Abstract
Wnt-mediated signal transduction pathways have long been recognized for their roles in regulating embryonic development, and have more recently been linked to cancer, neurologic diseases, inflammatory diseases, and disorders of endocrine function and bone metabolism in adults. Although therapies targeting Wnt signaling are attractive in theory, in practice it has been difficult to obtain specific therapeutics because many components of Wnt signaling pathways are also involved in other cellular processes, thereby reducing the specificity of candidate therapeutics. New technologies, and advances in understanding the mechanisms of Wnt signaling, have improved our understanding of the nuances of Wnt signaling and are leading to promising new strategies to target Wnt signaling pathways.
Wnt-mediated signaling is linked to disease processes (e.g., cancer). To fully realize the therapeutic potential of Wnt manipulation, more must be understood about its complex regulation.
As covered by other Wnt signaling investigators, the Wnt family of secreted glycoproteins act as ligands to activate multiple signal transduction pathways. The best understood is the Wnt/β-catenin pathway, which activates the function of β-catenin in the nucleus to regulate expression of genes by binding to T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, in addition to its role in regulating cadherin-dependent cell adhesion at the plasma membrane. The Wnt/β-catenin pathway acts in a context-dependent manner to regulate cell proliferation, migration, differentiation, and survival in both embryonic development and in adults.
Perturbations in the levels of Wnt/β-catenin signaling are linked to many disease processes. Elevation of Wnt/β-catenin signaling has been linked to cancer, whereas conversely attenuation of Wnt/β-catenin signaling has been linked to a distinct set of diseases including Alzheimer’s disease, familial exudative vitreoretinopathy (FEVR), and disorders of bone formation (Robitaille et al. 2002; reviewed in De Ferrari and Moon 2006; Hoeppner et al. 2009).
Reports have even shown that Wnt/β-catenin signaling can regulate aspects of human immunodeficiency virus (HIV) including gene expression and replication, further highlighting the ubiquitous function of this pathway in almost all cell types (Wortman et al. 2002; Carroll-Anzinger et al. 2007; Kumar et al. 2008; Kameoka et al. 2009). As the diverse roles for Wnt/β-catenin signaling in tissue homeostasis and disease continue to be elucidated, interest in therapeutic targeting of this pathway has expanded enormously.
In addition to the Wnt/β-catenin pathway, β-catenin-independent pathways such as the planar cell polarity (PCP) pathway and Wnt/ Ca2+ pathway have been described. These pathways are less well understood in part owing to the paucity of established reporter assays. In addition, β-catenin-independent Wnt signaling often inhibits the Wnt/β-catenin pathway, making the relative contribution of pathway activation versus inhibition to an observed phenotype difficult to distinguish. Studies in a variety of organisms have established that β-catenin-independent Wnt signaling is involved in regulating cell polarity during gastrulation in embryos, and in the polarized orientation of hair cells in the inner ear, as well as mesenchymal stem cell maintenance and renal development (reviewed in Sugimura and Li 2010). The β-catenin-independent Wnt pathway(s) are also linked to disease processes, notably cancer.
From a therapeutic point of view, the Wnt signaling pathways present several challenges to the development of a targeted drug, so it is not surprising that drug strategies specifically directed at this pathway are in a state of relative infancy. In addition to the existence of 19 Wnt ligands and 10 FZD receptor isoforms, specificity of targeting is further complicated by the convergence of downstream Wnt signaling events on promiscuous enzymes like glycogen synthase kinase 3 (GSK3) and on proteins that are central to fundamental and ubiquitous cellular structures such as the cytoskeleton and cell–cell junctions that are critical to all cells. Predictability of drug effects can be problematic with a pathway as ubiquitous as Wnt, particularly because it is quite likely that the majority of both somatic cells and stem cell niches in the body will show some effect with either Wnt activation or inhibition. Nevertheless, many studies in manipulation of Wnt signaling pathways have shown promise that we will examine in more detail in this review.
WNT/β-CATENIN SIGNALING IN CANCER
The Wnt/β-catenin pathway has been associated with cancer ever since the gene int-1 was identified as a mammary oncogene in mice (Nusse and Varmus 1982; Rijsewijk 1987). This relationship was solidified with the discovery that the adenomatous polyposis coli (APC) gene associated with familial adenomatous polyposis (FAP) is inactivated in ∼85% of colorectal carcinomas leading to constitutive nuclear translocation of β-catenin (Kinzler et al. 1991; Nishisho et al. 1991; Su et al. 1993). As a result, the therapeutic targeting of Wnt/β-catenin signaling has received considerable interest in the context of cancer.
Dysregulated Wnt/β-catenin signaling occurs in many solid tumors and hematologic malignancies even in the absence of documented mutations. Frequently, altered levels of expression of Wnt/β-catenin pathway regulators have been observed without mutations in the coding regions of the respective genes. For example, epigenetic silencing of genes that encode putative extracellular Wnt antagonists such as the secreted frizzled-related proteins (SFRPs) has been described in colon, breast, prostate, lung, and other cancers (Caldwell et al. 2004; Lee et al. 2004a; Suzuki et al. 2004; Fukui et al. 2005; Zou et al. 2005). Increased expression of other Wnt/β-catenin pathway members such as Wnt ligands (Rhee et al. 2002; Wong et al. 2002; Milovanovic et al. 2004) or dishevelled (DVL) have also been described (Okino et al. 2003; Uematsu et al. 2003a,b).
CONTEXT-DEPENDENT CORRELATIONS BETWEEN β-CATENIN AND PATIENT SURVIVAL
The role of Wnt/β-catenin activation via APC mutations in colorectal cancer progression as well as the initial discovery of int-1 in a mammary tumor screen has led to the dogmatic view that this pathway is oncogenic. Indeed, studies correlating increased Wnt/β-catenin signaling in tumors to worse prognosis for patients with colorectal cancer have helped solidify this viewpoint (Cheah et al. 2002; Miyamoto et al. 2004; Lugli et al. 2007). However, it is important to emphasize that elevated Wnt signaling does not correlate with reduced patient survival in all types of cancer. For example, in melanoma, active Wnt/β-catenin signaling as shown by nuclear β-catenin in tumors is associated with a lower proliferative index and correlates with a more favorable prognosis in melanoma patients (Kageshita et al. 2001; Maelandsmo et al. 2003; Bachmann et al. 2005; Chien et al. 2009b). Consistent with this observation, overexpression of Wnt3a in a murine melanoma model results in a less aggressive disease phenotype as well as an increase in melanocyte differentiation markers, which are often lost during melanoma progression (Chien et al. 2009b). Mechanistically, the effects of elevated β-catenin in melanoma differ from colorectal cancer because in the former the β-catenin promotes expression of the transcription factor MITF, which drives differentiation toward a melanocytelike cell fate and reduces cell movements (Dorsky et al. 2000; Takeda et al. 2000; Hornyak et al. 2001).
In some contexts, active Wnt signaling is correlated with less aggressive disease. Recently, discrete molecular subtypes of medulloblastoma were identified that included a subgroup characterized by constitutive Wnt/β-catenin pathway activation. The tumors in this subgroup universally harbor activating mutations in β-catenin (CTNNB1), and appear to derive from a distinct anatomic region within the brain. These Wnt/β-catenin-driven tumors are associated with a much better prognosis compared with those without active Wnt/β-catenin signaling (Gibson et al. 2010). Similarly, Wnt activation appears to function as a tumor suppressor in some sarcomas, as inhibition of Wnt in human mesenchymal stem cells leads to high-grade sarcoma formation in nude mice (Matushansky et al. 2007). In mouse models of pancreatic ductal adenocarcinoma (PDAC), the forced expression of a nondegradable constitutively active β-catenin can arrest Kras-driven transformation, resulting in a different tumor type that is much more benign compared with PDAC (Heiser et al. 2008; Morris et al. 2010). Other studies in prostate, ovarian, and even in colorectal cancer have also found that increased Wnt signaling in patient tumors is associated with a better prognosis in at least some stages of disease (Gamallo et al. 1999; Horvath et al. 2005; Elzagheid et al. 2008).
These findings suggest that the effects of Wnt/β-catenin signaling differ widely depending on the stage of disease progression, cell of origin, or context in which the signaling occurs. In our current model, dynamic regulation of Wnt signaling is tightly regulated in cells, resulting in an ongoing modulation of cellular Wnt activation levels by feedback mechanisms, which involve other diverse signaling pathways. The identification of putative Wnt inhibitors including AXIN2, DKK1, and TCF7L2 as direct transcriptional targets of β-catenin signaling supports the model of a tightly regulated pathway. To develop and successfully implement Wnt-directed therapies, several contextual considerations are essential. For one, understanding the mechanism of Wnt dysregulation, whether it be through ligand overexpression or mutational activation, will be central to developing appropriate strategies for targeting the pathway in different diseases. Second, the idea that Wnt activation may be an important part of maintaining homeostasis in normal, noncancerous cells must be considered in the bigger picture. Finally, the effects of targeting this pathway will undoubtedly vary across different cell types, which have broad implications for both predicting the efficacy of treatment as well as for predicting potential side effects in the context of an entire organism.
NONCANONICAL β-CATENIN-INDEPENDENT WNT SIGNALING IN CANCER
Although most studies have focused on the well-described Wnt/β-catenin pathway, Wnts can also activate pathways that act independently of β-catenin. These pathways, often referred to as “noncanonical” Wnt pathways, are currently known collectively as β-catenin-independent Wnt signaling. In both developmental models and in cultured mammalian cells, β-catenin-independent Wnt signaling is a key modulator of cellular adhesion and motility, and several studies have shown the involvement of this pathway in tumor cell migration and metastasis. Perhaps the best-studied activator of β-catenin-independent Wnt signaling is WNT5A, which has been shown to act both as a tumor suppressor as well as a promoter of tumor invasion depending on the model (Weeraratna et al. 2002; Kurayoshi et al. 2006; Pukrop 2006). In melanoma, WNT5A mediates a polarized redistribution of proteins such as myosin IIB to facilitate directional movement along a chemokine gradient (Witze et al. 2008). Other components of β-catenin-independent Wnt signaling such as VANGL1 and VANGL2 have also been implicated in tumor cell migration. These genes are mammalian homologs of the Drosophila PCP gene van gogh/strabismus and represent putative tetra-membrane-spanning proteins widely expressed in human cancers (reviewed in Katoh 2002). VANGL2 has recently been shown to form a Wnt-induced receptor complex with the receptor ROR2, which acts to sense Wnt dosage gradients in developing limb buds (Shafer et al. 2011). VANGL2 expression also appears to promote tumor cell migration and matrix metalloproteinase-dependent invasion in a human colon cancer line (Cantrell and Jessen 2010). VANGL1 also promotes metastases in murine models of colorectal and squamous cell carcinoma (Lee et al. 2004b, 2009; Kho et al. 2009). Interestingly, other roles for β-catenin-independent Wnt signaling besides migration are emerging. For example, WNT5A was found to potentiate androgen-mediated signaling through the androgen receptor in prostate cancer cells, thereby promoting tumor formation in a murine prostate cancer model (Takahashi et al. 2011).
Currently, the development of drugs targeting β-catenin-independent Wnt signaling has been limited by several key factors. First, assays for measuring activation of β-catenin-independent Wnt signaling are diverse, likely context dependent, and technically involved, thereby limiting the ability to perform large-scale exploratory studies such as high-throughput signaling. Second, our understanding of the mechanisms involved in β-catenin-independent Wnt signaling remains limited, even with regard to basic events such as the identification of receptors involved in ligand binding. Third, β-catenin-independent Wnt signaling can antagonize Wnt/β-catenin signaling, so even specific targeting of ligands such as Wnt5A could conceivably have implications for the broad effects of Wnt/β-catenin signaling in both cells and organisms. Nevertheless, there have been some efforts to target this pathway (Figs. 1 and 2; Tables 1 and 2) that have shown promise in early studies.
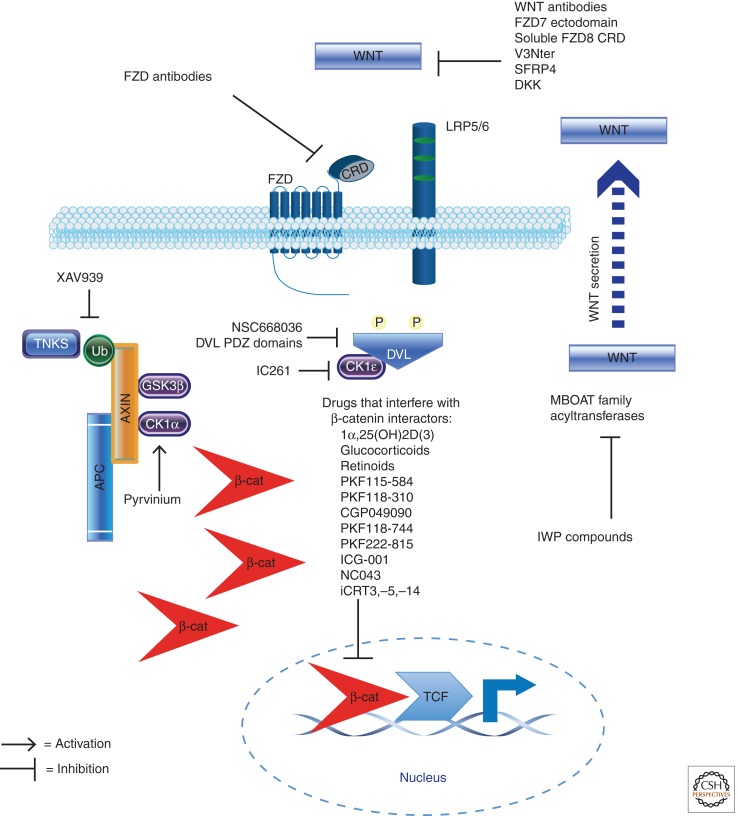
Figure 1.
Pharmacologic inhibitors of the Wnt/β-catenin signaling pathway and known interactions with pathway components (see Table 1 for references). This figure depicts the Wnt/β-catenin pathway in its active state, with cytoplasmic accumulation and nuclear translocation of β-catenin, along with the transactivation of target genes in the nucleus mediated by interaction between β-catenin and members of the TCF family of transcription factors. Note that in contexts in which downstream mutations in APC or β-catenin result in constitutive pathway activation, the use of upstream inhibitors that target ligand secretion, ligand binding, and even the APC-AXIN-GSK3β complex may not be sufficient to inhibit signaling. Also, targeting of β-catenin interactors can involve either the disruption of protein interactions or the direct inhibition of transcriptional activity (see also Table 1 for additional details and references).
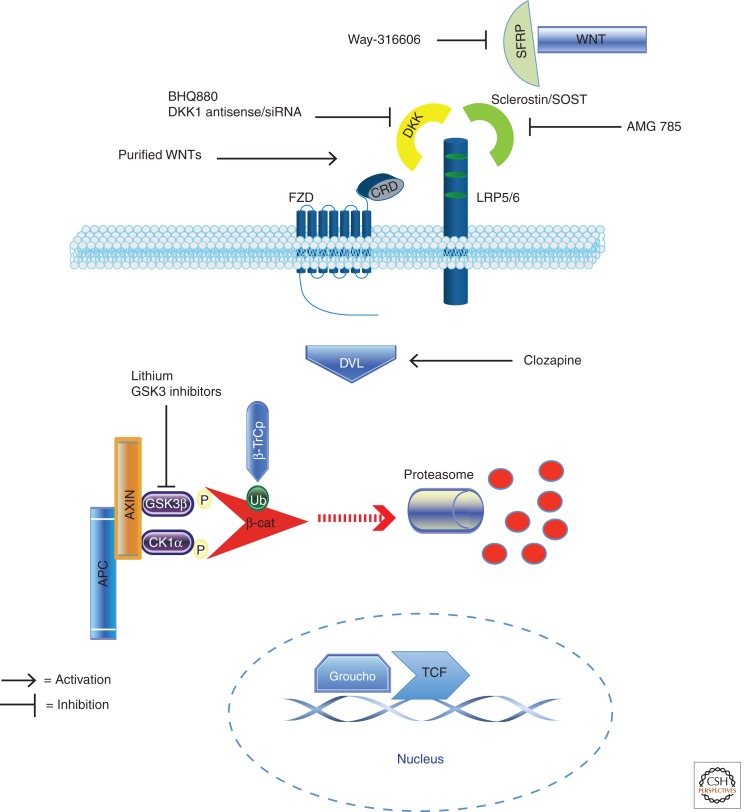
Figure 2.
Pharamcologic activators and enhancers of the Wnt/β-catenin signaling pathway and known interactions with pathway components (see Table 2 for references). This figure depicts the pathway in the inactive state, in which constitutive phosphorylation of β-catenin by GSK3β targets the protein for ubiquitylation and subsequent proteasomal degradation. In the absence of nuclear β-catenin, TCF-family transcription factors are bound by members of the Groucho family of transcriptional repressors, thus inhibiting target gene expression.
Table 1.
Drug candidates that inhibit Wnt signaling and diseases/disease models in which they have been studied
| Drug candidate | Target | Diseases or disease model(s) | References |
|---|---|---|---|
| Biologics | |||
| Wnt1 antibody | Wnt1 | NSCLC, head and neck cancer, sarcoma, colon cancer, breast cancer | He et al. 2004, 2005a,b; Mikami et al. 2005; Wei et al. 2009 |
| Wnt2 antibody | Wnt2 | Melanoma, mesothelioma, nonsmall cell lung cancer | You et al. 2004a,b; Mazieres et al. 2005b |
| Wnt5a antibody | Wnt5a | Rheumatoid arthritis | Sen et al. 2001 |
| Wnt16 antibody | Wnt16 | Acute lymphoblastic leukemia | Mazieres et al. 2005a |
| Fzd5 antibody | Fzd5 | Melanoma, rheumatoid arthritis | Sen et al. 2001; Weeraratna et al. 2002 |
| Soluble Fzd7 | Fzd7 | Hepatocellular carcinoma | Wei et al. 2011 |
| Fzd10 antibody (conjugated with Y90) | Fzd10 | Synovial sarcoma | Fukukawa et al. 2008 |
| Fzd7 ectodomain | Fzd7 | Colon cancer | Vincan et al. 2005 |
| Soluble Fzd8 CRD | Wnt binding | Teratocarcinoma | DeAlmeida et al. 2007 |
| V3Nter | Wnt binding | Colon cancer | Lavergne et al. 2011 |
| sFRP4 | Wnt binding | Renal injury | Surendran et al. 2005 |
| Small molecules | |||
| Glucocorticoids | β-catenin | Osteosarcoma | Takayama et al. 2006 |
| Retinoids | β-catenin | Colon cancer | Xiao et al. 2003 |
| Vitamin D(3) | β-catenin | Colon cancer | Pálmer et al. 2001 |
| IWP compounds | MBOAT family of acyltransferases | Inhibition of Wnt-dependent colon/prostate cancer proliferation | Chen et al. 2009 |
| IWR compounds | Axin stability | Inhibition of Wnt-dependent colon/prostate cancer proliferation | Chen et al. 2009 |
| XAV939 | TNKS1, TNKS2 | Colon cancer, cardimyogenesis | Huang et al. 2009; Wang et al. 2011a |
| PKF115-584 | TCF4/β-catenin | Colon cancer proliferation | Lepourcelet et al. 2004 |
| PKF118-310 | |||
| CGP049090 | |||
| PKF118-744 | |||
| PKF222-815 | |||
| ICG-001 | CBP | Pulmonary fibrosis, colon cancer |
Emami et al. 2004; Henderson et al. 2010 |
| NC043 | TCF4/β-catenin | Colon cancer | Wang et al. 2011b |
| iCRT3,-5,-14 | TCF/β-catenin | Breast cancer, colon cancer | Gonsalves et al. 2011 |
| IC261 | CKIε | Breast cancer | Kim et al. 2010 |
| Fumagillin, TNP-470 | MetAP-2 | Angiogenesis | Zhang et al. 2006; Cirone et al. 2008 |
| Phenylmethimazole | Wnt5a expression | Melanoma | Schwartz et al. 2009 |
| Pyrvinium | CK1α | Cardiac repair | Saraswati et al. 2010; Thorne et al. 2010 |
NSCLC, non-small-cell lung cancer; CRD, cysteine-rich domain; CBP, CREB-binding protein; CKIε, casein kinase-Iε.
Table 2.
Drug candidates that enhance Wnt signaling and diseases/disease models in which they have been studied
| Drug candidate | Target or class | Diseases or disease model(s) | References |
|---|---|---|---|
| Biologics | |||
| Purified Wnts | LPR5/6/ Fzd | Fracture repair, ES/iPS cell differentiation | Reya et al. 2003; Willert et al. 2003; Nostro et al. 2008; Lengerke et al. 2009; Vijayaragavan et al. 2009; Minear et al. 2010 |
| DKK1 antibody (BHQ880) | DKK1 | Osteoporosis, osteoarthritis, fracture healing, multiple myeloma | Wang et al. 2008; Fulciniti et al. 2009; Glantschnig et al. 2010; Komatsu et al. 2010 |
| DKK1 antisense oligonucleotides | DKK1 | Brain injury, osteoperosis | Cappuccio et al. 2005; Wang et al. 2007 |
| DKK1 siRNA | DKK1 | Alzheimer’s disease, brain injury | Caricasole et al. 2004; Cappuccio et al. 2005 |
| Sclerostin/SOST antibodies | Sclerostin | Osteoporosis, fracture healing | Li et al. 2009; Agholme et al. 2010; Ominsky et al. 2010; Padhi et al. 2011 |
| Foxy-5 | Wnt5a mimetic | Breast cancer | Säfholm et al. 2006; Ford et al. 2009 |
| Small molecules | |||
| Lithium | GSK3β | Bone formation, ES differentiation, Alzheimer’s disease | de Boer et al. 2004; Zamani et al. 2009; Toledo and Inestrosa 2010 |
| GSK3β inhibitors | GSK3β | Pancreatic transplant, bone formation, schizophrenia, multiple myeloma | Kulkarni et al. 2006; Roh et al. 2007; Freyberg et al. 2010; Graham et al. 2010; Gunn et al. 2010 |
| WAY-316606 | sFRP1 | Bone formation | Moore et al. 2009 |
| Iisoquercitrin and isorhamnetin | Unknown | Adipocyte inhibition | Lee et al. 2011 |
| Riluzole | GRM1 | Melanoma, bipolar disorder | Yip et al. 2009; Biechele et al. 2010; Le et al. 2010 |
ES, embryonic stem; iPS, induced pluripotent stem.
WNT PATHWAYS IN NEUROLOGIC DISEASE
Wnt-mediated pathways have a well-established role of the development of the central nervous system in embryonic development (reviewed in Salinas and Zou 2008). Therefore, it is not surprising that aberrations in Wnt signaling are common in neurologic diseases in the adult. One such disease that has received considerable attention is schizophrenia, in which genetic perturbations predicted to impact Wnt regulation have been recently identified. For example, a Scottish family with a high incidence of schizophrenia, depression, and bipolar disorder was found to carry a balanced chromosomal translocation involving disrupted in schizophrenia 1 (DISC1), encoding a protein subsequently found to have an important role in brain development and neural progenitor proliferation (Blackwood et al. 2001; Mao et al. 2009). DISC1 directly interacts with and inhibits GSK3β activity, resulting in enhanced β-catenin-mediated signaling (Mao et al. 2009). WNT1 was also observed at higher levels in the hippocampal region of brains of schizophrenic patients compared with controls on postmortem analysis (Miyaoka et al. 1999). Genetic linkage studies have also shown an association with schizophrenia and the Wnt receptor FZD3 in a subset of schizophrenic Chinese patients (Yang et al. 2003a).
Other neurologic diseases have been linked to aberrant Wnt signaling as well. For example, the presenilin proteins, which have been associated with early-onset Alzheimer’s disease, are negative regulators of canonical Wnt signaling (Kang et al. 2002). Additionally, variant alleles in the Wnt receptor LPR6 have been associated with Alzheimer’s disease in population-based linkage analyses. Functional analysis of one of these common alleles revealed decreased β-catenin signaling when this variant was expressed in HEK293T cells (De Ferrari et al. 2007). Wnt may also have a role in the metabolism of the amyloid precursor protein (APP) and inhibit phosphorylation of the tau protein, two events thought to be critical in the pathophysiology of the disease (Mudher et al. 2001). Wnt signaling may also participate in neuroprotection after brain injury. Enhanced Wnt/β-catenin was shown in newly derived glial progenitors and astrocytes in a model of traumatic brain injury (White et al. 2010). These observations establish the potential rationale for exploring the use of Wnt-directed therapeutics in these disease conditions.
WNT SIGNALING IN AUTOIMMUNE DISEASE AND INFLAMMATION
Wnt signaling has long been implicated in development of the immune system (reviewed in Yu et al. 2010) but the effects of Wnt signaling in adult immunity are only beginning to be appreciated. Studies of adult T cells have shown that the Wnt effector TCF1 appears to be required for the functional generation of memory CD8+ T cells (Gattinoni et al. 2009; Jeannet et al. 2010; Zhao et al. 2010; Zhou et al. 2010). Wnt signaling can also affect the differentiation of naïve CD4+ T lymphocytes. Blockade of Wnt by small interfering RNA (siRNA)-mediated knockdown of β-catenin or by treatment of CD4+ T cells with DKK1 inhibited the expression of Th2 cytokines (Notani et al. 2010). Wnt/β-catenin has also been shown to affect immunosuppressive T regulatory (Treg) cells that have been implicated in the pathogenesis of autoimmune disease, cancer, and infectious diseases (Ding et al. 2008). Enhanced Wnt signaling in gut-associated dendritic cells also led to immune suppression and tolerance in a model of inflammatory bowel disease, the effects of which were partially mediated through the induction of Tregs (Manicassamy et al. 2010).
Aberrant Wnt signaling has also been observed in autoimmune disorders. In rheumatoid arthritis (RA) patients, Wnt and FZD gene expression is higher in synovial membranes of affected joints compared with controls without RA (Sen et al. 2002). Increased expression of WNT7B has also been shown in RA patients and exogenous expression of WNT7B in human synovial cells induced inflammatory cytokines such as interleukin 1β, interleukin-6, and TNFα (Nakamura et al. 2005). β-catenin-independent Wnt signaling has also been implicated in the development of RA. WNT5A expression was noted to be higher in cultured synoviocytes from patients with RA compared with controls (Sen et al. 2000). The autoimmune disease systemic lupus erythematosus (SLE) has been associated with aberrant Wnt gene expression as well (Sheng et al. 2011). In a murine model of SLE, increased canonical Wnt pathway activity was shown in kidneys affected by lupus nephritis, suggesting a role for Wnt activity in mediating end organ damage associated with the disease (Tveita and Rekvig 2011).
In summary, manipulation of Wnt signaling may provide a novel means of shaping the immune response in autoimmune disease, tumor immunotherapy, transplantation, and vaccine development. Once again, the in-depth involvement of Wnt signaling pathways in immunological function highlights both the power of these pathways as regulators as well as the potential difficulties involved in the directed targeting of Wnt signaling in disease.
WNT SIGNALING IN TISSUE HOMEOSTASIS AND REGENERATIVE MEDICINE
Given the importance of Wnt pathways in development and stem cell biology, it should not be surprising that manipulating Wnt signaling has proven useful in the burgeoning field of regenerative medicine. One of the central problems that must be addressed in applying stem cell biology to regenerative medicine is how to specifically control stem cell renewal and differentiation to direct stem cells toward specific fates. There are numerous studies implicating Wnts as important components of the stem cell signaling network that can be used to direct specific cell fates (Nusse 2008). For example, Wnt signaling has been implicated in the self-renewal and maintenance of hematopoietic stem cells (Reya et al. 2003; Willert et al. 2003; Wang and Nakayama 2009). Also, canonical Wnt signaling appears to be required for differentiation of embryonic stem (ES) cells toward hematopoietic stem cells and primitive hematopoietic progenitors (Lengerke et al. 2008; Nostro et al. 2008; Wang and Nakayama 2009). β-Catenin-independent Wnt signaling has also been implicated in driving mesoderm specification and exit from the pluripotent state from ES cells (Vijayaragavan et al. 2009).
Wnt is important for the development and maintenance of other tissue types as well. For example, the maintenance of intestinal crypt stem cells requires the Wnt pathway transcription factor TCF7L2/TCF4 (Korinek et al. 1998). Accordingly, Wnt3a is one of a host of factors that can be used to derive human intestinal cells from induced pluripotent stem (iPS) cells (Spence et al. 2011). Wnt/β-catenin signaling has been implicated in cardiomyogenesis from human ES cells in a biphasic manner, promoting cardiomyocyte differentiation when triggered at early stages of differentiation, while inhibiting cardiogenesis at later stages (Paige et al. 2010; Yamauchi et al. 2010).
One of the most active fields in Wnt biology and regenerative medicine is in the field of bone metabolism. The initial discovery that Wnt pathways were related to bone formation in the adult was made when mutations in the Wnt coreceptor LRP5 were linked to the autosomal recessive disorder osteoporosis-pseudoglioma syndrome (OPPG) (Gong et al. 2001). Among other features of the syndrome, individuals with OPPG suffered with low bone mass and a predisposition to skeletal fractures. Carriers of the LRP5 mutation also had lower bone mass compared with age-matched controls. A different mutation in LRP5, G171V has been associated with increased muscle mass and skeletal strength in humans (Boyden et al. 2002). An indirect role for Wnt signaling in osteoblast biology has been suggested by the observation that Lrp5 deficiency in the duodenum has an endocrine effect on osteoblasts via gut-derived serotonin binding the receptor hydroxytryptamine (Htr1b) expressed on osteoblasts (Yadav et al. 2008), but a more recent study refutes this view and indicates a direct role of LRP5 in osteoblasts (Cui et al. 2011). Several other studies have also shown an important direct role for Wnt/β-catenin signaling in osteoblasts at multiple stages, including promoting osteoblast differentiation from progenitors as well as promoting osteoblast and osteocyte survival in vitro (Day et al. 2005; Rodda and McMahon 2006). In addition, β-catenin was found to regulate osteoprotegerin (OPG/TNFRSF11B) production in mature osteoblasts. Because OPG is an inhibitor of osteoclast differentiation, Wnt/β-catenin signaling in osteoblasts appears to inhibit bone resorption as well (Glass et al. 2005; Holmen et al. 2005).
As new models for regeneration are developed, the role of Wnt/β-catenin signaling as a therapeutic target will be further clarified. Because regeneration is a long-term process, the question of how persistent therapeutic activation or inactivation of Wnt signaling will affect an organism becomes more relevant. However, it should be noted that in developmental models, including embryonic development, the effects of Wnt/β-catenin signaling can be elicited through rather short exposure to Wnt ligand. During embryonic development, Wnt signaling over short time periods may completely alter the specification of cell fate, suggesting that prolonged treatment courses with activators or inhibitors may not be needed (Bakre et al. 2007; Ueno et al. 2007; Trowbridge et al. 2010). In zebrafish models, pulsed exposure to Wnt signaling can result in measurable improvements in fin regeneration, further supporting the idea that long-term, continuous activation or inhibition of the pathway may not be absolutely required to achieve the desired end result (Stoick-Cooper et al. 2007).
DRUGS THAT INHIBIT WNT SIGNALING
Given the aberrant Wnt pathway activation in cancer, it is not surprising that inhibition of Wnt signaling has been an active area of investigation in both academia and the biotechnology sector. One past limitation in identifying clinically useful drugs has been the lack of an obvious enzyme target to inhibit in the pathway. Another has been the complexity of Wnt pathway regulation. At least 19 Wnt isoforms have been identified in humans (Chien et al. 2009a), all of which share significant sequence homology. The mechanisms that determine the specificity of a particular Wnt isoform remain unresolved, even with regard to whether isoforms exclusively activate Wnt/β-catenin signaling or β-catenin-independent Wnt signaling.
Existing Patient-Experienced Drugs with Wnt Inhibitory Activity
Despite the inherent difficulties in developing novel Wnt inhibitors, many existing FDA-approved drugs inhibit Wnt pathways (Table 1). Nonsteroidal anti-inflammatory drugs (NSAIDs) were initially found to inhibit polyp formation both in patients with FAP as well as in the corresponding murine model, APCmin (Labayle et al. 1991; Giardiello et al. 1993; Mahmoud et al. 1998; Yang et al. 2003b; Boon et al. 2004). In FAP patients, reduced levels of β-catenin were shown in polyps of patients treated with the NSAID sulindac (Boon et al. 2004). The selective COX2 inhibitor celecoxib has been FDA approved in patients with FAP as it has been shown to reduce polyp formation as well (Steinbach et al. 2000; Phillips et al. 2002). It has also been shown to reduce nuclear β-catenin in colorectal cancer cell lines similarly to other NSAIDs (Steinbach et al. 2000). The R-enatiomer, the NSAID etodolac, whose structure is a nonsuperimposable mirror image to the parent drug but itself does not possess COX inhibitory activity, was found to inhibit Wnt/β-catenin target genes in HEK293 cells and enhance the apoptosis of chronic lymphocytic leukemia (CLL) cells in vitro (Jensen et al. 2008).
Several nuclear receptors have also been found to act, at least partially, through effects on the Wnt/β-catenin pathway. Retinoid-mediated signaling through retinoic acid receptors can compete with several TCF factors for β-catenin binding, thus inhibiting Wnt/β-catenin target genes (Xiao et al. 2003). Vitamin D derivatives can also inhibit Wnt signaling in colorectal cancer. Studies in colorectal carcinoma cell lines have shown that analogs of the active form allows the vitamin D receptor to bind β-catenin and compete with TCF7L2 to reduce Wnt target gene expression in these cells (Pálmer et al. 2001). Additionally, in a human osteosarcoma line that is sensitive to glucocorticoid-induced cell cycle arrest, glucocorticoid-mediated down-regulation of cyclin D1 was found to occur via interactions between the glucocorticoid receptor and the TCF/β-catenin complex (Takayama et al. 2006).
Other classes of drugs have been identified that can inhibit Wnt signaling by screening libraries of FDA-approved drugs. For example, the anti-helminthic drug pyrvinium was identified as an agent that could potentiate the activity of CK1α, thus leading to enhanced β-catenin and coactivator Pygo degradation and diminished signaling (Thorne et al. 2010). Pyrvinium has shown promise as a promoter of tissue repair in a mouse model of myocardial infarction, in which the inhibition of Wnt/β-catenin signaling led to global improvements in cardiac remodeling (Saraswati et al. 2010).
In summary, although the clinical use of drugs for the primary purpose of inhibiting Wnt signaling is still in early development, there are numerous drugs or compounds in current usage that affect Wnt/β-catenin signaling. Whether the effects of these drugs on inhibiting Wnt/β-catenin may contribute to some of the efficacy or side effects seen with the current use of these drugs will likely become more apparent with improvements in our understanding of Wnt signaling across various tissues and diseases.
Approaches to Identifying Novel Inhibitors of Wnt Signaling
Because Wnt pathways are subjected to extensive regulation at various levels, high-throughput screening technologies have greatly facilitated the identification of both biologics and small molecule pathway regulators. One promising approach has been the use of cell-based reporter assays to screen siRNA or chemical libraries. These cell-based reporters provide a rapid, reliable, and quantitative means of interrogating Wnt/β-catenin signaling, and have been critical tools in the identification and subsequent characterization of every Wnt-modulating small molecule identified to date (Biechele and Moon 2008). Genome-wide siRNA and drug library screening approaches are powerful but have many difficulties such as uneven transfection efficiency, the knockdown efficacy of siRNA sequences, or off-target effects leading to false hits. Such screens also offer little in the way of mechanistic data. These complications have been mitigated somewhat by improvements in assay automation and rigorous validation. Another approach to minimize the false hit rate and provide insights into mechanism is to integrate data from different platforms. For example, an integrated analysis from a genome-wide siRNA screen of modulators of Wnt signaling with protein interaction networks derived from mass spectrometry of 23 bait proteins or derived from known protein interactions in the literature identified 38 novel regulators, several of which were involved in chromatin remodeling (Major et al. 2008). Another siRNA-based loss-of-function screen of β-catenin-mediated transcription was integrated with gene copy number analysis in human colon cancer cells and identified cyclin-dependent kinase 8 (CDK8) as a key regulator of Wnt target gene expression (Firestein et al. 2008).
Strategies for Inhibiting Proximal Wnt Signaling
One attractive approach for inhibiting Wnt signaling has been targeting either ligands or extracellular receptors that are readily accessible to antibodies or small molecules (Fig. 1; Table 1). This biologics approach may be particularly relevant for diseases that display aberrant Wnt signaling in the absence of known downstream pathway mutations. In these cases, dysregulation of Wnt signaling is instead attributed to the aberrant expression of Wnt ligands. For example, WNT1 is overexpressed in numerous human cancers and a WNT1-blocking antibody both inhibited proliferation and induced apoptosis in many cancer cell types (He et al. 2004, 2005a,b; Mikami et al. 2005; Wei et al. 2009).
The FZD or LRP coreceptors are also potential targets for therapeutic manipulation. For example, synovial sarcomas show significant overexpression of FZD10 compared with normal tissue. In a mouse synovial sarcoma xenograft model, FZD10 antibody was used to target delivery of the radioisotope Yttrium-90 to tumors resulting in inhibition of tumor growth (Fukukawa et al. 2008). Using the FZD ectodomain to competitively bind Wnt ligand is another strategy that has been used. Overexpression of the FZD7 ectodomain attenuated proliferation in a colon cancer cell line in vitro as well as a murine xenograft assay (Vincan et al., 2005). Additionally, a soluble Wnt receptor consisting of a FZD8 cysteine rich domain (CRD) fused to a human Fc domain showed activity against teratoma lines in vitro and in vivo (DeAlmeida et al. 2007).
Another approach to target proximal events in Wnt signal transduction is to use endogenous extracellular modulators of Wnt such as Dickkopf (DKK), Wnt inhibitory factor-1 (WIF1), or SFRPs. SFRPs contain a cysteine-rich domain (CRD) similar to the extracellular Wnt-binding domain of the frizzled receptors, and have been postulated to act as a decoy receptor by directly binding Wnt ligands. WIF1 also directly binds Wnt ligands, whereas DKK binds to and inhibits transduction via the LRP coreceptor. Dysregulation of these endogenous inhibitors is common in many tumor types, typically by promoter hypermethylation (Suzuki et al. 2004, 2008; Fukui et al. 2005; Zou et al. 2005). Although SFRP is lost early in the progression of colon cancer, restoration of SFRP in colon cancer can attenuate Wnt signaling even in the presence of downstream activating mutations (Suzuki et al. 2004). In another study, a peptide containing a frizzled CRD derived from a cell-surface collagen VIII termed V3Nter was able to act in an SFRP-like fashion and inhibit canonical Wnt signaling in colorectal carcinoma lines as well as inhibit tumor growth in a xenograft model (Lavergne et al. 2011).
In addition to the use of Wnt inhibitors as soluble proteins, forced cellular overexpression has also been tested as a strategy. Forced DKK3 expression in renal cell carcinoma lines resulted in the induction of apoptosis, although this appeared to be mediated by alterations in the noncanonical JUN kinase (JNK) pathway rather than β-catenin inhibition (Ueno et al. 2011). Viral vector-mediated overexpression of DKK3 inhibits prostate and breast cancer growth in mice (Edamura et al. 2007; Kawasaki et al. 2009). Although the use of endogenous Wnt inhibitors seems to be a reasonable strategy, the finding that proteins like SFRPs can potentially enhance Wnt signaling in some contexts, perhaps by acting as chaperones, suggests that the therapeutic use of these particular proteins may be complicated (Uren et al. 2000; Chien et al. 2009a; von Marschall and Fisher 2010). Furthermore, DKK family members can themselves transduce receptor-mediated cellular signals that are not completely understood at this time (Wu et al. 2000; Mukhopadhyay et al. 2006; Yamamoto et al. 2008).
Another approach to inhibit upstream events in Wnt signaling is disruption of the interaction between the receptor FZD and DVL. For example, a structure-based virtual ligand screening identified a small molecule that is capable of binding to the PDZ domain of DVL required for its interaction with FZD receptors. This small molecule is able to inhibit Wnt3A-mediated signaling (Shan et al. 2005). Another study found that peptide binding to the DVL PDZ domain is more permissive compared with PDZ domains in other proteins and used peptide-phage display to identify peptide ligands, which bound to the PDZ domain of human DVL2. These peptide ligands were then shown to disrupt Wnt/β-catenin signaling in cell lines (Zhang et al. 2009).
Drugs that interfere with Wnt secretion have also been used as an approach to inhibit proximal signaling. For example, the drug IWP-2, identified in a screen of a synthetic chemical library, was found to inhibit the activity of Porcupine, a membrane-bound acyltransferase that modifies Wnt ligands with a palmitoyl group that is required for their secretion and signaling activity (Takada et al. 2006; Chen et al. 2009).
Drugs Inhibiting Downstream Wnt Signaling
Given the wide range of biologic effects of Wnt-mediated signaling in the adult, targeting more upstream signaling events may lead to unwanted side effects. Furthermore, in diseases such as colon cancer in which there frequently are mutations in APC, upstream inhibition at the level of the receptor may be ineffective for preventing the effects of a downstream activating mutation.
High-throughput screening approaches have been used to identify promising lead compounds that may one day lead to the development of bona fide clinical Wnt inhibitors (Fig. 1; Table 1). For example, HEK293 cells expressing the SuperTOPFLASH Wnt-responsive reporter were screened with a chemical library that led to the identification of XAV939 as a potent inhibitor of Wnt/β-catenin signaling. Mechanistic studies of this drug revealed a novel regulatory pathway by which the stability of Axin, a critical negative regulator of the pathway, was regulated by PARsylation and proteasomal degradation by tankyrases, TNKS1 and TNKS2 (Huang et al. 2009). This drug was able to inhibit colony formation of colorectal cancer cell lines and has also been used to inhibit β-catenin in a manner that promotes cardiomyocyte differentiation from embryonic stem cells (Huang et al. 2009; Wang et al. 2011a). Other substrates of tankyrases must be considered if tankyrase inhibitors are to be used clinically. For example, the dominantly inherited disorder cherubism is characterized by interosseous fibrocystic lesions resulting from hyperactive osteoclasts results from single missense mutations in a region of the adaptor protein 3BP2, which is part of a multiprotein signaling complex containing the SRC family kinases, SYK, and RHO family guanine nucleotide exchange factor VAV (Ueki et al. 2007; Guettler et al. 2011). These mutations have been found to disrupt the binding of tankyrase to 3BP2, inhibiting 3BP2 degradation and resulting in high levels of SRC, SYK, and VAV activity in osteoclasts (Guettler et al. 2011; Levaot et al. 2011). These observations may be relevant if tankyrase inhibitors are to be considered as cancer therapeutics, for instance, owing to not only potential side effects such as osteoporosis, but also concerns about potentiating the SRC pathway, which is already hyperactive in many human tumors.
As the β-catenin TCF/LEF complex is the ultimate downstream effector required for transcription of Wnt target genes, disruption of this complex has been studied as a therapeutic strategy. The ability to rationally design drugs to intervene in this interaction has been facilitated by characterization of the crystal structure of TCF/β-catenin complexes (Graham et al. 2000). One class of small molecules able to disrupt this interaction was identified by a high-throughput screening approach utilizing an assay based on the binding affinity of purified β-catenin to a TCF4 (TCF7L2) fragment (Lepourcelet et al. 2004). These compounds were able to inhibit growth of colorectal carcinoma cells in vitro (Lepourcelet et al. 2004). Another study used a small molecule secondary structure template library, which identified a compound, ICG-001 that was able to down-modulate β-catenin/TCF signaling by binding to the CREB response element-binding protein (CBP) and specifically inhibiting the CBP/β-catenin interaction (Emami et al. 2004). This compound was also shown to inhibit tumor formation in a mouse colon cancer xenotransplant model as well as attenuate pulmonary fibrosis induced by bleomycin, which is thought to be in part mediated by wnt/β-catenin signaling (Emami et al. 2004; Henderson et al. 2010). Another small molecule screen yielded the drug NC043, a diterpine alkaloid that can interrupt the β-catenin/TCF4 interaction and inhibit colorectal cancer growth in vivo (Wang et al. 2011b). Recently, several compounds were identified that inhibited β-catenin/TCF4 interactions utilizing a chemical library screen of Drosophila cells to mitigate functional genetic regulatory redundancies in mammalian cells. These compounds were able to inhibit β-catenin-dependent invasion of breast cancer cells and proliferation of colon cancer cell lines (Gonsalves et al. 2011). One conceptual difficulty in utilizing such drugs as therapeutics is that the structural basis for the interaction between β catenin and TCF is almost identical to that with E-cadherin, although there is some evidence for binding specificity mediated by β-catenin modifications (Graham et al. 2000; Huber and Weis 2001; Gottardi and Gumbiner 2004). Consequently, drugs designed to interrupt the β-catenin/TCF interaction may also disrupt the cadherins junction leading to unpredicted and potentially untoward side effects.
Other regulators of Wnt/β-catenin signaling have also emerged as potentially useful targets. For example, casein kinase-Iε (CKIε) was identified based on siRNA screening of breast cancer cell lines as a positive regulator of β-catenin-dependent transcription. Pharmacologic inhibition of CKIε with the drug IC261 inhibited breast cancer cell proliferation (Kim et al. 2010). Many other kinases that positively regulate Wnt have emerged as potential targets including phosphatidylinositol 4-kinase type IIa (PI4KIIa), phosphatidylinositol-4-phosphate 5-kinase type Ib (PIP5KIb), PAR-1, TNIK, and MAP3K1 (Sun et al. 2001; Pan et al. 2008; Mahmoudi et al. 2009; Sue Ng et al. 2010).
As the tools for interrogating Wnt signaling continue to evolve, novel pathways and promising compounds will continue to be identified as modulators of Wnt/β-catenin signaling. Because the effects of most of these compounds will be cell type/context-dependent, the challenge will be to understand the role of Wnt/β-catenin signaling across various tissues in such a way as to develop a practical therapeutic strategy.
STRATEGIES TO ACTIVATE WNT SIGNALING
Although much work has been performed identifying antagonists of Wnt signaling, particularly with regard to cancer therapy, drugs that activate Wnt pathways may be beneficial in many diseases including osteoporosis and certain cancers (Fig. 2; Table 2). Several drugs exist that act, at least partially, by enhancing Wnt pathways. For example, lithium has long been known as a powerful morphogen in embryonic development. Research into the mechanism for this property identified it as an inhibitor of GSK3β, which ultimately results in constitutive Wnt pathway activation (Klein and Melton 1996). Lithium is a well-known psychoactive drug and it has been postulated that its effects in the central nervous system (CNS) may be partially attributable to Wnt enhancement. Interestingly, many anti-psychotic drugs may act, at least to some degree, through interaction with Wnt pathway components. For example, the atypical anti-psychotic drug clozapine has been shown to increase levels of Dvl and phospho-Dvl in the rat brain, thereby enhancing β-catenin-mediated signaling (Roh et al. 2007).
Several studies have linked the use of lithium to increased Wnt/β-catenin signaling. For example, treatment of Lrp5-deficient mice with lithium significantly increased trabecular bone mass. Lithium also increased bone mass in animals with age-related osteoporosis and oophorectomy-induced osteoporosis (Clément-Lacroix et al. 2005). Furthermore, a retrospective clinical study comparing patients receiving lithium to age-matched controls noted increased bone density, lower serum ALP, and osteocalcin (markers of bone turnover) in patients who received lithium (Zamani et al. 2009). The directed clinical use of lithium specifically as a Wnt modulator is complicated by the substantial toxicities in vivo that accompany the very high doses needed to achieve Wnt activation in different contexts. More potent GSK3β inhibitors are available and may also be clinically useful, although these agents are still considered experimental at this time. In vivo effects on bone metabolism comparable to lithium have been seen with an orally available potent dual inhibitor of GSK3α/β (Engler et al. 2004). Pharmacologic inhibition of GSK3β has been shown to potentiate regulatory T-cell activity in vitro and extend allograft survival in a mouse model of pancreatic islet cell transplantation (Graham et al. 2010). Unfortunately, inhibition of an enzyme as ubiquitous as GSK3β has multiple consequences that are independent of the Wnt pathway, leading to significant side effects that have complicated the use of these otherwise potent compounds in human patients.
Other indirect means of enhancing Wnt signaling have been explored. For instance, knockdown of DKK1 by RNA interference (RNAi) significantly inhibited bone loss in a rat model of estrogen depletion as well as in a model of glucocorticoid-mediated bone loss (Wang et al. 2007, 2008). Investigators have also used anti-DKK1 monoclonal antibodies as a means to increase bone mass and mineralization in several small animal and nonhuman primate models (Glantschnig et al. 2010). DKK1 antibody has also been shown to inhibit degenerative osteoarthritis in the setting of inflammatory arthritis and to facilitate fracture healing (Diarra et al. 2007; Komatsu et al. 2010). Additionally, in a small animal model of ischemic brain injury, expression of DKK-1 was found to be strongly induced during injury. Treatment with antisense oligonucleotides against DKK-1 significantly inhibited neurotoxicity (Cappuccio et al. 2005). Interest in inhibiting the function of DKK has also emerged because it has been appreciated that tumor-derived DKK may inhibit Wnt-induced osteoblastic differentiation, thus facilitating osteolytic bone metastases in diseases such as breast cancer and multiple myeloma (Tian et al. 2003; Bu et al. 2008; Qiang et al. 2008). An anti-DKK1 antibody, BHQ880 was shown to increase osteoblasts and bone density in a (SCID)-hu murine model of human multiple myeloma as well as inhibit the growth of multiple myeloma cells on bone marrow stroma in vitro (Fulciniti et al. 2009).
Another extracellular regulator of Wnt signaling has been targeted as an approach to enhance bone formation. In Xenopus, a secreted cysteine-knot protein, Wise, was identified that acted as a context-dependent activator or inhibitor of Wnt signaling by interacting with LPR6 (Itasaki et al. 2003). Deficiency of a related mammalian gene product, sclerostin (SOST), is associated with the inherited disorder sclerosteosis, which results in hyperactive osteoblasts (Brunkow et al. 2001). SOST-deficient mice also showed markedly increased bone formation and bone strength (Li et al. 2008). Sclerostin was confirmed to bind LPR5/6 and inhibit Wnt/β-catenin signaling (Li et al. 2005; Semënov et al. 2005). Several anti-sclerostin antibodies have been developed that have shown significant activity in enhancing bone mass in small animal and nonhuman primate models (Li et al. 2009; Agholme et al. 2010; Ominsky et al. 2010). A single dose, placebo-controlled phase I clinical trial with a humanized sclerostin antibody, AMG 785, has been recently reported. In this study, patients receiving single doses of AMG 785 had increased markers of bone turnover and higher bone mineral density compared with placebo, and treatment was generally well tolerated (Padhi et al. 2011). A phase II study of this agent is currently ongoing.
Another putative secreted inhibitor of Wnt signaling, SFRP1 is also an attractive target to enhance Wnt signaling therapeutically. Sfrp1-deficient mice have increased trabecular bone density and mineralization, consistent with the observation that deletion of Sfrp1 inhibited age-associated bone loss in mice (Bodine et al. 2004). In one study, a small molecule inhibitor of SFRP1 was identified via a fluorescent polarization binding assay using purified human SFRP1 protein and a cell-based Wnt reporter assay. This small molecule was capable of stimulating ex vivo murine calvarial bone formation (Moore et al. 2009).
Although there has clearly been more work on identifying inhibitors, small molecule compounds that potentiate Wnt/β-catenin signaling have also been described. One study identified flavonoid components of the Persicaria hydropiper plant as enhancers of Wnt/β-catenin signaling that can block the differentiation of adipocytes from 3T3-L1 cells (Lee et al. 2011). In another study, a cell-based screen utilizing the BAR reporter of a chemical library of 1857 human-experienced drugs identified 44 compounds that enhanced Wnt3a-mediated stimulation (Pollock et al. 2003; Namkoong et al. 2007; Biechele et al. 2010). One of these activators, riluzole, is an FDA-approved drug for treating amyotrophic lateral sclerosis (ALS). Riluzole is thought to inhibit the metabotropic glutamate receptor GRM1 and is currently in clinical trials for melanoma based on its activity in melanoma cell lines, xenograft models, and a phase 0 patient trial (Pollock et al. 2003; Namkoong et al. 2007; Yip et al. 2009; Le et al. 2010). As Wnt/β-catenin signaling is associated with an improved prognosis in melanoma, it is intriguing to speculate that the mechanism of action of this drug may be at least partially owing to its effect on enhancing Wnt/β-catenin signaling (Chien et al. 2009b).
STRATEGIES FOR TARGETING β-CATENIN-INDEPENDENT WNT SIGNALING
Despite the mounting evidence for involvement of β-catenin-independent Wnt signaling in the pathogenesis of several disorders, approaches to target noncanonical Wnt signaling have been disappointingly limited. WNT5A antisense, dominant-negative WNT5A, and addition of a WNT5A antibody in vitro were shown to inhibit the production of proinflammatory cytokines such as IL-6, IL-15, and RANKL in cultured synoviocytes from RA patients (Sen et al. 2001). Small molecules represent another viable approach to targeting β-catenin-independent Wnt pathways. For example, the drug fumagillin, originally isolated from the fungus Aspergillus fumagatus, was initially identified as a compound that bound the protein methionine aminopeptidase 2 (MetAP-2) (Zhang et al. 2006). Fumagillin was subsequently found to disrupt noncanonical Wnt signaling in zebrafish embryos and to inhibit β-catenin-independent Wnt-mediated endothelial cell migration and angiogenesis (Zhang et al. 2006; Cirone et al. 2008). In another study, phenylmethimazole, an inhibitor of Toll-like receptor-3 (TLR3) was found to inhibit WNT5A expression in melanoma and pancreatic cell lines, thus inhibiting their growth and migration in vitro and in a murine xenograft model (Schwartz et al. 2009). A formylated hexapeptide derived from the primary WNT5A peptide sequence mimicked the ability of WNT5A to inhibit breast cancer cell migration, suggesting that peptide-based approaches may also be viable tools for modulating Wnt pathways (Säfholm et al. 2006). As more studies elucidate the specific roles of β-catenin-independent Wnt signaling in disease, more therapeutic targets and strategies are likely to emerge.
CONCLUSION
As research tools to understand the molecular mechanisms of human diseases continue to develop, so does our understanding of the prominent role that aberrant Wnt-mediated signaling plays in an ever-expanding number of human diseases. Apart from the examples described here, a role for Wnt is emerging in a multitude of other disease processes for which pharmacologic manipulation could prove clinically valuable. To fully realize the potential of Wnt manipulation, more must be understood about the complex regulation of both canonical and noncanonical pathways. As research tools to understand the molecular mechanisms of human disease continue to develop, so does our understanding of the nuances of Wnt regulation in disease as well as in the context of normal tissue homeostasis. Our ability to target these pathways for therapeutic benefit is only in its infancy. However, promising new technologies are paving the way to drive more effective therapeutic targeting of Wnt pathways in the future.
ACKNOWLEDGMENTS
Z.Z. is funded by a T32 training grant through the National Institutes of Health (T32 CA009515-2). R.T.M. is an Investigator of the Howard Hughes Medical Institute. A.J.C. is funded by a K08 Career Development Award from NIH/NCI (K08 CA128565). We are indebted to our funding agencies for their continued support of our research endeavors. As with any attempt to review a broad and dynamically changing field, we had to limit our scope and exclude certain studies, so we apologize in advance for any unintended omissions. The views detailed in this publication do not represent the official views of the National Institutes of Health or the Howard Hughes Medical Institute.
Footnotes
Editors: Roel Nusse, Xi He, and Renee van Amerongen
Additional Perspectives on Wnt Signaling available at www.cshperspectives.org
REFERENCES
- Agholme F, Li X, Isaksson H, Ke HZ, Aspenberg P 2010. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res 25: 2412–2418 [DOI] [PubMed] [Google Scholar]
- Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA 2005. Importance of P-cadherin, β-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res 11: 8606–8614 [DOI] [PubMed] [Google Scholar]
- Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW 2007. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem 282: 31703–31712 [DOI] [PubMed] [Google Scholar]
- Biechele TL, Moon RT 2008. Assaying β-catenin/TCF transcription with β-catenin/TCF transcription-based reporter constructs. Methods in molecular biology, Vol. 468, pp. 99–110 Humana Press, Clifton, NJ: [DOI] [PubMed] [Google Scholar]
- Biechele TL, Camp ND, Fass DM, Kulikauskas RM, Robin NC, White BD, Taraska CM, Moore EC, Muster J, Karmacharya R, et al. 2010. Chemical-genetic screen identifies riluzole as an enhancer of Wnt/β-catenin signaling in melanoma. Chem Biol 17: 1177–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DHR, Fordyce A, Walker MT, St. Clair DM, Porteous DJ, Muir WJ 2001. Schizophrenia and affective disorders—Cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: Clinical and P300 findings in a family. Am J Hum Genet 69: 428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine PVN, Zhao W, Kharode YP, Bex FJ, Lambert A-J, Goad MB, Gaur T, Stein GS, Lian JB, Komm BS 2004. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol (Baltimore, Md) 18: 1222–1237 [DOI] [PubMed] [Google Scholar]
- Boon EM.J, Keller JJ, Wormhoudt TA.M, Giardiello FM, Offerhaus GJ.A, van der Neut R, Pals ST 2004. Sulindac targets nuclear β-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Brit J Cancer 90: 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521 [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, et al. 2001. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Lu W, Liu C-C, Selander K, Yoneda T, Hall C, Keller ET, Li Y 2008. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: Implication for breast cancer osteolytic bone metastases. Int J Cancer 123: 1034–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell GM, Jones C, Gensberg K, Jan S, Hardy RG, Byrd P, Chughtai S, Wallis Y, Matthews GM, Morton DG 2004. The Wnt antagonist sFRP1 in colorectal tumorigenesis. Cancer Res 64: 883–888 [DOI] [PubMed] [Google Scholar]
- Cantrell VA, Jessen JR 2010. The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett 287: 54–61 [DOI] [PubMed] [Google Scholar]
- Cappuccio I, Calderone A, Busceti CL, Biagioni F, Pontarelli F, Bruno V, Storto M, Terstappen GT, Gaviraghi G, Fornai F, et al. 2005. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is required for the development of ischemic neuronal death. J Neurosci 25: 2647–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F 2004. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J Neurosci 24: 6021–6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L 2007. Human immunodeficiency virus-restricted replication in astrocytes and the ability of γ interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol 81: 5864–5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F 2002. A survival-stratification model of human colorectal carcinomas with β-catenin and 27kip1. Cancer 95: 2479–2486 [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, Wei S, Hao W, Kilgore J, Williams NS, et al. 2009. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Conrad WH, Moon RT 2009a. A Wnt survival guide: From flies to human disease. J Invest Dermatol 129: 1614–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT 2009b. Activated Wnt/β-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci 106: 1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone P, Lin S, Griesbach HL, Zhang Y, Slusarski DC, Crews CM 2008. A role for planar cell polarity signaling in angiogenesis. Angiogenesis 11: 347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssière B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G 2005. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci 102: 17406–17411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, et al. 2011. Lrp5 functions in bone to regulate bone mass. Nat Med 17: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y 2005. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8: 739–750 [DOI] [PubMed] [Google Scholar]
- DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B 2007. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res 67: 5371–5379 [DOI] [PubMed] [Google Scholar]
- de Boer J, Siddappa R, Gaspar C, van Apeldoorn A, Fodde R, van Blitterswijk C 2004. Wnt signaling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone 34: 818–826 [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT 2006. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene 25: 7545–7553 [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Sáez K, Henríquez JP, Zhao A, et al. 2007. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci 104: 9434–9439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. 2007. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13: 156–163 [DOI] [PubMed] [Google Scholar]
- Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ 2008. β-Catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med 14: 162–169 [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Raible DW, Moon RT 2000. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev 14: 158–162 [PMC free article] [PubMed] [Google Scholar]
- Edamura K, Nasu Y, Takaishi M, Kobayashi T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T, Watanabe M, et al. 2007. Adenovirus-mediated REIC/Dkk-3 gene transfer inhibits tumor growth and metastasis in an orthotopic prostate cancer model. Cancer Gene Ther 14: 765–772 [DOI] [PubMed] [Google Scholar]
- Elzagheid A, Buhmeida A, Korkeila E, Collan Y, Syrjanen K, Pyrhonen S 2008. Nuclear β-catenin expression as a prognostic factor in advanced colorectal carcinoma. World J Gastroentero 14: 3866–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo J-L, Oh SW, Kim HY, et al. 2004. A small molecule inhibitor of β-catenin/CREB-binding protein transcription. Proc Natl Acad Sci 101: 12682–12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler TA, Henry JR, Malhotra S, Cunningham B, Furness K, Brozinick J, Burkholder TP, Clay MP, Clayton J, Diefenbacher C, et al. 2004. Substituted 3-imidazo[1,2-a]pyridin-3-yl-4-(1,2,3,4-tetrahydro-[1,4]diazepino-[6,7,1-hi]indol-7-yl)pyrrole-2,5-diones as highly selective and potent inhibitors of glycogen synthase kinase-3. J Med Chem 47: 3934–3937 [DOI] [PubMed] [Google Scholar]
- Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, et al. 2008. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 455: 547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CE, Ekström EJ, Howlin J, Andersson T 2009. The WNT-5a derived peptide, Foxy-5, possesses dual properties that impair progression of ERα negative breast cancer. Cell Cycle (Georgetown, Tex) 8: 1838–1842 [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA 2010. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiat 167: 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, Yokoi K, Ueda Y, Shimokata K, Sekido Y 2005. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene 24: 6323–6327 [DOI] [PubMed] [Google Scholar]
- Fukukawa C, Hanaoka H, Nagayama S, Tsunoda T, Toguchida J, Endo K, Nakamura Y, Katagiri T 2008. Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci 99: 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai Y-T, Chauhan D, et al. 2009. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 114: 371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallo C, Palacios J, Moreno G, Calvo de Mora J, Suárez A, Armas A 1999. β-Catenin expression pattern in stage I and II ovarian carcinomas: Relationship with β-catenin gene mutations, clinicopathological features, and clinical outcome. Am J Pathol 155: 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. 2009. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15: 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ 1993. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 328: 1313–1316 [DOI] [PubMed] [Google Scholar]
- Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, et al. 2010. Subtypes of medulloblastoma have distinct developmental origins. Nature 468: 1095–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantschnig H, Hampton RA, Lu P, Zhao JZ, Vitelli S, Huang L, Haytko P, Cusick T, Ireland C, Jarantow SW, et al. 2010. Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 285: 40135–40147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DA II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, et al. 2005. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764 [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. 2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107: 513–523 [DOI] [PubMed] [Google Scholar]
- Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM.C, DasGupta R 2011. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci 108: 5954–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM 2004. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167: 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TA, Weaver C, Mao F, Kimelman D, Xu W 2000. Crystal structure of a β-catenin/Tcf complex. Cell 103: 885–896 [DOI] [PubMed] [Google Scholar]
- Graham JA, Fray M, de Haseth S, Lee KM, Lian MM, Chase CM, Madsen JC, Markmann J, Benichou G, Colvin RB, et al. 2010. Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3β inhibition. J Biol Chem 285: 32852–32859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettler S, Larose J, Petsalaki E, Gish G, Scotter A, Pawson T, Rottapel R, Sicheri F 2011. Structural basis and sequence rules for substrate recognition by tankyrase explain the basis for cherubism disease. Cell 147: 1340–1354 [DOI] [PubMed] [Google Scholar]
- Gunn WG, Krause U, Lee N, Gregory CA 2010. Pharmaceutical inhibition of glycogen-synthetase-kinase-3-β reduces multiple myeloma-induced bone disease in a novel murine plasmacytoma xenograft model. Blood 117: 1641–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM 2004. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 6: 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Barg RN, You L, Xu Z, Reguart N, Mikami I, Batra S, Rosell R, Jablons DM 2005a. Wnt signaling in stem cells and non-small-cell lung cancer. Clin Lung Cancer 7: 54–60 [DOI] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM 2005b. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 24: 3054–3058 [DOI] [PubMed] [Google Scholar]
- Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, Taketo MM, Biankin AV, Hebrok M 2008. Stabilization of β-catenin induces pancreas tumor formation. Gastroenterology 135: 1288–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson WR Jr, Chi EY, Ye X, Nguyen C, Tien Y-t, Zhou B, Borok Z, Knight DA, Kahn M 2010. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci 107: 14309–14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeppner LH, Secreto FJ, Westendorf JJ 2009. Wnt signaling as a therapeutic target for bone diseases. Expert Opin Ther Targets 13: 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO 2005. Essential role of β-catenin in postnatal bone acquisition. J Biol Chem 280: 21162–21168 [DOI] [PubMed] [Google Scholar]
- Hornyak TJ, Hayes DJ, Chiu LY, Ziff EB 2001. Transcription factors in melanocyte development: Distinct roles for Pax-3 and Mitf. Mech Dev 101: 47–59 [DOI] [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Lee C-S, Kench JG, Golovsky D, Brenner PC, O’Neill GF, Kooner R, Stricker PD, Grygiel JJ, et al. 2005. Lower levels of nuclear β-catenin predict for a poorer prognosis in localized prostate cancer. Int J Cancer 113: 415–422 [DOI] [PubMed] [Google Scholar]
- Huang S-MA, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. 2009. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620 [DOI] [PubMed] [Google Scholar]
- Huber AH, Weis WI 2001. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 105: 391–402 [DOI] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R 2003. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130: 4295–4305 [DOI] [PubMed] [Google Scholar]
- Jeannet G, Boudousquié C, Gardiol N, Kang J, Huelsken J, Held W 2010. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci 107: 9777–9782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M, Engert A, Weissinger F, Knauf W, Kimby E, Poynton C, Oliff IA, Rummel MJ, Osterborg A 2008. Phase I study of a novel pro-apoptotic drug R-etodolac in patients with B-cell chronic lymphocytic leukemia. Invest New Drugs 26: 139–149 [DOI] [PubMed] [Google Scholar]
- Kageshita T, Hamby CV, Ishihara T, Matsumoto K, Saida T, Ono T 2001. Loss of β-catenin expression associated with disease progression in malignant melanoma. Brit J Dermatol 145: 210–216 [DOI] [PubMed] [Google Scholar]
- Kameoka M, Kameoka Y, Utachee P, Kurosu T, Sawanpanyalert P, Ikuta K, Auwanit W 2009. Short communication: RNA interference directed against Axin1 upregulates human immunodeficiency virus type 1 gene expression by activating the Wnt signaling pathway in HeLa-derived J111 cells. AIDS Res Hum Retroviruses 25: 1005–1011 [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH 2002. Presenilin couples the paired phosphorylation of β-catenin independent of axin: Implications for β-catenin activation in tumorigenesis. Cell 110: 751–762 [DOI] [PubMed] [Google Scholar]
- Katoh M 2002. Strabismus (STB)/Vang-like (VANGL) gene family (Review). Int J Mol Med 10: 11–15 [PubMed] [Google Scholar]
- Kawasaki K, Watanabe M, Sakaguchi M, Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH, Kumon H, et al. 2009. REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther 16: 65–72 [DOI] [PubMed] [Google Scholar]
- Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Seo Y-W, Ahn KY, Chung IJ, Kim KK 2009. KITENIN recruits Dishevelled/PKCδ to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut 58: 509–519 [DOI] [PubMed] [Google Scholar]
- Kim SY, Dunn IF, Firestein R, Gupta P, Wardwell L, Repich K, Schinzel AC, Wittner B, Silver SJ, Root DE, et al. 2010. CK1epsilon is required for breast cancers dependent on β-catenin activity. PloS ONE 5: e8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D 1991. Identification of FAP locus genes from chromosome 5q21. Science (New York, NY) 253: 661–665 [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA 1996. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci 93: 8455–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu DE, Mary MN, Schroeder RJ, Robling AG, Turner CH, Warden SJ 2010. Modulation of Wnt signaling influences fracture repair. J Orthop Res 28: 928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383 [DOI] [PubMed] [Google Scholar]
- Kulkarni NH, Onyia JE, Zeng Q, Tian X, Liu M, Halladay DL, Frolik CA, Engler T, Wei T, Kriauciunas A, et al. 2006. Orally bioavailable GSK-3α/β dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J Bone Miner Res 21: 910–920 [DOI] [PubMed] [Google Scholar]
- Kumar A, Zloza A, Moon RT, Watts J, Tenorio AR, Al-Harthi L 2008. Active β-catenin signaling is an inhibitory pathway for human immunodeficiency virus replication in peripheral blood mononuclear cells. J Virol 82: 2813–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A 2006. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 66: 10439–10448 [DOI] [PubMed] [Google Scholar]
- Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P 1991. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology 101: 635–639 [DOI] [PubMed] [Google Scholar]
- Lavergne E, Hendaoui I, Coulouarn C, Ribault C, Leseur J, Eliat P-A, Mebarki S, Corlu A, Clément B, Musso O 2011. Blocking Wnt signaling by SFRP-like molecules inhibits in vivo cell proliferation and tumor growth in cells carrying active β-catenin. Oncogene 30: 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le MN, Chan JL-K, Rosenberg SA, Nabatian AS, Merrigan KT, Cohen-Solal KA, Goydos JS 2010. The glutamate release inhibitor Riluzole decreases migration, invasion, and proliferation of melanoma cells. J Invest Dermatol 130: 2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, He B, You L, Dadfarmay S, Xu Z, Mazieres J, Mikami I, McCormick F, Jablons DM 2004a. Expression of the secreted frizzled-related protein gene family is downregulated in human mesothelioma. Oncogene 23: 6672–6676 [DOI] [PubMed] [Google Scholar]
- Lee JH, Park SR, Chay K-O, Seo Y-W, Kook H, Ahn KY, Kim YJ, Kim KK 2004b. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res 64: 4235–4243 [DOI] [PubMed] [Google Scholar]
- Lee JK, Bae JA, Sun EG, Kim HD, Yoon TM, Kim K, Lee JH, Lim SC, Kim KK 2009. KITENIN increases invasion and migration of mouse squamous cancer cells and promotes pulmonary metastasis in a mouse squamous tumor model. FEBS Lett 583: 711–717 [DOI] [PubMed] [Google Scholar]
- Lee S-H, Kim B, Oh MJ, Yoon J, Kim HY, Lee KJ, Lee JD, Choi K-Y 2011. Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/β-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother Res 25: 1629–1635 [DOI] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JBA, et al. 2008. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell 2: 72–82 [DOI] [PubMed] [Google Scholar]
- Lengerke C, Grauer M, Niebuhr NI, Riedt T, Kanz L, Park I-H, Daley GQ 2009. Hematopoietic development from human induced pluripotent stem cells. Ann NY Acad Sci 1176: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepourcelet M, Chen Y-NP, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA 2004. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell 5: 91–102 [DOI] [PubMed] [Google Scholar]
- Levaot N, Voytyuk O, Dimitriou I, Sircoulomb F, Chandrakumar A, Deckert M, Krzyzanowski PM, Scotter A, Gu S, Janmohamed S, et al. 2011. Loss of tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism. Cell 147: 1324–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D 2005. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887 [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, et al. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869 [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, et al. 2009. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24: 578–588 [DOI] [PubMed] [Google Scholar]
- Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, Jass JR 2007. Prognostic significance of the wnt signalling pathway molecules APC, β-catenin and E-cadherin in colorectal cancer: A tissue microarray-based analysis. Histopathology 50: 453–464 [DOI] [PubMed] [Google Scholar]
- Maelandsmo GM, Holm R, Nesland JM, Fodstad Ø, Flørenes VA 2003. Reduced β-catenin expression in the cytoplasm of advanced-stage superficial spreading malignant melanoma. Clin Cancer Res 9: 3383–3388 [PubMed] [Google Scholar]
- Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, Newmark HL, Chadburn A, Bertagnolli MM 1998. The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis 19: 87–91 [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Li VSW, Ng SS, Taouatas N, Vries RGJ, Mohammed S, Heck AJ, Clevers H 2009. The kinase TNIK is an essential activator of Wnt target genes. EMBO J 28: 3329–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Roberts BS, Berndt JD, Marine S, Anastas J, Chung N, Ferrer M, Yi X, Stoick-Cooper CL, von Haller PD, et al. 2008. New regulators of Wnt/β-catenin signaling revealed by integrative molecular screening. Sci Signal 1: ra12. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang Y-C, Pulendran B 2010. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science (New York, NY) 329: 849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. 2009. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell 136: 1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, Singer S, Maki RG, Cordon-Cardo C 2007. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest 117: 3248–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazieres J, You L, He B, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM 2005a. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene 24: 5396–5400 [DOI] [PubMed] [Google Scholar]
- Mazieres J, You L, He B, Xu Z, Twogood S, Lee AY, Reguart N, Batra S, Mikami I, Jablons DM 2005b. Wnt2 as a new therapeutic target in malignant pleural mesothelioma. Int J Cancer 117: 326–332 [DOI] [PubMed] [Google Scholar]
- Mikami I, You L, He B, Xu Z, Batra S, Lee AY, Mazieres J, Reguart N, Uematsu K, Koizumi K, et al. 2005. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic T, Planutis K, Nguyen A, Marsh JL, Lin F, Hope C, Holcombe RF 2004. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol 25: 1337–1342 [PubMed] [Google Scholar]
- Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA 2010. Wnt proteins promote bone regeneration. Sci Transl Med 2: 29ra30. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Endoh Y, Hasebe T, Ishii G, Kodama K, Goya M, Ono M, Saitoh N, Chiba T, Ochiai A 2004. Nuclear β-catenin accumulation as a prognostic factor in Dukes’ D human colorectal cancers. Oncol Rep 12: 245–251 [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H 1999. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res 38: 1–6 [DOI] [PubMed] [Google Scholar]
- Moore WJ, Kern JC, Bhat R, Commons TJ, Fukayama S, Goljer I, Krishnamurthy G, Magolda RL, Nogle L, Pitts K, et al. 2009. Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides. J Med Chem 52: 105–116 [DOI] [PubMed] [Google Scholar]
- Morris JP IV, Cano DA, Sekine S, Wang SC, Hebrok M β-Catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest 120: 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, Pollard C, Killick R, Iqbal T, Raymond L, Varndell I, et al. 2001. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J Neurosci 21: 4987–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H 2006. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 133: 2149–2154 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Nawata M, Wakitani S 2005. Expression profiles and functional analyses of Wnt-related genes in human joint disorders. Am J Pathol 167: 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong J, Shin S-S, Lee HJ, Marín YE, Wall BA, Goydos JS, Chen S 2007. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res 67: 2298–2305 [DOI] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P 1991. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science (New York, NY) 253: 665–669 [DOI] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P 2008. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell 2: 60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S 2010. Global regulator SATB1 recruits β-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol 8: e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R 2008. Wnt signaling and stem cell control. Cell Res 18: 523–527 [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE 1982. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109 [DOI] [PubMed] [Google Scholar]
- Okino K, Nagai H, Hatta M, Nagahata T, Yoneyama K, Ohta Y, Jin E, Kawanami O, Araki T, Emi M 2003. Up-regulation and overproduction of DVL-1, the human counterpart of the Drosophila dishevelled gene, in cervical squamous cell carcinoma. Oncol Rep 10: 1219–1223 [DOI] [PubMed] [Google Scholar]
- Ominsky MS, Vlasseros F, Jolette J, Smith SY, Stouch B, Doellgast G, Gong J, Gao Y, Cao J, Graham K, et al. 2010. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25: 948–959 [DOI] [PubMed] [Google Scholar]
- Padhi D, Jang G, Stouch B, Fang L, Posvar E 2011. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26: 19–26 [DOI] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE 2010. Endogenous Wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PloS ONE 5: e11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, et al. 2001. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol 154: 369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Choi S-C, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, et al. 2008. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science (New York, NY) 321: 1350–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RKS, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L, et al. 2002. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 50: 857–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin S-S, et al. 2003. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet 34: 108–112 [DOI] [PubMed] [Google Scholar]
- Pukrop T 2006. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci 103: 5454–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang Y-W, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JDJ 2008. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: A potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 112: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423: 409–414 [DOI] [PubMed] [Google Scholar]
- Rhee C-S, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA 2002. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene 21: 6598–6605 [DOI] [PubMed] [Google Scholar]
- Rijsewijk F 1987. The Drosophila homology of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 50: 649–657 [DOI] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML.E, Kaykas A, Sheldahl LC, Zeisler J, Dubé M-P, Zhang L-H, Singaraja RR, Guernsey DL, Zheng B, et al. 2002. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32: 326–330 [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP 2006. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development (Cambridge, England) 133: 3231–3244 [DOI] [PubMed] [Google Scholar]
- Roh M-S, Seo MS, Kim Y, Kim SH, Jeon WJ, Ahn YM, Kang UG, Juhnn YS, Kim YS 2007. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Molec Med 39: 353–360 [DOI] [PubMed] [Google Scholar]
- Säfholm A, Leandersson K, Dejmek J, Nielsen CK, Villoutreix BO, Andersson T 2006. A formylated hexapeptide ligand mimics the ability of Wnt-5a to impair migration of human breast epithelial cells. J Biol Chem 281: 2740–2749 [DOI] [PubMed] [Google Scholar]
- Salinas PC, Zou Y 2008. Wnt signaling in neural circuit assembly. Annu Rev Neurosci 31: 339–358 [DOI] [PubMed] [Google Scholar]
- Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP 2010. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PloS ONE 5: e15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AL, Malgor R, Dickerson E, Weeraratna AT, Slominski A, Wortsman J, Harii N, Kohn AD, Moon RT, Schwartz FL, et al. 2009. Phenylmethimazole decreases Toll-like receptor 3 and noncanonical Wnt5a expression in pancreatic cancer and melanoma together with tumor cell growth and migration. Clin Cancer Res 15: 4114–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semënov M, Tamai K, He X 2005. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280: 26770–26775 [DOI] [PubMed] [Google Scholar]
- Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA 2000. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci 97: 2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Chamorro M, Reifert J, Corr M, Carson DA 2001. Blockade of Wnt-5A/Frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum 44: 772–781 [DOI] [PubMed] [Google Scholar]
- Sen M, Reifert J, Lauterbach K, Wolf V, Rubin JS, Corr M, Carson DA 2002. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum 46: 2867–2877 [DOI] [PubMed] [Google Scholar]
- Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y 2011. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell 20: 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Shi D-L, Wang J, Zheng J 2005. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry 44: 15495–15503 [DOI] [PubMed] [Google Scholar]
- Sheng Y-J, Gao J-P, Li J, Han J-W, Xu Q, Hu W-L, Pan T-M, Cheng Y-L, Yu Z-Y, Ni C, et al. 2011. Follow-up study identifies two novel susceptibility loci PRKCB and 8p11.21 for systemic lupus erythematosus. Rheumatology (Oxford, England) 50: 682–688 [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470: 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, et al. 2000. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 342: 1946–1952 [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT 2007. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development (Cambridge, England) 134: 479–489 [DOI] [PubMed] [Google Scholar]
- Su L, Vogelstein B, Kinzler K 1993. Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737 [DOI] [PubMed] [Google Scholar]
- Sue Ng S, Mahmoudi T, Li VSW, Hatzis P, Boersema PJ, Mohammed S, Heck AJ, Clevers H 2010. MAP3K1 functionally interacts with Axin1 in the canonical Wnt signalling pathway. Biol Chem 391: 171–180 [DOI] [PubMed] [Google Scholar]
- Sugimura R, Li L 2010. Noncanonical Wnt signaling in vertebrate development, stem cells, and diseases. Birth Defects Res C Embryo Today 90: 243–256 [DOI] [PubMed] [Google Scholar]
- Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT 2001. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol 3: 628–636 [DOI] [PubMed] [Google Scholar]
- Surendran K, Schiavi S, Hruska KA 2005. Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair K-W, Schuebel KE, Markowitz SD, Dong Chen W, Pretlow TP, Yang B, Akiyama Y, van Engeland M, et al. 2004. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36: 417–422 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Toyota M, Carraway H, Caraway H, Gabrielson E, Ohmura T, Fujikane T, Nishikawa N, Sogabe Y, Nojima M, et al. 2008. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Brit J Cancer 98: 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S 2006. Monounsaturated fatty acid modification of Wnt protein: Its role in Wnt secretion. Dev Cell 11: 791–801 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Watanabe T, Okada M, Inoue K, Ueda T, Takada I, Watabe T, Yamamoto Y, Fukuda T, Nakamura T, et al. 2011. Noncanonical Wnt signaling mediates androgen-dependent tumor growth in a mouse model of prostate cancer. Proc Natl Acad Sci 108: 4938–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Rogatsky I, Schwarcz LE, Darimont BD 2006. The glucocorticoid receptor represses cyclin D1 by targeting the Tcf-β-catenin complex. J Biol Chem 281: 17856–17863 [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S 2000. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem 275: 14013–14016 [DOI] [PubMed] [Google Scholar]
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. 2010. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol 6: 829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr 2003. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349: 2483–2494 [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC 2010. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Mol Psychiatry 15: 228, 272–285 [DOI] [PubMed] [Google Scholar]
- Trowbridge JJ, Guezguez B, Moon RT, Bhatia M 2010. Wnt3a activates dormant c-Kit− bone marrow-derived cells with short-term multilineage hematopoietic reconstitution capacity. Stem Cells (Dayton, Ohio) 28: 1379–1389 [DOI] [PubMed] [Google Scholar]
- Tveita AA, Rekvig OP 2011. Alterations in Wnt pathway activity in mouse serum and kidneys during lupus development. Arthritis Rheum 63: 513–522 [DOI] [PubMed] [Google Scholar]
- Ueki Y, Lin CY, Senoo M, Ebihara T, Agata N, Onji M, Saheki Y, Kawai T, Mukherjee PM, Reichenberger E, et al. 2007. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell 128: 71–83 [DOI] [PubMed] [Google Scholar]
- Uematsu K, He B, You L, Xu Z, McCormick F, Jablons DM 2003a. Activation of the Wnt pathway in non small cell lung cancer: Evidence of dishevelled overexpression. Oncogene 22: 7218–7221 [DOI] [PubMed] [Google Scholar]
- Uematsu K, Kanazawa S, You L, He B, Xu Z, Li K, Peterlin BM, McCormick F, Jablons DM 2003b. Wnt pathway activation in mesothelioma: Evidence of Dishevelled overexpression and transcriptional activity of β-catenin. Cancer Res 63: 4547–4551 [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE 2007. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci 104: 9685–9690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Hirata H, Majid S, Chen Y, Zaman MS, Tabatabai ZL, Hinoda Y, Dahiya R 2011. Wnt antagonist DICKKOPF-3 (Dkk-3) induces apoptosis in human renal cell carcinoma. Mol Carcinogen 50: 449–457 [DOI] [PubMed] [Google Scholar]
- Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS 2000. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem 275: 4374–4382 [DOI] [PubMed] [Google Scholar]
- Vijayaragavan K, Szabo E, Bossé M, Ramos-Mejia V, Moon RT, Bhatia M 2009. Noncanonical Wnt signaling orchestrates early developmental events toward hematopoietic cell fate from human embryonic stem cells. Cell Stem Cell 4: 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincan E, Darcy PK, Smyth MJ, Thompson EW, Thomas RJ.S, Phillips WA, Ramsay RG 2005. Frizzled-7 receptor ectodomain expression in a colon cancer cell line induces morphological change and attenuates tumor growth. Differentiation 73: 142–153 [DOI] [PubMed] [Google Scholar]
- von Marschall Z, Fisher LW 2010. Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem Biophys Res Commun 400: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nakayama N 2009. WNT and BMP signaling are both required for hematopoietic cell development from human ES cells. Stem Cell Res 3: 113–125 [DOI] [PubMed] [Google Scholar]
- Wang F-S, Ko J-Y, Lin C-L, Wu H-L, Ke H-J, Tai P-J 2007. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone 40: 485–492 [DOI] [PubMed] [Google Scholar]
- Wang F-S, Ko J-Y, Yeh D-W, Ke H-C, Wu H-L 2008. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 149: 1793–1801 [DOI] [PubMed] [Google Scholar]
- Wang H, Hao J, Hong CC 2011a. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem Biol 6: 192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Liu H, Wang S, Hao X, Li L 2011b. A diterpenoid derivative 15-oxospiramilactone inhibits Wnt/β-catenin signaling and colon cancer cell tumorigenesis. Cell Res 21: 730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM 2002. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1: 279–288 [DOI] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So SK 2009. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Chua M-S, Grepper S, So SK 2011. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer 10: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ 2010. β-Catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic gliogenesis in the adult brain. Stem Cells (Dayton, Ohio) 28: 297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR III, Nusse R 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452 [DOI] [PubMed] [Google Scholar]
- Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG 2008. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science (New York, NY) 320: 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SCC, Lo SFE, Lee KC, Yam JWP, Chan JK.C, Wendy Hsiao WL 2002. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol 196: 145–153 [DOI] [PubMed] [Google Scholar]
- Wortman B, Darbinian N, Sawaya BE, Khalili K, Amini S 2002. Evidence for regulation of long terminal repeat transcription by Wnt transcription factor TCF-4 in human astrocytic cells. J Virol 76: 11159–11165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Glinka A, Delius H, Niehrs C 2000. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/β-catenin signalling. Curr Biol 10: 1611–1614 [DOI] [PubMed] [Google Scholar]
- Xiao J-H, Ghosn C, Hinchman C, Forbes C, Wang J, Snider N, Cordrey A, Zhao Y, Chandraratna RAS 2003. Adenomatous polyposis coli (APC)-independent regulation of β-catenin degradation via a retinoid X receptor-mediated pathway. J Biol Chem 278: 29954–29962 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu J-H, Suda N, Tanaka KF, Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, et al. 2008. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135: 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Michiue T, Kikuchi A 2008. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of β-catenin signaling. Dev Cell 15: 37–48 [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Sumi T, Minami I, Otsuji TG, Kawase E, Nakatsuji N, Suemori H 2010. Cardiomyocytes develop from anterior primitive streak cells induced by β-catenin activation and the blockage of BMP signaling in hESCs. Genes Cells 15: 1216–1227 [DOI] [PubMed] [Google Scholar]
- Yang J, Si T, Ling Y, Ruan Y, Han Y, Wang X, Zhang H, Kong Q, Li X, Liu C, et al. 2003a. Association study of the human FZD3 locus with schizophrenia. Biol Psychiatry 54: 1298–1301 [DOI] [PubMed] [Google Scholar]
- Yang K, Fan K, Kurihara N, Shinozaki H, Rigas B, Augenlicht L, Kopelovich L, Edelmann W, Kucherlapati R, Lipkin M 2003b. Regional response leading to tumorigenesis after sulindac in small and large intestine of mice with Apc mutations. Carcinogenesis 24: 605–611 [DOI] [PubMed] [Google Scholar]
- Yip D, Le MN, Chan JL-K, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS 2009. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res 15: 3896–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, et al. 2004a. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res 64: 5385–5389 [DOI] [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F, et al. 2004b. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene 23: 6170–6174 [DOI] [PubMed] [Google Scholar]
- Yu Q, Sharma A, Sen JM 2010. TCF1 and β-catenin regulate T cell development and function. Immunol Res 47: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani A, Omrani GR, Nasab MM 2009. Lithium’s effect on bone mineral density. Bone 44: 331–334 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yeh JR, Mara A, Ju R, Hines JF, Cirone P, Griesbach HL, Schneider I, Slusarski DC, Holley SA 2006. A chemical and genetic approach to the mode of action of fumagillin. Chem Biol 13: 1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Appleton BA, Wiesmann C, Lau T, Costa M, Hannoush RN, Sidhu SS 2009. Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nat Chem Biol 5: 217–219 [DOI] [PubMed] [Google Scholar]
- Zhao D-M, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue H-H 2010. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol (Baltimore, MD, 1950) 184: 1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, Xue H-H 2010. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Molina JR, Harrington JJ, Osborn NK, Klatt KK, Romero Y, Burgart LJ, Ahlquist DA 2005. Aberrant methylation of secreted frizzled-related protein genes in esophageal adenocarcinoma and Barrett’s esophagus. Int J Cancer 116: 584–591 [DOI] [PubMed] [Google Scholar]