, Vos PD. 2008. Ecological diversification in the Bacillus cereus group. Environ. Microbiol. 10:851–865 [DOI] [PubMed] [Google Scholar]
- 14. Lecadet M-M, Sanchis V, Menou G, Rabot P, Lereclus D, Martouret D. 1988. Identification of a ∂-entoxin gene product specifically active against Spodoptera littoralis Bdv. among proteolysed fractions of the insecticidal crystals of Bacillus thuringiensis subsp. aizawai 7.29. Appl. Environ. Microbiol. 54:2689–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tran SL, Guillemet E, Gohar M, Lereclus D, Ramarao N. 2010. CwpFM (EntFM) is a Bacillus cereus potential cell wall peptidase implicated in adhesion, biofilm formation, and virulence. J. Bacteriol. 192:2638–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilois N, Ramarao N, Bouillaut L, Perchat S, Aymerich S, Nielsen-Leroux C, Lereclus D, Gohar M. 2007. Growth-related variations in the Bacillus cereus secretome. Proteomics 7:1719–1728 [DOI] [PubMed] [Google Scholar]
- 17. Guillemet E, Cadot C, Tran SL, Guinebretiere MH, Lereclus D, Ramarao N. 2010. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 192:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tran SL, Puhar A, Ngo-Camus M, Ramarao N. 2011. Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus. PLoS One 6:e22876 doi:10.1371/journal.pone.0022876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cell. Microbiol. 11:1034–1043 [DOI] [PubMed] [Google Scholar]
- 20. Auger S, Ramarao N, Faille C, Fouet A, Aymerich S, Gohar M. 2009. Biofilm formation and cell surface properties among pathogenic and nonpathogenic strains of the Bacillus cereus group. Appl. Environ. Microbiol. 75:6616–6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones SE, Knight KL. 2012. Bacillus subtilis-mediated protection from Citrobacter rodentium-associated enteric disease requires espH and functional flagella. Infect. Immun. 80:710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lemaitre B, Reichhart JM, Hoffmann JA. 1997. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. U. S. A. 94:14614–14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abi Khattar Z, Rejasse A, Destoumieux-Garzon D, Escoubas JM, Sanchis V, Lereclus D, Givaudan A, Kallassy M, Nielsen-Leroux C, Gaudriault S. 2009. The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. J. Bacteriol. 191:7063–7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramarao N, Nielsen-Leroux C, Lereclus D. 2012. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J. Vis. Exp. e4392 doi:10.3791/4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen-LeRoux C, Gaudriault S, Ramarao N, Lereclus D, Givaudan A. 2012. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr. Opin. Microbiol. 15:220–231 [DOI] [PubMed] [Google Scholar]
- 27. Bouillaut L, Ramarao N, Buisson C, Gilois N, Gohar M, Lereclus D, Nielsen-Leroux C. 2005. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 71:8903–8910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ramarao N, Lereclus D. 2005. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell. Microbiol. 7:1357–1364 [DOI] [PubMed] [Google Scholar]
- 29. Ramarao N, Lereclus D. 2006. Adhesion and cytotoxicity of Bacillus cereus and Bacillus thuringiensis to epithelial cells are FlhA and PlcR dependent, respectively. Microbes Infect. 8:1483–1491 [DOI] [PubMed] [Google Scholar]