Abstract
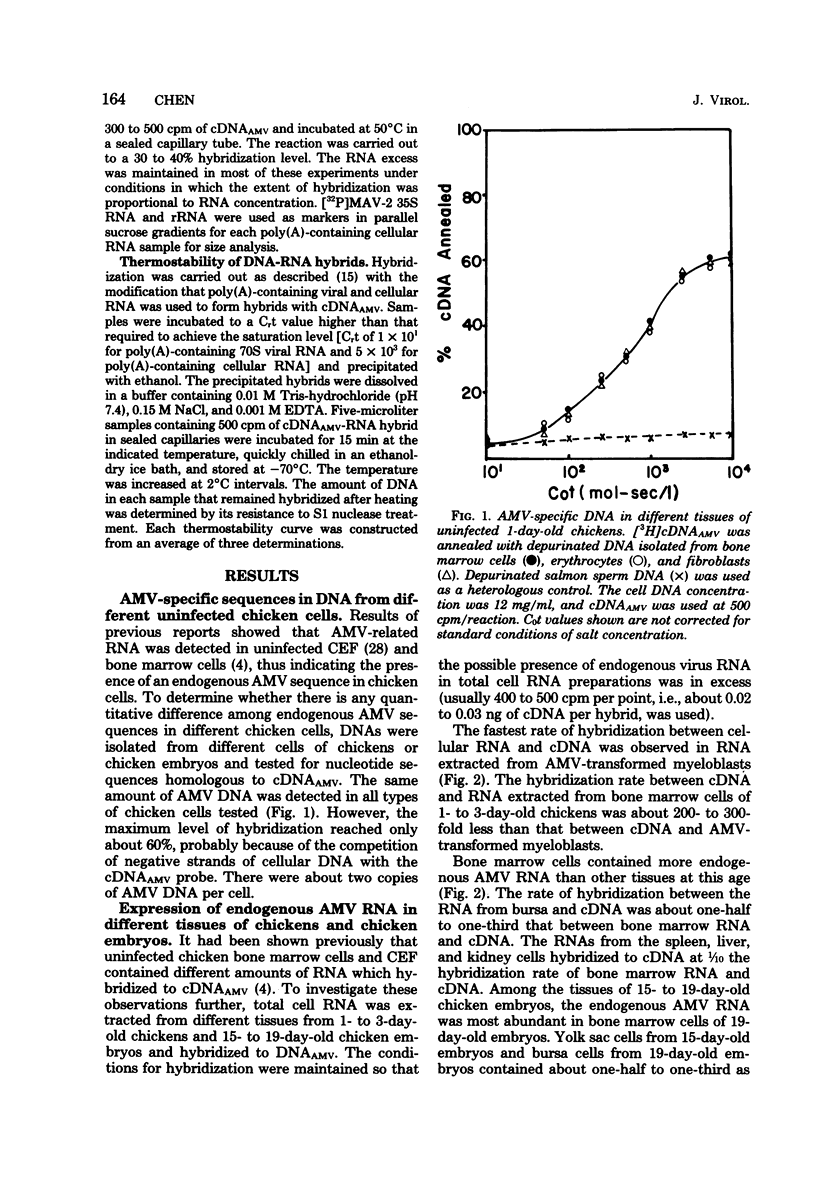
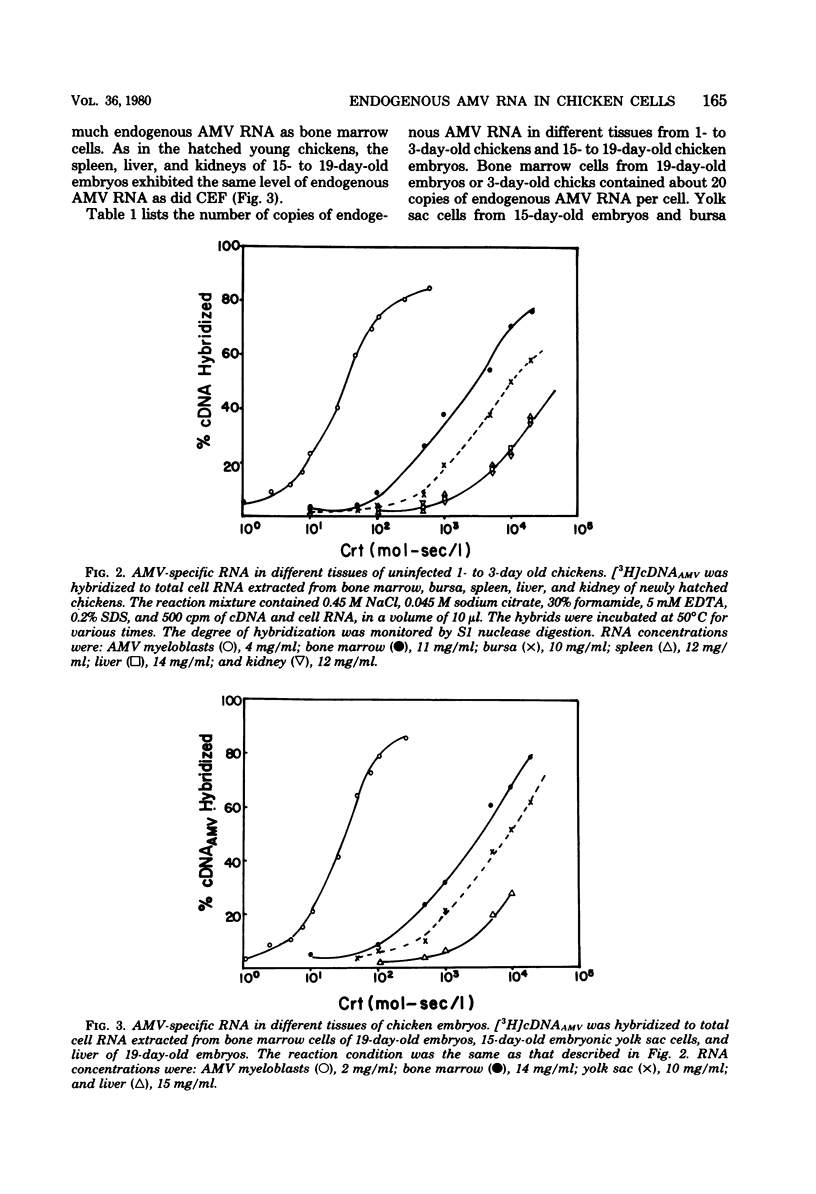
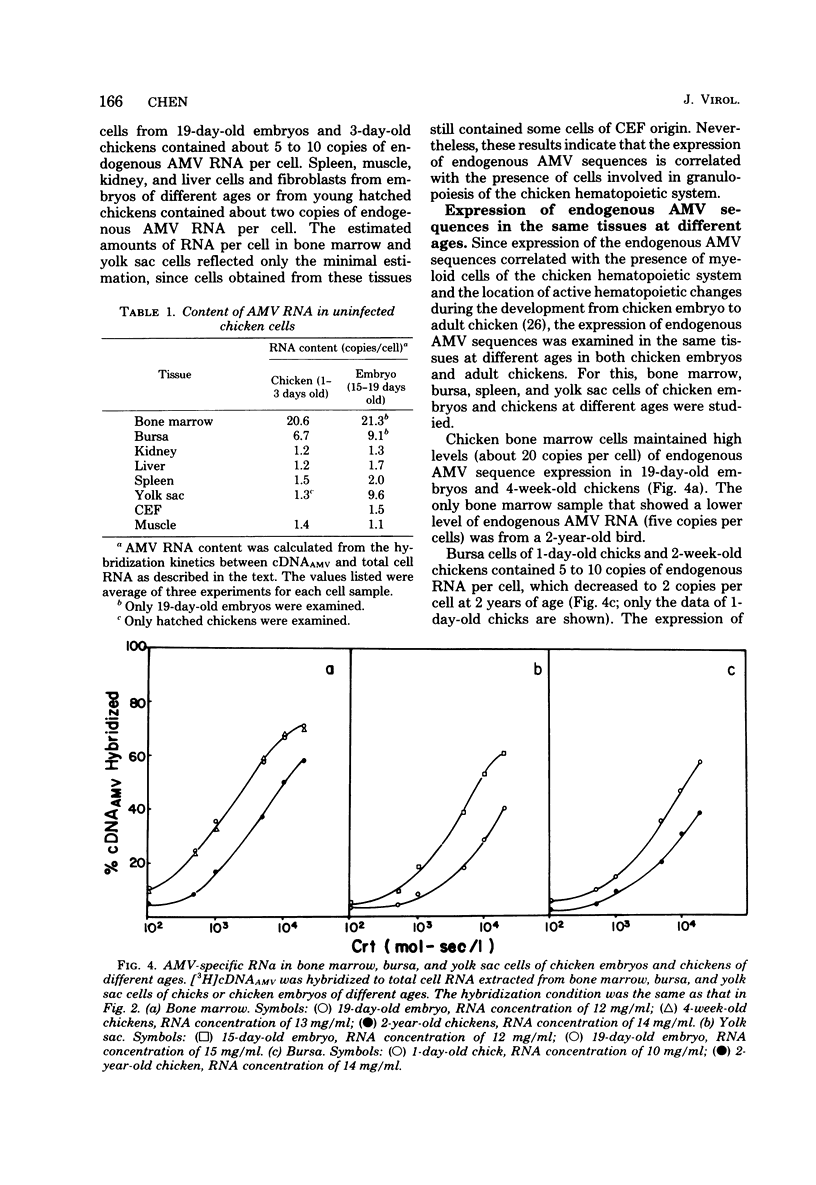
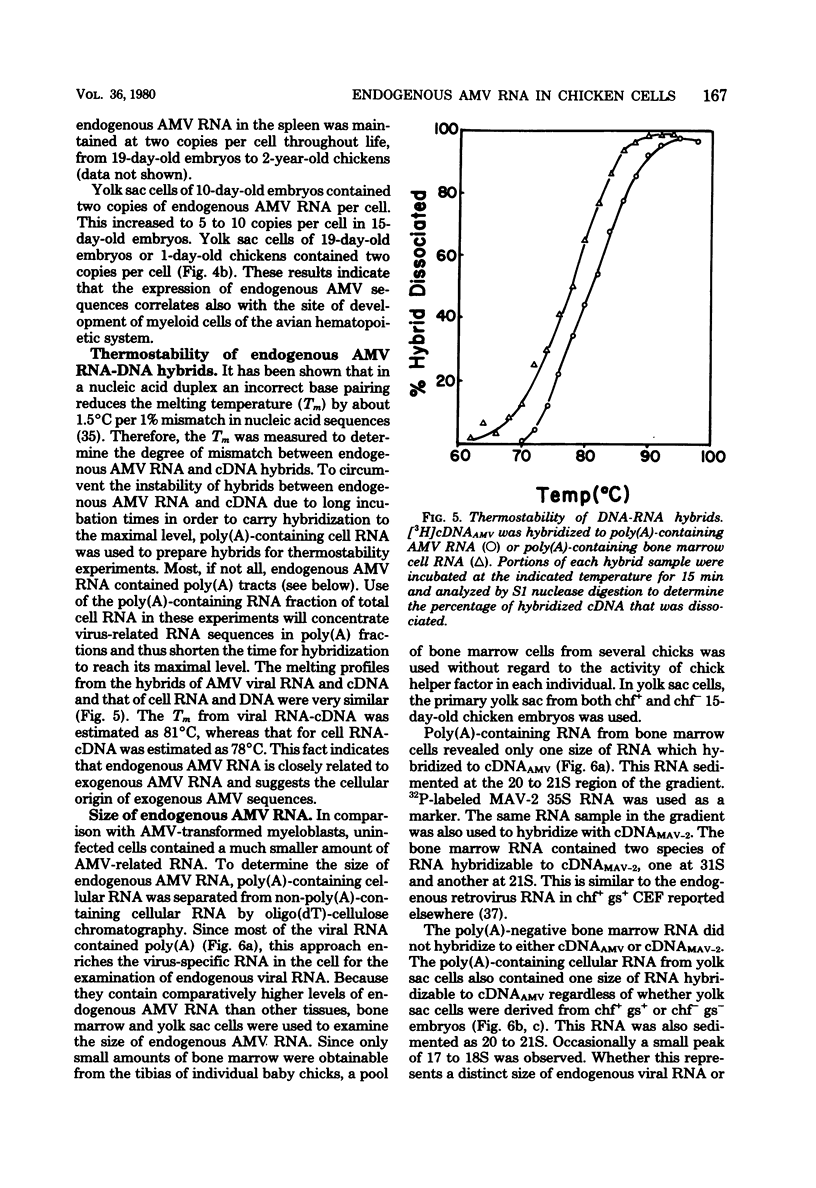
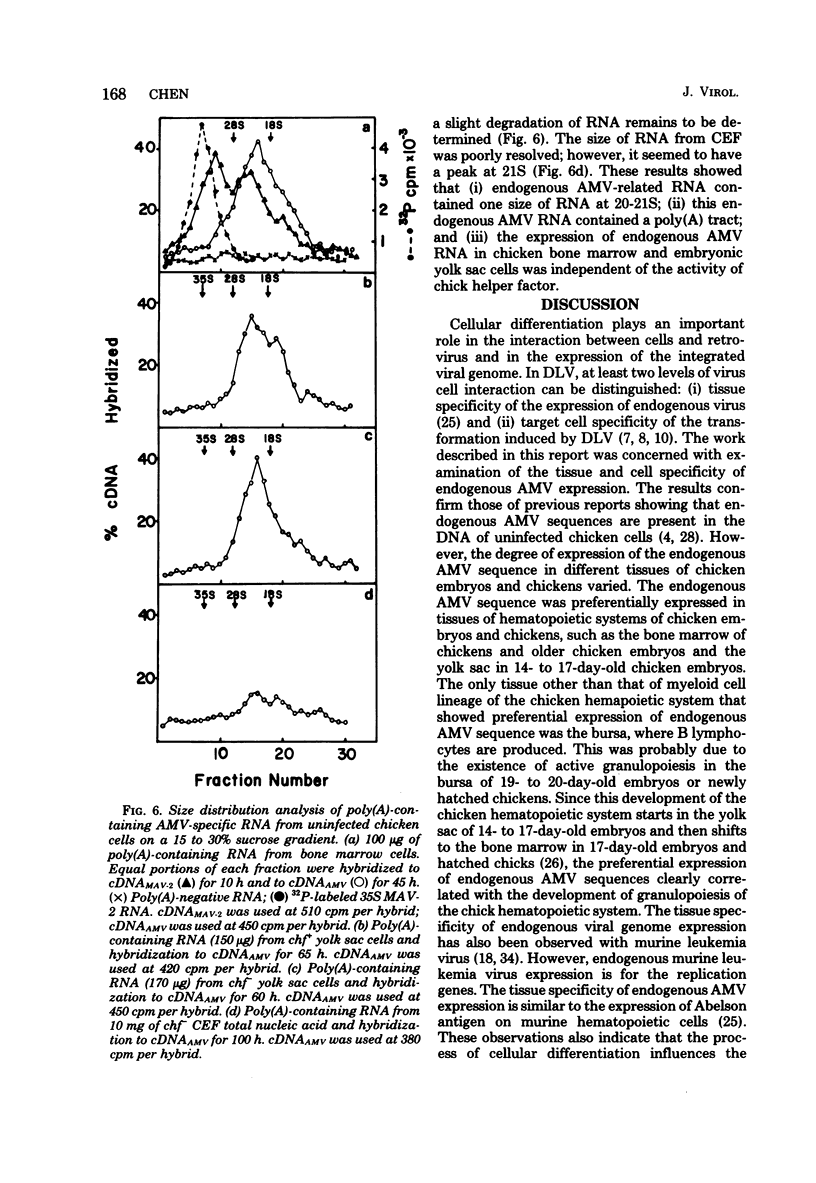
Uninfected chicken cells were found to contain endogenous avian myeloblastosis virus (AMV)-specific information. Different tissues from chicken embryos and chickens expressed different amounts of the AMV-specific information. The endogenous AMV-related RNA was most abundant in bone marrow cells, which contained about 20 copies per cell. About 5 to 10 copies of AMV endogenous RNA per cell were found in embryonic yolk sac cells and bursa cells. The spleen, muscle, liver, and kidney cells of chickens and the fibroblasts of chicken embryos contained about two copies per cell. The amounts of AMV endogenous RNA in bone marrow, yolk sac, and bursa varied with age. From 19-day-old embryos to 2-week-old chickens, the bone marrow contained 20 copies of AMV RNA per cell. Bone marrow cells from 2-year-old chickens contained five copies per cell. Yolk sac cells of 10-day-old embryos and 1-day-old chickens were found to contain two copies per cell, whereas in 15- to 17-day-old embryos, these cells contained 5 to 10 copies. These results indicate that the level of endogenous AMV expression correlates with the development of granulopoiesis of the chicken hemopoietic system. The results of experiments on the thermostability of RNA-DNA hybrids indicated that the endogenous AMV RNA is closely related to viral AMV RNA. The expression of endogenous AMV information is independent of the activity of the chick helper factor. This endogenous AMV information is expressed as 20 to 21S RNA in both bone marrow and yolk sac cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Hanafusa H. Detection of a protein of avian leukoviruses in uninfected chick cells by radioimmunoassay. J Virol. 1974 Feb;13(2):340–346. doi: 10.1128/jvi.13.2.340-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Hanafusa H. Avian tumor virus proteins and RNA in uninfected chicken embryo cells. J Virol. 1974 Dec;14(6):1419–1429. doi: 10.1128/jvi.14.6.1419-1429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Moscovici M. G., Moscovici C. Isolation of complementary DNA unique to the genome of avian myeloblastosis virus (AMV). Virology. 1980 May;103(1):112–122. doi: 10.1016/0042-6822(80)90130-0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Purchio A. F., Brugge J. S., Erikson R. L. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. doi: 10.1073/pnas.76.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge W. H., Moscovici C. Colony formation by chicken hematopoietic cells and virus-induced myeloblasts. J Cell Physiol. 1973 Jun;81(3):371–386. doi: 10.1002/jcp.1040810310. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici C., Moscovici M. G., Samarut J. Response of hemopoietic cells to avian acute leukemia viruses: effects on the differentiation of the target cells. Cell. 1979 Mar;16(3):627–638. doi: 10.1016/0092-8674(79)90036-9. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G. Tissue culture of avian hematopoietic cells. Methods Cell Biol. 1973;7:313–328. doi: 10.1016/s0091-679x(08)61784-7. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- Risser R. Abelson antigen is expressed on hematopoietic spleen colony-forming cells from mice carrying the Av-2S virus sensitivity gene. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5350–5354. doi: 10.1073/pnas.76.10.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. J., Stephenson J. R., Crittenden L. B., Aaronson S. A. Avian leukosis-sarcoma virus gene expression. Noncoordinate control of group-specific antigens in virus-negative avian cells. Virology. 1976 Apr;70(2):493–501. doi: 10.1016/0042-6822(76)90290-7. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Wilsnack R. E., Aaronson S. A. Radioimmunoassay for avian C-type virus group-specific antigen: detection in normal and virus-transformed cells. J Virol. 1973 Jun;11(6):893–899. doi: 10.1128/jvi.11.6.893-899.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T., Jaenisch R. Oncornavirus gene expression during embryonal development of the mouse. Virology. 1977 Feb;76(2):886–890. doi: 10.1016/0042-6822(77)90271-9. [DOI] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. II. Effects of deamination of cytosine. Biochim Biophys Acta. 1973 Feb 4;294(1):416–424. doi: 10.1016/0005-2787(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Hayward W. S., Hanafusa H. Genetic variation in the RNA transcripts of endogenous virus genes in uninfected chicken cells. J Virol. 1977 Oct;24(1):64–73. doi: 10.1128/jvi.24.1.64-73.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]


