Abstract
Many fungal species use glycerol as a compatible solute with which to maintain osmotic homeostasis in response to changes in external osmolarity. In Saccharomyces cerevisiae, intracellular glycerol concentrations are regulated largely by the high osmolarity glycerol (HOG) response pathway, both through induction of glycerol biosynthesis and control of its flux through the plasma membrane Fps1 glycerol channel. The channel activity of Fps1 is also controlled by a pair of positive regulators, Rgc1 and Rgc2. In this study, we demonstrate that Candida glabrata, a fungal pathogen that possesses two Fps1 orthologs and two Rgc1/-2 orthologs, accumulates glycerol in response to hyperosmotic stress. We present an initial characterization of mutants with deletions in the C. glabrata FPS1 (CAGL0C03267 [www.candidagenome.org]) and FPS2 (CAGL0E03894) genes and find that a double mutant accumulates glycerol, experiences constitutive cell wall stress, and is hypersensitive to treatment by caspofungin, an antifungal agent that targets the cell wall. This mutant is cleared more efficiently in mouse infections than is wild-type C. glabrata by caspofungin treatment. Finally, we demonstrate that one of the C. glabrata RGC orthologs complements an S. cerevisiae rgc1 rgc2 null mutant, supporting the conclusion that this regulatory assembly is conserved between these species.
INTRODUCTION
Under conditions of high osmolarity stress, many fungal species maintain osmotic equilibrium by producing and retaining high concentrations of glycerol as a compatible solute (18, 20, 30, 34). The intracellular glycerol concentration is regulated in Saccharomyces cerevisiae in part by the plasma membrane aquaglyceroporin, Fps1 (13, 26, 37, 39). Increased external osmolarity induces Fps1 closure, whereas decreased osmolarity causes channel opening, both within seconds of the change in external osmolarity (39). This channel, which functions as a homotetramer (2), is required for surviving a hypoosmotic shock, when yeast cells must export glycerol rapidly to prevent bursting (26, 39). Fps1 is also required for controlling turgor pressure during fusion of mating yeast cells (33).
Relative to other characterized aquaglyceroporins, Fps1 possesses N-terminal and C-terminal cytoplasmic extensions that are important for its regulation (11, 38). The pathway responsible for regulation of Fps1 in response to changes in osmolarity has not been fully delineated but involves the mitogen-activated protein (MAP) kinase Hog1 (high osmolarity glycerol response) (13, 39), which binds to the N-terminal domain of Fps1 (29). Hog1 is activated in response to hyperosmotic stress to mediate both the biosynthesis of glycerol and its retention within the cell (13). Fps1 activity is also controlled by a pair of positive regulators, named Rgc1 and Rgc2 (for regulator of the glycerol channel 1 and 2; YPR115W and YGR097W, respectively) (1). Rgc1 and Rgc2 are phosphorylation targets of Hog1, and their positive regulation of Fps1 is inhibited by Hog1 phosphorylation (1). Loss of either FPS1 or RGC1 and RGC2 function results in excess turgor pressure and consequent cell wall stress, to which the cell responds by fortifying the cell wall (1). Additional cell wall stress imposed upon these mutants results in cell lysis. Although the fungal kingdom is replete with Rgc orthologs, they are not represented in metazoans, suggesting that the Rgc-Fps pathway may be an attractive target for antifungal-drug development.
Candida species are the most common cause of systemic fungal infections in humans (4, 5). Although Candida albicans infections are the most prevalent among these, Candida glabrata is the second most common cause of such infections, accounting for approximately 15% of Candida bloodstream infections worldwide (17). Despite its status as an opportunistic human pathogen, C. glabrata is phylogenetically more closely related to the baker's yeast, S. cerevisiae, than it is to other Candida species associated with human disease (17).
The most recently introduced class of antifungal agents is the echinocandins, which include caspofungin, micafungin, and anidulafungin. These are lipopeptides that interfere with cell wall biosynthesis by inhibition of β-1,3-glucan synthase, the enzyme responsible for synthesizing the major structural polymer of the fungal cell wall (23). This class of antifungals is effective against Candida (particularly C. albicans and C. glabrata) and Aspergillus species (7). Significantly, S. cerevisiae rgc1 rgc2 null mutants are hypersensitive to caspofungin treatment because of their increased requirement for β-1,3-glucan synthase activity (1).
The C. glabrata genome possesses two orthologs of S. cerevisiae FPS1, hereinafter designated CgFPS1 and CgFPS2. These genes encode the only clear Fps1 orthologs that include the regulatory N-terminal and C-terminal extensions that have been identified among pathogenic fungal species. Additionally, C. glabrata possesses two RGC orthologs, hereinafter designated CgRGC1 and CgRGC2. As a first step toward understanding the functions of these genes in a fungal pathogen, we report here the initial characterization of C. glabrata mutants with deletions of FPS1 and FPS2.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The S. cerevisiae strains used in this study were all derived from the Research Genetics background S288c (Research Genetics, Inc., Huntsville, AL) and are listed in Table 1. Yeast cultures were grown in YPD (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose) or SD (synthetic dextrose; 0.67% yeast nitrogen base, 2% glucose) medium supplemented with the appropriate nutrients to select for plasmids. Chromosomal gene integrations were created by replacing endogenous open reading frames with cassettes created in integration vector pAG32 (hphMX4) (9). Cassettes were constructed to contain a C-terminally Flag-tagged version of the replacement gene ligated at its 3′ end to the hphMX4 gene, which confers hygromycin B resistance. Linear PCR products amplified from the engineered cassettes were transformed into the appropriate haploid yeast strain. Integrants were selected on plates containing hygromycin B, and gene replacement confirmed by PCR analysis across the integration junctions.
Table 1.
S. cerevisiae and C. glabrata strains
| Strain | Relevant genotype | Source |
|---|---|---|
| DL3187 | MATa S288c (BY4742) his3Δ leu2Δ ura3Δ lys2Δ | Research Genetics |
| DL3188 | MATα S288c rgc1Δ::KanMX rgc2Δ::KanMX | 1 |
| DL3207 | MATa S288c rgc1Δ::KanMX rgc2Δ::KanMX | 1 |
| DL3226 | MATa S288c fps1Δ::KanMX | Research Genetics |
| DL3229 | MATα S288c fps1Δ::KanMX | Research Genetics |
| DL3799 | MATα S288c fps1Δ::ScFPS1-Flag-HPH | This study |
| DL3800 | MATα S288c fps1Δ::CgFPS1-Flag-HPH | This study |
| DL3801 | MATα S288c fps1Δ::CgFPS2-Flag-HPH | This study |
| DL3802 | MATα S288c rgc1Δ::KanMX rgc2Δ::ScRGC2-Flag-HPH | This study |
| DL3803 | MATα S288c rgc1Δ::KanMX rgc2Δ::CgRGC1-Flag-HPH | This study |
| DL3804 | MATα S288c rgc1Δ::KanMX rgc2Δ::CgRGC2-Flag-HPH | This study |
| BG2 | C. glabrata wild type | 6 |
| DL3614 | C. glabrata BG14 ura3Δ::Tn903 G418r | 27 |
| DL3616 | C. glabrata BG14 fps1Δ::frt, no. 1 | This study |
| DL3618 | C. glabrata BG14 fps1Δ::frt, no. 2 | This study |
| DL3675 | C. glabrata BG14 fps2Δ::frt, no. 1 | This study |
| DL3677 | C. glabrata BG14 fps2Δ::frt, no. 2 | This study |
| DL3679 | C. glabrata BG14 fps1Δ::frt fps2Δ::frt, no. 1 | This study |
| DL3681 | C. glabrata BG14 fps1Δ::frt fps2Δ::frt, no. 2 | This study |
| BG3075 | C. glabrata BG14 fps1Δ::frt fps2Δ::frt URA3 | This study |
| BG3129 | C. glabrata BG14/pGRB2.0 | This study |
| BG3130 | C. glabrata BG14 fps1Δ::frt fps2Δ::frt/pGRB2.0 | This study |
| BG3123 | C. glabrata BG14 fps1Δ::frt fps2Δ::frt/pGRB2.0-CgFPS1 | This study |
All C. glabrata deletion strains were derived from a ura3 derivative (BG14) of the clinical isolate BG2 (6) and are listed in Table 1. Gene deletions were created in strain BG14 by homologous recombination. A cassette containing the C. albicans NAT1 (CaNAT1) gene (35), which confers nourseothricin resistance, and flanked by FLP recombination target (FRT) recombination sites was generated by PCR and cloned into pPCR2.1 (Life Technologies, Carlsbad, CA) to generate pNAT. To delete genes, the NAT-FRT cassette was amplified by PCR using primers that included 50 nucleotides of 5′ and 3′ noncoding sequence from the FPS1 or FPS2 gene. These fragments were used to target the cassette to the integration site. Deletions were selected on plates containing nourseothricin and confirmed by PCR analysis across the integration junctions. To create the fps1Δ fps2Δ double mutant, the NAT cassette was recombined out by transforming the fps1Δ::NAT mutant with a vector expressing FLP1 recombinase (pRD16) (28). Flp1-induced recombination at the FRT sites recombined out the NAT cassette, resulting in restoration of nourseothricin sensitivity and allowing the generation of a second deletion by the same method. Deletion strains lacking the NAT gene were created in this way and used for phenotypic analyses. For mouse infection studies, URA3 was restored to the fps1Δ::FRT fps2Δ::FRT ura3Δ::Tn903 strain either by transformation with the CgURA3 gene or by transformation with pGRB2.0-based plasmids (28) followed by selection for uracil prototrophy.
Plasmids.
Plasmids for the expression of S. cerevisiae FPS1 (ScFPS1), CgFPS1, and CgFPS2 were created by PCR amplification of genomic DNA of the coding regions of these genes with their respective promoters using forward primers containing a NotI site and reverse primers containing a SalI site. The amount of 5′ sequence included varied among these genes, being 800 bp for ScFPS1 and 650 bp for CgFPS1 and CgFPS2. DNA fragments were ligated into the C. glabrata expression vector pGRB2.0, a URA3 CEN/ARS plasmid (28). This yielded pGRB2.0-ScFPS1 (p3057), pGRB2.0-CgFPS1 (p3058), and pGRB2.0-CgFPS2 (p3059).
The C. glabrata cell wall stress reporter plasmid was constructed by amplifying the ScPRM5-lacZ cassette from p1366 (14) using a forward primer containing an XbaI site and a reverse primer containing an XhoI site. The cassette was ligated into pGRB2.0 to yield pGRB2.0-PRM5-lacZ (p3060). The plasmids used for gene integration were created by amplifying the coding regions of the genes indicated below, which were ligated into pAG32 (p2823). ScFPS1 was amplified using primers containing SalI and BglII sites (pAG32-ScFPS1; p3061), CgFPS1 with primers containing PvuII and SalI sites (pAG32-CgFPS1; p3062), CgFPS2 with primers containing SalI and BglII sites (pAG32-CgFPS2; p3063), ScRGC2 with primers containing HindIII and PacI sites (pAG32-ScRGC2; p3064), CgRGC1 with primers containing PacI and BglII sites (pAG32-CgRGC1; p3065), and CgRGC2 with primers containing SalI and BglII sites (pAG32-CgRGC2; p3066) of pAG32. Plasmids are listed in Table 2. Primer sequences are available upon request.
Table 2.
Plasmids
| Plasmid | Description | Source |
|---|---|---|
| p2769 | pGRB2.0 C. glabrata expression vector, URA3 | 8, 28 |
| pNAT | pPCR2.1 with NATR cassette, flanked by FRT sites | This study |
| p2823 | pAG32; integration vector, hphMX4 | 9 |
| p3055 | pRD16-FLP1 recombinase vector | 28 |
| p3057 | pGRB2.0-ScFPS1 C. glabrata vector, ScFPS1 | This study |
| p3058 | pGRB2.0-CgFPS1 C. glabrata vector, CgFPS1 | This study |
| p3059 | pGRB2.0-CgFPS2 C. glabrata vector, CgFPS2 | This study |
| p3060 | pGRB2.0-ScPRM5pro-lacZ | This study |
| p3061 | pAG32-ScFPS1 integration vector, ScFPS1 | This study |
| p3062 | pAG32-CgFPS1 integration vector, CgFPS1 | This study |
| p3063 | pAG32-CgFPS2 integration vector, CgFPS2 | This study |
| p3064 | pAG32-ScRGC2 integration vector, ScRGC2 | This study |
| p3065 | pAG32-CgRGC1 integration vector, CgRGC1 | This study |
| p3066 | pAG32-CgRGC2 integration vector, CgRGC2 | This study |
Quantitative reverse transcriptase (qRT)-PCR.
C. glabrata strain BG2 was grown to mid-log phase in YPD. A zero-time aliquot was centrifuged, washed once with diethyl pyrocarbonate (DEPC)-treated water, and flash frozen for subsequent RNA extraction. For hyperosmotic shock, cells were centrifuged, and pellets were resuspended in either YPD or YPD supplemented with 1.8 M sorbitol. These cultures were grown at 30°C, and samples were taken at 1-h, 2-h, 4-h, and 20-h intervals. Each sample was centrifuged and washed once with DEPC-treated water before being flash frozen for RNA extraction.
RNA was obtained from each sample by acid phenol extraction, followed by ethanol precipitation. Samples were treated twice with DNase (New England BioLabs) to remove any genomic DNA contamination. Samples of 8 μg of RNA were used for cDNA synthesis in 20-μl reaction mixtures, using 1× cDNA buffer, reverse transcriptase, and oligo(dT) primers. Reaction mixtures without reverse transcriptase were used to determine genomic DNA contamination in subsequent qRT-PCRs. Any remaining RNA was removed by RNase H (New England BioLabs) treatment. The resulting cDNA was diluted 1:10 in sterile water.
PCR primers (Eurofins Mwg Operon, Inc., Huntsville, AL) specific for CgFps1 and CgFps2 were designed to produce amplicon sizes of 158 and 131 bp, respectively, with sequences as follows: CgFps1_QRTFwd, TTGCCATCCACATACCATCC; CgFps1_QRTRev, GAGTCCTGTTGTGCTTTACC; CgFps2_QRTFwd, TTGTCGGCTGATATCTTCCC; and CgFps2_QRTRev, AAGGCCCATAGAGCAAGACG. Standard curves for each primer set were created using 10-fold serial dilutions of BG2 genomic DNA, and values were normalized to the total histone H3 transcript level in each sample. PCR products were amplified using 5 μl of cDNA diluted 10× in 25-μl reaction mixtures containing 1× polymerase buffer, 1 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 0.5 μM each primer, 1× EvaGreen nucleic acid dye (Biotium), and 0.25 units of Taq polymerase. The thermal cycling conditions were as follows (Bio-Rad CFX96 real-time system): initial denaturation at 95°C followed by 40 cycles of 95°C (10 s), 60°C (30 s), 72°C (30 s). A melting curve was performed starting at 65°C to test for the presence of nonspecific products.
Phenotypic analysis of the C. glabrata fpsΔ mutants.
Glycerol accumulation assays and β-galactosidase assays were carried out as described previously (1). Cell wall stress was imposed by exposing log-phase cells to 40 μg/ml calcofluor white for 5 h. Caspofungin was purchased from Merck.
Mouse infections.
Infection of mice with C. glabrata was carried out as described previously (3, 27). Briefly, mice were infected by tail vein injection with either wild-type (BG2) or fps1Δ fps2Δ (BG3075) cells suspended in 0.2 ml of phosphate-buffered saline (PBS). In a second experiment, mice were infected with either wild-type (BG14) or fps1Δ fps2Δ (DL3679) cells bearing a URA3-containing vector or a plasmid expressing CgFPS1. In both experiments, 4 days after infection, mice were treated by intraperitoneal injection with caspofungin (0.2 ml PBS containing 2 mg/kg of body weight or 25 mg/kg) or 0.2 ml PBS alone (mock treatment). Three days after caspofungin administration, the mice were sacrificed and their kidneys, livers, and spleens were recovered. The organs were homogenized, and dilutions of the samples from the kidney, spleen, and liver were plated on YPD plates for recovery of viable fungal cells. CFU per organ were counted after 48 h of growth at 30°C.
RESULTS
Candida glabrata FPS1 and FPS2.
The C. glabrata genome possesses two orthologs of the S. cerevisiae FPS1 gene. The most closely related of these (CAGL0C03267) encodes a predicted protein of 652 amino acids that shares 66% sequence identity with ScFps1 through the C-terminal two-thirds of the protein with reduced identity through the predicted N-terminal cytoplasmic domain. We designate this gene CgFPS1 herein. The other ortholog (CAGL0E03894) encodes a predicted protein of 602 amino acids that possesses a shorter N-terminal cytoplasmic domain than either ScFps1 or CgFps1 and shares 54% sequence identity with ScFps1 through the remainder of the protein. We designate this gene CgFPS2 herein.
We constructed single fps1Δ and fps2Δ mutants and a double fps1Δ fps2Δ mutant in the C. glabrata strain BG14 (27). We tested these mutants for several distinctive phenotypes displayed by S. cerevisiae fps1Δ mutants. First, mutants with mutations in ScFPS1 or its positive regulators, ScRGC1 and ScRGC2, retain excess intracellular glycerol, which causes elevated turgor pressure and constitutive cell wall stress (1, 26, 39). These mutants are consequently hypersensitive to growth at elevated temperature (35 to 40°C) and to growth inhibition by the cell wall biosynthesis inhibitor caspofungin (1).
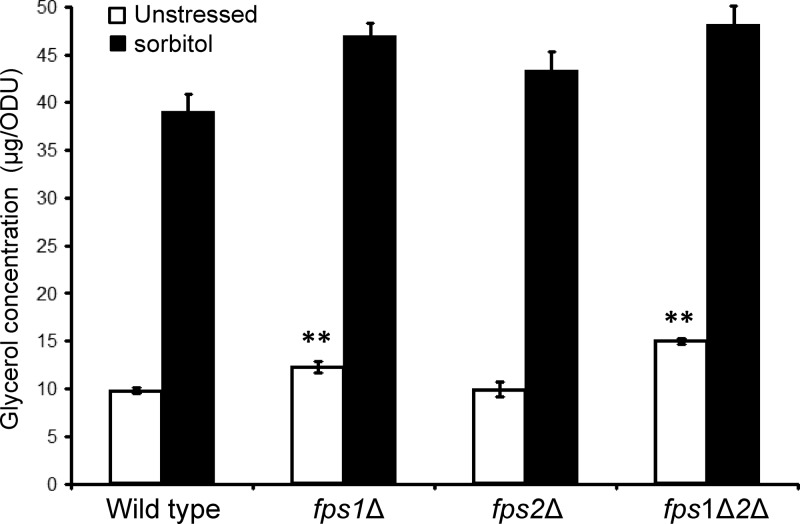
Although accumulation of glycerol in response to hyperosmotic shock has been detected in many fungal species, it has not been demonstrated in C. glabrata. Therefore, we first measured the intracellular glycerol levels in cells exposed to hyperosmotic shock with 1.8 M sorbitol. The wild-type (BG14) strain accumulated approximately 4-fold more intracellular glycerol after 15 min (Fig. 1). This is comparable to the increase observed for wild-type S. cerevisiae exposed to the same treatment (approximately 6-fold) (1). This result demonstrates that C. glabrata, like many other fungal species, accumulates glycerol as a compatible solute in response to hyperosmotic shock. We also compared basal glycerol retention in the mutants with single and double mutations of the CgFPS genes. The fps1Δ mutant retained a slightly greater glycerol concentration than the wild-type strain, whereas the fps2Δ mutant did not (Fig. 1). However, the effect of deleting both genes appeared to be additive, because the fps1Δ fps2Δ mutant retained approximately 50% more glycerol than the wild-type strain. This is a considerably smaller difference than that observed for an S. cerevisiae fps1Δ mutant (approximately 12-fold) (1). The S. cerevisiae fps1Δ mutant accumulates so much glycerol that it fails to increase its glycerol levels further in response to hyperosmotic shock (1). This was not the case for the C. glabrata fps1Δ fps2Δ mutant (Fig. 1). These results suggest that the CgFps1 and CgFps2 proteins function as glycerol channels that contribute to the maintenance of osmotic balance and that CgFps1 is more important than CgFps2 in this regard.
Fig 1.
Retention of glycerol by C. glabrata in response to hyperosmotic stress and in fpsΔ mutants. Glycerol concentrations of log-phase cells were determined in triplicate. C. glabrata strains were grown to mid-log phase in YPD and diluted into YPD with or without sorbitol (to 1.8 M) to induce hyperosmotic shock (15 min). Each value represents the mean and standard deviation from three independent experiments. Strains were the wild type (BG14) and fps1Δ (DL3616), fps2Δ (DL3675), and fps1Δ fps2Δ (fps1Δ2Δ) (DL3679) mutants. Statistical significance was determined using two-sample t tests with a two-tailed distribution. ODU, optical density units; **, P < 0.005.
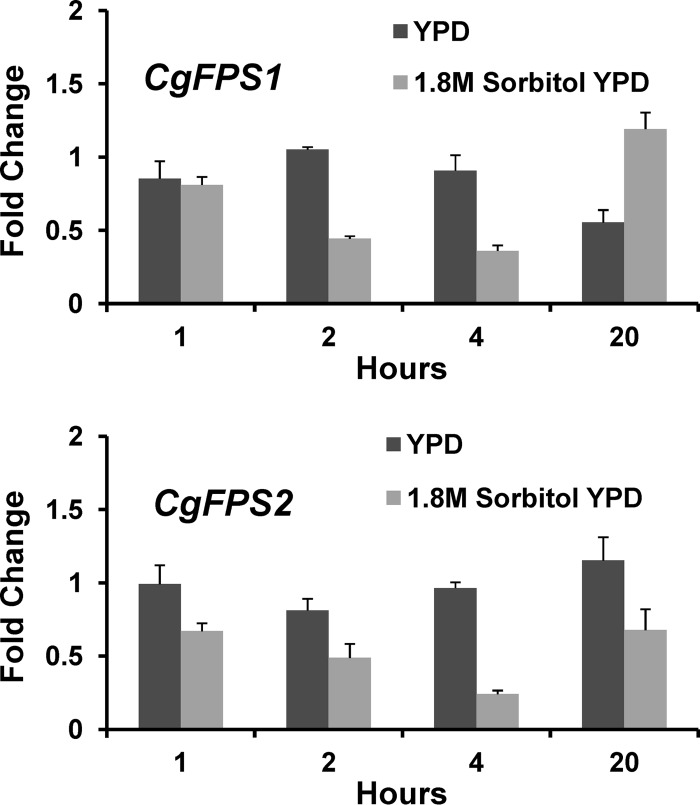
We examined the levels of CgFPS1 and CgFPS2 mRNA in response to treatment with 1.8 M sorbitol using quantitative RT-PCR. For both genes, we detected a modest decrease in mRNA levels at 2 h and 4 h after osmotic shock (Fig. 2). The mRNA levels of both genes returned to normal after 20 h in 1.8 M sorbitol-containing medium. These results suggest that C. glabrata may temporarily diminish the expression of the glycerol channels, as well as control their activity.
Fig 2.
Expression of C. glabrata FPS1 and FPS2. Wild-type (BG2) cells were grown to mid-log phase in YPD and diluted into YPD with or without sorbitol (to 1.8 M) to induce hyperosmotic shock for the indicated times. RNA was extracted and subjected to qRT-PCR analysis. Values are presented as relative to the value for the zero-time sample and represent the means and standard deviations of technical triplicate samples.
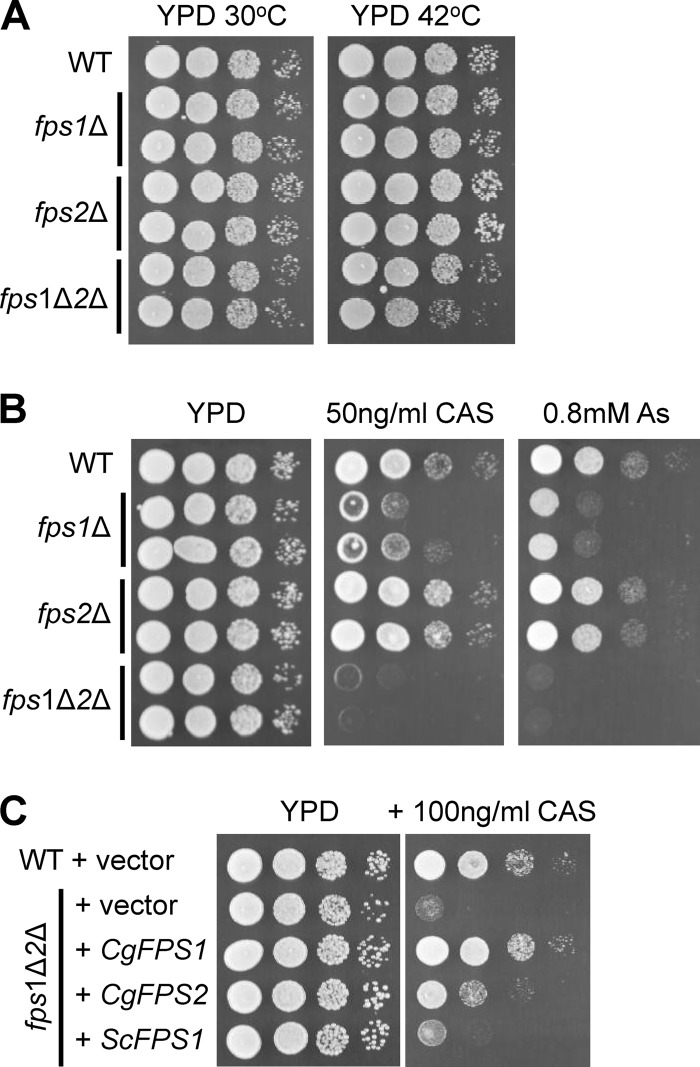
Despite retention of elevated intracellular glycerol, none of the C. glabrata mutants displayed strong temperature sensitivity for growth up to 42°C (Fig. 3A). Growth of the fps1Δ fps2Δ double mutant was only very slightly impaired at elevated temperature. However, the fps1Δ mutant was hypersensitive to caspofungin treatment (Fig. 3B). Moreover, the fps1Δ fps2Δ double mutant displayed an even greater degree of hypersensitivity to caspofungin than the fps1Δ mutant, supporting the conclusion that CgFPS2 is partially redundant with CgFPS1 but of lesser importance to the maintenance of proper turgor pressure. To confirm that the caspofungin sensitivity of the fps1Δ fps2Δ mutant was the consequence of loss of FPS1/-2 function, we reintroduced either CgFPS1 or CgFPS2 into the fps1Δ fps2Δ mutant on a centromeric plasmid. The CgFPS1 gene complemented the caspofungin sensitivity of the fps1Δ fps2Δ mutant fully, whereas the CgFPS2 gene partially complemented the mutant, supporting the conclusion that FPS1 is more important than FPS2 (Fig. 3C). The ScFPS1 gene failed to complement the C. glabrata fps1Δ fps2Δ mutant when provided in the context of its own promoter. However, we do not know if its failure to complement reflects a problem with the expression or function of the encoded protein. Collectively, our results support the conclusion that these putative channel proteins serve the same function with regard to glycerol transport in C. glabrata as in S. cerevisiae and that CgFPS1 is more important than CgFPS2 for the maintenance of glycerol homeostasis.
Fig 3.
Phenotypes of C. glabrata fpsΔ mutants. (A) Growth of mutants at elevated temperature. A wild-type (WT) C. glabrata strain (BG14) and pairs of independently derived fps1Δ (DL3616 and DL3618), fps2Δ (DL3675 and DL3677), and fps1Δ fps2Δ (fps1Δ2Δ) (DL3679 and DL3681) cells were grown to mid-log phase in YPD, and 10-fold serial dilutions were spotted onto YPD plates and incubated at 30°C or 42°C for 3 days. (B) Sensitivity of mutants to caspofungin and arsenite. The same strains as in the experiments whose results are shown in panel A were spotted onto YPD plates with or without caspofungin (CAS) or arsenite (As) prior to incubation at 30°C for 3 days. (C) Complementation of the caspofungin sensitivity of a C. glabrata fps1Δ fps2Δ mutant. Strain DL3679 was transformed with vectors expressing various FPS genes under the control of their endogenous promoters, and transformants were treated as described above.
The Fps1 glycerol channel of S. cerevisiae is also the principal point of entry for the toxic metalloid arsenite, and loss of function of this channel confers resistance to this agent (1, 40, 41). Surprisingly, not only were the C. glabrata fps1Δ and fps2Δ mutants not resistant to arsenite, but these mutations sensitized C. glabrata to the metalloid (Fig. 3B). This suggested both that the Fps1 and Fps2 channels are not significant ports for arsenite entry in C. glabrata and that their loss may induce the opening of channels that do allow the entry of arsenite.
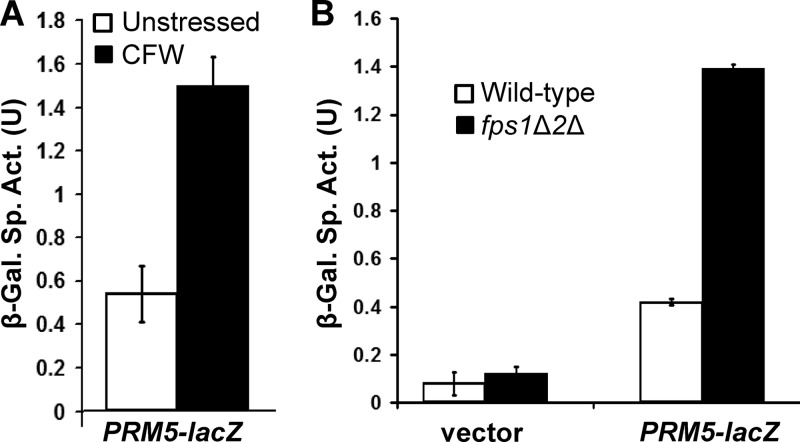
We next examined cell wall stress in the fps1Δ fps2Δ mutant. This was done using a cell wall stress-responsive reporter from S. cerevisiae (PRM5-lacZ) (14) cloned into a C. glabrata centromeric vector. Although not optimized for expression in C. glabrata, this reporter responded to cell wall stress induced by calcofluor white, a chitin antagonist (Fig. 4A). Basal cell wall stress was elevated in the fps1Δ fps2Δ mutant (Fig. 4B), as predicted by its hypersensitivity to caspofungin. These results, taken together, support the conclusion that CgFps1 and CgFps2, like ScFps1, function to maintain glycerol homeostasis.
Fig 4.
Cell wall stress in a C. glabrata fps1Δ fps2Δ mutant. (A) Activation of a cell wall stress reporter. An S. cerevisiae cell wall stress reporter, PRM5-lacZ, is induced in C. glabrata by the cell wall antagonist calcofluor white (CFW). Wild-type (BG14) cells, transformed with the pGRB2.0-PRM5-lacZ transcriptional reporter plasmid (p3060), were grown to mid-log phase in selective medium and treated with calcofluor white (40 μg/ml) for 5 h at 30°C, followed by cell lysis and measurement of β-galactosidase (β-Gal.) activity. Each value represents the mean and standard deviation from three independent transformants. (B) The C. glabrata fps1Δ fps2Δ (fps1Δ2Δ) mutant is under constitutive cell wall stress. Wild-type (BG14) cells and an fps1Δ fps2Δ mutant (DL3679) were transformed with pGRB2.0-PRM5-lacZ or pGRB2.0 (vector), and β-galactosidase activity was measured from log-phase cells growing at 30°C. Each value represents the mean and standard deviation of three separate experiments.
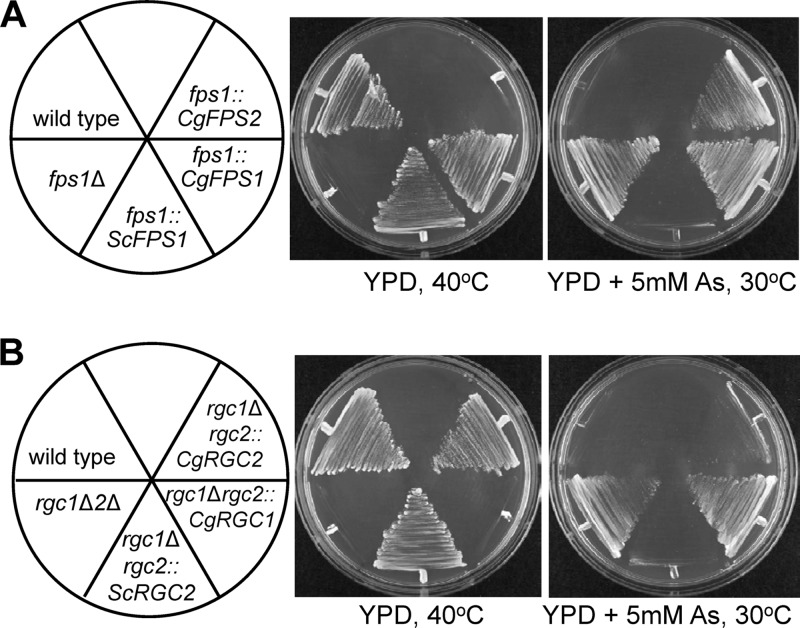
As a final test of the conservation of function of CgFps1 and CgFps2 with ScFps1, we integrated CgFPS1 or CgFPS2 at the S. cerevisiae FPS1 locus such that the coding region replaced that of ScFPS1 and was under the control of the endogenous ScFPS1 promoter. The CgFPS1 gene but not the CgFPS2 gene was able to complement the loss of ScFPS1 function for growth at 40°C (Fig. 5A), providing strong support for the conclusion that CgFPS1 functions as a glycerol facilitator. Interestingly, the expression of CgFPS1 did not restore arsenite sensitivity to the S. cerevisiae fps1Δ mutant (Fig. 5A), supporting the conclusion that arsenite does not enter through these C. glabrata channels.
Fig 5.
Heterologous complementation of S. cerevisiae fps1Δ and rgc1Δ rgc2Δ (rgc1Δ2Δ) mutant phenotypes by C. glabrata genes. (A) Complementation of an fps1Δ mutant. S. cerevisiae FPS1 was replaced by integration with the indicated FPS genes such that the integrated genes were expressed under the control of the endogenous ScFPS1 promoter. The indicated strains were streaked onto YPD plates with or without arsenite (As) and incubated at 40°C or 30°C, respectively, for 3 days. Strains were the wild type (DL3187) and fps1Δ (DL3226), fps1::ScFPS1 (DL3799), fps1::CgFPS1 (DL3800), and fps1::CgFPS2 (DL3801) mutants. (B) Complementation of an rgc1Δ rgc2Δ mutant. The RGC2 gene of S. cerevisiae was replaced by integration with the indicated RGC genes in an rgc1Δ mutant background such that the integrated genes were expressed under the control of the endogenous ScRGC2 promoter. Temperature and arsenite phenotypes were determined as described for panel A. Strains were the wild type (DL3187) and rgc1Δ rgc2Δ (DL3207), rgc1Δ rgc2::ScRGC2 (DL3802), rgc1Δ rgc2::CgRGC1 (DL3803), and rgc1Δ rgc2::CgRGC2 (DL3804) mutants.
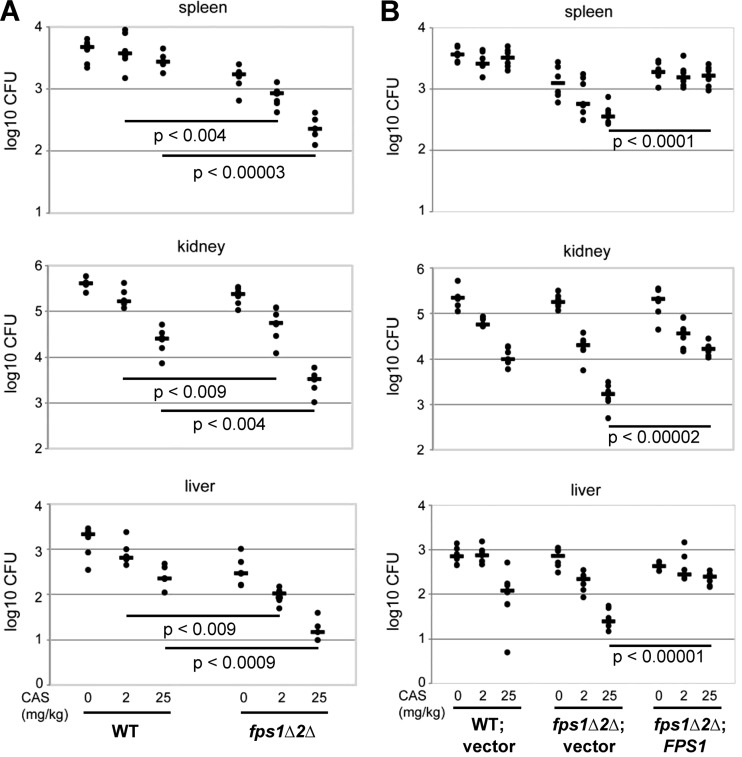
Because the fps1Δ fps2Δ mutation sensitized C. glabrata to caspofungin treatment, we asked if the fps1Δ fps2Δ mutant was cleared by caspofungin treatment more effectively than was the wild type in the context of a mouse infection. This experiment was carried out with two sets of strains. In one experiment, mice were infected by tail vein injection with wild-type C. glabrata (BG2) or the fps1Δ fps2Δ mutant (BG3075). Four days postinfection, mice were given a single dose of caspofungin (either 2 mg/kg or 25 mg/kg in PBS) by intraperitoneal injection or were mock treated with PBS. Three days posttreatment with caspofungin, mice were sacrificed and their spleens, kidneys, and livers were harvested and homogenized for detection of viable C. glabrata by measurement of CFU. Comparable numbers of wild-type and fps1Δ fps2Δ mutant cells were detected in the kidneys and spleens of untreated mice, with somewhat lower numbers of the fps1Δ fps2Δ mutant recovered from the liver (Fig. 6A). Importantly, treatment with either dose of caspofungin was more effective at clearing the fps1Δ fps2Δ mutant than the wild type from all three organs. We repeated the experiment using a second set of strains, to ensure that restoration of FPS1 would reverse the clearance phenotype (Fig. 6B). Mice were infected by tail vein injection with BG14 (ura3) cells transformed to Ura+ with empty pGRB2.0 vector, the fps1Δ fps2Δ mutant transformed with pGRB2.0 (BG3130), or the fps1Δ fps2Δ mutant transformed with pGRB2.0-CgFPS1 (BG3123). Four days postinfection, the mice were treated with caspofungin as described above, and they were sacrificed 3 days after the caspofungin treatment. Comparable numbers of wild-type, mutant, and restored cells were recovered from all three organs. As in the first experiment, treatment with caspofungin was noticeably more effective at clearing the fps1Δ fps2Δ mutant than the wild-type cells. The fps1Δ fps2Δ mutant transformed with pGRB2.0-CgFPS1 behaved like the wild type in this assay, demonstrating that loss of FPS1 and FPS2 sensitizes C. glabrata to the antifungal effect of caspofungin treatment (Fig. 6B).
Fig 6.
Caspofungin clearance of C. glabrata from mice infected with a wild-type strain or an fps1Δ fps2Δ mutant. (A) Groups of mice were infected by tail vein injection with either 2.2 × 107 wild-type (WT) cells (BG2) or 0.9 × 107 fps1Δ fps2Δ (fps1Δ2Δ) mutant cells (BG3075). Mice were treated with the indicated dose of caspofungin (CAS) by intraperitoneal injection (6 mice per group) 4 days postinfection. Three days posttreatment, viable fungal cells were measured from spleens, kidneys, and livers. (B) Groups of mice were infected with either 2.3 × 107 BG14 (wild type, ura3) cells transformed to Ura+ with empty vector pGRB2.0 (vector), 2.1 × 107 DL3679 (fps1Δ fps2Δ ura3) cells transformed to Ura+ with pGRB2.0, or 1.6 × 107 DL3679 cells transformed with the CgFPS1 expression plasmid (p3058, FPS1). Mice were treated with the indicated dose of caspofungin by intraperitoneal injection (7 mice per group) 4 days postinfection. Three days posttreatment, viable fungal cells were measured from spleens, kidneys, and livers. Statistical significance was determined using two-sample t tests with a two-tailed distribution. The ends of each bar indicate the sample pairs for which the significance value is presented. Bars within groups show the means.
Candida glabrata RGC1 and RGC2.
In S. cerevisiae, an rgc1Δ rgc2Δ mutant displays a phenotype similar to that of an fps1Δ mutant, because loss of function of these functionally redundant Fps1 regulators results in greatly diminished Fps1 channel activity (1). The C. glabrata genome also possesses two RGC orthologs, which we hereinafter designate CgRGC1 (CAGL0B04213) and CgRGC2 (CAGL0G03179), based both on respective sequence similarities and syntenic organization. The predicted CgRgc2 protein, at 1,062 amino acids, is similar in length to the S. cerevisiae Rgc proteins, whereas the predicted CgRgc1 protein is substantially shorter, at 901 amino acids. The ScRgc2 protein shares 45% sequence identity with CgRgc2 across the entire protein and 38% with CgRgc1 across the central 600 amino acids. The ScRgc1 protein shares 40% sequence identity with CgRgc2 across the entire protein and 41% with CgRgc1 over the central 600 amino acids.
We tested the C. glabrata RGC1 and RGC2 genes for their ability to complement the phenotypes of an S. cerevisiae rgc1Δ rgc12Δ mutant. As with the CgFPS genes, we integrated CgRGC1 or CgRGC2 at the ScRGC2 locus such that the coding region replaced that of ScRGC2 and was under the control of the endogenous ScRGC2 promoter. The CgRGC2 gene but not the CgRGC1 gene was able to complement the loss of ScRGC1 and ScRGC2 function for growth at 40°C and to restore arsenite sensitivity to this mutant (Fig. 5B). These results indicate that at least CgRgc2 is capable of activating the ScFps1 channel and suggest that it serves the same function in the regulation of the CgFps channels. It is possible that the shorter CgRgc1 protein has lost this function.
DISCUSSION
Many fungal species use glycerol as a compatible solute with which to maintain osmotic homeostasis in response to changes in external osmolarity. In S. cerevisiae, intracellular glycerol concentrations are regulated largely by the high osmolarity glycerol (HOG) response pathway, both through induction of glycerol biosynthesis and control of its flux through the plasma membrane Fps1 glycerol channel. Fps1 closes in response to hyperosmotic shock and opens in response to hypoosmotic shock (26, 39). Although the HOG signaling pathway is highly conserved among pathogenic fungi (10, 19, 20, 21, 34, 36), only the C. glabrata genome possesses clear orthologs of FPS1. However, it has not been demonstrated that C. glabrata retains glycerol in response to hyperosmotic stress. Here, we demonstrate that C. glabrata, like many other fungal species, elevates its intracellular glycerol concentration in response to hyperosmotic shock.
Candida glabrata FPS1 and FPS2.
In this work, we studied mutants with deletions of the two genes encoding the presumptive C. glabrata glycerol channels, Fps1 and Fps2. We found that the C. glabrata FPS1 and FPS2 genes encode functionally additive glycerol channels, with FPS1 playing a greater role than FPS2 for the maintenance of osmotic homeostasis. C. glabrata cells lacking both FPS1 and FPS2 retained excess intracellular glycerol in the absence of hyperosmotic stress and displayed constitutive cell wall stress as judged by a transcriptional reporter for cell wall stress and by hypersensitivity to caspofungin, an antifungal agent that targets cell wall biogenesis. The increase in glycerol concentration in the C. glabrata fps1 fps2 null mutant compared to its level in the wild-type was considerably smaller than that observed in an S. cerevisiae fps1 null mutant (1, 26, 39). It is not clear why loss of these C. glabrata proteins resulted in only a modest increase in basal glycerol retention, but that result suggests that either this species may have additional ports for glycerol efflux not possessed by S. cerevisiae or it has more efficient metabolic mechanisms for glycerol removal. Whatever the explanation, this comparatively weak effect may explain why the C. glabrata mutant was not sensitive to growth inhibition at elevated temperature, a cell wall stress condition in S. cerevisiae (15, 25).
We also tested the ability of the C. glabrata FPS genes to function in S. cerevisiae by expressing them under the control of the ScFPS1 promoter. CgFPS1 but not CgFPS2 was able to complement the turgor pressure-induced temperature sensitivity of an S. cerevisiae fps1 null mutant, perhaps reflecting the greater degree of conservation between S. cerevisiae FPS1 and CgFPS1 as compared to CgFPS2. The predicted N-terminal cytoplasmic domain of the CgFps2 protein is shorter than that of Fps1 from either species. Because the MAP kinase Hog1 binds to this domain of ScFps1 (29), it is possible that CgFps2 lacks an important regulatory site for control of osmotic homeostasis by Hog1.
In S. cerevisiae, Fps1 is also the port through which trivalent metalloids, such as arsenite and antimonite, enter the cell (1, 40, 41). Mutants lacking ScFps1 are consequently resistant to the toxic effects of these metalloids. Therefore, we were surprised to find that not only were the C. glabrata fps null mutants not more resistant than wild-type to arsenite, they were sensitized to this agent. Consistent with this, CgFPS1 failed to restore arsenite sensitivity to the S. cerevisiae fps1 null mutant, despite its ability to complement the growth defect. We conclude from these results that the C. glabrata Fps1 and Fps2 channels are not entry ports for arsenite. The enhanced sensitivity of the C. glabrata fps1 fps2 null mutant to arsenite may reflect compensatory activation of another glycerol efflux mechanism that may also allow entry of arsenite.
C. glabrata RGC1 and RGC2.
S. cerevisiae possesses two paralogous positive regulators of Fps1 channel activity, Rgc1 and Rgc2 (1). Their loss of function strongly impairs Fps1 activity, although the mechanism of their action is not yet known. Rgc proteins are found widely among fungal species, including those that do not possess Fps1 proteins, indicating that these proteins can serve other functions. Therefore, we tested the ability of the CgRGC1 and CgRGC2 genes to complement an S. cerevisiae rgc1 rgc2 null mutant. Our finding that CgRGC2 complemented the Fps1-related phenotypes of this mutant reveals that at least this ortholog is capable of regulating Fps1 and suggests that the roles of both the Rgc and Fps proteins in the maintenance of glycerol homeostasis are conserved between S. cerevisiae and C. glabrata.
Caspofungin sensitivity and clearance of C. glabrata infection.
Because the C. glabrata fps1 fps2 null mutant was hypersensitive to caspofungin treatment in vitro, we compared the efficacy of caspofungin treatment of this mutant to its efficacy against the wild type in the clearance of C. glabrata infections in mice. This mutant was reproducibly more effectively cleared from the spleen, kidney, and liver of infected animals by caspofungin.
Three echinocandins (caspofungin, micafungin, and anidulafungin) are currently available for clinical use. Although the first of these received FDA approval in 2002 (caspofungin) (32), clinical isolates of C. albicans and C. glabrata carrying mutations that confer reduced echinocandin sensitivity began to be reported shortly thereafter (12, 16, 22, 24, 31). In those cases examined, the mutations mapped to the β-1,3-glucan synthase catalytic subunit Fks1 or its paralog Fks2 (16, 32). Our results suggest the possibility of developing drugs that target Fps1 and Fps2 channel activity to enhance C. glabrata echinocandin sensitivity or to resensitize clinical isolates with reduced echinocandin sensitivity. Such drugs could target either the channel proteins directly or the Rgc1 and Rgc2 positive regulators. We have found recently that ScRgc2 maintains ScFps1 in an open conformation by binding to its C-terminal cytoplasmic domain (J. Lee and D. E. Levin, unpublished data). Pharmacological intervention with this interaction would be expected to induce closure of the glycerol channel.
ACKNOWLEDGMENTS
This work was supported by grants from NIH to D.E.L. (grant GM48533) and B.P.C. (grant AI046223).
Footnotes
Published ahead of print 19 October 2012
REFERENCES
- 1. Beese SE, Negishi T, Levin DE. 2009. Identification of positive regulators of the yeast fps1 glycerol channel. PLoS Genet. 5:e1000738 doi:10.1371/journal.pgen.1000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beese-Sims SE, Lee J, Levin DE. 2011. Yeast Fps1 glycerol facilitator functions as a homotetramer. Yeast 28:815–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brzankalski GE, et al. 2008. Evaluation of aminocandin and caspofungin against Candida glabrata including isolates with reduced caspofungin susceptibility. J. Antimicrob. Chemother. 62:1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calderone RA. (ed). 2002. Candida and Candidiasis. American Society for Microbiology, Washington, DC [Google Scholar]
- 5. Coleman DC, et al. 1998. Importance of Candida species other than Candida albicans as opportunistic pathogens. Med. Mycol. 36(Suppl 1):156–165 [PubMed] [Google Scholar]
- 6. Cormack BP, Falkow S. 1999. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowen LE, Steinbach WJ. 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot. Cell 7:747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frieman MB, McCaffery JM, Cormack BP. 2002. Modular domain structure in the Candida glabrata adhesin Epa1p, a beta1,6 glucan-cross-linked cell wall protein. Mol. Microbiol. 462:479–492. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein AL, McCusker JH. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541–1553 [DOI] [PubMed] [Google Scholar]
- 10. Gregori C, et al. 2007. The high-osmolarity glycerol response pathway in the human fungal pathogen Candida glabrata strain ATCC 2001 lacks a signaling branch that operates in baker's yeast. Eukaryot. Cell 6:1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedfalk K, et al. 2004. A regulatory domain in the C-terminal extension of the yeast glycerol channel Fps1p. J. Biol. Chem. 279:14954–14960 [DOI] [PubMed] [Google Scholar]
- 12. Hernandez S, et al. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hohmann S. 2009. Control of high osmolarity signaling in the yeast Saccharomyces cerevisiae. FEBS Lett. 583:4025–4029 [DOI] [PubMed] [Google Scholar]
- 14. Jung US, Sobering AK, Romeo MJ, Levin DE. 2002. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 46:781–789 [DOI] [PubMed] [Google Scholar]
- 15. Kamada Y, Jung US, Piotrowski J, Levin DE. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559–1571 [DOI] [PubMed] [Google Scholar]
- 16. Katiyar S, Pfaller M, Edlind T. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaur R, Domergue R, Zupancic ML, Cormack BP. 2005. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 8:378–384 [DOI] [PubMed] [Google Scholar]
- 18. Kayingo G, Kilian SG, Prior BA. 2001. Conservation and release of osmolytes by yeasts during hypo-osmotic stress. Arch. Microbiol. 177:29–35 [DOI] [PubMed] [Google Scholar]
- 19. Kayingo G, et al. 2009. A permease encoded by STL1 is required for active glycerol uptake by Candida albicans. Microbiology 155:1547–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kayingo G, Wong B. 2005. The MAP kinase Hog1p differentially regulates stress-induced production and accumulation of glycerol and D-arabitol in Candida albicans. Microbiology 151:2987–2999 [DOI] [PubMed] [Google Scholar]
- 21. Krantz M, Becit E, Hohmann S. 2006. Comparative genomics of the HOG-signaling system in fungi. Curr. Genet. 49:137–151 [DOI] [PubMed] [Google Scholar]
- 22. Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938–944 [DOI] [PubMed] [Google Scholar]
- 23. Kurtz MB, Douglas CM. 1994. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35:79–86 [DOI] [PubMed] [Google Scholar]
- 24. Laverdiere M, et al. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705–708 [DOI] [PubMed] [Google Scholar]
- 25. Levin DE. 2011. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luyten K, et al. 1995. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 7:1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma B, et al. 2009. High-affinity transporters for NAD+ precursors in Candida glabrata are regulated by Hst1 and induced in response to niacin limitation. Mol. Cell. Biol. 29:4067–4079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma B, Pan SJ, Zupancic ML, Cormack BP. 2007. Assimilation of NAD(+) precursors in Candida glabrata. Mol. Microbiol. 66:14–25 [DOI] [PubMed] [Google Scholar]
- 29. Mollapour M, Piper PW. 2007. Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol. Cell. Biol. 27:6446–6456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nevoigt E, Stahl U. 1996. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase [NAD+] levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 12:1331–1337 [DOI] [PubMed] [Google Scholar]
- 31. Park S, et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Philips J, Herskowitz I. 1997. Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J. Cell Biol. 138:961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. San Jose C, Monge A, Perez-Diaz R, Pla J, Nombela C. 1996. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 178:5850–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen J, Guo W, Kohler JR. 2005. CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect. Immun. 73:1239–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith DA, Nicholls S, Morgan BA, Brown AJP, Quinn J. 2004. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell 15:4179–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sutherland FCW, et al. 1997. Characteristics of Fps1-dependent and -independent glycerol transport in Saccharomyces cerevisiae. J. Bacteriol. 179:7790–7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tamas MJ, et al. 2003. A short regulatory domain restricts glycerol transport through yeast Fps1p. J. Biol. Chem. 278:6337–6345 [DOI] [PubMed] [Google Scholar]
- 39. Tamas MJ, et al. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31:1087–1104 [DOI] [PubMed] [Google Scholar]
- 40. Thorsen M, et al. 2006. The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol. Biol. Cell 17:4400–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wysocki R, et al. 2001. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 40:1391–1401 [DOI] [PubMed] [Google Scholar]