Abstract
Obesity is a common nutritional disorder associated with type 2 diabetes, cardiovascular diseases, dyslipidemia, and certain cancers. In this study, we investigated the effects of Citrus ichangensis peel extract (CIE) in high-fat (HF) diet-induced obesity mice. Female C57BL/6 mice were fed a chow diet or an HF diet alone or supplemented with 1% w/w CIE for 8 weeks. We found that CIE treatment could lower blood glucose level and improve glucose tolerance. In the HF+CIE group, body weight gain, serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) levels, and liver triglyceride (TG) and TC concentrations were significantly (P < 0.05) decreased relative to those in the HF group. To elucidate the mechanism of CIE on the metabolism of glucose and lipid, related genes expression in liver were examined. In liver tissue, CIE significantly decreased the mRNA expression levels of peroxisome proliferator-activated receptor γ (PPARγ) and its target genes, such as fatty acid synthase (FAS) and acyl-CoA oxidase (ACO). Moreover, CIE also decreased the expression of liver X receptor (LXR) α and β which are involved in lipid and glucose metabolism. These results suggest that CIE administration could alleviate obesity and related metabolic disorders in HF diet-induced obesity mice through the inhibition of PPARγ and LXR signaling.
1. Introduction
According to World Health Organization (WHO) estimates, there are 1.4 billion overweight adults worldwide and more than 500 million of these are obese [1]. Obesity is one of the most notorious symptoms of metabolic disorders throughout the world. It is considered to be a major risk factor for various chronic diseases, including type 2 diabetes, major cardiovascular diseases, hypertension, dyslipidemia, and certain cancers [2]. At present, only orlistat can be used for long-term weight reduction. However, these drugs are confounded by diminishing response in long-term treatment because of side effects and limited efficacies [3, 4]. Nutritional components may play a prominent role in the prevention and treatment of obesity and related metabolic disorders. Recently, there have been increasing efforts in research for new health-enhancing foods from natural products, and these findings also suggest that nutritional intervention could be an effective and promising strategy to inhibit obesity and obesity-related metabolic diseases [5, 6].
Citrus is one of the most important fruits in the world and is a rich source of nutrients and bioactive compounds. Citrus fruits not only provide ample vitamins, minerals, dietary fibers, and pectins but also provide an abundant of bioactive compounds, including flavonoids, coumarins, limonoids, and carotenoids. Currently, the study of bioactive compounds is one of the most active fields of food and medical science. Many epidemiological and experimental studies have provided convincing evidence that the intake of citrus fruits is beneficial to health [7–9]. Numerous prevention and treatment properties have been attributed to citrus fruits, like antioxidant, antiinflammatory, antitumor, anticardiovascular, and antiobesity properties [10–14]. Citrus fruits are usually consumed as fresh product or juice with peels and seeds discarded. Regretfully, these wastes are abundant sources of natural bioactive compounds [15]. In China, citrus peels like chenpi (dried peels of mature C. reticulate) or qingpi (dried peels of immature tangerine (C. reticulate)) have been commonly used in Chinese medicine for the treatment of a number of diseases, such as indigestion, bronchial asthma, vomiting, cough, skin inflammation, and muscle pain [16, 17]. Moreover, citrus peels have been extensively consumed as baked products, culinary seasonings, preserves, and food supplements in China for centuries. Recently, the prevention and treatment of obesity and obesity-related metabolic diseases of citrus peels have received increasing attention. Jung et al. found that Citrus unshiu peel extract inhibited lipid and triglyceride accumulation in 3T3-L1 adipocytes [18]. The extract from Citrus unshiu Mark induced the lipolysis in vitro [19]. A study by Bok et al. suggested that citrus peel diet reduced plasma and hepatic cholesterol in rats [20]. It has been reported that the immature Citrus sunki peel extract had an antiobesity effect by elevated β-oxidation and lipolysis in the adipose tissue of HF diet fed mice [21]. In addition, citrus phytochemicals, such as flavonoids, nomilin, synephrine, and auraptene, have exhibited antiobesity effects by increasing energy expenditure, improving metabolism, and enhancing lipolysis [22–25].
Emerging evidence suggests that PPARs are the targets of many citrus-derived flavonoids [26]. PPAR is the nuclear receptor transcription factor that is known to regulate carbohydrate and lipid metabolism in various tissues and cells [27]. The PPAR family includes three isoforms: PPARα, PPARγ, and PPARδ/β. PPARγ is an important regulator of adipocyte differentiation, lipogenesis, and glucose metabolism [28, 29]. Citrus flavonoids have been shown to inhibit adipogenesis and to decrease adiposity which can be explained in part by regulating the PPAR expression levels both in vivo and in vitro [30, 31]. It has been previously shown that citrus polymethoxylated flavones improve lipid and glucose homeostasis and restore insulin sensitivity through regulating the expression of PPARα and PPARγ [32, 33]. A recent study has suggested that Citrus aurantium flavonoids suppressed adipogenesis by inhibiting the expression of PPARγ in 3T3-L1 cells [31]. Studies have also identified that LXR is a target for metabolic diseases [34]. The citrus component naringin decreases serum lipid through the increase of PPARγ expression and inhibition of LXR expression in the liver of type 2 diabetic rats [34]. The grapefruit flavonoid naringenin has been reported to be an agonist of PPARα and PPARγ, and a partial agonist of LXRα [26].
Although citrus fruits are widely used in the pharmaceutical and food industries, researches on the functions of endemic citrus species remain insufficient. China has much abundant germplasm resources of the citrus fruits, but there is underutilization of these citrus resources. Citrus ichangensis Swingle is a unique citrus species grown in China and is known by its unusual hardiness and contains a wide range of bioactive compounds [35]. In horticulture, Citrus ichangensis was mainly used as rootstock of cultivated citrus due to its stress resistance and the fruit of Citrus ichangensis has been used in traditional Chinese medicine for a long history. It has been shown that Citrus ichangensis contains the complex pattern of flavones and a large amount of nonbitter deacetylnomilin [36]. Here, we investigate whether the long-term administration of Citrus ichangensis peel extract (CIE) would have beneficial effects on the prevention and treatment of obesity and its related metabolic diseases. In the present study, CIE was tested for body weight gain, lipid accumulation, and gene expression involved in glucose and lipid metabolism in HF diet-induced C57BL/6 mice.
2. Material and Methods
2.1. Preparation of Citrus ichangensis Peel Extract (CIE)
Citrus ichangensis Swingle was provided by the Citrus Research Institute, Chinese Academy of Agricultural Sciences, Chongqing China. Samples were prepared by adding 4 L of 95% ethanol to one kilogram of fresh citrus peel, extracting at 85°C for 2 h, cooling, and filtering the solution. The filtered solution was concentrated at 40°C with a rotary evaporator under reduced pressure, freeze-dried to a powder, and stored at −20°C until use. The frozen dried powder of CIE was added to the HF diet for the experiment.
2.2. HPLC Analysis
To determine the flavonoids content of CIE, high-performance liquid chromatography (HPLC) analysis was performed on an Agilent 1200 liquid chromatograph system. The flavonoid compounds were monitored at 280 nm using a Discovery C18 HPLC Column (250 × 4.6 mm, 5 μm). The column was operated at 30°C, and the injection volume was 10 μL. The mobile phase consisted of 100% acetonitrile (A) and water containing 0.5% acetic acid (B) at a flow rate of 1.0 mL/min. The gradient profile was as follows: 0–12 min, 85–75% B; 12–17 min, 75% B; 17–20 min, 75–50% B; 20–30 min, 50–25% B; 30–35 min, 25–5% B; and 35–40 min, back to 85% B.
2.3. Animals and Diets
The animal study protocols were approved by the Shanghai University of Traditional Chinese Medicine. Four-week-old female C57BL/6 mice were purchased from the SLAC Laboratory (Shanghai, China). Mice were kept under 22-23°C on a 12 h light/dark cycle. After a one-week adaptation period, C57BL/6 mice were randomly separated into three groups (n = 7) and were fed a chow diet (10% of calories derived from fat, Research Diets; D12450B), or an HF diet (60% of calories derived from fat, New Brunswick, NJ, Research Diets; D12492), either alone or supplemented with 1% CIE (HF+CIE) diet for 8 weeks. Food intake and body weight were measured every other day. The mice were given free access to food and water.
2.4. Intraperitoneal Glucose Tolerance Test
For intraperitoneal glucose tolerance test (ipGTT), all the mice were fasted for 12 h and a basal blood glucose levels (0 min) were determined from the tail vein. The mice were then intraperitoneally injected with glucose (1 g/kg body weight), and additional blood glucose levels were measured at 15, 30, 60, and 90 min.
2.5. Serum Chemistry Analysis
After overnight fasting, all mice were anesthetized with urethane before collecting blood samples for analysis. Blood samples were drawn from the heart into a vacuum tube and allowed to clot at room temperature for 30 min. Serum samples were separated from the blood and the serum triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) were analyzed using a Hitachi 7020 Automatic Analyzer. Serum lipid parameters were measured by Clinical Reagents following the manufacturer's instructions.
2.6. Liver and Fecal Lipid Content Analysis
The liver and other tissues were rapidly collected at the end of treatment, frozen in liquid nitrogen, and stored at −80°C for further experiments. 50 mg of frozen liver tissue was minced and homogenized in 1 mL of tissue lysis buffer (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton) and mixed with an equal volume of chloroform. The chloroform layer was separated, dried, and resuspended in 100 μL of isopropyl alcohol to measure the lipid levels as described above. Fecal lipids were extracted and measured as described above.
2.7. Morphological Analysis of Epididymal WAT
Scanning electron microscopy was used to examine the structure of epididymal white adipose tissue (WAT) by the method developed by Chun et al. [37]. The epididymal fat pads were fixed with 10% neutral formalin and postfixed in 1% osmium tetroxide. Samples were viewed using a Philip XL-30 scanning electron microscope with a magnifying power of ×200.
2.8. Quantitative Real-Time RT-PCR
Total RNA from liver tissue was extracted with a spin column (Qiagen, Germany) according to the manufacturer's protocol. The first-strand cDNA was synthesized using the cDNA synthesis kit (Fermentas, Madison, WI, USA). The gene expression levels were analyzed by quantitative real-time RT-PCR conducted using the ABI StepOnePlus real-time PCR system (Applied Biosystems, USA). The primers involved in the experiments were shown in Table 1. The cDNA was denatured at 95°C for 10 min followed by 40 cycles of PCR (95°C, 15 s, 60°C, 60 s). All results were obtained from at least three independent experiments. The expression levels of genes were normalized using β-actin as an internal control.
Table 1.
Sequences of the primers used in real-time PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β-Actin | TGTCCACCTTCCAGCAGATGT | AGCTCAGTAACAGTCCGCCTAGA |
| LXRα | GAGTGTCGACTTCGCAAATGC | CCTCTTCTTGCCGCTTCAGT |
| LXRβ | CAGGCTTGCAGGTGGAATTC | ATGGCGATAAGCAAGGCATACT |
| ABCA1 | GGCAATGAGTGTGCCAGAGTTA | TAGTCACATGTGGCACCGTTTT |
| ABCG1 | TCCCCACCTGTAAGTAATTGCA | TCGGACCCTTATCATTCTCTACAGA |
| ApoE | GAACCGCTTCTGGGATTACCT | TCAGTGCCGTCAGTTCTTGTG |
| CYP7A1 | GTGGTAGTGAGCTGTTGCATATGG | CACAGCCCAGGTATGGAATCA |
| SREBP1 | GGCTATTCCGTGAACATCTCCTA | ATCCAAGGGCATCTGAGAACTC |
| LPL | ATCGGAGAACTGCTCATGATGA | CGGATCCTCTCGATGACGAA |
| PGC-1β | GGGTGCGCCTCCAAGTG | TCTACAGACAGAAGATGTTATGTGAACAC |
| PPARγ | CGCTGATGCACTGCCTATGA | AGAGGTCCACAGAGCTGATTCC |
| aP2 | CATGGCCAAGCCCAACAT | CGCCCAGTTTGAAGGAAATC |
| ACC | GAATCTCCTGGTGACAATGCTTATT | GGTCTTGCTGAGTTGGGTTAGCT |
| ACO | CAGCACTGGTCTCCGTCATG | CTCCGGACTACCATCCAAGATG |
| UCP-2 | GGGCACTGCAAGCATGTGTA | TCAGATTCCTGGGCAAGTCACT |
| CD36 | GCTTGCAACTGTCAGCACAT | GCCTTGCTGTAGCCAAGAAC |
| FAS | CTGAGATCCCAGCACTTCTTGA | GCCTCCGAAGCCAAATGAG |
2.9. Statistical Analysis
All values are expressed as the mean ± SD unless otherwise indicated. Data analysis was performed by SPSS 12.0 software for Windows statistical program. Statistical analysis was programmed by one-way analysis of variance (ANOVA). Differences were defined as significant when P < 0.05.
3. Results
3.1. Flavonoid Contents in CIE
The flavonoid composition of CIE was detected through HPLC analysis. Figure 1 shows the levels of major citrus fruit flavonoids including neoeriocitrin, narirutin, naringin, hesperidin, neohesperidin, poncirin, naringenin, nobiletin, and tangeretin. The major flavonoids in CIE were naringin (8.12 mg/g), hesperidin (0.84 mg/g), and poncirin (1.33 mg/g). Naringin, the glycoside form of naringenin abundantly found in citrus fruits, possesses a wide range of pharmacological activities including antioxidative stress, anti-inflammatory, and anticancer effects.
Figure 1.

HPLC chromatograms of major flavonoids of citrus peel extract (CIE). (a) (1) Neoeriocitrin; (2) narirutin; (3) naringin; (4) hesperidin; (5) neohesperidin; (6) poncirin; (7) naringenin; (8) nobiletin; (9) tangeretin were analyzed as standards. (b) The major flavonoid components of CIE were determined to be compared to retention time of the chromatogram of standard: (3) naringin; (4) hesperidin; (6) poncirin.
3.2. CIE Blocks Body Weight Gain C57BL/6 Mouse Induced by HF Diet-Induced
To test the effects of CIE on the metabolic disorders, we fed the female C57BL/6 mice with a chow diet (Chow), or an HF diet alone or supplemented with 1% CIE (HF+CIE) diet for 8 weeks. The results showed that the mean body weight gain of the HF group was 91.9% more than those in the Chow group after 8 weeks of treatment, indicating the HF diet-induced obesity (Figure 2(a)). The body weight gain induced by HF diet was significantly suppressed by treatment with CIE from 2 weeks into the treatment period to the end of treatment (HF+CIE group). In this study, food intake in the HF+CIE group was roughly equivalent to that in the HF group (Figure 2(b)). As shown in Figures 2(e) and 2(f), the size of epididymal adipocytes was significantly elevated in the HF group compared to the Chow group after 8 weeks. Adipocyte size was markedly decreased in the HF+CIE group compared to the HF group. Furthermore, the concentrations of TG and TC in fecal material were slightly but not significantly higher in the HF+CIE diet-fed mice than the HF diet-fed mice (Figures 2(c) and 2(d)). This indicated that CIE slightly decreased lipid absorption or increased lipid excretion to antagonize diet-induced obesity. These results suggested that CIE could prevent diet-induced obesity independent of food intake inhibition and lipid absorption in the intestine.
Figure 2.
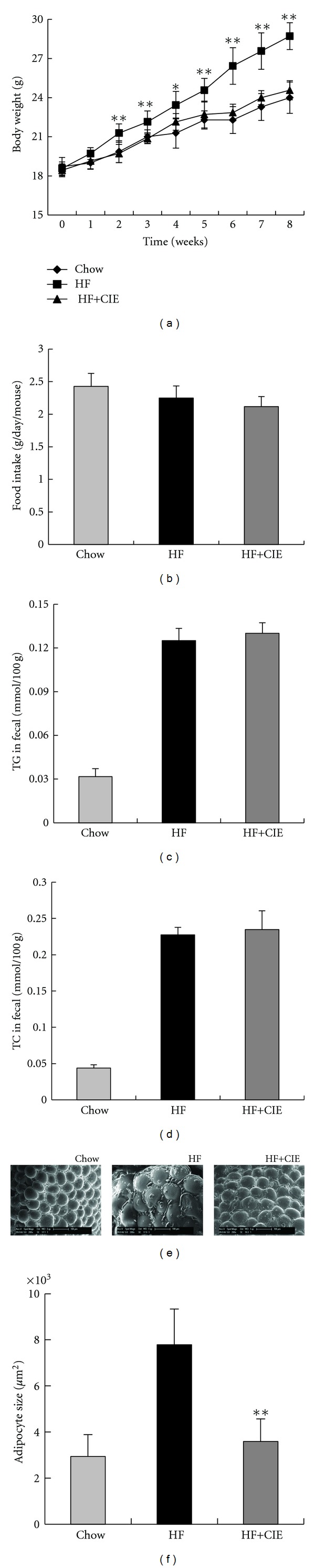
CIE prevents HF diet-induced weight gain. C57BL/6 mice were fed a chow diet (Chow) or an HF diet alone (HF) or supplemented with 1% of CIE (HF+CIE) for 8 weeks. (a) Body weight change; (b) food intake expressed as g/day/mouse; (c) TG concentrations in fecal material; (d) TC concentrations in fecal material; (e) epididymal WAT morphology are shown at 200x; (f) size of adipocytes. Values are expressed as means ± S.E. (n = 7; *P < 0.05, **P < 0.01 versus the HF group).
3.3. CIE Improves Glucose Tolerance and Attenuates Dyslipidemia
To understand the effects of CIE on the metabolic disorders, we analyzed the serum biochemical contents in the mice. HF fed mice showed a significant increase in fasting blood glucose levels compared to the Chow group mice (P < 0.05). In contrast, the CIE groups showed a statistically significant (P < 0.05) decrease in fasting blood glucose levels compared to the HF group (Figure 3(a)).
Figure 3.
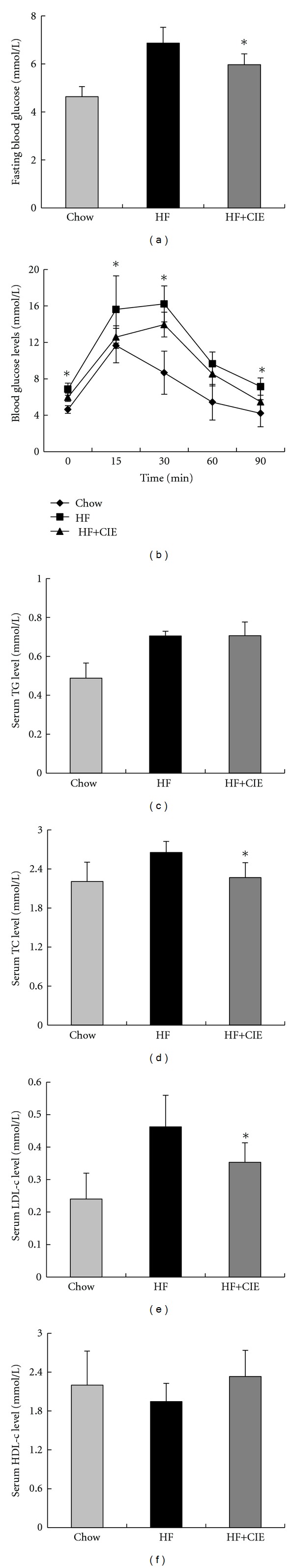
CIE decreases the blood glucose and serum lipid levels in HF diet-fed mice. Mice were fed a chow diet (Chow) or an HF diet alone (HF) or supplemented with 1% of CIE (HF+CIE) for 8 weeks. (a) Fasting blood glucose concentration. The mice were fasted for 12 hours and the tail vein blood was used to test the glucose level. (b) Glucose tolerance test was performed by intraperitoneal injection of glucose (1 g/kg body weight) into mice and blood glucoses were measured at 0, 15, 30, 60, and 90 min. ((c)–(f)) TG, TC, LDL-c and HDL-c concentrations in serum from fasted mice. Values are expressed as means ± S.E. (n = 7; *P < 0.05, **P < 0.01 versus the HF group).
We further tested ipGTT of the mice. As shown in Figure 3(b), the glucose levels were significantly increased in the HF group mice at 15, 30, 60, and 90 min following the injection of glucose, whereas the blood glucose levels in CIE-treated mice significantly decreased at 15, 30, and 90 min compared to the HF group. The total area under the curve of blood glucose levels between 0 to 90 min was 11.6 ± 1.7 mmol/L/min for the HF group and 9.8 ± 1.2 mmol/L/min for the HF+CIE group (P < 0.05). The results indicate that CIE treatment improved the glucose tolerance induced by an HF diet in the mouse.
The fasting serum TG, TC, and LDL-c concentrations of HF group were increased by 44.4%, 20.3%, and 91.1%, respectively, compared to the Chow group (Figures 3(c)–2(e)), whereas the level of HDL-c decreased by 8.7% when compared to the Chow group (Figure 3(f)). The levels of TC and LDL-c were markedly reduced by CIE treatment in HF diet-induced mice. However, a slight increase was observed in HDL-c in the HF+CIE groups when compared to the HF group. We did not observe the differences of the TG levels between the HF and HF+CIE groups (Figure 3(f)). These results indicate that CIE is effective in attenuating high-fat diet-induced risk for dyslipidemia in vivo.
3.4. CIE Prevents Hepatic Lipid Accumulation
The liver, one of the insulin-sensitive tissues, plays a pivotal role in the processes of hyperglycemia and dyslipidemia [38, 39]. Therefore, we examined the lipid contents in the liver of the mice. As shown in Figure 4, the levels of TG and TC in liver of HF group were increased 2.1 and 2.2 times, respectively, compared to the Chow group (P < 0.05). Supplementation with CIE significantly reduced TG and TC accumulation in liver compared to the HF group (P < 0.05). This finding suggests that CIE could block the HF diet-induced lipid accumulation in the liver.
Figure 4.
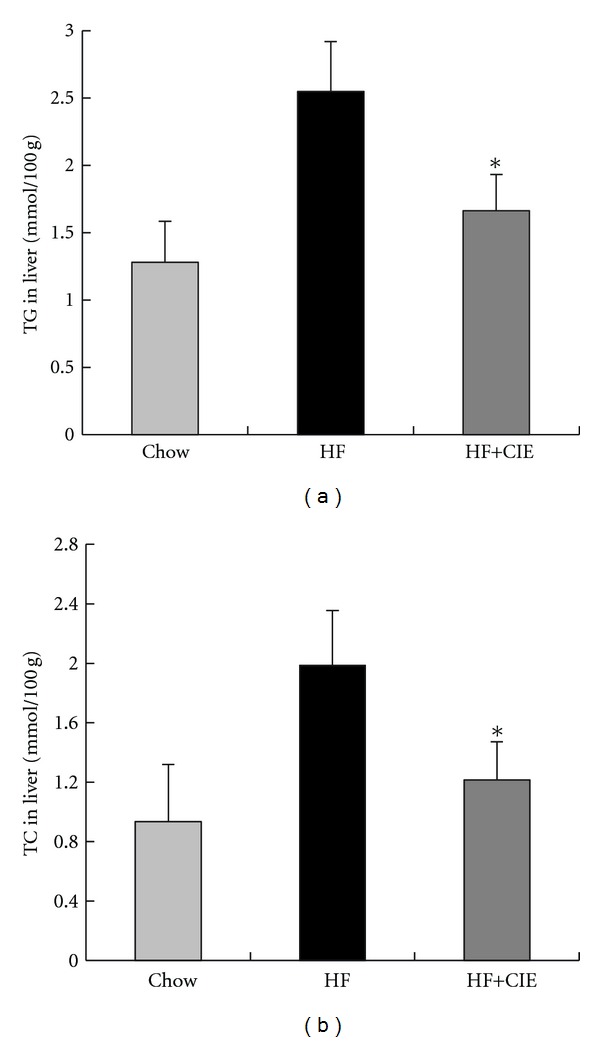
Effects of CIE on hepatic lipid levels in HF diet-fed mice. (a) TG concentrations in liver; (b) TC concentrations in liver. Values are expressed as means ± S.E. (n = 7; *P < 0.05, versus the HF group).
3.5. CIE Inhibits the Transactivities of PPARγ, LXRα, and LXRβ
To determine the mechanism of CIE ameliorates disorders of glucose and lipid metabolism, related genes' expressions in the liver were examined, as shown in Figures 5(a) and 5(b). PPARγ, one of the most important nuclear receptor transcription factors, regulates the expression of a group of genes involved in lipid and glucose metabolism. Compared with the HF group, CIE treatment notably decreased the mRNA expression of PPARγ in the mouse liver. Moreover, the mRNA levels of PPARγ target genes, including fatty acid synthase (FAS), acyl-CoA oxidase (ACO), and uncoupling protein 2 (UCP2), were also significantly decreased in the livers of HF+CIE group compared with the HF group. However, the expression of adipose fatty acid-binding protein (aP2), acetyl-CoA carboxylase (ACC), and PPAR coactivator1-β (PGC1β) were not changed significantly by CIE treatment. CIE increased the levels of CD36 mRNA, which is involved in low-density lipoprotein oxidation. These results indicate that CIE plays a role in the regulation of lipid and glucose homeostasis by regulating the expression of PPARγ and its target genes.
Figure 5.
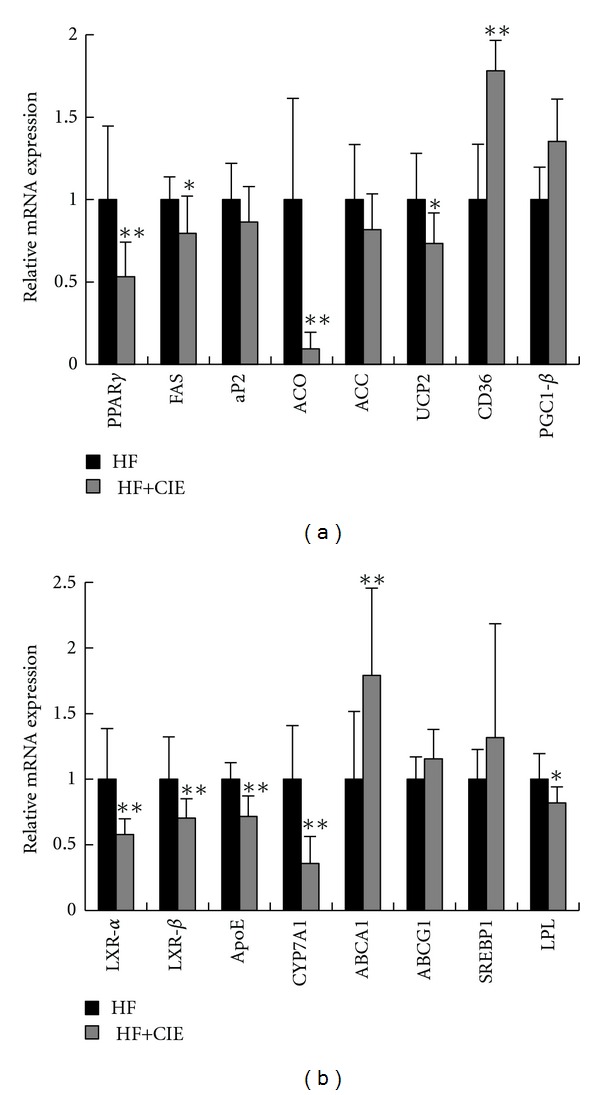
Effects of CIE on the relative mRNA expression in liver tissue. (a) PPARγ and its target genes. (b) LXR and its target genes. Beta-actin was used as an internal control. Values are expressed as means ± S.E. (n = 7; *P < 0.05, **P < 0.01, versus the HF group).
Next, we analyzed the mRNA abundance of LXR and its target genes that regulate fatty acids, cholesterol synthesis, and glucose metabolism, such as apolipoprotein E (ApoE), cytochrome P450 7A1 (CYP7A1), lipoprotein lipase (LPL), ATP-binding cassette subfamily G member 1 (ABCG1), ATP-binding cassette transporter A1 (ABCA1), and sterol regulatory element-binding transcription factor 1 (SREBP1), in the liver tissue of CIE-treated and untreated mice. As shown in Figure 5(b), the expression levels of ApoE, CYP7A1, LPL, LXRα, and LXRβ were significantly decreased in CIE-treated mice. There was no significant difference in the mRNA expression levels of ABCG1 and SREBP1 in the liver of the HF group compared with the HF+CIE group. These results indicate that CIE plays a role in metabolic disorders partly through LXR signaling.
4. Discussion
Citrus peels are rich in flavonoids that have various biological activities. We found that the major flavonoids in CIE were naringin, hesperidin, and poncirin, of which the highest was naringin. This is different from the findings from Citrus unshiu peel extract, which showed that the flavonoid compositions were hesperidin, narirutin, and naringin, with hesperidin being the highest [18]. Both hesperidin and naringin exhibit various biological and pharmacological effects, including antitumor, antiinflammatory, and antioxidant activities, and the potential to improve hyperglycemia, dyslipidemia, and hepatic steatosis in Type 2 diabetes [21, 40–42]. Hesperidin also exhibited hypoglycemic activity in STZ-induced diabetic rats [43]. In a clinical trial, it was demonstrated in hypercholesterolemia patients that naringin supplementation reduces LDL-c by 17% and TC by 14% [7]. Yoon et al. suggested that poncirin promotes osteoblast differentiation and prevents adipogenesis in mesenchymal stem cells [44]. Our results suggest that CIE may be used in the prevention and treatment of the metabolic disorders.
Our animal study showed that CIE could lower blood glucose levels and improve glucose tolerance. In the HF group, body weight gain, serum TC and LDL-c levels, and liver TG and TC levels were significantly increased relative to those in the Chow group and were improved by CIE supplementation. Similar to Citrus unshiu peel extract, the CIE also significantly reduced hepatic lipid content as well as blood glucose level. Furthermore, dietary intake of CIE effectively reduced body weight gain and epididymal WAT size in the experimental mice. These positive effects were due to the citrus flavanones such as naringin and hesperidin. We noticed that the food intake was not changed between the groups of HF and HF mixed with CIE, suggesting that the weight-reducing effects of CIE are not caused by suppressing appetite. Also, the fecal lipids were not altered in the CIE-treated mice, suggesting that the body weight reducing effect of CIE is not caused by the inhibition of lipid absorption in the intestine. These results provide convincing evidence that the extract of Citrus ichangensis has prevented HF diet-induced obesity and related metabolic disorders.
Citrus aurantium flavonoids show antiadipogenesis activity by downregulating the expression of PPARγ in 3T3-L1 cells. In the present study, CIE treatment decreased the gene expression of major glucose and lipid metabolism regulators, including PPARγ and LXRs. The active element of Citrus ichangensis probably is naringin, that is, the richest flavonoid in the extract. PPARγ is a major nuclear receptor transcription factor for adipogenesis and lipogenesis. It regulates the expression of a group of genes, including CD36, ACC, ACO, UCP2, FAS, and aP2, which are related to fatty acid synthesis, oxidation, and adipogenesis. It has been shown that PPARγ antagonists can prevent and treat HF diet-induced obesity [45]. Gong et al. reported that suppressing PPARγ-activity can inhibit adipocyte differentiation in vitro [46]. Our in vivo studies have showed that CIE efficiently suppressed the gene expression of PPARγ in the liver tissue. The expressions of FAS, aP2, ACO, and UCP2 were also significantly decreased in the liver of mice treated with CIE. Moreover, the lipid levels of the liver were significantly lower in the HF+CIE group than in the HF group. These results indicate that CIE may reduce fat weight through the regulation of PPARγ signaling.
To detect other prospective molecular targets through which CIE exhibits anti-metabolic disorders effects, we examined the transactivity of LXR in liver tissue. LXR is known to play a major role in regulation of cholesterol, fatty acid, and glucose homeostasis metabolism [47]. It has been demonstrated that naringenin may decrease serum lipid levels by inhibiting the activation of LXRα [26], indicating that the inhibition of LXR also has a therapeutic role. In this study, both LXRα and LXRβ transactivities were inhibited by CIE. The mRNA expression of LXR target genes such as ApoE, CYP7A1, ABCA1, ABCG1, SREBP1, and LPL were further confirmed. The expression levels of ApoE, CYP7A1, and LPL were significantly decreased in the CIE+HF group in comparison to the HF group. However, ABCG1 mRNA expression was not changed significantly by CIF treatment. These results demonstrate that CIE regulates cholesterol and glucose metabolism partly through LXR antagonism in high-fat diet-induced obese mice.
In conclusion, we found that CIE prevents the development of obesity induced by a HF diet and lowers hyperlipidemia and hyperglycemia, while protecting against the lipid accumulation in the liver. These effects may involve multi-molecular targets, including the inhibition of PPARγ and LXRs, in the liver tissue. Our results suggest that Citrus ichangensis could be used as a dietary supplement for antiobesity and hyperlipidemia-lowering therapy. However, the mechanism needs to be further investigated.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (31171930).
References
- 1.W.H. Organization. Obesity and overweight. 2011, http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. Risk factor, paradox, and impact of weight loss. Journal of the American College of Cardiology. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 3.Fernstrom JD, Choi S. The development of tolerance to drugs that suppress food intake. Pharmacology and Therapeutics. 2008;117(1):105–122. doi: 10.1016/j.pharmthera.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Annals of Internal Medicine. 2005;142(7):532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 5.Vasudeva N, Yadav N, Sharma SK. Natural products: a safest approach for obesity. Chinese Journal of Integrative Medicine. 2012;18(6):473–480. doi: 10.1007/s11655-012-1120-0. [DOI] [PubMed] [Google Scholar]
- 6.Choudhary M, Grover K. Development of functional food products in relation to obesity. Functional Foods in Health and Disease. 2012;2:188–197. [Google Scholar]
- 7.Jung UJ, Kim HJ, Lee JS, et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clinical Nutrition. 2003;22(6):561–568. doi: 10.1016/s0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 8.Gorinstein S, Caspi A, Libman I, et al. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. Journal of Agricultural and Food Chemistry. 2006;54(5):1887–1892. doi: 10.1021/jf058171g. [DOI] [PubMed] [Google Scholar]
- 9.Okwu DE. Citrus fruits: a rich source of phytochemicals and their roles in human health. International Journal of Chemical Sciences. 2008;6:451–471. [Google Scholar]
- 10.Tanaka T, Tanaka M, Kuno T. Cancer chemoprevention by citrus pulp and juices containing high amounts of beta-cryptoxanthin and hesperidin. Journal of Biomedicine and Biotechnology. 2012;2012:10 pages. doi: 10.1155/2012/516981.516981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada T, Hayasaka S, Shibata Y, et al. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi medical school cohort study. Journal of Epidemiology. 2011;21(3):169–175. doi: 10.2188/jea.JE20100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preuss HG, DiFerdinando D, Bagchi M, Bagchi D. Citrus aurantium as a thermogenic, weight-reduction replacement for eprhedra: an overview. Journal of Medicine. 2002;33(1–4):247–264. [PubMed] [Google Scholar]
- 13.Wu SJ, Ng CC, Tzeng WS, Ho KC, Shyu YT. Functional antioxidant and tyrosinase inhibitory properties of extracts of Taiwanese pummelo (Citrus grandis Osbeck) African Journal of Biotechnology. 2011;10(39):7668–7674. [Google Scholar]
- 14.Shin HS, Kang SI, Ko HC, et al. Anti-inflammatory effect of the immature peel extract of Jinkyool (Citrus sunki Hort. ex Tanaka) Food Science and Biotechnology. 2011;20(5):1235–1241. [Google Scholar]
- 15.Manthey JA, Grohmann K. Phenols in citrus peel byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. Journal of Agricultural and Food Chemistry. 2001;49(7):3268–3273. doi: 10.1021/jf010011r. [DOI] [PubMed] [Google Scholar]
- 16.Neville F, Pchelintsev NA, Broderick MJF, Gibson T, Millner PA. Novel one-pot synthesis and characterization of bioactive thiol-silicate nanoparticles for biocatalytic and biosensor applications. Nanotechnology. 2009;20(5) doi: 10.1088/0957-4484/20/5/055612.055612 [DOI] [PubMed] [Google Scholar]
- 17.S.P. Committee. Chinese Pharmacopoeia. Beijing, China: Chemical Industry Press; 2005. [Google Scholar]
- 18.Jung HK, Jeong YS, Park CD, Park CH, Hong JH. Inhibitory effect of citrus peel extract on lipid accumulation of 3T3-L1 adipocytes. Journal of Applied Biological Chemistry. 2011;54(2):169–176. [Google Scholar]
- 19.Tsujita T, Takaku T. Lipolysis induced by segment wall extract from Satsuma mandarin orange (Citrus unshu Mark) Journal of Nutritional Science and Vitaminology. 2007;53(6):547–551. doi: 10.3177/jnsv.53.547. [DOI] [PubMed] [Google Scholar]
- 20.Bok SH, Lee SH, Park YB, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3- methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. Journal of Nutrition. 1999;129(6):1182–1185. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- 21.Kang SI, Shin HS, Kim HM, et al. Immature Citrus sunki peel extract exhibits antiobesity effects by beta-oxidation and lipolysis in high-fat diet-induced obese mice. Biological and Pharmaceutical Bulletin. 2012;35(2):223–230. doi: 10.1248/bpb.35.223. [DOI] [PubMed] [Google Scholar]
- 22.Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean-Chaney S, Allison DB. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obesity Reviews. 2006;7(1):79–88. doi: 10.1111/j.1467-789X.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 23.Ono E, Inoue J, Hashidume T, Shimizu M, Sato R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochemical and Biophysical Research Communications. 2011;410(3):677–681. doi: 10.1016/j.bbrc.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 24.Nagao K, Yamano N, Shirouchi B, et al. Effects of citrus auraptene (7-Geranyloxycoumarin) on hepatic lipid metabolism in vitro and in vivo . Journal of Agricultural and Food Chemistry. 2010;58(16):9028–9032. doi: 10.1021/jf1020329. [DOI] [PubMed] [Google Scholar]
- 25.Mulvihill EE, Huff MW. Protection from metabolic dysregulation, obesity, and atherosclerosis by citrus flavonoids: activation of hepatic PGC1α-mediated fatty acid oxidation. PPAR Research. 2012;2012:9 pages. doi: 10.1155/2012/857142.857142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARα, PPARγ and LXRα . PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012399.e12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144(6):2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 28.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nature Medicine. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 29.Pakala R, Rha SW, Kuchulakanti PK, Cheneau E, Baffour R, Waksman R. Peroxisome proliferator-activated receptor γ: its role in atherosclerosis and restenosis. Cardiovascular Radiation Medicine. 2004;5(1):44–48. doi: 10.1016/j.carrad.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Cho KW, Kim YO, Andrade JE, Burgess JR, Kim YC. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor α protein expression and decreases plasma triglyceride and adiposity in rats. European Journal of Nutrition. 2011;50(2):81–88. doi: 10.1007/s00394-010-0117-8. [DOI] [PubMed] [Google Scholar]
- 31.Kim GS, Park HJ, Woo JH, et al. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complementary and Alternative Medicine. 2012;12, article 31 doi: 10.1186/1472-6882-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li RW, Theriault AG, Au K, et al. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sciences. 2006;79(4):365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Kurowska EM, Manthey JA, Casaschi A, Theriault AG. Modulation of HepG2 cell net apolipoprotein B secretion by the citrus polymethoxyflavone, tangeretin. Lipids. 2004;39(2):143–151. doi: 10.1007/s11745-004-1212-8. [DOI] [PubMed] [Google Scholar]
- 34.Sharma AK, Bharti S, Ojha S, et al. Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. British Journal of Nutrition. 2011;106(11):1713–1723. doi: 10.1017/S000711451100225X. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel-Roy P, Goldschmidt EE. Biology of Citrus. New York, NY, USA: Cambridge University Press; 1996. [Google Scholar]
- 36.Herman Z, Hasegawa S, Fong CH, Ou P. Limonoids in Citrus ichangensis . Journal of Agricultural and Food Chemistry. 1989;37(4):850–851. [Google Scholar]
- 37.Chun TH, Inoue M, Morisaki H, et al. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010;59(10):2484–2494. doi: 10.2337/db10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annual Review of Biochemistry. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 39.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. The Journal of Biological Chemistry. 2000;275(12):8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 40.Choi MS, Do KM, Park YB, et al. Effect of naringin supplementation on cholesterol metabolism and antioxidant status in rats fed high cholesterol with different levels of vitamin E. Annals of Nutrition and Metabolism. 2001;45(5):193–201. doi: 10.1159/000046729. [DOI] [PubMed] [Google Scholar]
- 41.Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. International Journal of Biochemistry and Cell Biology. 2006;38(7):1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. Journal of Nutrition. 2004;134(10):2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 43.Akiyama S, Katsumata SI, Suzuki K, Ishimi Y, Wu J, Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. Journal of Clinical Biochemistry and Nutrition. 2010;46(1):87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon HY, Yun SI, Kim BY, et al. Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells. European Journal of Pharmacology. 2011;664(1–3):54–59. doi: 10.1016/j.ejphar.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Fan S, Hu N, et al. Rhein reduces fat weight in db/db mouse and prevents diet-induced obesity in C57Bl/6 mouse through the inhibition of PPARγ signaling. PPAR Research. 2012;2012:9 pages. doi: 10.1155/2012/374936.374936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong Z, Huang C, Sheng X, et al. The role of Tanshinone IIA in the treatment of obesity through peroxisome proliferator-activated receptor γ antagonism. Endocrinology. 2009;150(1):104–113. doi: 10.1210/en.2008-0322. [DOI] [PubMed] [Google Scholar]
- 47.Mitro N, Mak PA, Vargas L, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445(7124):219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]


