Abstract
Morphogenetic conversions contribute to the pathogenesis of Candida albicans invasive infections. Many studies to date have convincingly demonstrated a link between filamentation and virulence; however, relatively little is known regarding the role of the filament-to-yeast transition during the pathogenesis of invasive candidiasis. We previously identified the C. albicans pescadillo homolog (PES1) as essential during yeast growth and growth of lateral yeast on hyphae but not during hyphal growth. Furthermore, we demonstrated that PES1 is required for virulence in vivo in a Galleria mellonella larva model of candidiasis. Here, we have used a regulatable tetO-PES1/pes1 strain to assess the contribution of C. albicans PES1 to pathogenesis in the commonly used and clinically relevant murine model of hematogenously disseminated candidiasis. Our results indicate that a physiologically controlled level of PES1 expression is required for full virulence in this animal model, with virulence defects observed both when PES1 is overexpressed and and when it is depleted. The pathogenetic defect of cells depleted of PES1 is not due to a general growth defect, as demonstrated by the fact that PES1-depleted cells still kill Caenorhabditis elegans as efficiently as the wild type due to hyphal outgrowth through worm tissues. Our results suggest a critical role of lateral yeast growth in the ability of C. albicans to normally proliferate within tissues, as well as a pivotal role for Pes1 in the normal developmental cycle of C. albicans within the mammalian host during infection.
INTRODUCTION
Candida albicans is an opportunistic pathogenic fungus and the most common cause of invasive fungal disease in humans (15). Candidemia ranks as the fourth-most-common nosocomial bloodstream infection, with high mortality rates (2, 8, 23). C. albicans is a pleomorphic fungus capable of undergoing morphological transitions between budding yeasts and pseudohyphal and hyphal filaments. Under yeast-inducing conditions, yeast do not produce filamentous cells; however, under filament-inducing conditions, apical segments of hyphae and pseudohyphae produce filamentous daughter cells while their subapical sister filaments produce yeast cells (lateral yeasts). Multiple genetic regulators involved in the yeast-to-filament transition have been identified (3); however, little is known about the regulation of the reverse process, the transition from filament to yeast. We have recently identified the C. albicans pescadillo homolog (PES1) as pivotal for the completion of the C. albicans developmental cycle (20). pescadillo is essential in all eukaryotes studied (1, 6, 9). In C. albicans, PES1 is required for normal growth of cells induced as yeast and for lateral yeast growth on filamentous cells; however, under filamenting-inducing conditions, C. albicans can tolerate loss of PES1 and hyphal cells grow normally in the absence of PES1 (20).
It is now well established that morphogenetic conversions contribute to the pathogenesis of C. albicans invasive infections (7). In the last few years, this concept has evolved from the belief that filaments are responsible for C. albicans virulence (10) to a more dynamic concept of C. albicans pathogenesis in which both yeast and filamentous forms play critical roles at different steps of the infectious process (13, 19). For example, yeast are likely to play an important role during circulation in the bloodstream and dissemination to distal sites, while filamentous forms are capable of penetrating tissue planes and therefore are damaging to tissue in deep organs (7, 19). Notably, lateral yeast growth likely occurs in vivo, since filamentous forms are essentially never seen without yeast in deep organs during invasive candidiasis (14).
Our previous report demonstrated that PES1 is required for virulence in vivo in a Galleria mellonella larva model of candidiasis (20). In the present study, we examined the contribution of Pes1 to C. albicans virulence in a murine model of hematogenously disseminated candidiasis to reassess the role of lateral yeast growth and yeast proliferation in the pathogenesis of invasive candidiasis.
MATERIALS AND METHODS
C. albicans strains and culture conditions.
The C. albicans tetO-PES1/pes1 strain was used in these experiments and has been described before (20). In this strain, production of lateral yeast cells from hyphae is known to be reduced during repression of PES1 (growth in the presence of doxycycline) and increased when PES1 is overexpressed (growth in the absence of doxycycline) (20). A PES1/PES1 parental strain (HIS1/his1::tetR::FRT) (20) was used as a control for comparison purposes. The strains were routinely maintained and grown on yeast extract-peptone-dextrose (YPD) medium.
Murine model of hematogeneously disseminated candidiasis.
Cultures of C. albicans strains for injection were grown overnight in YPD medium without doxycycline and incubated at 30°C. Under these conditions, the cells grew solely as yeast cells. Cells were harvested by centrifugation and washed three times in sterile pyrogen-free saline solution and counted using a hemocytometer. Cells (2 × 105 cells in 200 μl of pyrogen-free saline solution per mouse) of the C. albicans tetO-PES1/pes1 strain were delivered by tail vein injection into two groups of mice, each consisting of eight 6-to-8-week-old female BALB/c mice, with or without doxycycline in their drinking water (2 mg/ml in 5% sucrose). Cells of the control PES1/PES1 strain were injected at the same infecting dose into another group of animals (n = 8) without doxycycline in their drinking water. We have previously demonstrated that the pathogenicity of wild-type strains not containing any tetracycline-regulatable element is not affected by the presence or absence of doxycycline (5). Confirmation of the number and viability of cells present in the infecting inocula was performed by plate count. Days on which mice died were recorded, and moribund animals were euthanized and recorded as dying the following day.
To determine fungal burden at different times postinfection, groups of mice (n = 5 per group) receiving either sucrose alone or sucrose containing doxycycline were sacrificed 3 or 5 days after infection with the C. albicans tetO-PES1/pes1 strain (1 × 106 cells per mouse administered intravenously in 200 μl of pyrogen-free saline solution). In all experiments, one kidney was processed for histology, the other kidney (as well as the brain and the spleen in the case of time-scheduled sacrifices) was homogenized, and fungal loads were determined by plating dilutions onto Sabouraud agar plates. For histology, kidneys retrieved from deceased or sacrificed mice were fixed in 10% buffered formalin and embedded in paraffin, and thin tissue slices were obtained and stained with Grocott-Gomori methenamine-silver (GMS) stain prior to microscopic evaluation. All animal experimentation was conducted in an AAALAC-certified facility at The University of Texas at San Antonio (UTSA) following the National Institutes of Health guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after pertinent review and approval by the Institutional Animal Care and Use Committee at The University of Texas at San Antonio. Mice were allowed a 1-week acclimatization period before experiments were started.
C. albicans-Caenorhabditis elegans pathogenesis assay.
A previously described protocol for infecting C. elegans with C. albicans in a liquid medium pathogenesis assay was used in these studies (16). Briefly, adult glp-4(bn2);sek-1(km4) C. elegans animals were exposed to C. albicans strains of the indicated genotype on solid brain heart infusion (BHI) agar containing kanamycin (45 μg/ml) for 4 h at 25°C. A total of 30 to 40 animals per condition were then added to 2 ml of liquid infection medium (80% M9 buffer, 20% BHI agar) with or without doxycycline (20 μg/ml). Each condition was studied in duplicate. Worms were considered to be dead if they did not move in response to mechanical stimulation with a pick. In this assay, all animals that die within the first 48 h after infection have hyphae protruding from their bodies (16). Dead worms were removed from the assay. Microscopy of nematodes was performed by using Nomarski optics on a Zeiss AxioImager microscope.
Statistical analyses.
Survival data and differences between groups were analyzed using the Kaplan-Meier and log rank tests. Organ fungal burden was monitored by determining the total CFU per gram of organ in kidney, brain, and spleen. Thereafter, logarithmic values for the different groups were obtained and results expressed as geometric means and standard deviations. The Mann-Whitney test was used to determine statistical significance for CFU data. Analyses were performed using GraphPad Prism version 4.03 for Windows (GraphPad, San Diego, CA).
RESULTS AND DISCUSSION
Physiologic PES1 expression is required for full virulence of C. albicans in a murine model of hematogenously disseminated candidiasis.
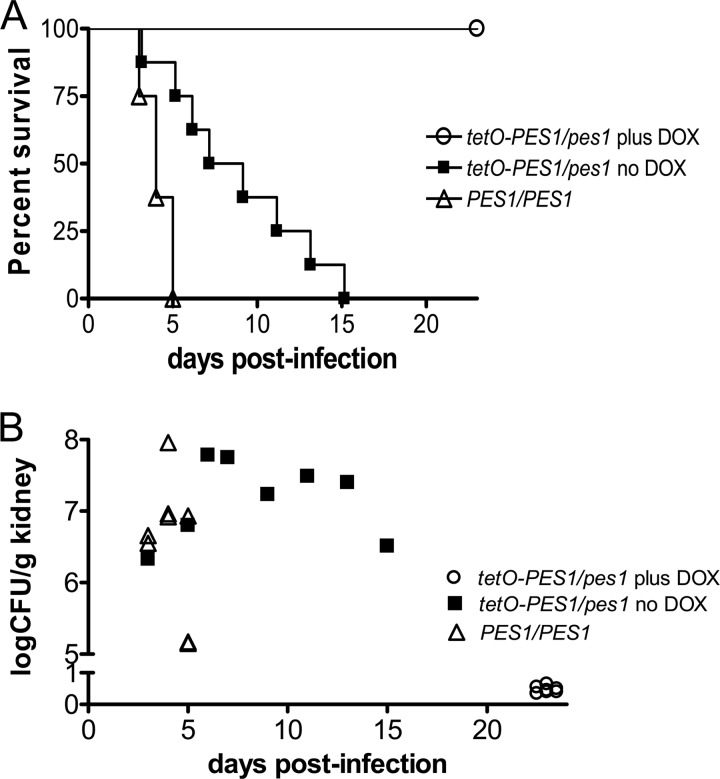
To determine the effect of PES1 expression on virulence of C. albicans in a murine model of disseminated candidiasis, we used a C. albicans tetO-PES1/pes1 strain that allows the external manipulation of PES1 function. When this strain is grown in the presence of doxycycline, PES1 transcription is repressed, the production of lateral yeast cells from hyphae is reduced, and proliferation of yeast cells ceases (20). Hyphae grow robustly during repression of PES1 (20). In the absence of doxycycline, PES1 is overexpressed, lateral yeast formation is increased, and yeast proliferation in vitro is indistinguishable from that of the wild type (20). We infected mice with the tetO-PES1/pes1 strain and the PES1/PES1 isogenic parental control strain. The tetracycline-regulatable promoter system was manipulated within the animal by the addition of doxycycline to the drinking water or the omission of it from the water (4, 5, 12, 17–19). As shown in Fig. 1A, mice infected with the parental PES1/PES1 control strain died within 5 days of infection (median survival time, 4 days). Interestingly, 100% of the mice treated with doxycycline and infected with tetO-PES1/pes1 survived (Fig. 1A; P = 0.0002 versus control), indicating that loss of PES1 expression renders C. albicans avirulent in mice. We note that in mammalian tissue, hypha-inducing conditions prevail; thus, PES1 is not expected to be essential for growth and proliferation of all C. albicans cells (20). In fact, under hypha-inducing conditions, the “in vitro” growth curve for cells in which PES1 expression is repressed by the addition of doxycycline is essentially identical to that for the wild-type strain (see supplementary material in reference 20). We also found that the virulence of the tetO-PES1/pes1 strain was attenuated under conditions where PES1 was overexpressed (i.e., in the absence of doxycycline) compared to that of control strains (median survival time, 8 days) (P = 0.0038 versus control). To assess the burden of infection, kidneys were retrieved from all animals by necropsy at the time of death or sacrifice (Fig. 1B). The organ loads in the kidneys retrieved from mice that succumbed to the infection with the PES1/PES1 strain or the tetO-PES1/pes1 strains in the absence of doxycycline were similar (averages of 2.52 × 107 and 1.56 × 107 CFU/g, respectively; P = 0.2786), whereas all mice that survived the infection with the tetO-PES1/pes1 strain in the presence of doxycycline demonstrated negligible fungal loads that were mostly below the detection limits.
Fig 1.
(A) Survival curves for the different groups of mice (8 animals per group) infected with the C. albicans PES1/PES1 isogenic parental strain or with the C. albicans tetO-PES1/pes1 strain in both the presence and the absence of doxycycline (DOX). Statistically significant differences were observed between the comparison groups of mice (P < 0.01 for all comparisons). (B) Fungal loads in kidneys recovered from mice at the time of death on the indicated day postinfection or after euthanasia for mice surviving the infection.
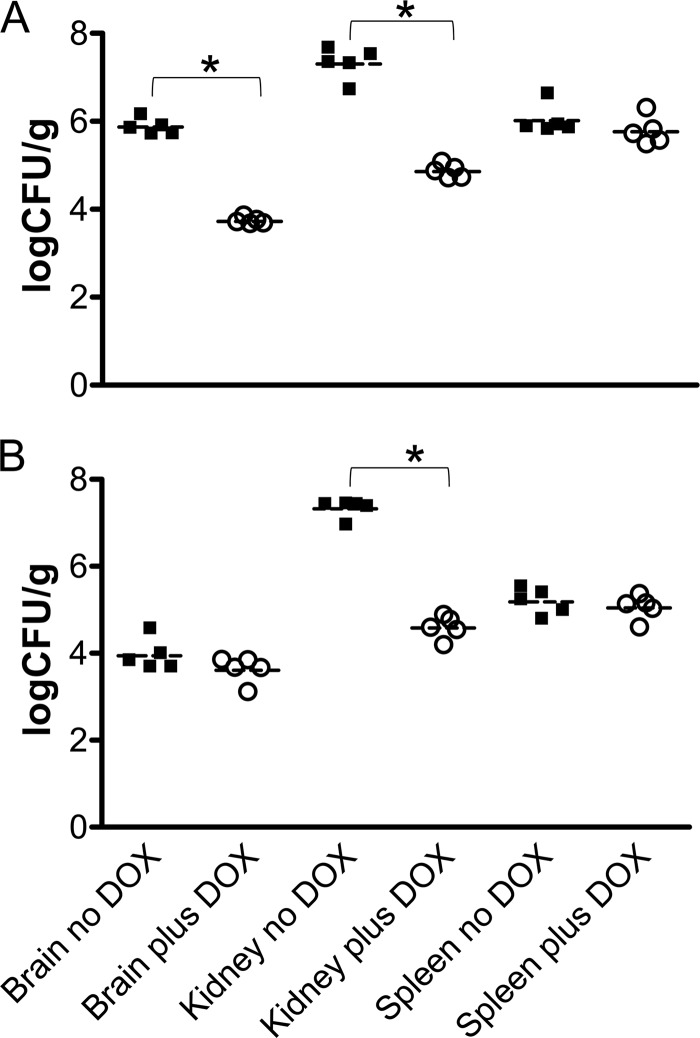
To better understand the role of Pes1 in virulence, a second set of experiments was performed to determine the fungal loads in different organs after 3 and 5 days of infection with the C. albicans tetO-PES1/pes1 strain in the presence or absence of doxycycline. Dissemination of C. albicans to all organs is achieved immediately in this model of tail vein injection with a yeast cell inoculum; the inoculated yeast enter the systemic circulation directly and are carried to all deep organs within minutes (11, 19). Interestingly, even in the presence of doxycycline, which results in the complete depletion of PES1, we were able to detect significant levels of C. albicans in brain, kidney, and spleen at these early time points, although at levels that were somewhat lower than those detected for organs retrieved from animals in the absence of doxycycline, particularly in the case of kidney samples (Fig. 2). Therefore, these results indicate that the lack of virulence associated with PES1 depletion is not due to the inability of the cells to extravasate early during infection or to a complete growth defect once they have reached their target organs. It is likely to be due to a defect in production of lateral yeast on hyphae in deep tissues and in their subsequent proliferation.
Fig 2.
Fungal burdens in brains, kidneys, and spleens retrieved 3 days (A) and 5 days (B) postinfection from two groups of mice (5 animals per group) challenged with the C. albicans tetO-PES1/pes1 strain in the presence or absence of doxycycline (DOX). Results are expressed as log CFU per gram for each individual mouse, with horizontal lines indicating the geometric means. Asterisks indicate statistically significant differences (P < 0.05).
Fungal morphology in kidney tissues retrieved from animal hosts with invasive candidiasis.
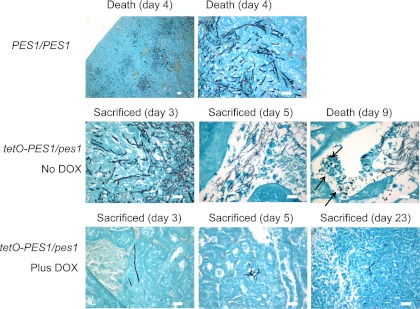
To gain further insight into the role of Pes1 during infection, we next performed histological analyses to observe the morphology of fungal elements in kidneys retrieved from animals infected with the different strains at the time of death or sacrifice. As shown in Fig. 3, mice infected with the PES1/PES1 isogenic parent strain showed characteristic kidney lesions of mostly hyphal and pseudohyphal filaments. The observations were similar for kidneys recovered from mice that were infected with the tetO-PES1/pes1 strain in the absence of doxycycline, although a somewhat higher proportion of yeast cells were detected in some instances. For example, groups of abundant yeast cells were observed in the calices of a mouse that succumbed to infection with this strain in the absence of doxycycline, which is unusual for this specific site of infection in which the hyphal morphology predominates during infections with wild-type strains. The results of histological analyses of kidney sections retrieved from animals infected with the tetO-PES1/pes1 strain in the presence of doxycycline were dramatically different, showing minimal lesions consisting mostly of isolated cells or groups of cells with a somewhat elongated morphology.
Fig 3.
Morphology of fungal cells present in kidneys retrieved from the different groups of mice (infected with the C. albicans PES1/PES1 control strain or with the C. albicans tetO-PES1/pes1 strain in both the presence and the absence of doxycycline [DOX]) at the time of death or sacrifice as revealed by GMS staining. Arrows point to groups of cells with yeast morphology in the calices of a mouse that succumbed to infection with the C. albicans tetO-PES1/pes1 strain in the absence of doxycycline. Bar, 20 μm (all panels).
C. albicans PES1-depleted cells are fully virulent in a Caenorhabditis elegans infection model.
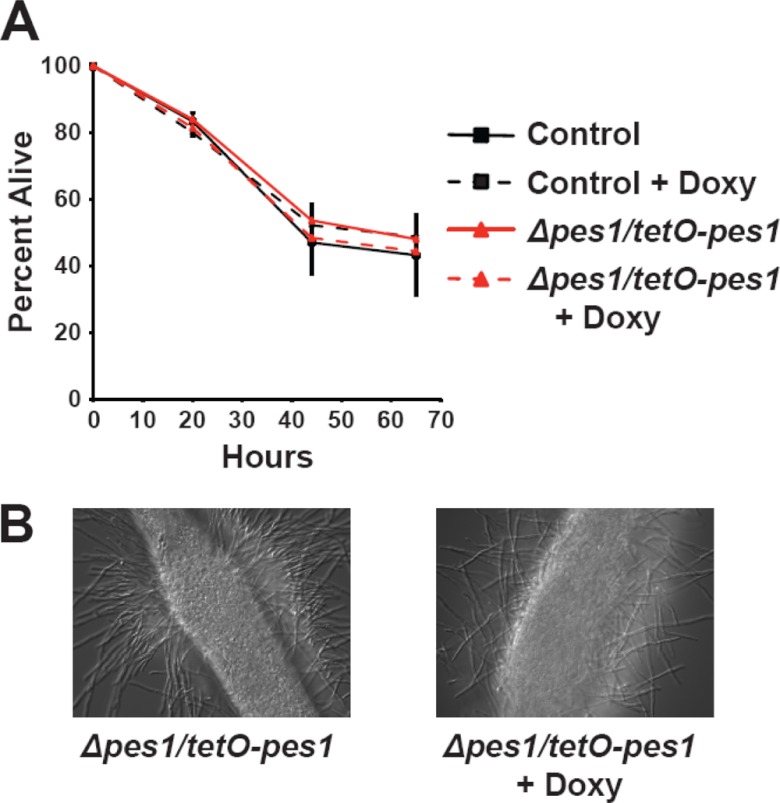
We have previously demonstrated that PES1-depleted hyphae grow robustly whereas PES1-depleted yeast arrest and eventually die, resulting in dramatically reduced virulence in a Galleria mellonella infection model (20). Our current results in the murine intravenous injection model of infection confirm the importance of PES1 and yeast growth for virulence in both the murine and Galleria models. However, as pescadillo homologs are essential in all other eukaryotes examined, we still considered the possibility that the virulence of all PES1-depleted C. albicans cells, hyphal as well as yeast, could be diminished. In the nematode Caenorhabditis elegans, hyphal growth and normal stress responses have been shown to be critical for the virulence of C. albicans (16). We therefore chose the previously described C. elegans model of C. albicans pathogenesis (16) to examine whether PES1-depleted C. albicans cells, with their intact ability to grow as hyphae, are capable of lethal infection of the worm. We found that in C. elegans, PES1-overexpressing as well as PES1-depleted cells were fully as virulent as the wild type (Fig. 4A). Under PES1 expression-inducing conditions (no doxycycline in the liquid medium) as well as expression-repressing conditions (20 μg/ml doxycycline in the medium), yeast cells ingested by the worms formed hyphae which penetrated worm tissues and broke through the outer cuticle (Fig. 4B). Thus, results from this set of experiments support the notion that PES1 depletion does not under all circumstances render C. albicans cells avirulent but that their lack of virulence in the murine model is due to defective lateral yeast growth and yeast proliferation.
Fig 4.
Nematodes were infected with the C. albicans PES1/PES1 isogenic parental strain or with the C. albicans tetO-PES1/pes1 strain and transferred to liquid medium either with or without doxycycline (Doxy; 20 μg/ml). (A) The conditional tetO-PES1/pes1 strain was able to kill the worms at rates similar to those of the control strain, irrespective of the presence or absence of doxycycline. Error bars represent the standard deviations of the results determined with two replicate experiments. The data in panel A are from a single experiment representative of two independent biological replicates. (B) Microscopic observations indicated that killing of nematodes by cells of the C. albicans tetO-PES1/pes1 strain was associated with hyphal formation, in both the presence and the absence of doxycycline.
Overall, these results are consistent with those previously reported for Pes1 in the insect model of infection (20) and further elaborate on an important role of lateral yeast growth and yeast proliferation during the pathogenesis of invasive candidiasis, this time in a mammalian model. Taken together, our results indicate that physiologic regulation of PES1 expression is required for the display of full virulence of C. albicans, with virulence defects observed both when PES1 is overexpressed and when it is depleted. This suggests that the organism must be able to fine-tune its morphogenetic transitions between yeast and hyphal growth during pathogenesis, perhaps in response to diverse microenvironments. Overexpression of PES1 in vivo, as observed with the tetO-PES1/pes1 strain in the absence of doxycycline, leads to attenuated virulence that is associated with an increased proportion of cells displaying a yeast morphology in infected tissues. This is also consistent with the increased yeast growth and elevated levels of lateral yeast produced from hyphae seen in vitro during biofilm development (22). Filamentous growth still occurs under these conditions, allowing penetration through tissue planes. In contrast, PES1 depletion, as it occurs with the tetO-PES1/pes1 strain in the presence of doxycycline, renders C. albicans completely avirulent in this model.
However, under these conditions (complete depletion of PES1) we were still able to detect significant levels of fungal burdens in different organs at earlier time points during the infection (3 and 5 days postinfection) and in some cases even 23 days postinfection (see Fig. 3), providing further evidence for the growth and persistence of Pes1-depleted strains in vivo. Furthermore, our experiments performed here using the invertebrate model of infection suggest that the lack of virulence observed when PES1 is depleted is not secondary to an overall lack of growth. Altogether, these observations clearly indicate that the lack of virulence observed with the tetO-PES1/pes1 strain in the presence of doxycycline cannot be simply attributed to a growth defect. Rather, it supports the idea of a critical role of lateral yeast growth in the ability of C. albicans to normally proliferate within tissues as well as a pivotal role for Pes1 in the normal developmental cycle of C. albicans within the mammalian host during infection.
Our results also suggest that different animal models may be able to separately assess the contributions of distinct virulence determinants to pathogenesis, thus aiding in the analysis of the highly complex host-pathogen interactions of invasive candidiasis. Here, the C. elegans model indicates that the contribution of hyphal growth to virulence is still intact during loss of PES1, while the loss of virulence in the Galleria and mouse models can best be explained by a significant requirement for lateral yeast growth and yeast proliferation during pathogenesis in these systems. We posit that during invasive disease, C. albicans completes multiple rounds of a life cycle in which yeast disseminate through the bloodstream and then extravasate at metastatic foci of infection. There, they give rise to hyphae which penetrate through tissues and in turn produce lateral yeast cells which can then proliferate and regain access to blood vessels to course through the bloodstream. This model is also consistent with the fact that sepsis and renal failure are the main causes of death in the murine model (11, 19, 21). The morphogenetic steps required for this life cycle are orchestrated by key regulators, including PES1, and are all required for full virulence of C. albicans in vivo. Thus, it would seem that PES1 depletion results in a less aggressive infection that can be easily contained by the mammalian host. Our results also reinforce the need for animal experimentation, as other, simpler in vitro and in vivo models, even though still useful to study specific aspects or stages of the infectious process (i.e., filamentation, penetration, and damage of tissues), fail to faithfully recapitulate the high degree of complexity associated with the pathogenesis of invasive candidiasis.
ACKNOWLEDGMENTS
This work was funded by Public Health Service grants RO1AI064562 and R21 AI064715 from the National Institute of Allergy and Infectious Diseases to J.L.L.R. and J.R.K., respectively. P.U. is supported by a postdoctoral fellowship, 10POST4280033, from the American Heart Association. R.P.-W. was supported by the Irvington Institute Fellowship Program of the Cancer Research Institute and K08 award AI081747 from NIAID/NIH. Additional support was provided by the Army Research Office of the Department of Defense under contract no. W911NF-11-1-0136. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The content is solely our responsibility and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, the American Heart Association, or the Department of Defense.
Footnotes
Published ahead of print 26 October 2012
P.U. and A.K.C. contributed equally to this article.
REFERENCES
- 1. Allende ML, et al. 1996. Insertional mutagenesis in zebrafish identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Genes Dev. 10: 3141–3155 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee S. N., et al. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am. J. Med. 91: 86S–89S [DOI] [PubMed] [Google Scholar]
- 3. Berman J, Sudbery PE. 2002. Candida Albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3: 918–930 [DOI] [PubMed] [Google Scholar]
- 4. Carlisle PL, et al. 2009. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc. Natl. Acad. Sci. U. S. A. 106: 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaturvedi AK, et al. 2011. Validation of the tetracycline regulatable gene expression system for the study of the pathogenesis of infectious disease. PLoS One 6: e20449 doi:10.1371/journal.pone.0020449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du YC, Stillman B. 2002. Yph1p, an ORC-interacting protein: potential links between cell proliferation control, DNA replication, and ribosome biogenesis. Cell 109: 835–848 [DOI] [PubMed] [Google Scholar]
- 7. Gow NA, Brown AJ, Odds FC. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5: 366–371 [DOI] [PubMed] [Google Scholar]
- 8. Gudlaugsson O, et al. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37: 1172–1177 [DOI] [PubMed] [Google Scholar]
- 9. Kinoshita Y, et al. 2001. Pescadillo, a novel cell cycle regulatory protein abnormally expressed in malignant cells. J. Biol. Chem. 276: 6656–6665 [DOI] [PubMed] [Google Scholar]
- 10. Lo HJ, et al. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949 [DOI] [PubMed] [Google Scholar]
- 11. MacCallum DM, Odds FC. 2005. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48: 151–161 [DOI] [PubMed] [Google Scholar]
- 12. Nakayama H, et al. 2000. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect. Immun. 68: 6712–6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odds FC, Gow NA, Brown AJ. 2001. Fungal virulence studies come of age. Genome Biol. 2: reviews1009–reviews1009.4 doi:10.1186/gb-2001-2-3-reviews1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odds FC, Van Nuffel L, Gow NA. 2000. Survival in experimental Candida albicans infections depends on inoculum growth conditions as well as animal host. Microbiology 146(Pt 8): 1881–1889 [DOI] [PubMed] [Google Scholar]
- 15. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20: 133–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pukkila-Worley R, Peleg AY, Tampakakis E, Mylonakis E. 2009. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell 8: 1750–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saville SP, et al. 2006. Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob. Agents Chemother. 50: 3312–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, Lopez-Ribot JL. 2008. Use of a genetically engineered strain to evaluate the pathogenic potential of yeast cell and filamentous forms during Candida albicans systemic infection in immunodeficient mice. Infect. Immun. 76: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2: 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen J, Cowen LE, Griffin AM, Chan L, Kohler JR. 2008. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc. Natl. Acad. Sci. U. S. A. 105: 20918–20923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spellberg B, Ibrahim AS, Edwards JE, Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192: 336–343 [DOI] [PubMed] [Google Scholar]
- 22. Uppuluri P, et al. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 6: e1000828 doi:10.1371/journal.ppat.1000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wisplinghoff H, et al. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39: 309–317 [DOI] [PubMed] [Google Scholar]