Abstract
We characterized 12 Exophiala strains isolated from patients over a 15-year period to the species level using phenotypic tests and internal transcribed spacer (ITS) and Rpb1 sequencing and described the clinical spectrum of the 12 patients. Eight patients had nail or skin infections, two had invasive infections, and two had colonization of the gastrointestinal tract. ITS and Rpb1 sequencing showed that 11 of the 12 strains were known Exophiala species (E. oligosperma [n = 3], E. jeanselmei [n = 2], E. lecanii-corni [n = 2], E. bergeri [n = 1], E. cancerae [n = 1], E. dermatitidis [n = 1], and E. xenobiotica [n = 1]), which included the first reported cases of onychomycosis caused by E. bergeri and E. oligosperma. The 12th strain (HKU32T), isolated from the nail clipping of the right big toe of a 68-year-old female patient with onychomycosis, possessed unique morphological characteristics distinct from other Exophiala species. It grew very slowly and had a velvety colony texture after 28 days, short conidiophores of the same olivaceous color as the supporting hyphae, numerous spores, and no chlamydospore-like cells. ITS, Rpb1, β-tubulin, and β-actin gene sequencing unambiguously showed that HKU32T was clustered with but formed branches distinct from other Exophiala species in phylogenetic trees. We propose the new species Exophiala hongkongensis to describe this novel fungus.
INTRODUCTION
Exophiala is a genus of saprophytic fungi that have been isolated from environments rich in hydrocarbons (1–3) or from hot, humid, and oligotrophic environments, such as dishwashers (4), steam bath facilities (2), and bathrooms (5). This genus belongs to the family Herpotrichiellaceae and currently consists of more than 30 species. Traditionally, these fungi are considered dematiaceous molds (6). Due to their phenotypic characteristics at the beginning of colony formation, these fungi are often also referred to as “black yeasts” (7), a misnomer which sometimes may mislead the choice of antifungal agents. When the cultures mature, brown hyphae are formed which bear conidiogenous cells referred to as annellides, a typical characteristic of this genus of fungi (8).
Although Exophiala species are environmental fungi, they should not be disregarded as contaminants when they are isolated from clinical specimens. These fungi are causative agents of skin and subcutaneous tissue infections and of systemic infections, such as prosthetic valve endocarditis, dialysis-associated peritonitis, and disseminated infections, especially in immunocompromised patients (9–17). In this study, we report the clinical spectrum of Exophiala infections in our hospital, characterized by phenotypic examination and sequencing of the internal transcribed spacer 1 (ITS1)-5.8S-ITS2 rRNA gene cluster (referred to hereinafter as ITS) and RNA polymerase II's largest subunit gene (Rpb1). During the process, we also discovered a potentially novel Exophiala species from the toe nail clipping of a patient with onychomycosis. The strain, named HKU32T, exhibited phenotypic characteristics that do not fit into the pattern of any known Exophiala species. Amplification and sequencing of four independent DNA regions showed that it is distinct from all other Exophiala species. On the basis of these studies, we propose a new species, Exophiala hongkongensis sp. nov., to describe this fungus.
MATERIALS AND METHODS
Patients and strains.
All Exophiala strains isolated from patients in this study were retrieved from the collection in the clinical microbiology laboratory at the Queen Mary Hospital in Hong Kong during a 15-year period (1998 to 2012). All clinical data were collected as described in our previous publication (18). Clinical specimens were collected and handled according to standard protocols. The reference strains, including E. jeanselmei (CBS 507.90T), E. nishimurae (CBS 101538T), E. oligosperma (CBS 265.49T), E. spinifera (CBS 899.68T), and E. xenobiotica (CBS 118157T), were purchased from The Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre.
Phenotypic characterization.
All strains were inoculated onto Sabouraud dextrose agar (SDA) (Sigma-Aldrich, St. Louis, MO) for fungal culture. Slides for microscopic examination were prepared using the agar block smear method we described recently (19). The enzyme activity test was performed using the API-ZYM system (bioMérieux SA, Marcy l'Etoile, France). All tests were performed in triplicate. The effects of different temperatures on growth on potato dextrose agar (PDA) (Becton, Dickinson and Company, Franklin Lakes, NJ) and comparison of growth rates on PDA, brain heart infusion (BHI) agar (Becton, Dickinson and Company, Franklin Lakes, NJ), cornmeal agar (CMA) (Sigma-Aldrich, St. Louis, MO), and oatmeal agar (OMA) (Becton, Dickinson and Company, Franklin Lakes, NJ) at 24°C were studied using published protocols, with slight modifications (20–22). Briefly, conidia were harvested in distilled water from 7-day cultures on SDA. Concentrations of conidia were determined using a hemocytometer. A circular cavity was made at the center of each agar plate using a Pasteur pipette. Five thousand spores were inoculated into the central cavity of each agar plate. PDA plates were incubated at temperatures ranging from 24 to 37°C, and other agar plates were incubated at 24°C. The radii of colonies were measured in four directions after 14 days of incubation. All assays were performed in triplicate, and the mean radii were calculated. Scanning electron microscopy of strain HKU32T was performed according to the protocol described in our previous publication (23).
DNA extraction and ITS, Rpb1, β-tubulin, and β-actin gene sequencing.
Fungal DNA extraction, PCR amplification, and DNA sequencing of the ITS and partial Rpb1 gene for all 12 Exophiala strains isolated from our patients and the reference strains and of the partial β-tubulin and β-actin genes for HKU32T and the reference strains were performed according to the protocols described in our previous publication (24). Briefly, fungal cells on SDA were harvested with a sterilized cotton swab and suspended in 1 ml of autoclaved distilled water. DNA was then extracted from the fungal cells using the DNeasy plant minikit according to the manufacturer's instructions (Qiagen, Hilden, Germany). The extracted DNA was eluted in 40 μl of distilled water, and 1 μl of the extracted DNA was used for PCR. For PCR, each 20-μl PCR mixture contained diethyl pyrocarbonate (DEPC)-treated water (Invitrogen, Carlsbad, CA), fungal DNA, PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, and 3.125 mM MgCl2) (Applied Biosystems, Foster City, CA), 1 mM each primer (Invitrogen, Carlsbad, CA) (Table 1), 200 μM each deoxyribonucleoside triphosphate (dNTP), and 0.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA). The PCR mixtures were first heated at 95°C for 10 min, then heated in 45 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and finally incubated at 72°C for 10 min in an automated thermal cycler (GeneAmp PCR System 9700; Applied Biosystems, Foster City, CA). Preparation of PCR master mix, addition of DNA samples into the reaction tubes, and post-PCR steps, including gel electrophoresis, purification of PCR products, and DNA sequencing were performed in separate rooms to avoid possible contamination. Autoclaved distilled water was used as the negative control in each run of PCR. Ten microliters of each amplified product was electrophoresed in 1.5% (wt/vol) agarose gel (SeaKem LE agarose), and the PCR products were purified using the QIAquick gel extraction kit (QIAgen, Hilden, Germany). Both strands of the PCR products were sequenced twice with an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA), using the PCR primers. The sequences of the PCR products were processed using BioEdit 7.1.3.0 (25) and compared with sequences of closely related species in GenBank by multiple sequence alignment using MUSCLE 3.8 (26).
Table 1.
Primer sequences used for PCR amplification in this study
| Target region | Primer, sequence |
|
|---|---|---|
| Forward | Reverse | |
| ITS | ITS1, 5′-TCCGTAGGTGAACCTGCGG-3′ | ITS4, 5′-TCCTCCGCTTATTGATATGC-3′ |
| Rpb1 gene | LPW20506, 5′-TYMTGRSNARRGTCAAGAAGAT-3′ | LPW20507, 5′-GGNAGBGMVGABARRATCATCCA-3′ |
| β-tubulin gene | LPW17603, 5′-SWVRTCTCDGGMGAACAYGGTCT-3′ | LPW17604, 5′-HRTKKCTTACCAGCACCGCT-3′ |
| β-actin gene | LPW17499, 5′-CGTGTCGAYATGGCTGGTCG-3′ | LPW17500, 5′-GGHGCRATRATCTTGACCTTCAT-3′ |
Phylogenetic characterization.
Poorly aligned or divergent regions of the aligned DNA sequences were removed using Gblocks 0.91b (27, 28) with relaxed parameters. Testing for the substitution model and phylogenetic tree construction, by the maximum likelihood method, were performed using MEGA 5.0.5 (29). Phylogenetic analyses included 468 nucleotide positions of the ITS, 371 nucleotide positions of the partial Rpb1 gene, and 641 nucleotide positions of the concatenated partial β-tubulin gene and the partial β-actin gene sequence.
Nucleotide sequence accession numbers.
The ITS and partial Rpb1 gene sequences of the Exophiala strains and the partial β-tubulin and β-actin gene sequences of HKU32T and E. nishimurae CBS 101538T have been deposited in GenBank under the accession numbers listed in Table 2.
Table 2.
GenBank accession numbers of the ITS and partial Rpb1, β-tubulin, and β-actin gene sequences of the 12 Exophiala strains from patients and the control strains
| Strain (case) | GenBank accession no. |
|||
|---|---|---|---|---|
| ITS | Partial |
|||
| Rpb1 gene | β-Tubulin gene | β-Actin gene | ||
| PW2461 (1) | JX473275 | JX498926 | ||
| PW2482 (2) | JX473282 | JX498933 | ||
| PW2462 (3) | JX473276 | JX498927 | ||
| PW2464 (4) | JX473278 | JX498929 | ||
| PW2534 (5) | JX473283 | JX498934 | ||
| PW2468 (6) | JX473281 | JX498932 | ||
| PW2465 (7) | JX473279 | JX498930 | ||
| PW2535 (8) | JX473284 | JX498935 | ||
| PW2466 (9) | JX473280 | JX498931 | ||
| HKU32T (10) | JN625231 | JX498924 | JN625236 | JN625241 |
| PW2642 (11) | JX473285 | JX498936 | ||
| PW2643 (12) | JX473286 | JX498937 | ||
| CBS 101538T | JX473274 | JX498925 | JX482552 | JX482553 |
RESULTS
Phenotypic characterization and ITS and Rpb1 sequencing of Exophiala strains and identification of a novel Exophiala species.
On SDA, the colonies of all 12 strains were initially brown to black, moist, and yeastlike. All mature colonies became velvety, with the front color gray-to-black-olivaceous and the reverse black. Microscopic examination of the young cultures of all the 12 strains showed subspherical, budding, yeastlike cells. As the cultures became mature, septate hyphae which bore tubular and rocket-shaped annellides that tapered to form narrow elongated tips were observed. Ellipsoidal conidia, in clusters at the apices of the annellides or at the sides of the conidiophores, were produced from the annellides.
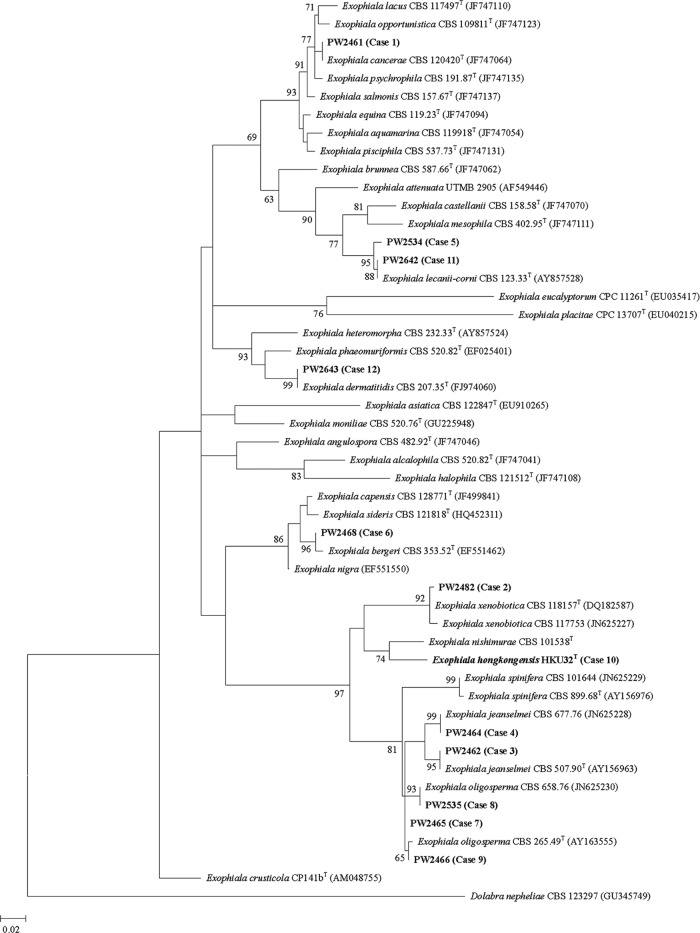
PCR of the ITS and partial Rpb1 gene of the 12 strains and the reference strains showed bands at about 600 bp and 500 bp, respectively. Sequencing of the ITS showed that 11 of the 12 strains were known Exophiala species (Fig. 1). Sequencing of the partial Rpb1 gene revealed results concurring with those of ITS sequencing (data not shown). As for HKU32T (case 10), although it is most closely related to E. nishimurae CBS 101538T, there was a 46 (8.0%)-base difference between the ITS of HKU32T and that of E. nishimurae CBS 101538T and a 37 (8.1%)-base difference between the partial Rpb1 gene of HKU32T and that of E. nishimurae CBS 101538T.
Fig 1.
Phylogenetic trees showing the relationships of the 12 strains of Exophiala species in this study to known Exophiala species. The tree was inferred from ITS sequence data by the maximum likelihood method with the substitution model GTR+G+I (general time-reversible model, with gamma-distributed rate variation [G] and an estimated proportion of invariable sites [I]). The scale bar indicates the estimated number of substitutions per 50 bases. Numbers at nodes indicate levels of bootstrap support calculated from 1,000 trees. All names and accession numbers are given as cited in the GenBank database.
Clinical spectrum of Exophiala infections.
The clinical characteristics of the 12 patients with Exophiala species isolated are shown in Table 3. The male-to-female ratio was 1:1. The median age was 66 years (range, 3 to 88). Eight patients had infections of the nails or skin (four had onychomycosis, two had skin nodules, and two had chronic skin infections), two patients had invasive infections (one had continuous ambulatory peritoneal dialysis [CAPD] peritonitis and one had pneumonia), and two had colonization of the gastrointestinal tract. All patients recovered from the Exophiala infections.
Table 3.
Characteristics of patients with Exophiala species isolated in the present study
| Case (reference) | Yr of isolation | Sexa/age (yr) | Underlying condition(s)a | Diagnosis | Clinical specimen | Identification by ITS and Rpb1 sequencing |
|---|---|---|---|---|---|---|
| 1 | 1998 | F/3 | Cord blood transplant recipient for β-thalassemia major | Colonization of gastrointestinal tract | Stool | E. cancerae |
| 2 (13) | 2000 | M/66 | End-stage renal failure on CAPD | CAPD peritonitis | Peritoneal dialysate | E. xenobiotica |
| 3 | 2002 | F/79 | Tuberculous cervical lymphadenitis, hypertension | Right wrist nodule for 5 years | Wrist nodule | E. jeanselmei |
| 4 | 2008 | M/86 | DM, carcinoma of rectum, hypertension, ischemic heart disease, COPD | Right middle finger nodule for 3 years | Finger nodule | E. jeanselmei |
| 5 | 2008 | F/87 | Bullous pemphigoid, DM, hypertension, Alzheimer's disease | Tinea pedis | Skin scrapping | E. lecanii-corni |
| 6 | 2009 | M/37 | None | Onychomycosis | Toe nail | E. bergeri |
| 7 | 2009 | M/23 | None | Onychomycosis | Big toe nail | E. oligosperma |
| 8 | 2009 | M/66 | None | Pneumonia | Bronchoalveolar lavage fluid | E. oligosperma |
| 9 | 2009 | F/51 | None | Onychomycosis | Thumb nail | E. oligosperma |
| 10 | 2010 | F/68 | Hypertension | Onychomycosis | Big toe nail | Novel species |
| 11 | 2012 | M/88 | DM, gout, recurrent cellulitis | Chronic skin infection | Skin scrapping | E. lecanii-corni |
| 12 | 2012 | F/43 | AML, PBSCT | Colonization of gastrointestinal tract | Stool | E. dermatitidis |
F, female; M, male; CAPD, continuous ambulatory peritoneal dialysis; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; AML, acute myeloid leukemia; PBSCT, peripheral blood stem cell transplant.
Enzymatic activities of HKU32T.
The API-ZYM test for HKU32T showed that it was positive for alkaline phosphatase, esterase (C4), esterase lipase (C8), leucine arylamidase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, β-glucosidase, and N-acetyl-β-glucosaminidase in all replicates.
Effects of temperature and medium on growth of HKU32T.
On PDA, HKU32T grew at 24°C, 30°C, 33°C, and 37°C, with optimal growth at 30°C. HKU32T also grew on CMA, OMA, and BHI agar at 24°C, with the fastest and the slowest growth observed on CMA and BHI agar, respectively.
Partial β-tubulin and β-actin gene sequencing of HKU32T.
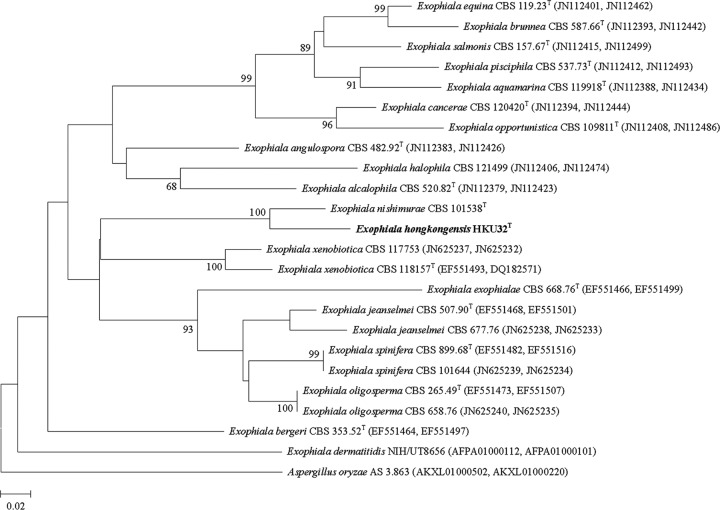
PCR of the partial β-tubulin and β-actin genes of HKU32T and the reference strains showed bands at about 300 bp and 500 bp, respectively. Sequencing and phylogenetic analysis showed that HKU32T is most closely related to E. nishimurae CBS 101538T, concurring with the results of ITS and Rpb1 gene sequencing (Fig. 2). Pairwise alignment showed that there was a 28 (9.5%)-base difference between the partial β-tubulin gene of HKU32T and that of E. nishimurae CBS 101538T and a 36 (7.6%)-base difference between the partial β-actin gene of HKU32T and that of E. nishimurae CBS 101538T.
Fig 2.
Concatenated phylogenetic tree showing the relationship of E. hongkongensis HKU32T to closely related species. The tree was inferred from actin and β-tubulin sequence data by the maximum likelihood method with the substitution model K2+G+I (K2 referring to the Kimura two-parameter model). The scale bar indicates the estimated number of substitutions per 50 bases. Numbers at nodes indicate levels of bootstrap support calculated from 1,000 trees. All names and accession numbers are given as cited in the GenBank database, with the first accession numbers in the parentheses referring to the β-actin gene sequences and the second accession numbers in the parentheses referring to the β-tubulin gene sequences.
TAXONOMY
Description of Exophiala hongkongensis Woo, Ngan, Tsang, Ling, Chan, Leung, Yuen, Lau, sp. nov. MycoBank accession no. MB563298. Teleomorph: unknown. Known distribution: Hong Kong. Etymology: hong.kong.en′sis. N.L. fem. adj., named after Hong Kong, where the type strain was isolated. Specimen examined: Hong Kong; from the big toe nail of a human presenting with onychomycosis in 2010 (holotype: dried culture in NBRC Herbarium H-13132; ex-type cultures: HKU32T [= NBRC 109366T = JCM 18697T = CBS 131511T]).
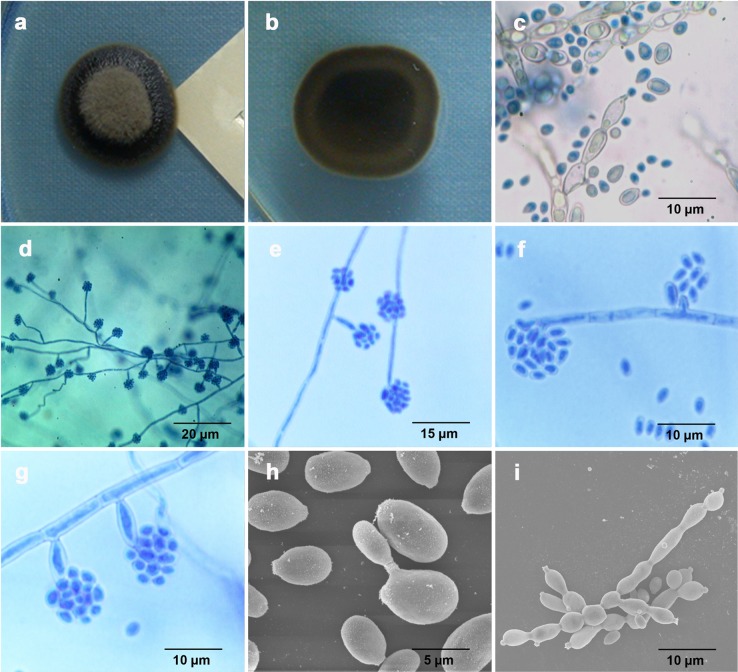
On SDA, HKU32T initially grew slowly as black, slimy, yeast-like colonies with a diameter of 2 mm after incubation at 25°C for 14 days. Subsequently, the centers of the colonies became velvety, gray-olivaceous, and dome-shaped with black reverse, but the margin remained slimy and yeastlike after 28 days of incubation at 25°C (Fig. 3a and b). Microscopic examination of the yeastlike colonies at 14 days of incubation after lactophenol cotton blue staining revealed brown yeast cells that were ellipsoidal, had an average size of 6 by 3.5 μm, and possessed short annellated zones to form budding cells (Fig. 3c). Mycelia were usually swollen and formed torulose hyphae with annellated zones found at their ends (Fig. 3c). At the subsequent velvety stage, septate mycelia were not swollen and conidia were formed distinctively in clusters (Fig. 3d and e). The clusters of conidia were either intercalary or found at the tips of free conidiophores (Fig. 3d and e). Under high-power magnification, intercalary annellides were observed in the form of short annellated pegs (Fig. 3f). These intercalary annellides were present along the creeping septate hyphae. Free conidiophores consisting of 1 or 2 cells with inconspicuous short annellated tips were also observed (Fig. 3g). They possessed the same olivaceous color as the creeping septate hyphae and were often constricted at their bases (Fig. 3g). At both stages, conidia were formed by percurrent growth through annellated zones. Most of them were oval or subglobose, with an average size of 3 by 2 μm, and some with truncated scars. No synanamorphs of Phialophora or Rhinocladiella types were observed. Scanning electron microscopic examination of HKU32T at 14 days of incubation revealed budding yeast cells and torulose hyphae with distinct annellated zones, consistent with the results observed on light microscopy (Fig. 3h and i). In addition, yeast cells with truncated scars were also observed (Fig. 3h).
Fig 3.
Culture of HKU32T on SDA after 28 days of incubation at 25°C showed a black slimy colony with gray olivaceous center (a) and black reverse (b). At the initial yeast stage (14 days of incubation), yeast and torulose hyphae with annellated zones were observed with light microscopy (c) and scanning electron microscopy (h and i). Yeast cells with truncated scars were observed (h), and distinct annellated zones were well demonstrated (h and i). At the subsequent velvety stage (28 days of incubation), conidia in clusters were either intercalary or found at the tips of free conidiophores under both low-power (d) and high-power magnification (e). Intercalary annellides with short pegs (f) and free conidiophores with constricted bases (g) were observed.
DISCUSSION
Using the polyphasic approach with a combination of phenotypic and genotypic techniques, we describe a wide variety of Exophiala species associated with different forms of clinical infections. Although Exophiala can often be identified to the genus level by morphological characteristics, identification of Exophiala to the species level by phenotypic characterization alone is very difficult. This difficulty is exemplified by our description of the first case of CAPD peritonitis caused by Exophiala in 2000 (Table 3, case 2) (13). At that time, we could not identify the Exophiala to the species level. Due to the advancement of molecular techniques and availability of DNA sequences of different gene loci in sequence databases such as GenBank, identification of Exophiala to the species level has been made possible. In this study, by sequencing two independent gene loci (ITS and Rpb1), eight different Exophiala species, including E. oligosperma, E. jeanselmei, E. lecanii-corni, E. bergeri, E. cancerae, E. dermatitidis, E. xenobiotica, and a novel Exophiala species, were isolated from 12 patients (Table 3 and Fig. 1). These Exophiala species were found to be associated with both superficial infections and invasive infections, including the case of CAPD peritonitis caused by E. xenobiotica (Table 3, case 2) and another case of pneumonia caused by E. oligosperma (Table 3, case 8). In two patients with cord blood and peripheral blood stem cell transplant, respectively, E. cancerae and E. dermatitidis were the colonizers of their gastrointestinal tracts (Table 3, cases 1 and 12).
Phenotypic and genotypic analysis revealed that HKU32T is a novel species in the genus Exophiala, which we propose to name E. hongkongensis. Using four independent DNA regions widely used for phylogenetic analysis, including ITS and three housekeeping genes (Rpb1, β-tubulin, and β-actin genes), it was shown unambiguously that E. hongkongensis is closely related to but distinct from other Exophiala species (Fig. 1 and 2). Among the Exophiala species, E. hongkongensis is most closely related to E. nishimurae, as shown in all phylogenetic trees with high bootstrap supports. Comparison of phenotypic characteristics between E. hongkongensis and those of other closely related Exophiala species shown by ITS and Rpb1 sequencing also revealed unique phenotypic characteristics of E. hongkongensis (Table 4). E. hongkongensis grew very slowly and had a velvety colony texture after 28 days, short conidiophores of the same olivaceous color as the supporting hyphae, numerous spores, and no chlamydospore-like cells.
Table 4.
Comparison of phenotypic features of E. hongkongensis and closely related Exophiala species
| Species | Colony texture after 28 days of incubation at 25°C on SDA | Colony diam after 14 days of incubation at 25°C on SDA | Average length of conidiophores (μm) | Darkening of conidiophores | Abundance of spores | Presence of chlamydospore-like cells |
|---|---|---|---|---|---|---|
| E. hongkongensis HKU32T | Velvety | 2 mm | 10 | Same color as supporting hyphae | Numerous | Absence |
| E. jeanselmei CBS 507.90T | Velvety | 60 mm | 12 | Inconspicuous | Normal | Absence |
| E. nishimurae CBS 101538T | Velvety | 5 mm | 12 | Same color as supporting hyphae | Numerous | Presence |
| E. oligosperma CBS 265.49T | Velvety | 50 mm | >100, branched | Same color as supporting hyphae | Few | Absence |
| E. spinifera CBS 899.68T | Slimy | 40 mm | 50 | Conspicuous | Normal | Absence |
| E. xenobiotica CBS 118157T | Slimy | 20 mm | 15, branched | Same color as supporting hyphae | Normal | Absence |
This study includes the first reported cases of onychomycosis associated with E. hongkongensis, E. bergeri, and E. oligosperma. Although Exophiala species are relatively common causes of subcutaneous and skin infections in the forms of phaeohyphomycosis, chromoblastomycosis, and mycetoma, they have only occasionally been reported to cause onychomycosis. Among the seven cases reported in the literature, four were caused by E. dermatitidis and three were caused by E. jeanselmei (Table 5), whereas for the four patients with Exophiala onychomycosis in the present study, two were caused by E. oligosperma, one was caused by E. bergeri, and one was caused by E. hongkongensis (Table 3). Exophiala species are global pathogens of onychomycosis, with cases reported from Asia, Europe, America, and Africa. Among the seven cases reported in the literature, underlying diseases leading to immunosuppressive states were present in three patients, including diabetes mellitus in two patients and renal transplantation in one (Table 5), whereas for the four patients with Exophiala onychomycosis in the present study, only the patient with E. hongkongensis onychomycosis had underlying hypertension (Table 3). In all 11 patients, the nails were infected by the fungi for one to a few years before presentation, indicating that the disease was a very indolent process with relatively mild symptoms and, hence, patients tended to observe them for a time and delay in seeking medical advice. The nails of the big toes were affected in eight patients (Tables 3 and 5). Due to underreporting and difficulty in making the microbiological diagnosis, the number of cases of Exophiala onychomycosis is probably underestimated. This is in line with the results of a recent study, which also suggested that the incidence of superficial infections caused by black yeastlike fungi could be underestimated (37). Another study also noted that nonthermophilic Exophiala species may expand in diabetic patients with poor blood circulation (38). A combination of phenotypic and genotypic techniques using the polyphasic approach in microbiology laboratories will facilitate the understanding of the epidemiology of Exophiala onychomycosis, as well as other infections associated with Exophiala species.
Table 5.
Cases of onychomycosis caused by Exophiala species reported in the literature
| Reference | Geographical location | Sex/age (yr)a | Underlying disease | Nail(s) involved | Clinical presentation | Exophiala species isolated |
|---|---|---|---|---|---|---|
| Matsumoto et al. (30) | Japan | F/51 | Diabetes mellitus | Both big toes | Black discoloration and subungual hyperkeratosis of nails for 8 years | E. dermatitidis |
| Krajden et al. (31) | Canada | M/NA | Diabetes mellitus | Middle finger | Black discoloration of nail for 1.5 years | E. dermatitidis |
| Hata et al. (32) | Japan | F/61 | None | Big toe | Discoloration of nail | E. dermatitidis |
| Boisseau-Garsaud et al. (33) | France | M/60 | Post-renal transplant | Two thumbs and two big toes | Hyperkeratosis and black coloration of nails for 4 years | E. jeanselmei |
| Oudaina et al. (34) | Morocco | F/39 | None | Big toe | Discoloration of distal side of nail for 2 years | E. jeanselmei |
| Park et al. (35) | South Korea | M/42 | None | Big toe | Linear longitudinal ridging with yellowish pigmentation of nail for 1 year | E. dermatitidis |
| Sharma et al. (36) | India | M/50 | None | Big toe | Black discoloration and hyperkeratosis of nail for 5 years | E. jeanselmei |
F, female; M, male; NA, not available.
ACKNOWLEDGMENTS
This work is partly supported by the HKSAR Health and Medical Research Fund, a Research Grants Council grant, the University Development Fund, and the Committee for Research and Conference Grants, The University of Hong Kong.
Footnotes
Published ahead of print 14 November 2012
REFERENCES
- 1. Seyedmousavi S, Badali H, Chlebicki A, Zhao J, Prenafeta-Boldú FX, de Hoog GS. 2011. Exophiala sideris, a novel black yeast isolated from environments polluted with toxic alkyl benzenes and arsenic. Fungal Biol. 115:1030–1037 [DOI] [PubMed] [Google Scholar]
- 2. Sudhadham M, Prakitsin S, Sivichai S, Chaiyarat R, Dorrestein GM, Menken SBJ, de Hoog GS. 2008. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 61:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao J, Zeng J, de Hoog GS, Attili-Angelis D, Prenafeta-Boldú FX. 2010. Isolation and identification of black yeasts by enrichment on atmospheres of monoaromatic hydrocarbons. Microb. Ecol. 60:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zalar P, Novak M, de Hoog GS, Gunde-Cimerman N. 2011. Dishwashers—a man-made ecological niche accommodating human opportunistic fungal pathogens. Fungal Biol. 115:997–1007 [DOI] [PubMed] [Google Scholar]
- 5. Lian X, de Hoog GS. 2010. Indoor wet cells harbour melanized agents of cutaneous infection. Med. Mycol. 48:622–628 [DOI] [PubMed] [Google Scholar]
- 6. Harris JE, Sutton DA, Rubin A, Wickes B, de Hoog GS, Kovarik C. 2009. Exophiala spinifera as a cause of cutaneous phaeohyphomycosis: case study and review of the literature. Med. Mycol. 47:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsumoto T, Padhye A, Ajello L. 1987. Medical significance of the so-called black yeasts. Eur. J. Epidemiol. 3:87–95 [DOI] [PubMed] [Google Scholar]
- 8. Lief MH, Caplivski D, Bottone EJ, Lerner S, Vidal C, Huprikar S. 2011. Exophiala jeanselmei infection in solid organ transplant recipients: report of two cases and review of the literature. Transpl. Infect. Dis. 13:73–79 [DOI] [PubMed] [Google Scholar]
- 9. Fothergill AW. 1996. Identification of dematiaceous fungi and their role in human disease. Clin. Infect. Dis. 22:S179–S184 [DOI] [PubMed] [Google Scholar]
- 10. Gold WL, Vellend H, Salit IE, Campbell I, Summerbell R, Rinaldi M, Simor AE. 1994. Successful treatment of systemic and local infections due to Exophiala species. Clin. Infect. Dis. 19:339–341 [DOI] [PubMed] [Google Scholar]
- 11. Greig J, Harkness M, Taylor P, Hashmi C, Liang S, Kwan J. 2003. Peritonitis due to the dermatiaceous mold Exophiala dermatitidis complicating continuous ambulatory peritoneal dialysis. Clin. Microbiol. Infect. 9:713–715 [DOI] [PubMed] [Google Scholar]
- 12. Hiruma M, Kawada A, Ohata H, Ohnishi Y, Takahashi H, Yamazaki M, Ishibashi A, Hatsuse K, Kakihara M, Yoshida M. 1993. Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36:1–7 [DOI] [PubMed] [Google Scholar]
- 13. Lau SKP, Woo PCY, Chiu S-K, Leung K-W, Yung RWH, Yuen K-Y. 2003. Early diagnosis of Exophiala CAPD peritonitis by 18S ribosomal RNA gene sequencing and its clinical significance. Diagn. Microbiol. Infect. Dis. 46:95–102 [DOI] [PubMed] [Google Scholar]
- 14. Martínez-González MC, Verea MM, Velasco D, Sacristán F, Del Pozo J, García-Silva J, Fonseca E. 2008. Three cases of cutaneous phaeohyphomycosis by Exophiala jeanselmei. Eur. J. Dermatol. 18:313–316 [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto T, Padhye AA, Ajello L, Standard PG, McGinnis MR. 1984. Critical review of human isolates of Wangiella dermatitidis. Mycologia 76:232–249 [Google Scholar]
- 16. Nachman S, Alpan O, Malowitz R, Spitzer ED. 1996. Catheter-associated fungemia due to Wangiella (Exophiala) dermatitidis. J. Clin. Microbiol. 34:1011–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. 2007. Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 45:3713–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woo PCY, Lau SKP, Teng JLL, Que T-L, Yung RWH, Luk W-K, Lai RWM, Hui W-T, Wong SSY, Yau H-H, Yuen K-Y. 2004. Association of Laribacter hongkongensis in community-acquired gastroenteritis with travel and eating fish: a multicentre case-control study. Lancet 363:1941–1947 [DOI] [PubMed] [Google Scholar]
- 19. Woo PCY, Ngan AHY, Chui H-K, Lau SKP, Yuen K-Y. 2010. Agar block smear preparation: a novel method of slide preparation for preservation of native fungal structures for microscopic examination and long-term storage. J. Clin. Microbiol. 48:3053–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao C, Li R, Wan Z, Liu W, Wang X, Qiao J, Wang D, Bulmer G, Calderone R. 2007. The effects of temperature, pH, and salinity on the growth and dimorphism of Penicillium marneffei. Med. Mycol. 45:401–407 [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann K, Discher S, Voigt K. 2007. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol. Res. 111:1169–1183 [DOI] [PubMed] [Google Scholar]
- 22. Woo PCY, Lau SKP, Ngan AHY, Tung ETK, Leung S-Y, To KKW, Cheng VCC, Yuen K-Y. 2010. Lichtheimia hongkongensis sp. nov., a novel Lichtheimia spp. associated with rhinocerebral, gastrointestinal, and cutaneous mucormycosis. Diagn. Microbiol. Infect. Dis. 66:274–284 [DOI] [PubMed] [Google Scholar]
- 23. Woo PCY, Tam EWT, Chong KTK, Cai JJ, Tung ETK, Ngan AHY, Lau SKP, Yuen K-Y. 2010. High diversity of polyketide synthase genes and the melanin biosynthesis gene cluster in Penicillium marneffei. FEBS J. 277:3750–3758 [DOI] [PubMed] [Google Scholar]
- 24. To KKW, Lau SKP, Wu AKL, Lee RA, Ngan AHY, Tsang CCC, Ling IWH, Yuen K-Y, Woo PCY. 2012. Phaeoacremonium parasiticum invasive infections and airway colonization characterized by agar block smear and ITS and β-tubulin gene sequencing. Diagn. Microbiol. Infect. Dis. 74:190–197 [DOI] [PubMed] [Google Scholar]
- 25. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 26. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540–552 [DOI] [PubMed] [Google Scholar]
- 28. Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56:564–577 [DOI] [PubMed] [Google Scholar]
- 29. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsumoto T, Matsuda T, Padhye AA, Standard PG, Ajello L. 1992. Fungal melanonychia: ungual phaeohyphomycosis caused by Wangiella dermatitidis. Clin. Exp. Dermatol. 17:83–86 [DOI] [PubMed] [Google Scholar]
- 31. Krajden S, Summerbell RC, Woo FC, McGouch DA, Rinaldi MG. 1994. Wangiella dermatitidis melanonychia acquired in Mauritius, abstr F-77. Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1994. American Society for Microbiology, Washington, DC. [Google Scholar]
- 32. Hata Y, Naka W, Nishikawa T. 1999. A case of melanonychia caused by Exophiala dermatitidis. Jpn. J. Med. Mycol. 40:231–234 [DOI] [PubMed] [Google Scholar]
- 33. Boisseau-Garsaud AM, Desbois N, Guillermin ML, Ossondo M, Gueho E, Cales-Quist D. 2002. Onychomycosis due to Exophiala jeanselmei. Dermatology 204:150–152 [DOI] [PubMed] [Google Scholar]
- 34. Oudaina W, Tligui H, Boughaidi A, Agoumi A. 2009. Onychomycosis due to Exophiala jeanselmei. J. Med. Mycol. 19:126–128 [Google Scholar]
- 35. Park KY, Kim HK, Suh MK, Seo SJ. 2011. Unusual presentation of onychomycosis caused by Exophiala (Wangiella) dermatitidis. Clin. Exp. Dermatol. 36:418–419 [DOI] [PubMed] [Google Scholar]
- 36. Sharma A, Chauhan S, Gupta P, Guleria RC. 2012. A case of onychomycosis which was caused by Exophiala Jeanselmei. J. Clin. Diagn. Res. 6:1081–1082 [Google Scholar]
- 37. Saunte DM, Tarazooie B, Arendrup MC, de Hoog GS. 2012. Black yeast-like fungi in skin and nail: it probably matters. Mycoses 55:161–167 [DOI] [PubMed] [Google Scholar]
- 38. de Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, Seyedmousavi S. 2011. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27:46–72 [DOI] [PMC free article] [PubMed] [Google Scholar]