Abstract
Protein import into complex plastids of red algal origin is a multistep process including translocons of different evolutionary origins. The symbiont-derived ERAD-like machinery (SELMA), shown to be of red algal origin, is proposed to be the transport system for preprotein import across the periplastidal membrane of heterokontophytes, haptophytes, cryptophytes, and apicomplexans. In contrast to the canonical endoplasmic reticulum-associated degradation (ERAD) system, SELMA translocation is suggested to be uncoupled from proteasomal degradation. We investigated the distribution of known and newly identified SELMA components in organisms with complex plastids of red algal origin by intensive data mining, thereby defining a set of core components present in all examined organisms. These include putative pore-forming components, a ubiquitylation machinery, as well as a Cdc48 complex. Furthermore, the set of known 20S proteasomal components in the periplastidal compartment (PPC) of diatoms was expanded. These newly identified putative SELMA components, as well as proteasomal subunits, were in vivo localized as PPC proteins in the diatom Phaeodactylum tricornutum. The presented data allow us to speculate about the specific features of SELMA translocation in contrast to the canonical ERAD system, especially the uncoupling of translocation from degradation.
INTRODUCTION
Organelles such as plastids, including those of secondary origin, almost completely rely on protein import from the host cytosol (46, 65). The structure of complex plastids, surrounded by three or four membranes required, in contrast to primary plastids, the evolution of several additional protein transport mechanisms. Complex plastids arose through secondary endosymbiosis, a process which describes the engulfment of a former free-living eukaryotic alga into a eukaryotic host cell (32, 33). During evolution, the symbiont was subsequently reduced in terms of compartmentalization and genome size to an organelle strictly dependent on the host cell (16, 32). Different types of secondary plastids exist in a very broad range of algae and protists, which can be distinguished based on their evolutionary origin (e.g., a red or green alga derived symbiont), as well as on the amount of cellular reduction inside the host cell. Our understanding of the evolution of organisms harboring a secondary plastid of red algal origin has changed in the last few years. According to the chromalveolate hypothesis, six major lineages were grouped together to be of monophyletic origin: cryptophytes, haptophytes, heterokontophytes, peridinin-containing dinoflagellates, apicomplexans, and the non-plastid-containing ciliates, as well as several smaller lineages related to some chromalveolate members (15, 41). However, recent phylogenetic analyses have given rise to extended theories about the evolution of the lineages with a red algal endosymbiont, including serial endosymbiotic events with secondary, as well as tertiary, endosymbioses (21, 22, 26, 27, 56, 61, 71, 75).
It has been shown that the lineages with an endosymbiont of red algal origin share common plastid protein import mechanisms despite remarkable differences resulting from specific features in plastid ultrastructure (10, 37, 65). Import into complex plastids starts cotranslationally at the endoplasmic reticulum (ER) membrane where nascent precursor proteins are synthesized into the ER lumen. This transport step requires a canonical N-terminal signal peptide (SP). In heterokontophytes, cryptophytes, and haptophytes, the outermost plastid membrane, termed the chloroplast ER (cER) membrane, is continuous with the endomembrane system of the host cell; therefore, the Sec61-mediated import already represents transport across the first membrane of complex plastids. In contrast, the plastids of apicomplexans and peridinin-containing dinoflagellates are not connected to the endomembrane system. Thus, after import into the ER lumen, proteins are likely to be transported to the plastid via vesicle transport mechanisms directly from the ER or via the Golgi apparatus (47, 57).
After the preprotein has entered the cER lumen, the SP is thought to be cleaved off, and a transit peptide-like sequence (TPL) is exposed at the new N terminus. The TPL is required for further transport into the periplastidal compartment (PPC), which resembles the naturally reduced cytoplasm of the endosymbiont, and further into the stroma of the plastid. Such transit peptide-like sequences thereby fulfill an additional function in contrast to transit peptides (TP) in primary plastids. Detailed characterization of the TPL revealed a difference between stroma- and PPC-localized proteins. Stromal proteins possess an aromatic (Phe, Tyr, and Trp) or bulky (Leu) amino acid at the +1 position of their TPL, in contrast to PPC proteins (4, 31, 34, 42). However, the observed AXA-FAP motif at the transition between SP and TPL of stromal proteins is not as well conserved in haptophytes, apicomplexans, and dinoflagellates as it is the case for heterokontophytes and cryptophytes (58). Furthermore, some membrane proteins of apicomplexan plastids (apicoplasts) seem to carry intrinsic targeting signals instead of a bipartite targeting signal consisting of SP and TPL (1, 20).
For transport across the second outermost membrane, the periplastidal membrane, a translocon model was proposed to consist of a recycled ER-associated degradation (ERAD) machinery of symbiont origin (67). Support for this model came from the detection of symbiont-specific ERAD components encoded on the nucleomorph of the cryptophyte Guillardia theta, this being the remnant nucleus of the former red algal endosymbiont in the cryptophytes PPC (23). The canonical ERAD removes aberrant or misfolded luminal (ERAD-L) and membrane (ERAD-M and ERAD-C) ER proteins and tags them after retrotranslocation in the cytosol with polyubiquitin moieties for subsequent proteasomal degradation (7, 40, 66). However, in the symbiont-specific ERAD-like pathway (SELMA) the retrotranslocation machinery of ERAD-L is postulated to be maintained and possesses the capacity to transport proteins from an ER luminal compartment into a cytoplasmic compartment, the PPC. This process is supposed to be uncoupled from degradation. SELMA is conserved in all secondary evolved organisms with a red algal endosymbiont, for which genomic data are available (26, 67, 68). Proteins of the derlin family are still controversially discussed elements of the ERAD-specific translocon. In the diatom Phaeodactylum tricornutum, two symbiont-localized derlins (PtsDer1-1/PtsDer1-2) are expressed which form hetero-oligomers as well as homo-oligomers and show interaction with transit peptide-like sequences of PPC-localized proteins (38). These components are indeed involved in the transport of proteins into the plastid as indicated by a conditional knock-down mutant of the Toxoplasma gondii sDer1 protein which showed impairment in plastid protein import (2). The translocation process is predicted to be dependent on ubiquitylation, further supported by the presence of a set of ubiquitylation enzymes (39, 67). Additional factors proposed to be involved in SELMA are a symbiont-specific Cdc48 AAA-ATPase with its cofactor Ufd1 and adaptor proteins (55, 67). After translocation, the precursor proteins are likely to undergo deubiquitylation and are either passed on to the translocon in the third outermost membrane or folded in the PPC (13, 39, 55). Although a residual set of 20S proteasomal components was identified in the PPC of diatoms, there is currently no link between SELMA and proteasomal degradation (55).
Having passed through the PPC, transport across the innermost plastid membranes seems to be comparable to primary plastids with a translocon at the inner membrane of chloroplasts (TIC) and a recently identified Omp85 protein which belongs to the family of Toc75 proteins, the core components of the translocon at the outer membrane of chloroplasts (TOC) (1, 10, 13, 73).
Here, we present an update on the SELMA translocation model in organisms with a red algal endosymbiont with focus on five heterokontophytes and apicomplexan parasites. In particular, we mined the genomes of organisms that carry secondary plastids, including recently published full genome sequences, for SELMA proteins. With this collected data set one would expect to define the degree of factor conservation and identify main components of the SELMA system which evolved to function in protein transport at a plastid membrane. Our results are compared to the respective host ERAD system, as well as to red algal ERAD components, from which SELMA originated. In addition, four new PPC-localized proteins similar to factors involved in ERAD could be identified in the diatom P. tricornutum. We also extended the set of core proteasomal components in the PPC of heterokontophytes and discuss their putative function in relation to SELMA.
MATERIALS AND METHODS
Bioinformatic analysis.
The protein sequences of ERAD and SELMA, as well as proteasomal components, were collected from published data or retrieved via blastp and tblastn searches. As queries, sequences from the Saccharomyces cerevisiae ERAD system and the P. tricornutum SELMA system were used to search the genomic databases for Phaeodactylum tricornutum v2.0 (12), Thalassiosira pseudonana (5), Fragilariopsis cylindrus (http://genome.jgi-psf.org/Fracy1/Fracy1.home.html), Aureococcus anophagefferens (30), Emiliania huxleyi CCMP1516 main genome assembly v1.0 (http://genome.jgi-psf.org/Emihu1/Emihu1.home.html), and Guillardia theta CCMP2712 v1.0 (http://genome.jgi-psf.org/Guith1/Guith1.home.html). Sequences from Ectocarpus siliculosus (19) and Babesia bovis were searched at the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov/guide/). Apicomplexan sequences were retrieved from the Plasmodium Genomics Resource version 9.0 (6) for Plasmodium, the Toxoplasma Genomics Resource v7.2 (29) for T. gondii and Neospora caninum, TparvaDB version 1.0 (74) and the NCBI server (http://www.ncbi.nlm.nih.gov/guide/) for Theileria parva, and the Cryptosporidium Genomics Resource v4.6 (36) for Cryptosporidium parvum. ERAD sequences for red algae were either retrieved from the genome projects of Cyanidioschyzon merolae (53) and Galdieria sulphuraria (Michigan State University Galdieria Database [http://genomics.msu.edu/galdieria/about.html]) or by local BLAST (blast-2.2.10-ia32-win32) using expressed sequence tags (EST) of Porphyridium cruentum and partial genome data from Calliarthron tuberculosum (http://dbdata.rutgers.edu/data/plantae/) generated by Chan et al. (17).
In general, a minimal e-value of 1e−04 was set as threshold for the identification of ERAD/SELMA components on the protein level. However, in cases of weak query sequence significance, matches with a lower e-value were also inspected. In addition, criteria such as domain structure and composition similarity (NCBI Conserved Domain search) were applied for the identification of relevant proteins (51). For proteasomal components, all S. cerevisiae 20S protein sequences were used as queries to collect a data set of putative proteasomal components, which were then classified according to the NCBI Conserved Domain Database (51), which differs from the S. cerevisiae nomenclature (detailed information on different classifications can be found in reference 60).
All gene models of the identified proteins were aligned to genomic and EST sequences, if available. Thereby, missing N and C termini were identified by searching for putative start and stop codons in frame, respectively. If possible, intron borders of the gene models were checked to be in agreement with EST data. The protein sequences were additionally examined for N-terminal targeting sequences to discriminate symbiont proteins from host factors. PPC directed proteins are characterized by the presence of a SP and a TPL. The SignalP 3.0 Server (24) was used for the prediction of a SP with a cutoff of >0.5 by the HMM algorithm. The sequences were then analyzed with the TargetP 1.1 Server (25) with default settings to define the SP as a secretory signal sequence and exclude mitochondrial targeting. In general, the TPL of PPC (symbiont) proteins cannot be predicted accurately with available tools. For this reason, besides performing the prediction with the TargetP 1.1 Server (25) using signal peptide truncated sequences in “plant” mode, the criteria defined in reference 55 were applied. In some cases, a protein model was identified with high similarity to a known symbiont protein of the diatom P. tricornutum or the apicomplexan parasite P. falciparum but without SP prediction. This can be caused by an incorrect gene model prediction due to the lack of EST data or the presence of several putative start codons. Therefore, these proteins were assigned as symbiont but marked to lack a signal peptide prediction.
Analyses of transmembrane-spanning regions were performed with TOPCONS (9); analyses of domain and coiled-coil prediction were done using SMART (45). Protein sequence alignments were performed with GENEDOC Software (version 2.6.002 [http://www.psc.edu/biomed/genedoc]).
Plasmid construction and transfection of P. tricornutum.
The predicted PPC proteins were cloned and transfected into the diatom P. tricornutum. The sequences of genes containing introns or without EST support were amplified from cDNA, the rest from gDNA, cloned in front of egfp into P. tricornutum transfection vectors. ptsubx, ptspng1, ptsubq were cloned into the nitrate-inducible pPha-NR vector (GenBank accession no. JN180663), ptsnpl4, ptsβ1, ptsα3, pthβ7, and pthrpn10 into the light-inducible pPha-T1 vector (GenBank accession no. AF219942). For further information about the sequences of in vivo-localized proteins, as well as the primer sequences, see File S1 in the supplemental material. Biolistic transfection into P. tricornutum cells was performed as described previously (67, 77). Positive transformants were cultured under standard conditions as described before (3) with 1.5 mM NH4+ in permanent cultivation. Protein expression under the control of the nitrate reductase promoter (pPha-NR vector) was induced by cultivation on 0.9 mM NO3− for 2 days.
Fluorescence microscopy.
P. tricornutum transformants were fixed with 4% paraformaldehyde–0.0075% glutaraldehyde in 1× phosphate-buffered saline buffer and analyzed with a confocal laser scanning microscope Leica TCS SP2 using a HCX PL APO 40×/1.25 to 0.75 oil CS objective lens. The fluorescence of enhanced green fluorescent protein (eGFP) and chlorophyll was excited with an argon laser at 488 nm and detected with two photomultiplier tubes at a bandwidths of 500 to 520 nm and 625 to 720 nm for eGFP and chlorophyll fluorescence, respectively.
RESULTS
Identification of ERAD and SELMA components in red algae and organisms with a red algal endosymbiont.
In order to identify new ERAD and SELMA components, all available genomic sequences of red algae and organisms with a red algal endosymbiont were screened via BLAST search with queries from the best-studied ERAD system of Saccharomyces cerevisiae (40, 66). The recently published genomes of heterokontophytes (the diatom Fragilariopsis cylindrus, the brown alga Ectocarpus siliculosus, the harmful alga Aureococcus anophagefferens), the nuclear genome of the cryptophyte Guillardia theta, and the apicomplexan Neospora caninum were included in these analyses. Because SELMA was shown to be phylogenetically derived from the ERAD system of the red algal endosymbiont (26), we also included sequences from the red algae Cyanidioschyzon merolae, Porphyridium cruentum, Calliarthron tuberculosum, and Galdieria sulphuraria in our analyses (see Materials and Methods for a detailed description of the genome data used). In contrast to the other chromalveolate groups, dinoflagellate plastids have only three surrounding membranes, and very little is known about the mechanisms that transport proteins across these membranes (10, 65). Due to the paucity of genomic data for these organisms, we have not included peridinin-containing dinoflagellates in the present study.
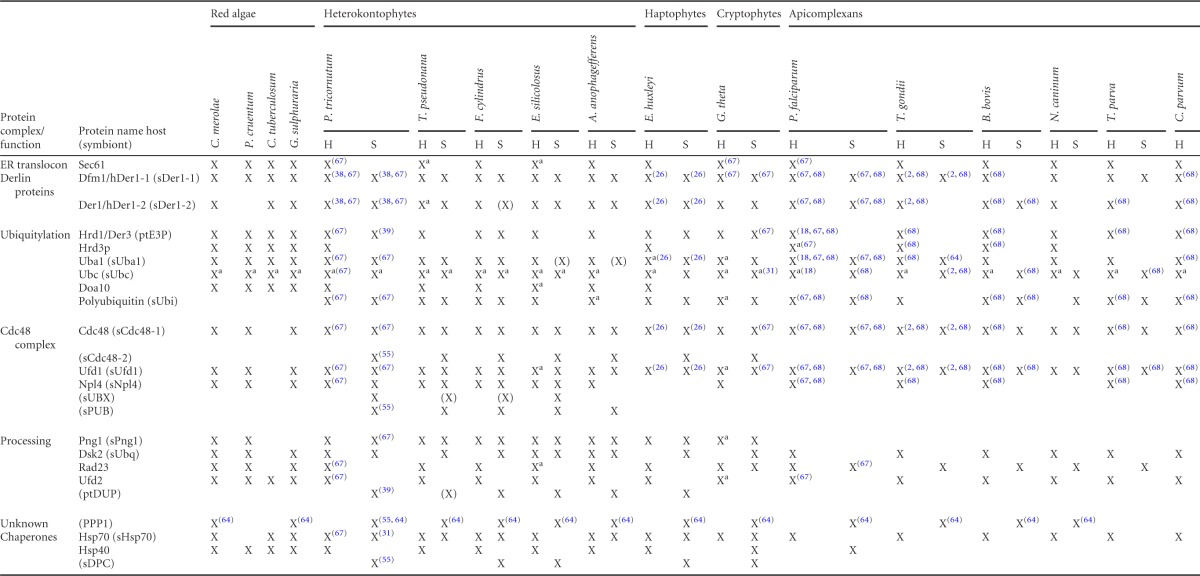
We identified genes for conserved ERAD components in all investigated red algal genomes (Table 1). However, due to incomplete data for Porphyridium cruentum (EST data) and especially Calliarthron tuberculosum (partial genome data), only a subset of ERAD factors could be identified. The collected data set for red algae implicates that the progenitor from which the SELMA machinery originated was capable of ERAD-L via the Hrd1 complex, as well as ERAD-C, via the Doa10 complex in the ER membrane. In addition, all proteins required for ubiquitylation and efficient proteasomal substrate delivery after ERAD retrotranslocation are present in red algae.
Table 1.
Overview of all identified host ERAD and symbiont SELMA components in organisms with secondary plastids of red algal origin compared to ERAD proteins of four red algal speciesa
Proteins similar to ScUsa1p, ScCue1p, and ScUbx4p could not be identified in the host or symbiont version and are therefore not included in the table. Ubc proteins are further assigned to the most similar S. cerevisiae Ubc enzyme in File S2 in the supplemental material (see Table S1 in the supplemental material). X, detected; (X), symbiont gene detected by homology but without targeting sequence; a, more than one gene detected. Superscript numbers in parentheses indicate the corresponding reference(s). Protein identifiers can be found in File S2 (Table S1) in the supplemental material.
In organisms with a red algal endosymbiont, the SELMA system exists in parallel with the host ERAD machinery. The discrimination between proteins of both systems is based on the targeting signal of the PPC localized SELMA proteins in contrast to the mostly cytosolic ERAD components (see Materials and Methods). Identification of a SELMA protein is more reliable if a respective host protein with the same putative function can be found. Therefore, a detailed analysis of the host ERAD system of the investigated organisms was included, and almost all ERAD proteins known from S. cerevisiae could be identified in the genomes (Table 1; for detailed information, see File S2 and Table S1 in the supplemental material). All organisms encode for the ER membrane proteins Sec61α, Hrd1, the derlin proteins and, with the exception of apicomplexans, also for Doa10. In addition, a cytosolic ubiquitylation machinery, the Cdc48 complex with its cofactors (Npl4 and Ufd1) and all proteasomal substrate delivery factors (Rad23, Dsk2, Png1) were identified.
Our inspection of the SELMA system in secondary evolved algae and apicomplexan parasites showed a high degree of conserved components for this putative protein translocation machinery (Table 1). However, there can be different reasons for failures in the identification of certain proteins. If a protein is present in most of the organisms of one group but lacking in one specific organism, this is likely caused by incomplete genome sequencing and assembly or by incorrect protein model prediction (e.g., for Aureococcus anophagefferens). In contrast, a protein not identified in a whole group of organisms may have been lost completely during evolution. Haptophytes and cryptophytes are represented only by one organism, hindering a final conclusion about the presence or absence of specific proteins but allowing considerations of whole protein complexes. The analysis of the newly available genome sequence of the cryptophyte G. theta shows that the partially nucleomorph-encoded SELMA system is supplemented with nucleus-encoded factors (Table 1).
Interestingly, from the three ER membrane protein classes—Sec61α, derlin proteins, and the ubiquitin ligase Hrd1, which are discussed as putative ERAD channel proteins—only the derlin proteins are found in the complex plastids of these organisms as membrane proteins with several transmembrane domains, with the exception of a nucleomorph-encoded Hrd1 in cryptophytes (see below). In respect to derlins, two symbiotic representatives are present in heterokontophytes, haptophytes, and cryptophytes, as is the case for yeast (ScDer1p and ScDfm1p). Ubiquitylation requires a cascade of three enzymes starting with a ubiquitin-activating enzyme (Uba1) which is present in all organisms. At least one symbiont ubiquitin-conjugating enzyme (sUbc) can also be found, but not all putative PPC-targeted sUbc proteins can be assigned to the same S. cerevisiae Ubc protein. While heterokontophytes share a sUbc similar to ScUbc6p and at least one other sUbc protein, apicomplexans seem to encode only for one sUbc protein with the highest similarity to ScUbc4p. The ubiquitin ligase sHrd1 of heterokontophytes differs in protein structure from the symbiont ubiquitin ligase of cryptophytes. Several transmembrane domains are predicted for the GtsHrd1 protein. Therefore, it more resembles the yeast ScHrd1p structure than the heterokontophyte E3 ligase which contains only one predicted transmembrane domain.
The symbiont Cdc48-complex together with sUfd1 can be found in all organisms investigated, and we were now able to identify a sNpl4 protein in the diatom P. tricornutum which is conserved among heterokontophytes. The same is the case for three other newly identified putative symbiont proteins, sUBX, sUbq, and sPng1. These share similarity to ERAD factors and are present in addition to the host version in the diatom P. tricornutum and other heterokontophytes. None of these proteins is conserved in apicomplexans (Table 1).
Newly identified putative SELMA components: the sCdc48 cofactor sNpl4, a UBX domain-containing protein, a symbiont ubiquilin-like protein and a peptide N-glycanase.
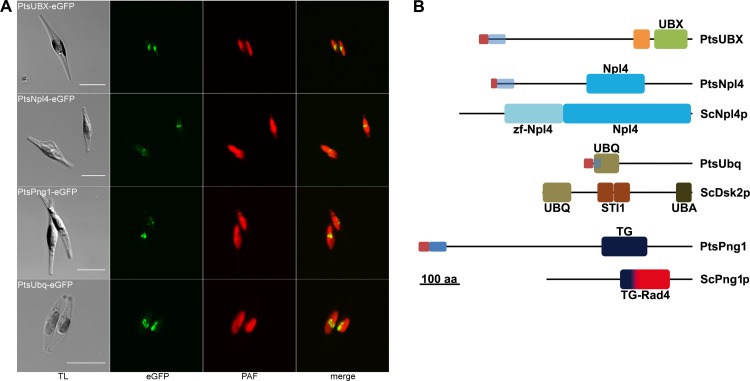
Among the newly identified putative SELMA components are two Cdc48 binding proteins, the UBX domain-containing protein sUBX (symbiont UBX) and the Ufd1 cofactor sNpl4, as well as proteins with sequence similarity to the de-glycosylation enzyme ScPng1p (sPng1) and the polyubiquitin-binding protein ScDsk2p (sUbq). Not all four proteins are predicted to have a TPL in the diatom P. tricornutum. In such a case, a signal peptide on usually cytosolic proteins is indicative for a PPC localization but remained to be verified in localization experiments. Therefore, PtsNpl4, PtsUBX, PtsUbq, and PtsPng1 were expressed as eGFP fusions in P. tricornutum and their localization was examined in vivo. All constructs showed the typical fluorescence pattern of PPC-localized proteins in the middle of the two plastid lobes (Fig. 1A).
Fig 1.
In vivo localization and domain organization of new SELMA proteins of the diatom P. tricornutum. (A) In vivo localizations of PtsUBX, PtsNpl4, PtsPng1, and PtsUbq as eGFP fusion proteins in P. tricornutum show the characteristic PPC fluorescence in the middle of the two plastid lobes (scale, 10 μm; TL, transmission light; PAF, plastid autofluorescence). (B) Structural overview of the domain composition of PtsNpl4, PtsUbq, and PtsPng1 in comparison to the respective S. cerevisiae ERAD protein (ScNpl4p, ScDsk2p and ScPng1p). Whereas PtsNpl4 and PtsPng1 share the conserved domains of their yeast counterparts, PtsUbq lacks the UBA domain for polyubiquitin binding. PtsUBX cannot be assigned to a specific ERAD protein. Red, signal peptide; blue, TPL predicted; light blue, no TPL predicted; UBX, domain present in ubiquitin-regulatory proteins; orange, coiled coil region; UBQ, ubiquitin homologues; STI1, heat shock chaperonin-binding motif; UBA, ubiquitin associated domain; TG, transglutaminase/protease-like homologues; Rad4; Rad4 transglutaminase-like domain.
A comparison of the protein sequences to well-known ERAD components of S. cerevisiae and their domain composition points to their putative function (Fig. 1B). The identified symbiont UBX domain-containing protein, PtsUBX, shares sequence similarity to other proteins only in its UBX domain which has been shown to be a general Cdc48 binding module (63). Preceding the UBX domain, the protein harbors a coiled-coil region for homo- or heterotypic protein interaction (52). The second identified Cdc48 binding protein, PtsNpl4, now completes the sCdc48 complex, together with the previously described cofactor sUfd1 (67). In comparison to its yeast ERAD counterpart the protein lacks the N-terminal Npl4 zinc finger domain. The symbiont ubiquilin-like protein PtsUbq shares the N-terminal UBQ domain known from mammalian ubiquilins and ScDsk2p (28, 69) but lacks the C-terminal UBA domain for polyubiquitin binding as well as the internal STI1 domains. PtsPng1 as a symbiont deglycosylation enzyme was initially annotated as the host protein (67) despite the presence of a weakly predicted signal peptide, which can be explained by a missing second copy of this protein at that time. In the present study an additional Png1 protein was identified in its place, lacking an N-terminal targeting sequence and leading to reevaluation of the former prediction. PtsPng1 has a transglutaminase/protease-like domain with the conserved catalytic residues of cytoplasmic PNGase (data not shown) (70).
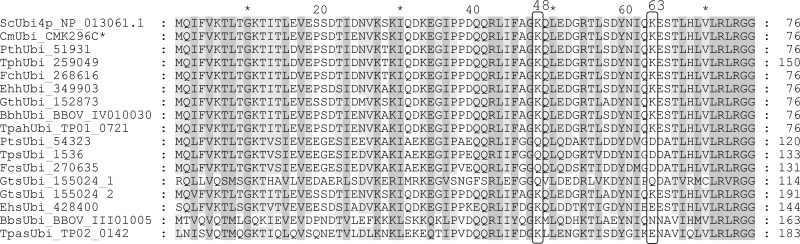
The symbiont ubiquitin in diatoms lacks the conserved lysine residues Lys48 and Lys63.
The SELMA model proposes ubiquitylation of the precursor proteins during transport and subsequent removal of the ubiquitin moiety via a PPC-specific deubiquitinating enzyme (38, 67). The PPC-targeted ubiquitin of P. tricornutum (PtsUbi) was shown to lack the specific lysine residue (Lys48) as the most prominent linker for polyubiquitylation involved in degradation (67). With the identification of symbiont ubiquitins from T. pseudonana and F. cylindrus, this feature becomes even more apparent, as the Lys48 is also absent in these diatom sequences (Fig. 2). The TpsUbi protein model (ID 1539) can hardly be recognized and has to be modified according to the available EST sequences in order to obtain the full sequence (see File S1 in the supplemental material). In the genome of the cryptophyte Guillardia theta, a diubiquitin protein sequence with a signal peptide prediction could be identified with the less conserved first ubiquitin domain sharing the diatom lysine mutation. In contrast, sUbi sequences from the haptophyte E. huxleyi and the apicomplexans B. bovis and T. parva still contain lysine 48. The previously identified symbiont ubiquitin of P. falciparum shows only weak conservation with the symbiont ubiquitins from diatoms (68). Unfortunately, it was not possible to detect a symbiont ubiquitin in the other newly investigated organisms.
Fig 2.
Alignment of the ubiquitin domain of hUbi and sUbi sequences with the S. cerevisiae and C. merolae ubiquitins. The host ubiquitin sequences are derived from polyubiquitins consisting of multiple ubiquitin domains of the same sequence. Protein sequences of sUbi from P. tricornutum, F. cylindrus, and T. pseudonana share the same lysine mutations at positions 48 and 63. E. huxleyi, T. parva, and B. bovis only show an altered Lys63 position. Both ubiquitin domains of the F. cylindrus symbiont diubiquitin have identical protein sequences; in contrast, the diubiquitin of G. theta is depicted as two independent ubiquitin domains (GtsUbi_155024_1/2). (*, There is no polyubiquitin in C. merolae; therefore, a ubiquitin-ribosomal fusion protein was included in the alignment. For detailed information on the protein sequences, see File S1 in the supplemental material).
Interestingly, the position Lys63, usually used for polyubiquitin linkages related to modifications of protein function, is also no longer present in all symbiont ubiquitins except the second domain of GtsUbi.
Identification and localization of proteasomal components in the PPC of heterokontophytes and cryptophytes.
We previously reported on the presence of relict 20S proteasomal components in the P. tricornutum PPC (55). Here, we expand the model of a symbiont core proteasome by extensive in silico analyses.
The cryptophyte Guillardia theta, which is still able to synthesize proteins in the PPC, encodes for an almost complete set of PPC-localized proteasomal degradation components on its nucleomorph genome (23). This is in contrast to other organisms with a red algal endosymbiont and, among them, only in heterokontophytes could residual 20S subunits be identified. Importantly, we did not detect a symbiont 19S regulatory particle in the PPC of heterokontophytes, although it is present in cryptophytes. We could not identify a complete set of 20S subunits, including seven alpha and seven beta subunits, for any of the organisms studied (Table 2). Instead, the putative 20S core particle seems to vary in subunit composition, with the exception of conserved sα2, sα3, and sβ6. The putative catalytically active subunits β6 and β7 could be identified in almost all heterokontophytes in a PPC-directed version; a second β5 gene is only detectable as a gene fusion with a 5′-3′ exonuclease, also lacking a signal peptide. In A. anophagefferens and E. siliculosus in addition to a symbiont sβ5 with signal peptide prediction, several putative symbiont subunits exist but an exact defining of the gene model is difficult. Therefore, a classification into host or symbiont protein cannot yet conclusively be determined.
Table 2.
Overview of host and symbiont 20S proteasomal components of heterokontophytes and cryptophytes compared to those from red algaea
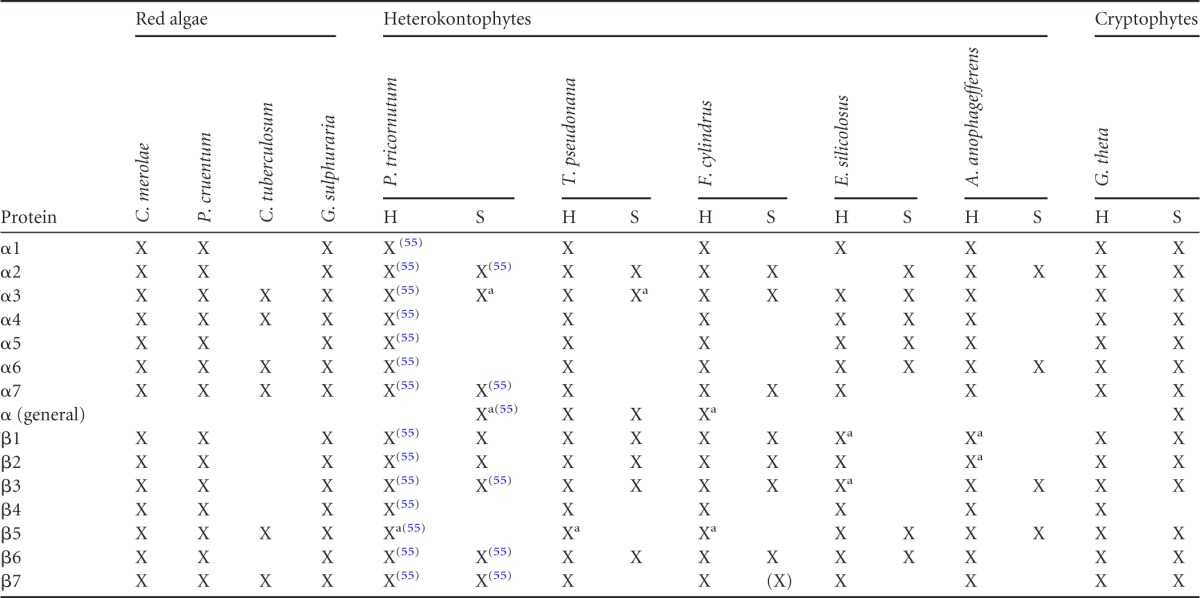
X, detected; a, more than one gene detected; (X), symbiont gene detected by homology but without targeting sequence. Superscript numbers in parentheses indicate the corresponding reference. Protein identifiers can be found in File S2 (Table S2) in the supplemental material.
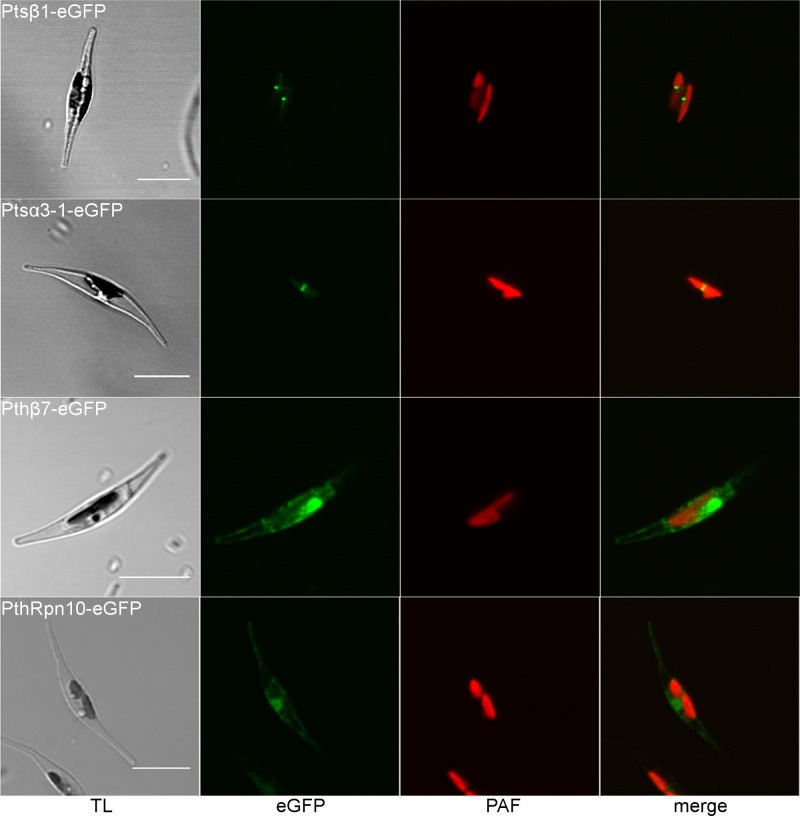
In addition to the already-reported symbiont 20S proteasomal subunits Ptsβ2, Ptsβ6, Ptsβ7, Ptsα7-1, and Ptsα7-2 (55), we successfully localized two additional subunits in the PPC of P. tricornutum. Both Ptsα3-1 and Ptsβ1 showed the typical PPC fluorescence pattern (Fig. 3). As a comparison, two subunits of the host proteasome were also localized, Pthβ7 and PthRpn10, which resulted in a different fluorescence pattern outside of the plastid.
Fig 3.
In vivo localization of new symbiont 20S proteasomal components in the PPC of the diatom P. tricornutum. eGFP fusion proteins Ptsβ1 and Ptsα3-1 show fluorescence in the middle of the two plastid lobes, indicating a PPC localization in contrast to the host proteins Pthβ7 and PthRpn10, which localize in areas around the plastid (scale, 10 μm; TL, transmission light; PAF, plastid autofluorescence).
DISCUSSION
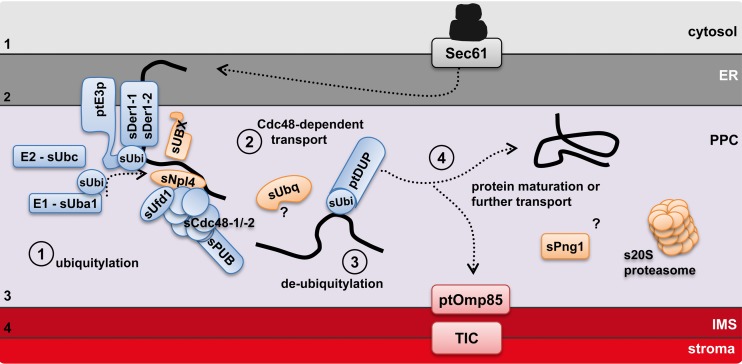
For protein transport across the periplastidal membrane of complex plastids of red algal origin, an ERAD-derived mechanism (SELMA) was proposed as the protein translocation machinery (67). The SELMA model, originally based on our findings in cryptophytes, was shown to be conserved in organisms with a red algal endosymbiont (2, 26, 67, 68), and SELMA components can be identified in all available genomes in addition to the host ERAD machinery (Table 1). However, the exact mechanism of this transport step, the pore-forming proteins, and the minimal required components remain an open question. The proposed SELMA components show often a minimized structure, as domains, known from ERAD proteins of other organisms, are missing. Thus, the SELMA complex should indicate a minimized version of the retro-translocation activity of ERAD in general. Different extents of ERAD to SELMA reduction can be found in the investigated organisms according to the amount of reduction of the former endosymbiont. The cryptophyte G. theta represents the most extended set of SELMA and proteasomal components, resulting most probably from its transcriptionally and translationally active nucleomorph in the PPC. Apicomplexans instead have the smallest set of identified SELMA components and seem to lack a symbiont proteasome. Thus far, all investigated organisms share the following as SELMA components in the PPC: derlins as membrane and putative channel proteins, a ubiquitylation machinery, and a Cdc48 complex. It remains to be determined whether the recently identified conserved PPC protein PPP1 provides a new crucial function for protein transport (64). All other identified proteins with functions related to SELMA or the proteasome in heterokontophytes might represent lineage specific adaptations (Fig. 4).
Fig 4.
Schematic model of protein import into the PPC across the SELMA translocation complex in diatoms. Upon recognition of a SELMA substrate in the cER lumen, precursor proteins are translocated across the membrane complex composed of sDer1-1/-2 and the ubiquitin ligase ptE3p. As soon as they reach the PPC, the precursors are ubiquitylated via a PPC-located ubiquitylation machinery. This leads to a recruitment of the Cdc48 complex with its cofactors sUfd1 and sNpl4, and translocation is completed. The ubiquitin moiety is most likely removed prior to protein maturation for PPC resident proteins or further transport for plastid proteins. The identified 20S proteasomal components function according to current knowledge independent of ubiquitylation. cER, chloroplast ER; PPC, periplastidal compartment; IMS, intermembrane space. Orange indicates proteins identified in the present study. (Modified from reference 39.)
Of all ER membrane proteins that are candidates for a translocation channel in ERAD (66), only the derlin proteins could be identified as SELMA components. Apart from that, the Sec61 channel and the ubiquitin ligase Hrd1 are in discussion as potentially being capable of fulfilling this function. On the one hand, we could not identify additional symbiont Sec61 subunits; on the other hand, the diatom symbiont E3 ubiquitin ligase is predicted to have only one transmembrane domain and is therefore most likely not capable of homotypic channel formation. However, the derlin proteins and the ubiquitin ligase might form a membrane complex which connects translocation to ubiquitylation. The presence of the ubiquitylation enzymes sUba and sUbc and ubiquitin itself in almost all investigated organisms, including apicomplexans, suggests the presence of a ubiquitin-dependent mechanism in the PPC. Once the preprotein is ubiquitylated in the PPC, it can be recognized by the Cdc48 complex (76). We could identify at least one symbiont-specific sCdc48 protein in all organisms investigated. The Cdc48-ATPase has been shown to be a central component of ERAD, acting specifically in concert with its cofactors Ufd1 and Npl4 (54, 76). Although Cdc48 is known to have various cellular functions, the identification and localization of a symbiont Npl4 protein of P. tricornutum presented here now define the sCdc48-sUfd1-sNpl4 complex as a SELMA component. However, other functions unrelated to protein transport together with thus-far-unidentified cofactors cannot be excluded. The new PPC-localized protein PtsUBX, most likely a sCdc48 binding protein due to its UBX domain, might also be involved in SELMA translocation, akin to the case for UBX proteins in ERAD. These proteins can direct the Cdc48-ATPase to a specific protein complex in the context of ERAD to ubiquitin ligases at the ER membrane (50, 62, 63).
After translocation is completed, ERAD substrates are recognized by a set of cytosolic proteins and processed for degradation by the proteasome (59, 76). The presence of a relict symbiont proteasome in heterokontophytes (Table 2) raises the question of a functional link between the ERAD derived SELMA machinery and degradation in the PPC of these organisms. Several features of both machineries in the PPC argue against such a connection. On the one hand, the PPC of apicomplexan parasites and haptophytes lacks symbiont 20S subunits and therefore harbors a SELMA system which seems to be completely independent of a proteasomal function. On the other hand, proteasomal substrates are not only delivered by the ERAD system but can also be degraded independently of ubiquitylation (8). The 20S core particle was shown to have basal proteolytic activity toward unstructured or oxidized proteins (60). It is also implicated in maturation and specific cleavage of various proteins, which gain access to the proteolytic chamber through an interaction with N termini of the α-subunits (8, 49). In the PPC of heterokontophytes, we identified proteasomal subunits of the 20S core particle, including proteolytic active subunits. It was not possible to detect a complete set of 20S subunits in any of the heterokontophyte species. Either the remaining α- and β-subunits in the genomes are too divergent to be recognized or the putative reduced 20S particle in the PPC can vary in subunit composition, replacing some subunits by other ones.
In addition, the recognition and unfolding of ubiquitylated proteasomal substrates is mediated by the 19S regulatory particle of the proteasome, which was not identified in a PPC-targeted version in heterokontophytes. However, the two newly identified PPC proteins sUbq and sPng1 are counterparts to ScDsk2p and ScPng1p, which are known from ERAD to function between retrotranslocation and degradation (43). The symbiont ubiquilin-like protein PtsUbq lacks the C-terminal ubiquitin-associated (UBA) domain for polyubiquitin binding present in ScDsk2p and mammalian ubiquilins for recognition of proteasomal substrates (28, 48). In addition, the ubiquitin-like domain (UBQ) at the N terminus of ScDsk2p was shown to bind proteasomal components of the 19S regulatory particle, as well as to ScUfd2p (35, 69), both not present in a symbiont version in the PPC. Most likely, both proteins had to adapt to new functions. PtsPng1, the PPC-localized peptide N-glycanase, might either be involved in the maturation of PPC-localized proteins or be required for efficient removal of glycan moieties of plastid precursor proteins added in the ER lumen before transport across the third outermost plastid membrane. In contrast to heterokontophytes, haptophytes, and cryptophytes, apicomplexans encode neither a host nor a symbiont Png1 protein. This is likely due to a reduction of N-glycosylation capacities in these organisms, especially for apicoplast proteins (14).
Another important feature of SELMA is the symbiont ubiquitin, which shows alterations at specific lysine residues. In heterokontophytes, the PPC-localized ubiquitin (sUbi) does not possess the conserved lysine residues Lys48 and Lys63, whereas haptophyte and apicomplexan (except P. falciparum) sUbi sequences still contain Lys48 but show mutations at Lys63. Lys48 was shown to represent the most prominent position for polyubiquitylation leading to proteasomal degradation (72). Although recent work suggests a more complex interplay between different ubiquitin linkages on various lysine residues also in degradation (44), the loss of Lys48 in the symbiont ubiquitins of heterokontophytes might be an evolutionary adaptation required for the establishment of the symbiont ERAD as a preprotein translocation system. This is supported by the finding that only organisms with a symbiont proteasome (Table 2) show this ubiquitin Lys48 modification. An exception is the cryptophyte G. theta with two ubiquitin domains in the predicted GtsUbi sequence, one overall conserved and another having Lys mutations at both positions. One might speculate about a separation of SELMA and proteasomal degradation in the cryptophytes PPC based on different ubiquitins, which might be caused by the different morphology, since cryptophytes—in contrast to all other organisms with a secondary red algal symbiont—still synthesize proteins in the PPC. Ubiquitin Lys63 is implicated in ubiquitylation processes related to functional modifications of target proteins (72). Remarkably, loss of Lys63 in all organisms with a secondary plastid of red algal origin leads to reduced ubiquitylation possibilities in the PPC in contrast to the manifold mechanisms regulated by ubiquitylation in the host cytosol. Thus, it remains to be determined whether the symbiont ubiquitins can be used for both mono- and polyubiquitylation on the remaining lysine residues.
The SELMA translocation machinery (Fig. 4) provides an interesting view into evolutionary rearrangements and modifications of already existing mechanisms. During the establishment of a red alga as an organelle, the symbiont ER-associated degradation machinery was split into a translocation complex on the one hand and a presumed degradation machinery on the other one. The former now represents the second step of protein import into complex plastids across the periplastidal membrane, whereas the latter one might be required for protein homeostasis in the PPC of only certain groups of organisms with a red algal endosymbiont. Such modularization of the well conserved ERAD translocation not only gave rise to SELMA but also was shown to be the principle mechanism of the peroxisomal importomer, again a ubiquitin-dependent translocation independent of proteasomal degradation (11).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft (Collaborative Research Centre 593 for S.S., I.W., S.Z., and U.-G.M. and SFB TR1 for J.M.P.). D.M. is a fellow of the International Max Planck Research School for Environmental, Cellular and Molecular Microbiology (IMPRS-MIC).
Footnotes
Published ahead of print 5 October 2012
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Agrawal S, Striepen B. 2010. More membranes, more proteins: complex protein import mechanisms into secondary plastids. Protist 161:672–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrawal S, van Dooren GG, Beatty WL, Striepen B. 2009. Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins. J. Biol. Chem. 284:33683–33691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apt KE, Grossman A, Kroth-Pancic P. 1996. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. Gen. Genet. 252:572–579 [DOI] [PubMed] [Google Scholar]
- 4. Apt KE, et al. 2002. In vivo characterization of diatom multipartite plastid targeting signals. J. Cell Sci. 115:4061–4069 [DOI] [PubMed] [Google Scholar]
- 5. Armbrust EV, Berges JA, Bowler C, Green BR. 2004. The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86 [DOI] [PubMed] [Google Scholar]
- 6. Aurrecoechea C, et al. 2009. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 37:D539–D543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagola K, Mehnert M, Jarosch E, Sommer T. 2011. Protein dislocation from the ER. Biochim. Biophys. Acta 1808:925–936 [DOI] [PubMed] [Google Scholar]
- 8. Baugh JM, Viktorova EG, Pilipenko EV. 2009. Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J. Mol. Biol. 386:814–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernsel A, Viklund H, Hennerdal A, Elofsson A. 2009. TOPCONS: consensus prediction of membrane protein topology. Nucleic Acids Res. 37:W465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolte K, et al. 2009. Protein targeting into secondary plastids. J. Eukaryot. Microbiol. 56:9–15 [DOI] [PubMed] [Google Scholar]
- 11. Bolte K, et al. 2011. Making new out of old: recycling and modification of an ancient protein translocation system during eukaryotic evolution: mechanistic comparison and phylogenetic analysis of ERAD, SELMA, and the peroxisomal importomer. Bioessays 33:368–376 [DOI] [PubMed] [Google Scholar]
- 12. Bowler C, et al. 2008. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244 [DOI] [PubMed] [Google Scholar]
- 13. Bullmann L, et al. 2010. Filling the gap, evolutionarily conserved Omp85 in plastids of chromalveolates. J. Biol. Chem. 285:6848–6856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bushkin GG, et al. 2010. Suggestive evidence for Darwinian selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii. Eukaryot. Cell 9:228–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cavalier-Smith T. 1999. Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree. J. Eukaryot. Microbiol. 46:347–366 [DOI] [PubMed] [Google Scholar]
- 16. Cavalier-Smith T. 2000. Membrane heredity and early chloroplast evolution. Trends Plant Sci. 5:174–182 [DOI] [PubMed] [Google Scholar]
- 17. Chan CX, et al. 2011. Red and green algal monophyly and extensive gene sharing found in a rich repertoire of red algal genes. Curr. Biol. 21:328–333 [DOI] [PubMed] [Google Scholar]
- 18. Chung D-WD, Ponts N, Prudhomme J, Rodrigues EM, Le Roch KG. 2012. Characterization of the ubiquitylating components of the human malaria parasite's protein degradation pathway. PLoS One 7:e43477 doi:10.1371/journal.pone.0043477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cock JM, et al. 2010. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465:617–621 [DOI] [PubMed] [Google Scholar]
- 20. Derocher AE, Karnataki A, Vaney P, Parsons M. 2012. Apicoplast targeting of a Toxoplasma gondii transmembrane protein requires a cytosolic tyrosine-based motif. Traffic 13:694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deschamps P, Moreira D. 2012. Re-evaluating the green contribution to diatom genomes. Genome Biol. Evol. 4:683–688 doi:10.1093/gbe/evs053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dorrell RG, Smith AG. 2011. Do red and green make brown? : perspectives on plastid acquisitions within chromalveolates. Eukaryot. Cell 10:856–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douglas S, et al. 2001. The highly reduced genome of an enslaved algal nucleus. Nature 410:1091–1096 [DOI] [PubMed] [Google Scholar]
- 24. Dyrløv Bendtsen J, Nielsen H, von Heijne G, Brunak S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783–795 [DOI] [PubMed] [Google Scholar]
- 25. Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300:1005–1016 [DOI] [PubMed] [Google Scholar]
- 26. Felsner G, et al. 2011. ERAD components in organisms with complex red plastids suggest recruitment of a preexisting protein transport pathway for the periplastid membrane. Genome Biol. Evol. 3:140–150 doi:10.1093/gbe/evq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frommolt R, et al. 2008. Ancient recruitment by chromists of green algal genes encoding enzymes for carotenoid biosynthesis. Mol. Biol. Evol. 25:2653–2667 [DOI] [PubMed] [Google Scholar]
- 28. Funakoshi M, Sasaki T, Nishimoto T, Kobayashi H. 2002. Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc. Natl. Acad. Sci. U. S. A. 99:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gajria B, et al. 2008. ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res. 36:D553–D556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gobler CJ, et al. 2011. Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proc. Natl. Acad. Sci. U. S. A. 108:4352–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gould SB, et al. 2006. Nucleus-to-nucleus gene transfer and protein retargeting into a remnant cytoplasm of cryptophytes and diatoms. Mol. Biol. Evol. 23:2413–2422 [DOI] [PubMed] [Google Scholar]
- 32. Gould SB, Waller RF, McFadden GI. 2008. Plastid evolution. Annu. Rev. Plant Biol. 59:491–517 [DOI] [PubMed] [Google Scholar]
- 33. Green BR. 2011. After the primary endosymbiosis: an update on the chromalveolate hypothesis and the origins of algae with Chl c. Photosynth. Res. 107:103–115 [DOI] [PubMed] [Google Scholar]
- 34. Gruber A, Vugrinec S, Hempel F, Gould SB, Maier Kroth U-GPG. 2007. Protein targeting into complex diatom plastids: functional characterisation of a specific targeting motif. Plant Mol. Biol. 64:519–530 [DOI] [PubMed] [Google Scholar]
- 35. Hänzelmann P, Stingele J, Hofmann K, Schindelin H, Raasi S. 2010. The yeast E4 ubiquitin ligase Ufd2 interacts with the ubiquitin-like domains of Rad23 and Dsk2 via a novel and distinct ubiquitin-like binding domain. J. Biol. Chem. 285:20390–20398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heiges M, et al. 2005. CryptoDB: a Cryptosporidium bioinformatics resource update. Nucleic Acids Res. 34:D419–D422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hempel F, et al. 2007. Transport of nuclear-encoded proteins into secondarily evolved plastids. Biol. Chem. 388:899–906 [DOI] [PubMed] [Google Scholar]
- 38. Hempel F, Bullmann L, Lau J, Zauner S, Maier UG. 2009. ERAD-derived preprotein transport across the second outermost plastid membrane of diatoms. Mol. Biol. Evol. 26:1781–1790 [DOI] [PubMed] [Google Scholar]
- 39. Hempel F, Felsner G, Maier UG. 2010. New mechanistic insights into pre-protein transport across the second outermost plastid membrane of diatoms. Mol. Microbiol. 76:793–801 [DOI] [PubMed] [Google Scholar]
- 40. Hoseki J, Ushioda R, Nagata K. 2010. Mechanism and components of endoplasmic reticulum-associated degradation. J. Biochem. 147:19–25 [DOI] [PubMed] [Google Scholar]
- 41. Keeling PJ. 2009. Chromalveolates and the evolution of plastids by secondary endosymbiosis. J. Eukaryot. Microbiol. 56:1–8 [DOI] [PubMed] [Google Scholar]
- 42. Kilian O, Kroth PG. 2005. Identification and characterization of a new conserved motif within the presequence of proteins targeted into complex diatom plastids. Plant J. 41:175–183 [DOI] [PubMed] [Google Scholar]
- 43. Kim I, et al. 2006. The Png1-Rad23 complex regulates glycoprotein turnover. J. Cell Biol. 172:211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kravtsova-Ivantsiv Y, Ciechanover A. 2012. Non-canonical ubiquitin-based signals for proteasomal degradation. J. Cell Sci. 125:539–548 [DOI] [PubMed] [Google Scholar]
- 45. Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40:D302–D305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H-M, Chiu C-C. 2010. Protein transport into chloroplasts. Annu. Rev. Plant Biol. 61:157–180 [DOI] [PubMed] [Google Scholar]
- 47. Lim L, Kalanon M, McFadden GI. 2009. New proteins in the apicoplast membranes: time to rethink apicoplast protein targeting. Trends Parasitol. 25:197–200 [DOI] [PubMed] [Google Scholar]
- 48. Lim PJ, et al. 2009. Ubiquilin and p97/VCP bind erasin, forming a complex involved in ERAD. J. Cell Biol. 187:201–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin Y-C, et al. 2012. Plastidial starch phosphorylase in sweet potato roots is proteolytically modified by protein-protein interaction with the 20S proteasome. PLoS One 7:e35336 doi:10.1371/journal.pone.0035336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madsen L, et al. 2011. The tissue-specific Rep8/UBXD6 tethers p97 to the endoplasmic reticulum membrane for degradation of misfolded proteins. PLoS One 6:e25061 doi:10.1371/journal.pone.0025061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marchler-Bauer A, et al. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mason JM, Arndt KM. 2004. Coiled coil domains: stability, specificity, and biological implications. Chembiochem 5:170–176 [DOI] [PubMed] [Google Scholar]
- 53. Matsuzaki M, et al. 2004. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428:653–657 [DOI] [PubMed] [Google Scholar]
- 54. Meyer H, Bug M, Bremer S. 2012. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 14:117–123 [DOI] [PubMed] [Google Scholar]
- 55. Moog D, Stork S, Zauner S, Maier U-G. 2011. In silico and in vivo investigations of proteins of a minimized eukaryotic cytoplasm. Genome Biol. Evol. 3:375–382 doi:10.1093/gbe/evr031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moustafa A, et al. 2009. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 324:1724–1726 [DOI] [PubMed] [Google Scholar]
- 57. Nassoury N, Cappadocia M, Morse D. 2003. Plastid ultrastructure defines the protein import pathway in dinoflagellates. J. Cell Sci. 116:2867–2874 [DOI] [PubMed] [Google Scholar]
- 58. Patron NJ, Waller RF. 2007. Transit peptide diversity and divergence: a global analysis of plastid targeting signals. Bioessays 29:1048–1058 [DOI] [PubMed] [Google Scholar]
- 59. Richly H, et al. 2005. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120:73–84 [DOI] [PubMed] [Google Scholar]
- 60. Saeki Y, Tanaka K. 2012. Assembly and function of the proteasome. Methods Mol. Biol. 832:315–337 [DOI] [PubMed] [Google Scholar]
- 61. Sanchez-Puerta MV, Delwiche CF. 2008. A hypothesis for plastid evolution in chromalveolates. J. Phycol. 44:1097–1107 [DOI] [PubMed] [Google Scholar]
- 62. Schuberth C, Buchberger A. 2005. Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat. Cell Biol. 7:999–1006 [DOI] [PubMed] [Google Scholar]
- 63. Schuberth C, Buchberger A. 2008. UBX domain proteins: major regulators of the AAA ATPase Cdc48/p97. Cell. Mol. Life Sci. 65:2360–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sheiner L, et al. 2011. A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathol. 7:e1002392 doi:10.1371/journal.ppat.1002392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sheiner L, Striepen B. 2012. Protein sorting in complex plastids. Biochim. Biophys. Acta doi:10.1016/j.bbamcr.2012.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith MH, Ploegh HL, Weissman JS. 2011. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334:1086–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sommer MS, et al. 2007. Der1-mediated preprotein import into the periplastid compartment of chromalveolates? Mol. Biol. Evol. 24:918–928 [DOI] [PubMed] [Google Scholar]
- 68. Spork S, et al. 2009. An unusual ERAD-like complex is targeted to the apicoplast of Plasmodium falciparum. Eukaryot. Cell 8:1134–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Su V, Lau AF. 2009. Ubiquitin-like and ubiquitin-associated domain proteins: significance in proteasomal degradation. Cell. Mol. Life Sci. 66:2819–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Suzuki T, Park H, Lennarz WJ. 2002. Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. FASEB J. 16:635–641 [DOI] [PubMed] [Google Scholar]
- 71. Teich R, Zauner S, Baurain D, Brinkmann H, Petersen J. 2007. Origin and distribution of Calvin cycle fructose and sedoheptulose bisphosphatases in plantae and complex algae: a single secondary origin of complex red plastids and subsequent propagation via tertiary endosymbioses. Protist 158:263–276 [DOI] [PubMed] [Google Scholar]
- 72. Trempe J-F. 2011. Reading the ubiquitin postal code. Curr. Opin. Struct. Biol. 21:792–801 [DOI] [PubMed] [Google Scholar]
- 73. van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. 2008. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc. Natl. Acad. Sci. U. S. A. 105:13574–13579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Visendi P, et al. 2011. TparvaDB: a database to support Theileria parva vaccine development. Database (Oxford) 2011:bar015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Woehle C, Dagan T, Martin WF, Gould SB. 2011. Red and problematic green phylogenetic signals among thousands of nuclear genes from the photosynthetic and apicomplexa-related Chromera velia. Genome Biol. Evol. 3:1220–1230 doi:10.1093/gbe/evr100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wolf DH, Stolz A. 2012. The Cdc48 machine in endoplasmic reticulum associated protein degradation. Biochim. Biophys. Acta 1823:117–124 [DOI] [PubMed] [Google Scholar]
- 77. Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE. 2000. Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J. Phycol. 36:379–386 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.