Abstract
Autophagy is a vesicular trafficking pathway that regulates the degradation of aggregated proteins and damaged organelles. Initiation of autophagy requires several multiprotein signaling complexes, such as the ULK1 kinase complex and the Vps34 lipid kinase complex, which generates phosphatidylinositol 3-phosphate [PtdIns(3)P] on the forming autophagosomal membrane. Alterations in autophagy have been reported for various diseases, including myopathies. Here we show that skeletal muscle autophagy is compromised in mice deficient in the X-linked myotubular myopathy (XLMTM)-associated PtdIns(3)P phosphatase myotubularin (MTM1). Mtm1-deficient muscle displays several cellular abnormalities, including a profound increase in ubiquitin aggregates and abnormal mitochondria. Further, we show that Mtm1 deficiency is accompanied by activation of mTORC1 signaling, which persists even following starvation. In vivo pharmacological inhibition of mTOR is sufficient to normalize aberrant autophagy and improve muscle phenotypes in Mtm1 null mice. These results suggest that aberrant mTORC1 signaling and impaired autophagy are consequences of the loss of Mtm1 and may play a primary role in disease pathogenesis.
INTRODUCTION
The autophagy-lysosomal pathway regulates the degradation of bulk cytosol, protein aggregates, and mitochondria. Nutrient limitation represents one of the major ways in which autophagy is activated, and in this context, the recycling of cellular components provides the cell with a source of ATP and amino acids to maintain normal homeostatic processes (1). Tissue-specific deletion of essential autophagy genes (ATG) such as Atg5 or Atg7 has revealed that autophagy plays a cytoprotective role by degrading potentially toxic aggregated proteins and damaged organelles (2–9). The regulation of autophagy is complex but can be categorized into three major phases: initiation, maturation and, degradation (10). The ULK1-Atg13-FIP200 complex plays an essential role in certain nucleating events during initiation (11). This complex is regulated by mTOR (12–14), which itself assembles into two multiprotein complexes termed mTORC1 and mTORC2 (15). The two complexes can be distinguished on the basis of unique components, namely, Raptor and Rictor, which associate with mTORC1 and mTORC2, respectively (16–18). mTORC1 suppresses autophagy and in parallel promotes cell growth via the activation of eIF4E and ribosomal S6 protein kinase (S6K) (15). Inhibition of mTORC1 by nutrient deprivation or pharmacological inhibitors such as rapamycin results in the activation of ULK1 and autophagy (11). In addition to ULK1, the class III phosphatidylinositol 3-kinase Vps34 is required for the formation of autophagosomes during pathway initiation. It is believed that following activation of the ULK1 complex, ATG14L recruits Vps34 to the surface of the endoplasmic reticulum, where it catalyzes the production of phosphatidylinositol 3-phosphate [PtdIns(3)P] (19–21). The exact role of PtdIns(3)P in autophagy is unclear, but studies suggest that PtdIns(3)P recruits specific effector proteins such as Atg18/WIPI (22, 23) and DFCP1 (double FYVE domain-containing protein 1) (19), both of which may play a role in autophagosome formation. Autophagy inactivation by PtdIns(3)P phosphatases is poorly understood but is likely because wortmannin, which inhibits Vps34, also inhibits autophagy (24). MTM1 and related phosphatases can dephosphorylate PtdIns(3)P in vitro (25) and may therefore oppose the action of Vps34.
MTM1 is the archetypal member of the MTM family of phosphatases and is mutated in 90% of X-linked myotubular myopathy (XLMTM) patients (26). XLMTM is a severe form of centronuclear myopathy that is present at birth and is clinically characterized by muscle weakness and respiratory failure (26). Muscle biopsy specimens from patients have revealed the presence of small, rounded myofibers and central nuclei (27, 28). The most severe cases of XLMTM are associated with mutations that abolish MTM1 phosphatase activity (29, 30). Since MTM1 can dephosphorylate PtdIns(3)P (25), one might expect that MTM1 deficiency would lead to overactivation of autophagy, similar to the AKT pathway gain of function in cells lacking phosphatase and tensin homolog, a PtdIns(3,4,5)P3 phosphatase (31). In fact, recent studies have reported that the myotubularin-related (MTMR) family members Jumpy (MTMR14) and MTMR3 negatively regulate autophagy (32–34). In this study, we sought to determine if autophagy is altered in XLMTM. Using Mtm1-deficient mice, we made the unexpected finding that autophagy is blocked in Mtm1-null muscle. Interestingly, mTORC1 signaling is hyperactivated in this context and this coincides with inhibitory phosphorylation on ULK1. Administration of an mTOR-selective antagonist lowers ULK1 phosphorylation and normalizes autophagy in Mtm1-deficient muscle, reducing the abnormal accumulation of the intermediate filament (IF) protein desmin and leading to increased muscle mass.
MATERIALS AND METHODS
Antibodies and inhibitors.
Anti-phospho-S6K1(Thr389), anti-phospho-S6 (Ser240/244), anti-phospho-ULK1 (Ser757), anti-phospho-Akt (Ser473), anti-S6K1, anti-S6, anti-4EBP1, anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH), and antiubiquitin antibodies (9234, 4838, 6888, 9271, 9202, 2217, 9452, 2118, and 3936, respectively) were from Cell Signaling Technologies (Danvers, MA). Anti-β-actin antibody (ab-6276) was from Abcam (Cambridge, MA). Anti-LC3 antibody (NB100-2220) was from Novus Biologics (Littleton, CO), and anti-GAB(A) receptor-associated protein (anti-GABARAP) and anti-GATE16 (anti-GABARAPL2) antibodies (18723-1-AP and 18724-1-AP, respectively) were from Proteintech (Chicago, IL). Anti-p62 antibody (H00008878-M01) was from Abnova (Taiwan), and anti-Nbr1 antibody (sc-130380) was from Santa Cruz Biotechnologies (Santa Cruz, CA). Antidesmin antibody (M0760) was from Dako (Carpinteria, CA). Anti-Lamp2 antibody (1921-01) was from Southern Biotech. A C-terminal peptide corresponding to amino acid residues 589 to 603 of mouse myotubularin was used to generate a polyclonal antibody (Covance Research Products Inc., Denver, PA) (35). The anti-phospho-4EBP1 (Thr36/47) polyclonal antibody was generated by using a peptide corresponding to amino acid residues 32 to 51 of human 4EBP1 (Covance Research Products Inc., Denver, PA). The antiubiquitin antibody (Z0458) used for immunofluorescence assay was from Dako (Carpinteria, CA). The antilaminin antibody (L9393) used for muscle morphometry was from Sigma-Aldrich (St. Louis, MO). The ATP-competitive mTORC1/C2 inhibitor AZD8055 (CT-A8055) was from ChemieTek (Indianapolis, IN).
Mtm1gt/y mice.
Mtm1 gene trap (Mtm1gt/y) mice were obtained from Taconic (Hudson, NY). The gene trap was inserted into intron 1 of the Mtm1 gene, upstream of the ATG site. Mtm1gt/y mice were backcrossed to C57BL/6 mice for three generations. Gene trap insertion was confirmed by PCR using genomic DNA isolated from tails of hemizygous mice. The animal procedures used were approved by the Institutional Animal Care and Use Committee of Novartis Institutes for Biomedical Research (NIBR).
Drug treatments.
Mice were subjected to treatment with RAD001 (Novartis) or AZD8055 (ChemieTek). RAD001 was formulated as a 2% microemulsion concentrate diluted to 10 mg/kg and administered once daily for 1 h or 5 days via oral gavage. For analysis of mTORC1 signaling in wild-type (WT) mice, AZD8055 was diluted in the vehicle at a concentration of 25 mg/kg and administered via oral gavage (one dosing) for 1 h or once daily for 5 days. For biochemical studies, WT or Mtm1gt/y mice were administered AZD8055 at a concentration of 25 mg/kg by oral gavage twice daily for 3 days (six dosings) or at a concentration of 5 mg/kg twice daily for 2 weeks.
Myofiber morphometry.
Frozen tibialis anterior (TA) or soleus muscle was cut into serial sections (8 μm) and stained for laminin to determine fiber cross-sectional area. Images of the tissue sections were acquired by using Scanscope (Aperio). The mean myofiber cross-sectional area of all fibers in the section was determined by using custom software developed at NIBR. Using this method, more than 3,000 fibers in each section were measured.
Forelimb grip strength.
Forelimb grip strength was measured weekly by using a Chatillon Grip Strength Meter (Columbus Instruments International, Columbus, OH). Mice were placed on a horizontal grid and gently pulled in the opposite direction in the horizontal plane. The maximal strength exerted by the mouse before it lost its grip was recorded. Three independent measurements per mouse were recorded, allowing 1 min of recovery after each recording.
Muscle tissue preparation for protein analysis.
Frozen TA, soleus, and gastrocnemius muscles were homogenized in lysis buffer containing 1% Triton X-100 in phosphate-buffered saline (PBS), 10 mM sodium pyrophosphate (Na4O7P2), 50 mM NaF, 1 mM sodium orthovanadate, 2 mM β-glycerophosphate, and Roche Complete protease inhibitor cocktail using a TissueLyser II (Qiagen). The homogenates were incubated at 4°C for 1 h and then centrifuged (13,000 rpm) for 15 min at 4°C, and supernatants were collected (1% Triton X-100-soluble fraction). To obtain the insoluble fraction, pellets were resuspended in 1% SDS in PBS. Protein concentrations were determined by using a detergent-compatible (DC) protein assay kit (Bio-Rad).
Immunoblotting.
Protein samples were separated on 4 to 12% Bis-Tris gels (Bio-Rad) and transferred to nitrocellulose membranes (Protran), which were probed with the indicated primary antibodies for either 1 h at room temperature or overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (Millipore) for 1 h at room temperature. Proteins were visualized by using enhanced chemiluminescence (Pierce).
PtdIns(3)P measurement.
Levels of PtdIns(3)P were measured in lipid extracts from TA muscle by using a competitive enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (Echelon Biosciences). In brief, the muscle was isolated from 5-week-old WT or Mtm1 null mice. Tissue weight was recorded, and then tissue was snap-frozen in liquid nitrogen. To extract lipids, muscle samples were ground and homogenized in a mortar and pestle under liquid nitrogen. Samples were then incubated in ice-cold 5% trichloroacetic acid to extract lipids. Lyophilized lipids were resuspended in PBS-Tween 20 containing 3% protein stabilizer. The ELISA was performed in triplicate. The quantities of PtdIns(3)P in the samples were determined by comparison with the values on the PtdIns(3)P standard curve. Concentrations were corrected for body weight.
Transmission electron microscopy.
TA muscle was dissected and placed in fixative containing 5% glutaraldehyde and 2.5% paraformaldehyde (PFA) plus 0.06% picric acid in 0.2 M sodium cacodylate buffer (pH 7.4) for 2 h at room temperature, washed in 0.1 M cacodylate buffer, and postfixed with 1% osmium tetroxide–1.5% potassium ferrocyanide for 1 h. Specimens were then washed in water and incubated in 1% aqueous uranyl acetate for 1 h; this was followed by two washes in water and subsequent dehydration in gradients of alcohol (10 min each at 50, 70, and 90%, 2 × 10 min at 100%). The samples were then put in propylene oxide for 1 h and infiltrated overnight with a 1:1 mixture of propylene oxide and TAAB Epon (Marivac Canada Inc., St. Laurent, Canada). The following day, the samples were embedded in TAAB Epon and polymerized at 60°C for 48 h. Ultrathin sections (about 60 nm) were cut on a Reichert Ultracut-S microtome, picked up on copper grids, stained with lead citrate, and examined in a Tecnai G2 Spirit BioTWIN transmission electron microscope, and images were recorded with an AMT 2k charge-coupled device camera.
Histology and immunofluorescence assay.
TA or soleus muscle was snap-frozen in 2-methylbutane precooled in liquid nitrogen for histological and immunofluorescence analysis. Serial sections were cut from the frozen muscles of WT or Mtm1gt/y mice and stained with hematoxylin and eosin (H&E). For immunofluorescence assay, frozen tissue cut into cross sections was fixed in 4% PFA, permeabilized in 0.1% Triton X-100 in PBS for 15 min, and then incubated with antibodies against ubiquitin (Dako Z0458) or p62 (Abnova H00008878-M01). Samples were incubated with a goat anti-rabbit antibody conjugated to Cy5 (Chemicon International) or a goat anti-mouse antibody coupled to Alexa Fluor 568 (Invitrogen) and imaged on a Zeiss LSM 510 confocal microscope.
Enzyme activity staining.
Skeletal muscle specimens were harvested at necropsy, frozen in isopentane (2-methylbutane), cooled in liquid nitrogen, and cryostat cross-sectioned at 8.0 μm for subsequent cytochrome c oxidase (COX) and succinate dehydrogenase (SDH) enzyme activity staining by standard histochemical staining methods (36). Quantitative image analysis for enzyme activity staining was performed by scanning stained tissues on glass slides using a digital slide scanning system (ScanScope XT; Aperio Technologies, Inc., Vista, CA). Quantification of enzyme activity staining on digital images was performed by using a color deconvolution algorithm software application (Aperio Technologies, Inc., Vista, CA) with the area and relative intensity of enzyme activity staining calculated and expressed as the percentage of weakly or strongly positive pixels in the total stained area (cumulative total area of combined positive and negative pixels). The thresholds for determination of boundaries between weak and strong staining areas were set by a veterinary pathologist (R. A. Valdez) board certified by the American College of Veterinary Pathologists as input parameters to the color deconvolution algorithm and establishment of a macro for subsequent analyses.
COX enzyme activity assay.
Mitochondrial complex IV (COX) activity was measured in whole muscle tissue with the microplate assay kit (MS444; MitoSciences) according to the manufacturer's protocol. TA muscle was homogenized in 1× PBS containing Roche Complete protease inhibitor cocktail (Roche Applied Science) by using an Omni tissue homogenizer (Omni International). Tissue homogenate was resuspended to 2 mg/ml in the extraction buffer provided, and 50 μg of protein was used for the assay. Activity was expressed as the change in absorbance per minute.
Quantitative PCR.
Frozen TA muscle or liver was pulverized and total RNA was isolated by using the RNeasy Fibrous Tissue kit (Qiagen, Valencia, CA). RNA was reverse transcribed to cDNA by using TaqMan reverse transcription reagent (Applied Biosystems, Foster City, CA), and the cDNA was subjected to quantitative real-time PCR by using the ABI Prism 7900 sequence detection system and Assay-on-Demand primer probes (Applied Biosystems, Foster City, CA). All data were normalized to GAPDH.
TaqMan Assay-on-Demand primer probes.
The TaqMan Assay-on-Demand primer probes used were as follows: NAD(P)H dehydrogenase quinone 1 (Nqo1), Mm00500821_m1; sulfiredoxin 1 homolog (Srxn1), Mm00769566_m1; metallothionein 1 (Mt1), Mm00496660_g1; metallothionein 4 (Mt4), Mm00485227_m1; glutathione S-transferase α1/α2 (GSTa1/a2), Mm04207463_mH; GAPDH, Mm_99999915_g1.
Microarray and GSEA.
RNAs isolated from TA muscle and liver tissues from 2- and 5-week-old WT or Mtm1gt/y mice were used for microarray analysis. RNA was hybridized to the GeneChip Mouse Genome 430 2.0 array (Affymetrix, Santa Clara, CA) in triplicate. The Bioconductor Limma open-source software was used to analyze microarray data. The data were then analyzed by gene set enrichment analysis (GSEA) to identify pathways that are differentially regulated. The false-discovery rate (FDR) multiple-testing correction method was applied to the data to obtain the FDR-adjusted P value. Pathways that were enriched in Mtm1gt/y muscle from 5-week-old mice were considered significantly changed if they had an FDR-adjusted P value of <0.001.
Statistical analysis.
Statistical analysis was performed by using the unpaired two-tailed Student t test, one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparison post hoc test, or two-way ANOVA followed by the Bonferroni post hoc test where appropriate. Differences were considered statistically significant if the P value was <0.05.
RESULTS
Impaired autophagy in Mtm1 null muscle.
Aberrant autophagy has been reported in several myopathies, particularly those associated with inclusions or dysfunctional mitochondria (1). Muscle-specific deletion of Atg5 or Atg7 results in muscle atrophy and a decrease in muscle strength (8, 9). To investigate the role of MTM1 in autophagy, Mtm1-null mice (Mtm1gt/y) were generated by using a gene trap strategy (Fig. 1A to C). To determine if loss of Mtm1 modulates PtdIns(3)P in vivo, we measured levels of PtdIns(3)P in lipid extracts from muscle. As expected, Mtm1-deficient muscle had significantly increased PtdIns(3)P levels compared to those of WT muscle (Fig. 1D). At an early age, Mtm1gt/y mice were indistinguishable from age-matched WT controls, but starting at about 4 weeks of age, these mice exhibited a significant and progressive decrease in body weight (Fig. 2A). Similar to reports for Mtm1 knockout mice (37), morphological analysis of fast (TA) and slow (soleus) skeletal muscles showed a progressive myopathy in Mtm1gt/y mice with a general decrease in myofiber size (Fig. 2B and C) and the presence of central or, more often, paracentral nuclei (Fig. 2D and E). The myofiber cross-sectional area was significantly reduced by 59% in TA muscle and 52% in soleus muscle (Fig. 2F and G). Furthermore, we observed several ultrastructural abnormalities in Mtm1gt/y muscle, including wavy, misaligned Z lines and a dilated sarcoplasmic reticulum (Fig. 2H). Loss of Mtm1 leads to mislocalization and aggregation of the IF protein desmin (38), which is the major muscle-specific IF protein. Desmin localizes mainly in the Z disc of striated muscles and has an essential role in muscle architecture maintenance (39). Here we also show that desmin accumulates in Mtm1-deficient muscle (Fig. 2I). Consistent with the histological observations, muscle weight was decreased (Fig. 2J) and Mtm1gt/y mice developed a progressive decrease in forelimb grip strength (Fig. 2K).
Fig 1.
Generation of Mtm1gt/y mice. (A) Diagram representing the Mtm1 gene trapped mutant allele and resulting mutant mRNA transcript. The arrows indicate the primers used for reverse transcription-PCR analysis. (B) Genotyping of WT and Mtm1gt/y mice. PCR analysis of genomic DNA from mouse tails using primers A, B, and C shown in panel A. The Mtm1 gene trap specific band (mutant allele) is present only in Mtm1gt/y mice. (C) Mtm1gt/y (gt/y) mice are null for Mtm1 expression. Protein lysates from muscle, heart, liver, and brain tissues were subjected to immunoblot analysis with the indicated antibodies. (D) PtdIns(3)P levels were measured in lipid extracts from muscle tissue by ELISA. Concentrations were corrected for body weight. Values are means ± standard deviations (n ≥ 3 per group).
Fig 2.
Muscle phenotype of Mtm1gt/y mice. (A) Growth curves of WT and Mtm1gt/y (gt/y) mice. A significant difference between Mtm1gt/y and WT mice was observed after 29 days. (B to E) H&E staining of cross sections of soleus (B) and TA (C) muscles of 4-week-old mice reveals a smaller fiber size in Mtm1gt/y muscle and fibrosis with inflammatory cell infiltration in the soleus muscle (arrowheads). Centralized nuclei (arrows) can be found in TA muscles of 6-week-old Mtm1gt/y mice (D). Longitudinal sections of TA muscles of 5-week-old mice show disruption of nuclear positioning along muscle fibers (E). (F) Quantification of the cross-sectional area (CSA) of myofibers from TA and soleus muscles. Values are means ± standard deviations (***, P < 0.001). The numbers of animals are indicated in the respective bars. (G) Histograms showing the distribution of cross-sectional areas (μm2) in panel F. (H) Electron micrographs of WT and MTM1gt/y TA muscles. In longitudinal sections, Z-line misalignment and a dilated sarcoplasmic reticulum (arrowheads) are evident in Mtm1gt/y mice. Scale bars, 100 nm. (I) Protein lysates were extracted from TA or soleus muscles and analyzed by Western blotting for levels of desmin and Mtm1. Immunoblot assays for GAPDH are shown to verify equal protein loading. (J) Weights of TA and soleus muscles. The number of animals per group is indicated in each respective bar. Values are means ± standard deviations (***, P < 0.001). (K) The forelimb grip strength of 3- and 4-week-old WT and Mtm1gt/y mice was measured and normalized to their weight. Values are means ± standard deviations (WT, n = 10 mice; Mtm1gt/y, n = 6 mice;**, P < 0.01; ***, P < 0.001).
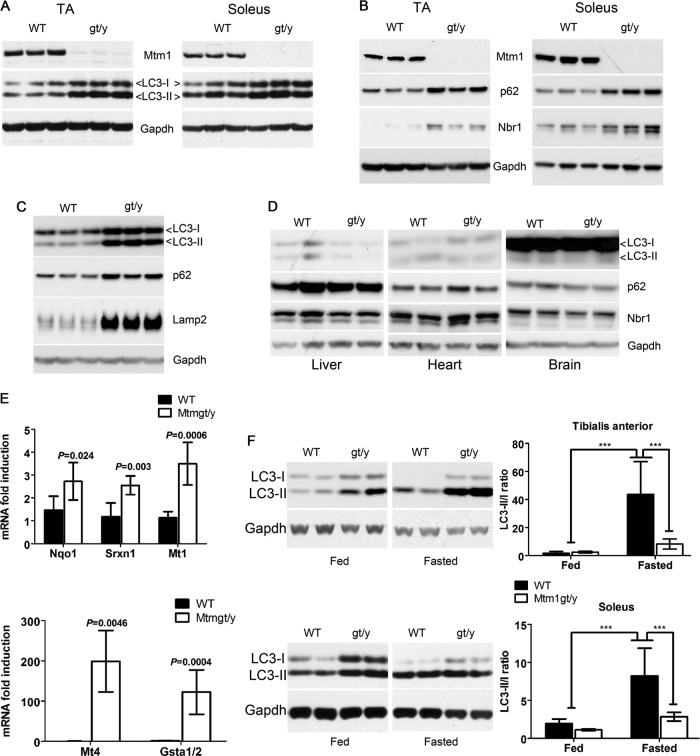
To examine autophagy status in Mtm1gt/y mice, Western blot analysis was used to measure LC3-I and LC3-II in extracts of TA and soleus skeletal muscles. Marked increases in the levels of LC3-I and LC3-II were observed in Mtm1gt/y muscle compared to the WT (Fig. 3A). The expression of the LC3 homologs GATE16 and GABARAP were similarly altered in Mtm1gt/y muscle (data not shown). p62 and Nbr1 are LC3-interacting proteins that are degraded via autophagy (4, 40, 41), and their expression was elevated in Mtm1gt/y muscle (Fig. 3B). In addition, there was a marked elevation of the lysosomal marker Lamp2 in Mtm1-deficient muscle (Fig. 3C). Together, the accumulation of lipidated LC3, p62, Nbr1, and Lamp2 likely suggests a late-stage block in autophagy, leading to a failure of efficient autophagy substrate turnover. The alterations in LC3, p62, and Nbr1 were not observed in other tissues (Fig. 3D), which is consistent with previous reports showing that skeletal muscle is the primary tissue affected in this disease (37). Robust induction of oxidative stress response enzymes in Mtm1gt/y muscle was also noted (Fig. 3E). This observation is consistent with recent reports showing that reduced autophagic turnover of p62 leads to deleterious activation of Nrf2 (nuclear factor erythroid 2-related factor 2), the transcription factor that regulates the expression of enzymes induced in response to oxidative stress (42). To determine if Mtm1 is required for starvation-induced autophagy in vivo, mice were fasted for 20 h as previously described (43). Fasting markedly increased the conversion of LC3-I to LC3-II in both TA and soleus muscles from WT mice (Fig. 3F), but only partial conversion was observed in Mtm1gt/y muscle. Collectively, these data show that both basal and starvation-induced autophagy is impaired in Mtm1-deficient muscle.
Fig 3.
Defective autophagy in Mtm1-deficient mice. (A) Immunoblot analysis of LC3 and Mtm1 in muscle homogenates from TA and soleus muscles of 4-week-old Mtm1gt/y (gt/y) and WT mice. (B) Immunoblot analysis of p62 and Nbr1 in TA and soleus muscles of WT and Mtm1gt/y (gt/y) mice. (C) Protein lysates were extracted from either WT or Mtm1gt/y (gt/y) muscles and subjected to Western blotting for LC3, p62, and Lamp2. (D) Immunoblot analysis for LC3, p62, and Nbr1 in brain, heart, and liver homogenates from WT and Mtm1gt/y mice. (E) Quantitative PCR analysis of stress response enzymes in WT and Mtm1gt/y TA muscles. Expression values were normalized to GAPDH. Data are expressed as fold induction relative to that in the WT. Values are means ± standard deviations (P values are indicated in the graph; n = 5 mice per group). Nqo1, NAD(P)H dehydrogenase quinone 1; Srxn1, sulfiredoxin 1; Mt1, metallothionein 1; Mt4, metallothionein 4; GSTa1/2, glutathione S-transferase α1/α2. (F) Western blot assay for LC3 in protein lysates from TA and soleus muscles of WT or Mtm1gt/y (gt/y) mice either fed or fasted for 20 h. Relative band intensities were quantified and used to determine the ratio of LC3-II to LC3-I and are represented in the graph. Values are means ± standard deviations (n ≥ 4 mice per group; ***, P < 0.001 [relative to fasted WT mice]).
Accumulation of polyubiquitin proteins and dysfunctional mitochondria in Mtm1-deficient muscle.
Accumulation of protein aggregates and damaged mitochondria are hallmarks of autophagy deficiency (44). There was a profound accumulation of soluble ubiquitinated proteins in TA and soleus muscles from Mtm1gt/y mice (Fig. 4A) but not in liver, heart, or brain tissue (data not shown). Levels of ubiquitinated proteins in the Triton-insoluble fraction of muscle were enhanced, indicating the presence of protein aggregates in Mtm1gt/y muscle (Fig. 4A). Expression of p62 and Nbr1 was markedly higher in the Triton-insoluble muscle fraction from Mtm1gt/y mice than in that from WT mice (Fig. 4B). Immunofluorescence analysis revealed the presence of both ubiquitin- and p62-positive aggregates in these myofibers (Fig. 4C and D). These observations are consistent with previous reports showing that elevated levels of p62, a key molecule involved in the targeting of ubiquitinated proteins to the autophagosome, contribute to the aggregation of ubiquitinated proteins when autophagic flux is reduced (4, 41).
Fig 4.
Accumulation of ubiquitin aggregates in Mtm1gt/y muscle. (A) Immunoblot analysis of ubiquitin in 1% Triton X-100 detergent-soluble and insoluble fractions of muscle homogenates from 5-week-old mice. GAPDH was used as a loading control for the soluble fraction, and β-actin was used as a loading control for the insoluble fraction. (B) Accumulation of p62 and Nbr1 in both the detergent-soluble and insoluble fractions. Homogenates from mice were fractionated and analyzed by immunoblotting as described for panel A. (C and D) Immunofluorescent staining for ubiquitin (C) and p62 (D) shows the presence of aggregates in Mtm1gt/y but not in WT muscles. DAPI, 4′,6-diamidino-2-phenylindole.
Transcriptional profiling and GSEA revealed that genes representing oxidative phosphorylation, mitochondrial translation, and the tricarboxylic acid (TCA) cycle were among the most significantly downregulated in Mtm1gt/y TA muscle from 5-week-old mice (Table 1). These data suggest that a failure to degrade mitochondria via autophagy (referred to as mitophagy) might result in the accumulation of defective mitochondria in Mtm1gt/y muscle. Electron microscopic analysis confirmed the presence of abnormal-appearing mitochondria with fewer, decondensed, and swollen cristae compared to those of the WT (Fig. 5A to D). We additionally performed histochemical analysis of SDH (complex II) and COX (complex IV), which can provide information on the oxidative capacity and thus the mitochondrial content. SDH and COX staining exhibited the expected checkerboard staining pattern in WT TA (Fig. 5E to G) and soleus muscles (data not shown) but not in Mtm1gt/y samples, where many fibers appeared nearly devoid of staining and some showed a distinct concentration of subsarcolemmal staining. Direct measurement of COX enzyme activity showed that activity was significantly decreased in Mtm1 null muscle (Fig. 5H), which correlates with the reduced COX staining pattern in these mice (Fig. 5F). Taken together, these data suggest that Mtm1 deficiency reduces autophagic capacity in muscle, leading to reduced clearance of protein aggregates and damaged mitochondria.
Table 1.
Mitochondrial gene sets are differentially enriched in Mtm1gt/y muscle as determined by GSEA
| Class system | Subclass title | P valuea |
|---|---|---|
| GO | Mitochondrial respiratory chain complex I | 4.04E-13 |
| GO | Mitochondrial electron transport, NADH to ubiquinone | 4.04E-13 |
| GO | Mitochondrial matrix | 4.04E-13 |
| MetaCore GeneGo processes | Translation in mitochondria | 4.04E-13 |
| MetaCore pathway maps | Oxidative phosphorylation | 4.04E-13 |
| GO | NADH dehydrogenase (ubiquinone) activity | 7.20E-12 |
| MetaCore pathway maps | Ubiquinone metabolism | 1.44E-11 |
| GO | Electron transport chain | 2.61E-10 |
| GO | TCA cycle | 5.74E-07 |
| KEGG pathways | Citrate cycle (TCA cycle), Homo sapiens (human) | 1.62E-05 |
| GO | NADH dehydrogenase activity | 2.06E-05 |
| KEGG pathways | Oxidative phosphorylation, Homo sapiens (human) | 2.14E-05 |
The P value is the FDR-adjusted P value derived from the Kolmogorov-Smirnov statistic.
Fig 5.
Abnormal mitochondria in Mtm1 null muscle. (A to D) Electron micrographs of TA muscles from 5-week-old WT (A and B) and Mtm1gt/y (C and D) mice. Scale bars, 500 nm (A and C) and 100 nm (B and D). (E and F) Cross sections of TA muscle stained for SDH (E) and COX (F) activities. Fibers with dense subsarcolemmal (“ring”) staining in Mtm1-deficient TA are indicated (arrows). (G) Quantification (see Materials and Methods) of relative differences in the intensity of enzyme activity staining for SDH (E) and COX (F) in TA muscles from WT and Mtm1gt/y mice. Data are means ± standard deviations (P values are indicated in the graph; n = 3 mice per group). (H) COX enzyme activity of whole muscle homogenates from WT and Mtm1gt/y mice. Values are means ± standard deviations (n = 3 mice per genotype).
Activation of mTORC1 signaling in Mtm1gt/y muscle.
Given the key role that mTOR plays in the control of autophagy, we analyzed the activation status of mTOR signaling in Mtm1 null mice. Surprisingly, upregulation of phosphorylated S6K1Thr389, S6Ser240/244, and 4EBP1Thr37/46 was observed in Mtm1gt/y muscle (Fig. 6A) but not in other tissues (Fig. 6B). Interestingly, muscle mTORC1 signaling was sustained after fasting conditions in Mtm1gt/y mice whereas these readouts were either absent or significantly reduced in WT mice, as expected (Fig. 6A). We also assessed the status of Akt phosphorylation and found that under fed conditions there was a small but statistically insignificant increase in AktSer473 phosphorylation in Mtm1gt/y muscle (Fig. 6C). However, under fasting conditions, Akt phosphorylation was reduced in both WT and Mtm1gt/y muscles, whereas phosphorylation of S6Ser240/244 and 4EBP1Thr37/46 remained elevated (Fig. 6C). Together, these observations suggest that mTORC1 hyperactivation in Mtm1 null muscle occurs independently of changes in key upstream inputs to mTOR such as Akt.
Fig 6.
Aberrant mTORC1 signaling in Mtm1gt/y muscle. (A) TA muscles from WT and Mtm1gt/y mice fed or fasted for 20 h were subjected to immunoblot analysis with the indicated antibodies. (B) Immunoblot analysis for P-S6 and P-4EBP1 in brain, heart, or liver homogenates from WT and Mtm1gt/y mice. (C) TA muscles from WT and Mtm1gt/y mice fed or fasted for 24 h were subjected to immunoblot analysis for S6Ser240/244, S6, 4EBP1Thr37/46, and AKTSer473. Relative band intensities were quantified and are represented in the graph. Values are means ± standard deviations (*, P < 0.05 [relative to fed WT mice]; **, P < 0.01 [relative to fed WT mice]; ***, P < 0.001 [relative to fed WT mice]; ++, P < 0.01 [relative to fed Mtm1gt/y mice]; +++, P < 0.001 [relative to fed Mtm1gt/y mice]; ##, P < 0.01 [relative to fasted WT mice]; ###, P < 0.001 [relative to fasted WT mice]). AU, arbitrary units. (D) TA muscles from WT and Mtm1gt/y mice fed or fasted for 10 h were subjected to immunoblot analysis with the indicated antibodies.
One way in which mTOR is believed to inhibit autophagy is direct phosphorylation of ULK1 (12–14), and a recent study identified ULK1Ser757 as an mTOR-regulated phosphorylation site (45). In Mtm1 null muscle, ULK1Ser757 phosphorylation is increased under normal fed conditions, and similar to S6K1 and 4EBP1 phosphorylation, ULK1Ser757 remains elevated under fasting conditions in Mtm1 null muscle (Fig. 6D). These data suggest that hyperactive mTORC1 signaling contributes to the altered autophagy observed in these mice via direct phosphorylation of ULK1.
We sought to determine whether pharmacologic inhibition of mTOR can restore autophagy in Mtm1gt/y mice. Rapamycin and semisynthetic rapamycin analogues such as RAD001 are allosteric inhibitors of mTOR and are used clinically to treat cancer and prevent organ transplant rejection (46). Several small-molecule inhibitors that act as ATP-competitive inhibitors have been recently developed (46). These inhibitors have been reported to be more effective inhibitors than rapamycin because they modulate mTORC1 outputs such as 4EBP1 and autophagy, which are only modestly affected by rapamycin (47, 48). To determine the potential of these compounds to activate autophagy in muscle, we treated mice with the allosteric inhibitor RAD001 or the ATP-competitive inhibitor AZD8055 (49). We found that 1 h of treatment with either RAD001 or AZD8055 was effective at inhibiting the phosphorylation of S6K1 (Fig. 7A), but only mice treated with AZD8055 showed a decrease in 4EBP1 phosphorylation and robust LC3 conversion (Fig. 7A). The differential effects of RAD001 and AZD8055 persisted even when either compound was administered for 5 days (Fig. 7B). On the basis of these data and our previous findings (47), we used the catalytic mTOR inhibitor AZD8055 to evaluate whether aberrant mTORC1 pathway activity contributes to the autophagy defects in Mtm1 null muscle. Following a single treatment with AZD8055, aberrant phosphorylation of S6K1, ULK1, and 4EBP1 was blocked and, remarkably, autophagy was activated in Mtm1gt/y muscle to an extent similar to that seen in WT muscle (Fig. 7C). More prolonged treatment with AZD8055 significantly reduced the levels of p62 and Lamp2, suggesting an increase in autophagy-mediated degradation (Fig. 7D). Despite the fact that starvation could neither fully activate autophagy (Fig. 3D) nor reduce mTORC1 signaling (Fig. 6A) in Mtm1gt/y muscle, mTOR inhibition using AZD8055 was sufficient (Fig. 7C and D). Together, these data indicate that aberrant mTORC1 activation contributes to the autophagy defect in Mtm1gt/y mice and that this defect is not permanent and can be corrected in vivo.
Fig 7.
Inhibition of mTOR corrects the autophagy defect in Mtm1gt/y muscle. Mice were treated with the vehicle, RAD001 (10 mg/kg), or AZD8055 (25 mg/kg) for 1 h (A) or 5 consecutive days (B). Muscle protein was isolated for immunoblot analysis for P-S6K1, P-4EBP1, and LC3. (C) Immunoblot analysis for LC3, P-ULK1, P-S6K1, and P-4EBP1 in gastrocnemius muscles of WT and Mtm1gt/y mice treated with the vehicle control or AZD8055 (10 mg/kg four times a day) for the indicated times. Values are means ± standard deviations (n ≥ 2 mice per group; **, P < 0.01; ***, P < 0.001 [relative to the vehicle-treated control]). (D) Immunoblot analysis for p62 and Lamp2 in muscles of WT and Mtm1gt/y mice treated with AZD8055 for 3 days. Relative band intensities were quantified and are represented in the graph. Values are means ± standard deviations (*, P < 0.05; ***, P < 0.001 [relative to vehicle-treated Mtm1gt/y mice]; #, P < 0.05; ##, P < 0.01 [relative to AZD8055-treated Mtm1gt/y mice]; +++, P < 0.001 [relative to AZD8055-treated WT mice]; n ≥ 3 mice per group).
Furthermore, compared to vehicle-treated mice, inhibition of mTOR in Mtm1 null mice significantly restores muscle mass to a level similar to that of WT muscle (Fig. 8A). In addition, desmin accumulation is much reduced in muscle from Mtm1gt/y mice treated with AZD8055 (Fig. 8B). These data suggest that inhibition of mTOR pathway activity is sufficient to both increase autophagy-mediated degradation and alleviate abnormalities in Mtm1-deficient muscle.
Fig 8.

AZD8055 improves muscle mass and reduces abnormal desmin accumulation in Mtm1gt/y muscle. (A) Weights of muscles from WT or Mtm1gt/y mice treated with AZD8055 for 3 days. The number of animals per group is indicated in the graph. Values are means ± standard deviations (*, P < 0.05; ***, P < 0.001 [relative to vehicle-treated Mtm1gt/y mice]; ##, P < 0.01 [relative to AZD8055-treated Mtm1gt/y mice]; n ≥ 3 mice per group). MW, muscle weight; BW, body weight. (B) Mice were treated with the vehicle or AZD8055 for 2 weeks. Protein lysates were extracted from muscle for immunoblot analysis for desmin. Relative band intensities were quantified and are represented in the graph. Values are means ± standard deviations (**, P < 0.01 [relative to vehicle-treated Mtm1gt/y mice]).
DISCUSSION
Autophagy has a critical role in maintaining cellular quality control of proteins and organelles, and accumulation of ubiquitin, p62, and Nbr1 protein aggregates is observed in tissue-specific Atg gene knockout mouse models, as is the accumulation of deformed or dysfunctional mitochondria (44). Not surprisingly, failure to degrade protein aggregates and mitochondria via autophagy has been associated with the pathogenesis of certain diseases, including neurodegeneration, cancer, and muscle weakness (1). The PtdIns(3)P-specific phosphatase MTM1 is causally mutated in XLMTM patients, but the underlying disease mechanisms have not been described. Using an Mtm1-deficient mouse model, we observed an accumulation of LC3, p62, Nbr1, and polyubiquitinated proteins, as well as abnormal and dysfunctional mitochondria, in skeletal muscle. These phenotypes are reminiscent of those described for muscle-specific Atg5 and Atg7 knockout mice (8, 9), but interestingly, disease onset is more rapid in Mtm1 null mice. This suggests that a block in autophagic degradation is at least partially responsible for the muscle myopathy in XLMTM patients, but there are potentially additional autophagy-independent functions that are affected by loss of MTM1 that contribute to the aggressive nature of the disease.
How might defective autophagy contribute to this disease? The presence of abnormal mitochondria and reduced COX activity indicates a decrease in skeletal muscle mitophagy in Mtm1 null mice, which is consistent with recent findings (38). Therefore, the muscle weakness seen in XLMTM patients might be due to persistent mitochondrial dysfunction, as this can contribute to tissue damage (50), and changes in mitochondrial content and function are critical to the maintenance of muscle function (51, 52). Intriguingly, transcriptional profiling of Mtm1gt/y muscle shows a trend toward defective mitochondrial function pathways in asymptomatic 2-week-old mice (data not shown), while no such change was observed in liver tissue from either 2- or 5-week-old Mtm1gt/y mice. Thus, alterations in autophagy within muscle may precede disease pathology.
The levels of PtdIns(3)P were significantly elevated in Mtm1 null muscle, and on the basis of current models (10, 32), this should overactivate autophagy. In fact, this was not observed, which is surprising because one might expect that an increase in PtdIns(3)P would promote excessive autophagy by increasing the recruitment of Vps34 effector proteins Atg18/WIPI and DFCP1 to autophagosome formation sites. Instead, loss of Mtm1 leads to a failure to undergo efficient autophagy, likely because of persistent mTORC1 activation, which represses ULK1 and therefore blocks autophagy. The observation that an mTOR-selective inhibitor such as AZD8055 could normalize autophagy supports the idea that there is an imbalance between mTOR and autophagy in the absence of Mtm1. Since the ULK1-Atg13-FIP200 complex is involved in initiating the formation of autophagosomes, inhibition of ULK1 would be expected to lead to impaired autophagy at an early stage. In Mtm1gt/y muscle, the increased abundance of both LC3-I and -II levels is not consistent with a block in autophagy induction. Importantly, similar increases in both LC3-I and -II were also reported in mice with liver-specific knockout of TSC1 (tuberous sclerosis complex 1) (53), an upstream inhibitor of mTORC1 activation. Thus, it is possible that chronic activation of mTORC1 has two effects on autophagy. First, direct phosphorylation of ULK1 would suppress initiation and lead to the buildup of LC3-I. Second, mTORC1-regulated inhibition of transcription factor EB (54, 55), a master regulator of lysosome biogenesis, would lead to decreased synthesis of lysosomal hydrolases and a reduced number of lysosomes, resulting in a failure to degrade autophagic cargo and any existing LC3-II. Although the precise mechanism by which MTM1 regulates autophagy needs further investigation, our study suggests that loss of Mtm1 leads to inefficient autophagic degradation and that suppression of mTORC1 restores autophagic flux, as indicated by the conversion of LC3-I to LC3-II and decreased p62 and Lamp2 levels following treatment with AZD8055. Importantly, we show that AZD8055 treatment has positive effects on muscle mass and reduces the abnormal accumulation of the IF protein desmin, suggesting that impaired autophagy and aberrant mTORC1 pathway activity contribute to the disease phenotypes.
The observation that Mtm1 impacts mTORC1 under starvation conditions is intriguing, as it suggests that this phosphatase might play a central role in coordinating nutrient-induced signals to mTOR and the regulation of autophagy. Previous work has shown that amino acid withdrawal or starvation rapidly inhibits mTORC1 and activates autophagy (15). In the present study, overnight fasting was sufficient to robustly activate autophagy in mouse skeletal muscle (43) but this response was significantly blunted in Mtm1gt/y mice. A question that remains is: how might MTM1 regulate mTORC1 activation? Recent reports suggest that activation of mTOR in response to amino acids requires redistribution of mTOR to lysosomes (56), where it encounters its upstream activator Rheb (56, 57). When mTORC1 is artificially targeted to the lysosome, mTOR pathway activity is protected from amino acid starvation (57). Since MTM1 regulates endosomal membrane trafficking (58, 59), further work is needed to determine whether MTM1 negatively impacts the ability of nutrients to drive mTOR distribution toward lysosomes.
In conclusion, our data provide evidence that MTM1 is involved in the regulation of mTORC1 and autophagy and that loss of MTM1 leads to the accumulation of ubiquitinated proteins and defective mitochondria. Autophagy inhibition is thought to play a role in many myopathies with inclusions or abnormal mitochondria (1, 60, 61), and the present observations indicate that correction of defects in autophagy may improve muscle defects associated with XLMTM.
ACKNOWLEDGMENTS
We thank our colleagues at NIBR, particularly David J. Glass, Chikwendu Ibebunjo, Dmitri Wiederschain, Lloyd Klickstein, Ronenn Roubenoff, and Beat Nyfeler, for their help and advice with this study and preparation of the manuscript. We also thank members of the NIBR Transgenic Group for support with animal breeding and care.
Footnotes
Published ahead of print 29 October 2012
REFERENCES
- 1. Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132: 27– 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Mishima K, Saito I, Okano H, Mizushima N. 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885– 889 [DOI] [PubMed] [Google Scholar]
- 3. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441: 880– 884 [DOI] [PubMed] [Google Scholar]
- 4. Komatsu M, Waguri S, Koike M, Sou Y, Ueno T, Hara T, Mizushima N, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. 2007. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131: 1149– 1163 [DOI] [PubMed] [Google Scholar]
- 5. Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. 2007. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 13: 619– 624 [DOI] [PubMed] [Google Scholar]
- 6. Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. 2008. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 8: 325– 332 [DOI] [PubMed] [Google Scholar]
- 7. Jung HS, Chung KW, Won KJ, Kim J, Komatsu M, Tanaka K, Nguyen YH, Yoon K-H, Kim J-W, Jeong YT, Han MS, Lee M-K, Kim K-W, Lee M-S. 2008. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 8: 318– 324 [DOI] [PubMed] [Google Scholar]
- 8. Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, Ralston E. 2008. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum. Mol. Genet. 17: 3897– 3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Schiaffino S, Sandri M. 2009. Autophagy is required to maintain muscle mass. Cell Metab. 10: 507– 515 [DOI] [PubMed] [Google Scholar]
- 10. Simonsen A, Tooze SA. 2009. Coordination of membrane events during autophagy by multiple class III PI3-kinase complexes. J. Cell Biol. 186: 773– 782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. 2010. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 90: 1383– 1435 [DOI] [PubMed] [Google Scholar]
- 12. Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S-I, Takehana K, Yamada N, Guan J-L, Oshiro N, Mizushima N. 2009. Nutrient-dependent mTORCl association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 20: 1981– 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, Kundu M, Kim D-H. 2009. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 20: 1992– 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganley IG, Lam H, Wang J, Ding X, Chen S, Jiang X. 2009. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 284: 12297– 12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wullschleger S, Loewith R, Hall MN. 2006. TOR signaling in growth and metabolism. Cell 124: 471– 484 [DOI] [PubMed] [Google Scholar]
- 16. Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. 2002. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177– 189 [DOI] [PubMed] [Google Scholar]
- 17. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163– 175 [DOI] [PubMed] [Google Scholar]
- 18. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14: 1296– 1302 [DOI] [PubMed] [Google Scholar]
- 19. Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. 2008. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182: 685– 701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, Noda T, Yoshimori T. 2010. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J. Cell Biol. 190: 511– 521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Itakura E, Mizushima N. 2010. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 6: 764– 776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obara K, Sekito T, Niimi K, Ohsumi Y. 2008. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem. 283: 23972– 23980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Proikas-Cezanne T, Waddell S, Gaugel A, Frickey T, Lupas A, Nordheim A. 2004. WIPI-1alpha (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene 23: 9314– 9325 [DOI] [PubMed] [Google Scholar]
- 24. Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. 1997. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 243: 240– 246 [DOI] [PubMed] [Google Scholar]
- 25. Robinson FL, Dixon JE. 2006. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 16: 403– 412 [DOI] [PubMed] [Google Scholar]
- 26. Jungbluth H, Wallgren-Pettersson C, Laporte J. 2008. Centronuclear (myotubular) myopathy. Orphanet J. Rare Dis. 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallgren-Pettersson C, Clarke A, Samson F, Fardeau M, Dubowitz V, Moser H, Grimm T, Barohn RJ, Barth PG. 1995. The myotubular myopathies: differential diagnosis of the X linked recessive, autosomal dominant, and autosomal recessive forms and present state of DNA studies. J. Med. Genet. 32: 673– 679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pierson CR, Agrawal PB, Blasko J, Beggs AH. 2007. Myofiber size correlates with MTM1 mutation type and outcome in X-linked myotubular myopathy. Neuromuscul. Disord. 17: 562– 568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laporte J, Biancalana V, Tanner SM, Kress W, Schneider V, Herger F, Buj-Bello A, Blondeau F, Liechti G, Mandel J-L. 2000. MTM1 mutations in X-linked myotubular myopathy. Hum. Mutat. 15: 393– 409 [DOI] [PubMed] [Google Scholar]
- 30. Taylor GS, Maehama T, Dixon JE. 2000. Inaugural article: myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc. Natl. Acad. Sci. U. S. A. 97: 8910– 8915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wymann MP, Marone R. 2005. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr. Opin. Cell Biol. 17: 141– 149 [DOI] [PubMed] [Google Scholar]
- 32. Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas C, Laporte J, Deretic V. 2009. Control of autophagy initiation by phosphoinositide 3-phosphatase jumpy. EMBO J. 28: 2244– 2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taguchi-Atarashi N, Hamasaki M, Matsunaga K, Omori H, Ktistakis NT, Noda T. 2010. Modulation of local Ptdins3P levels by the PI phosphatase MTMR3 regulates constitutive autophagy. Traffic 11: 468– 478 [DOI] [PubMed] [Google Scholar]
- 34. Dowling JJ, Low SE, Busta AS, Feldman EL. 2010. Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy. Hum. Mol. Genet. 19: 2668– 2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buj-Bello A, Fougerousse F, Schwab Y, Messaddeq N, Spehner D, Pierson CR, Durand M, Kretz C, Danos O, Douar AM, Beggs AH, Schultz P, Montus M, Denefle P, Mandel JL. 2008. AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis. Hum. Mol. Genet. 17: 2132– 2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanji K, Bonilla E. 2008. Light microscopic methods to visualize mitochondria on tissue sections. Methods 46: 274– 280 [DOI] [PubMed] [Google Scholar]
- 37. Buj-Bello A, Laugel V, Messaddeq N, Zahreddine H, Laporte J, Pellissier J-F, Mandel J-L. 2002. The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc. Natl. Acad. Sci. U. S. A. 99: 15060– 15065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hnia K, Tronchere H, Tomczak KK, Amoasii L, Schultz P, Beggs AH, Payrastre B, Mandel JL, Laporte J. 2011. Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J. Clin. Invest. 121: 70– 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paulin D, Li Z. 2004. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res. 301: 1– 7 [DOI] [PubMed] [Google Scholar]
- 40. Kirkin V, Lamark T, Sou YS, Bjorkoy G, Nunn JL, Bruun JA, Shvets E, McEwan DG, Clausen TH, Wild P, Bilusic I, Theurillat JP, Overvatn A, Ishii T, Elazar Z, Komatsu M, Dikic I, Johansen T. 2009. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33: 505– 516 [DOI] [PubMed] [Google Scholar]
- 41. Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. 2007. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282: 24131– 24145 [DOI] [PubMed] [Google Scholar]
- 42. Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. 2010. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 12: 213– 223 [DOI] [PubMed] [Google Scholar]
- 43. Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. 2004. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15: 1101– 1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mizushima N, Levine B. 2010. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 12: 823– 830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim J, Kundu M, Viollet B, Guan KL. 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol.. 13: 132– 141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benjamin D, Colombi M, Moroni C, Hall MN. 2011. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 10: 868– 880 [DOI] [PubMed] [Google Scholar]
- 47. Nyfeler B, Bergman P, Triantafellow E, Wilson CJ, Zhu Y, Radetich B, Finan PM, Klionsky DJ, Murphy LO. 2011. Relieving autophagy and 4EBP1 from rapamycin resistance. Mol. Cell. Biol. 31: 2867– 2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. 2009. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284: 8023– 8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R, Jacobsen M, Smith GCM, Guichard S, Pass M. 2010. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 70: 288– 298 [DOI] [PubMed] [Google Scholar]
- 50. Wallace DC. 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39: 359– 407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. 2010. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280– 289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del PP, Foretz M, Scorrano L, Rudolf R, Sandri M. 2010. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 29: 1774– 1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, Bronson RT, Kwiatkowski DJ, Manning BD. 2012. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci. Signal. 5: ra24 doi:10.1126/scisignal.2002739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martina JA, Chen Y, Gucek M, Puertollano R. 2012. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 8:903– 914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Settembre C, Di MC, Polito VA, Garcia AM, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. 2011. TFEB links autophagy to lysosomal biogenesis. Science 332: 1429– 1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. 2008. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496– 1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. 2010. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290– 303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cao C, Backer JM, Laporte J, Bedrick EJ, Wandinger-Ness A. 2008. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol. Biol. Cell 19: 3334– 3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Velichkova M, Juan J, Kadandale P, Jean S, Ribeiro I, Raman V, Stefan C, Kiger AA. 2010. Drosophila Mtm and class II PI3K coregulate a PI(3)P pool with cortical and endolysosomal functions. J. Cell Biol. 190: 407– 425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, Rabinovitch PS, Kaeberlein M, Kennedy BK. 2012. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 4: 144ra103 doi:10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, Blaauw B, Urciuolo A, Tiepolo T, Merlini L, Maraldi NM, Bernardi P, Sandri M, Bonaldo P. 2010. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 16:1313– 1320 [DOI] [PubMed] [Google Scholar]