Abstract
Varicella-zoster virus (VZV) is a ubiquitous, highly cell-associated, and exclusively human neurotropic alphaherpesvirus. VZV infection is initiated by membrane fusion, an event dependent in part on VZV glycoproteins gH and gL. Consistent with its location on the virus envelope, the gH/gL complex is a target of neutralizing antibodies produced after virus infection. One week after immunizing a 59-year-old VZV-seropositive man with Zostavax, we sorted his circulating blood plasma blasts and amplified expressed immunoglobulin variable domain sequences by single-cell PCR. Sequence analysis identified two plasma blast clones, one of which was used to construct a recombinant monoclonal antibody (rec-RC IgG). The rec-RC IgG colocalized with VZV gE on the membranes of VZV-infected cells and neutralized VZV infection in tissue culture. Mass spectrometric analysis of proteins immunoprecipitated by rec-RC IgG identified both VZV gH and gL. Transfection experiments showed that rec-RC IgG recognized a VZV gH/gL protein complex but not individual gH or gL proteins. Overall, our recombinant monoclonal anti-VZV antibody effectively neutralizes VZV and recognizes a conformational epitope within the VZV gH/L protein complex. An unlimited supply of this antibody provides the opportunity to analyze membrane fusion events that follow virus attachment and to identify multiple epitopes on VZV-specific proteins.
INTRODUCTION
Varicella-zoster virus (VZV), the etiologic agent of varicella (chickenpox) and zoster (shingles), is an exclusively human pathogen and a member of the Herpesviridae family of enveloped DNA viruses. Herpesviruses cause both lytic and latent infections. Lytic infection requires membrane fusion, an event governed by a core complex consisting of conserved glycoproteins gB, gH, and gL. Along with membrane fusion, VZV gH and gL are also involved in virus egress and are essential for virus replication (1–4). In addition to mediating virus entry, VZV glycoproteins can traffic from infected cells to uninfected cells (5). VZV glycoproteins induce strong humoral immune responses both in naturally infected individuals and in varicella or zoster vaccine recipients (6–10). While VZV gE is the most immunogenic and predominant glycoprotein in VZV-infected cell membranes, antibodies to VZV gH are the major neutralizing antibodies (11–16). It is likely that neutralizing activity by the gH/gL complex effectively prevents cell-to-cell virus spread (5, 17–20).
Analysis of VZV attachment and membrane fusion requires highly specific neutralizing antibodies. Hybridoma cell lines and phage display libraries produce human anti-VZV gE and gH monoclonal antibodies (MAbs) that neutralize virus infection (21–24). Monoclonal antibodies that recognize the VZV gH/gL protein complex hold promise in therapies involving passive transfer of neutralizing VZV antibodies (15, 16, 25, 26). Here we present a new method for constructing a recombinant human monoclonal anti-VZV antibody and show that this antibody detects a conformational epitope on the gH/gL complex and neutralizes virus.
MATERIALS AND METHODS
Cells and virus.
Human lung fibroblast (HFL) and human embryonic kidney (HEK-EBNA 293) cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium supplemented with 4 mM l-glutamine (DMEM; Sigma-Aldrich, St. Louis, MO) and 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA). VZV was propagated by cocultivating infected cells with uninfected cells as described previously (27). Infected HFL cultures were harvested at the height of virus-induced cytopathic effect, usually at 3 days postinfection (dpi).
Construction of recombinant antibody.
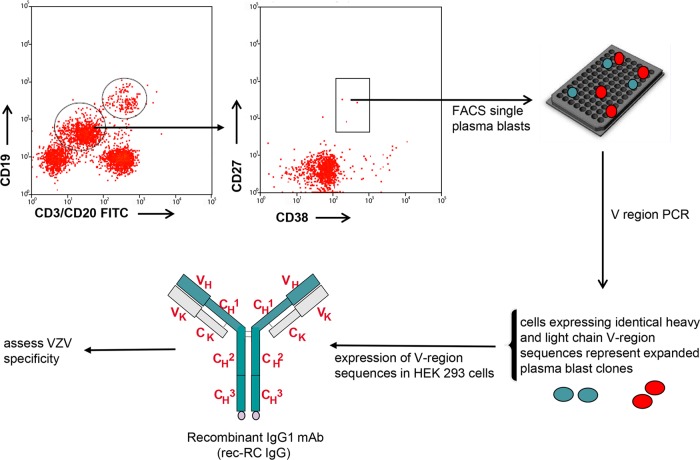
Blood was collected 7 days after immunization with zoster vaccine (Zostavax; Merck, Whitehouse Station, NJ) from a consenting healthy 59-year-old man with a history of varicella at age 7 and who was not taking any immunosuppressive drugs. White blood cells were isolated using Vacutainer CPT tubes (Becton-Dickinson, Franklin Lakes, NJ). B cell subsets and circulating CD19+ CD20− CD38++ CD27++ plasma blasts were sorted using specific fluorescent antibodies against CD3 (fluorescein isothiocyanate [FITC]), CD20 (FITC), CD14 (FITC), CD19 (Pac-blue), CD27 (R-phycoerythrin [R-PE]), and CD38 (allophycocyanin [APC]-Cy5.5), as described previously (28) and as diagrammed in Fig. 1. Plasma blasts were deposited at 1 cell per well in 96-well plates and lysed, and cDNA was synthesized immediately as described previously (29). Heavy and light chain variable (V) region sequences expressed by single plasma blasts were amplified using a set of family-based leader and framework primers (29). V region sequences were analyzed using DNAsis software (San Francisco, CA), and sequences were aligned to immunoglobulin (Ig) databases (V base [http://www2.mrc-lmb.cam.ac.uk/vbase]) to identify the closest human VH and VL germ line sequences. Flag-tagged bivalent human IgG1 antibodies were produced in HEK293 cells from a single plasma blast clone, using established vectors for expression (30), affinity purified on protein A-Sepharose beads, concentrated to about 500 μl using Centricon YM 30 centrifugal filter devices (Millipore, Bedford, MA), and dialyzed overnight at 4°C in phosphate-buffered saline (PBS). The antibody, named rec-RC IgG, was quantified (bicinchoninic acid [BCA] protein assay; Pierce Chemical, Rockford, IL), supplemented with 0.1% protease-free and IgG-free bovine serum albumin–0.002% NaN3, and stored at 4°C (30).
Fig 1.
Cloning of VZV-specific monoclonal antibodies from the circulating plasma blast pool after Zostavax immunization. Seven days after Zostavax immunization, circulating plasma blasts (CD19+ CD27+ CD38+ CD3− CD20−) were sorted (1 cell/well), and heavy and light chain variable regions from each cell were amplified. One pair of identical heavy and light chain variable regions was expressed in HEK293 cells, producing the monoclonal recombinant antibody rec-RC IgG. Dot plots show the expression of CD27 and CD38 among CD19-gated cells that were CD3 and CD20 negative. FITC, fluorescein isothiocyanate; FACS, fluorescence-activated cell sorting; VH, heavy chain variable region; CH, heavy chain constant region; VK, kappa light chain variable region; CK, kappa light chain constant region.
Immunohistochemistry.
Immunostaining was performed on fixed and unfixed VZV-infected and control HFL cells at 3 dpi. To immunostain fixed cells, samples were incubated in 4% paraformaldehyde (PFA) for 20 min and permeabilized with 0.3% Triton X solution for 10 min. Cells were blocked with 5% normal goat serum (NGS) for 30 min, washed with PBS, and incubated with primary antibody (1:5,000 dilution of rabbit polyclonal anti-VZV 63 antibody [31], 0.2 μg/ml mouse monoclonal anti-VZV gE antibody [Santa Cruz Biotechnologies, Santa Cruz, CA], or 1 μg/ml rec-RC IgG). After a PBS wash, samples were incubated with 2 μg/ml secondary antibody conjugated to the Alexa 488 or Alexa 594 fluorochrome (goat anti-rabbit IgG, goat anti-mouse IgG, donkey anti-mouse IgG, goat anti-human IgG, or donkey anti-human IgG) and sealed with mounting medium containing DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA). To immunostain proteins expressed on cell surface membranes, samples were first incubated for 45 min with rec-RC IgG (1.25 μg/ml) or mouse anti-VZV gE MAb (0.2 μg/ml), fixed with 4% PFA, blocked with 5% NGS, incubated with secondary antibodies, and sealed as described above. All samples were viewed using a Nikon Eclipse E800 microscope, and images were captured with a Carl Zeiss Axio Cam microscope using Axiovision digital imaging software. The final magnification was ×360.
Plaque reduction antibody neutralization assay.
Aliquots of VZV-infected HFL cells were incubated for 60 min at 37°C in 50 μl DMEM containing increasing amounts of rec-RC IgG (0.0125, 0.125, 1.25, and 12.5 μg). Surviving virus was titrated on subconfluent HFL monolayers propagated in 1 ml of 10% FBS-DMEM in 12-well plates. VZV plaques were quantified after staining with rabbit polyclonal anti-VZV 63 (31). Plaque reduction is expressed as percent virus survival for triplicate experiments.
Immunoprecipitation and protein identification.
Proteins located on the cell membrane surface were immunoprecipitated as described previously (32). Briefly, VZV-infected and uninfected HFL cells were incubated for 1 h at 37°C in DMEM containing 5 μg/ml rec-RC IgG. Cells were washed with PBS, lysed for 1 h on ice in 150 mM NaCl, 1 mM EDTA, 100 mM Tris-HCl (pH 7.5), 0.5% deoxycholate acid, and 1% Triton X-100 supplemented with protease inhibitors (Roche Diagnostics, Mannheim, Germany), and centrifuged (16,100 × g for 20 min). The supernatant was incubated with protein A/G agarose beads (Pierce, Rockford, IL) overnight at 4°C and collected by centrifugation (1,000 × g for 60 s). Beads containing the immunocomplex were washed with PBS, boiled in Laemmli buffer (Bio-Rad Laboratories, Hercules, CA), and separated by electrophoresis on 4 to 15% gradient SDS-polyacrylamide gels, and proteins were visualized with EZBlue gel staining (Sigma-Aldrich, St. Louis, MO). Distinct VZV-specific protein bands were excised, digested with trypsin, and identified by mass spectrometry at the Proteomics Facility of the University of Colorado School of Medicine.
Cloning of VZV ORF 37 (gH) and ORF 60 (gL).
VZV ORF 37- and ORF 60-specific primers were designed based on the prototype VZV DNA sequence (33) and were synthesized by Integrated DNA Technologies (Coralville, IA). ORF 37 primers were as follows: forward, 5′-GCT AGC ATG TTT GCG CTA GTT TTA GC-3′; and reverse, 5′-CTC GAG TGT CAG AGG TAT TTT ATT ATA TTC TCG-3′. VZV ORF 60 primers were as follows: forward, 5′-GCT AGC ATG GCA TCA CAT AAA TGG TTA CTG-3′; and reverse, 5′-GCG GCC GCT CAG TCG ACT CAA TGA TGG TGG TGA TGG TGC TCG AGT TGG CAT ACG CGT TGG-3′. PCR products were verified by gel electrophoresis, T-A ligated into Topo-TA vector (Invitrogen, Carlsbad, CA), and subcloned into the eukaryotic expression plasmid PCI-Neo (Invitrogen) at unique XhoI and NheI restriction endonuclease recognition sites. Final plasmid construction was verified by DNA sequence analysis, and the plasmid was maintained in Escherichia coli XL1-Blue (Stratagene, La Jolla, CA).
Plasmid transfection.
VZV ORF 37 and/or ORF 60 expression plasmid DNA was transfected into HEK293 cells seeded on glass coverslips by use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Cells were harvested for immunostaining at 2 to 3 dpi. Each transfection was performed 3 times. Fixed HEK293 cells were immunostained as described above, using rec-RC IgG at 1.25 μg/ml as the primary antibody.
RESULTS
Flow cytometry and construction of rec-RC monoclonal IgG.
Fluorescence-activated cell sorter (FACS) and single-cell PCR analyses of the V region repertoire of circulating blood plasma blasts obtained at 7 days post-Zostavax immunization identified two clones with identical V region cDNA sequences among 33 cells (Table 1). The paired heavy and light chain V regions of both clones utilized VH1 and VK1 family gene segments, respectively, and were rearranged IgG1 molecules showing extensive somatic hypermutation. Clone 1 (Table 1) expressed a heavy chain derived from the DP-8 germ line (locus 1-02), with 93.7% sequence homology; the paired light chain was derived from the DPK 8 germ line (locus L8), with 94.0% homology. The second clone (Table 1) had a heavy chain derived from the DP-14 germ line (locus 1-18), with 91.1% homology, and a light chain derived from the DPK 1 germ line (locus O18), with 94.6% homology. V regions from clone 1 were inserted into pCEP4 expression vectors, and recombinant antibody (rec-RC) was purified from the supernatant of HEK-EBNA 293 cells following transient transfection. The complete amino acid sequences of the heavy and light chain variable regions are presented in Table 2.
Table 1.
CDR3 sequences of heavy and light chains amplified from single plasma blasts
| Chain and sequencea | Family |
|---|---|
| CDR3-VH | |
| GGMTMVRGVMMDb | VH1 |
| GGMTMVRGVMMD | VH1 |
| VFYYDSLLWSFDYd | VH1 |
| VFYYDSLLWSFDY | VH1 |
| AKVLGYCSGDNCYCQYYGLDV | VH4 |
| GGALRGTYSLGLNPPRKGYFDY | VH4 |
| DNYDLWSANYGGMDV | VH4 |
| ND | ND |
| DRCDTTRCYLGRFDP | VH3 |
| TLLYNWNYYYGMDV | VH4 |
| VAAVYFTYYYYYTMDV | VH3 |
| SPRAYDILTGYLSREYYYYYGMDV | VH3 |
| GAHRLALRYFRY | VH4 |
| ND | VH3 |
| TVKPSQYDYINWFDP | VH1 |
| GRAGRKYCRGSTCYTTHYFDY | VH4 |
| GRRGVYALQRACYFDY | VH4 |
| DRTYGYPTFDF | VH1 |
| HLRYSGSSLDS | VH4 |
| DSRQVFGLMKGDYNYGIDV | VH3 |
| IQGTFYYDSNGYYSHHYLLLPVGR | VH2 |
| DRGVICSAGTCYLASFDI | VH4 |
| DHSGSATYYRDYYDMDV | VH3 |
| ND | ND |
| ND | ND |
| KGESSGSYPLAY | VH3 |
| MRGIWDYFDH | VH3 |
| HFPAQTTLFGAAPDI | VH1 |
| DLKPTPPYSTFGRVIRPEKYIYSHGLDV | VH3 |
| SLSQITSEIVAVIPATVDYYGMDVM | VH1 |
| SVLLYSLLWSFDY | VH1 |
| DRAAWVVIAVFDS | VH3 |
| DGLRYPVRPYGMDV | VH1 |
| CDR3-VL | |
| QQLNSYSLTc | VK1 |
| QQLNSYSLT | VK1 |
| QQYDVPPPSe | VK1 |
| ND | ND |
| QQSFTTLFS | VK1 |
| QQYSSYPYYT | VK1 |
| LQHNSYPYT | VK1 |
| QQYGSLPYT | VK3 |
| QQYLDTPF | VK4 |
| QQYGGARPLT | VK3 |
| QQSFCTRTWT | VK1 |
| MQTLQTLT | VK2 |
| QQGYTPPFT | VK1 |
| QQRSKWPVT | VK3 |
| QQVKSYPVH | VK1 |
| QQYNNWPSYT | VK3 |
| QQYNSYSPWT | VK1 |
| QQSYSSVCT | VK1 |
| ND | ND |
| LQYNIYPRT | VK1 |
| QQSYSTPPT | VK1 |
| QQYNNYSPWT | VK1 |
| QQSHSPPLT | VK1 |
| MQGLQTHT | VK2 |
| MQSIQLPLT | VK2 |
| QQYGSSPPWT | VK3 |
| QQRTNWPVT | VK3 |
| FQAMSPRT | VK3 |
| QQTDSVPLT | VK1 |
| QQTDSVPLT | VK1 |
| ND | ND |
| ND | ND |
| QQSYSPLSYT | VK1 |
CDR, complementarity-determining region; VH, heavy chain variable region; VL, light chain variable region ; ND, not determined.
Amino acid sequence of CDR3-VH clone 1.
Amino acid sequence of CDR3-VL clone 1.
Amino acid sequence of CDR3-VH clone 2.
Amino acid sequence of CDR3-VL clone 2.
Table 2.
Amino acid sequences of heavy and light chain variable regions of rec-RC IgG
| IgG chain | Amino acid sequence of variable regiona |
|---|---|
| Heavy | QVQLVQSGAEMKKPGASVKVSCKASGYTFIGYHLHWVRQAPGQGLEWMGWINPNSGETNYAQKFQDWVTMTRDTSINTAYMELRLRSDDTAVYYCARGGMTMVRGVMMDWGQGTLVTVSSASTKGb |
| Light | DIQMTQSPSFLSASVGDRVTITCRASQGLDNFLAWYQQKPGKAPKLLIYAASTLQRGVPSRFGGSGSGTEFTLTISSLQPEDFATYYCQQLNSYSLTFGPGTKVEIKRTVAAPSc |
Conserved cysteines involved in disulfide binding are shown in bold.
The heavy chain CDR3 region is underlined, and the start of the heavy chain constant region is double underlined.
The light chain CDR3 region is underlined, and the start of the light chain constant region is double underlined.
rec-RC IgG antibody immunostains VZV-infected human lung fibroblasts.
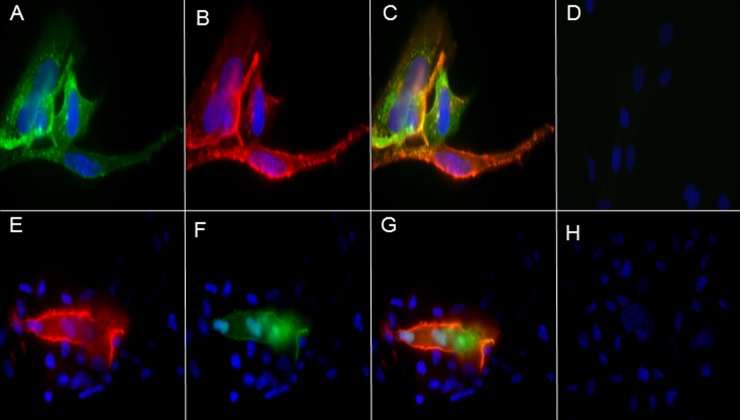
Recombinant antibody was used to immunostain uninfected and VZV-infected cells. Only infected cells were positive for viral antigen (Fig. 2). Colocalization of VZV antigen predominantly at the cell membrane was seen when fixed infected cells were immunostained with rec-RC IgG and anti-VZV gE (Fig. 2C). Immunostaining of unfixed VZV-infected cells with rec-RC IgG revealed VZV antigen in the membranes of multinucleated giant cells (Fig. 2E), and staining was colocalized when anti-VZV gE antibody and rec-RC IgG were used (Fig. 2G); nuclear staining was also seen when unfixed infected cells were stained with anti-VZV gE antibody (Fig. 2F).
Fig 2.
VZV antigen detected by immunostaining with rec-RC IgG on fixed and unfixed human lung fibroblasts. VZV-infected HFLs cultured on coverslips were fixed in 4% paraformaldehyde at the height of cytopathic effect (CPE) and then stained with rec-RC IgG and anti-VZV gE. Infected cells stained positive with rec-RC IgG (green) (A) and anti-VZV gE (red) (B); the merged image (C) shows colocalization of both stains. (D) Uninfected cells showed no staining with either antibody. At the height of CPE, unfixed VZV-infected HFLs also showed positive membrane staining of multinucleated infected cells with rec-RC IgG (red) (E) and anti-VZV gE (green) (F), with positive staining also seen in merged images (G), while uninfected cells showed no antibody staining (H). Magnification, ×360.
rec-RC IgG neutralizes VZV infection.
VZV was incubated with multiple dilutions of rec-RC IgG, and surviving virus was determined by plaque formation. A linear pattern of decreased plaque formation was seen with increasing concentrations of rec-RC IgG (Fig. 3). The concentration of rec-RC IgG that produced 50% plaque reduction was 0.6 μg/ml.
Fig 3.
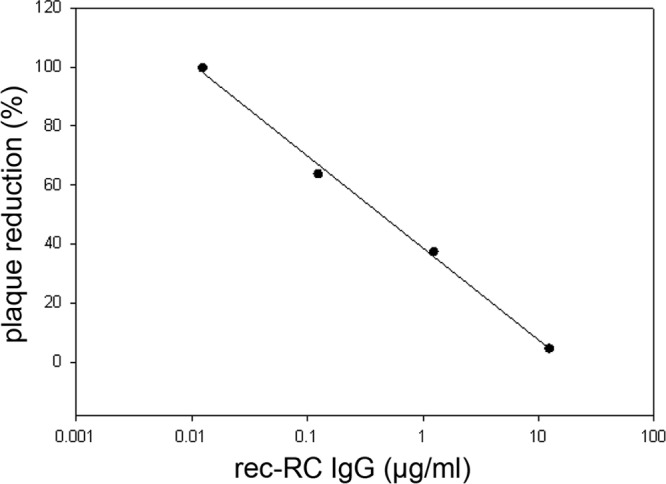
rec-RC IgG neutralizes VZV infection. VZV-infected HFLs were incubated with multiple dilutions of rec-RC IgG. Surviving virus was titrated on monolayers of HFLs, and plaques were counted. Data are given as the percent plaque reduction after antibody incubation (average for 3 replicates) at each of 4 different antibody concentrations compared to the plaque formation of non-antibody-treated virus. Virus infection was reduced by 50% at an antibody concentration of 0.6 μg/ml.
Mass spectrometric identification of proteins immunoprecipitated with rec-RC IgG.
VZV-infected or uninfected cells were incubated with rec-RC IgG, lysed, and coupled with agarose beads, and the eluate was resolved by SDS-PAGE. Two distinct bands, at ∼110 kDa (Fig. 4, band A) and ∼18 kDa (Fig. 4, band B), were seen for VZV-infected cells but not uninfected cells. Two additional bands, at ∼50 kDa and 25 kDa, corresponding to the sizes of antibody heavy and light chains, respectively (Fig. 4, bands HC and LC), were seen in VZV-infected but not uninfected cells. Mass spectrometric analysis of bands A and B unequivocally identified VZV gH (ORF 37) and gL (ORF 60) as the proteins precipitated by the rec-RC IgG antibody (Table 3); no specific viral proteins were found in corresponding bands cut from uninfected sample lanes. Bands at ∼250 kDa and 42 kDa seen for both VZV-infected and uninfected cells were not analyzed by mass spectrometry.
Fig 4.
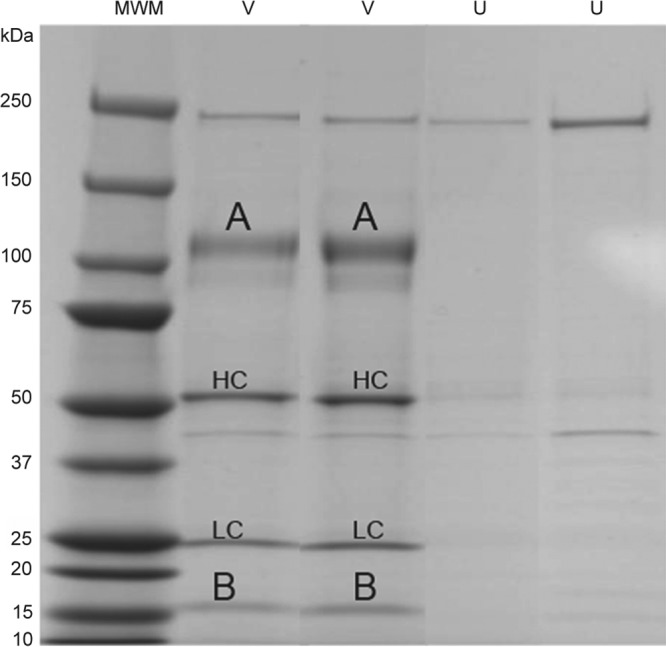
Immunoprecipitation of membrane antigen with rec-RC IgG. VZV-infected cells were immunoprecipitated with rec-RC IgG and analyzed by SDS-PAGE. Two prominent bands, of ∼110 kDa (A) and ∼18 kDa (B), were seen in duplicate lanes for infected cells (V) but not uninfected cells (U). Also seen were bands corresponding to the heavy chain (HC) and light chain (LC) of rec-RC IgG. MWM, molecular size marker.
Table 3.
Identification by mass spectrometry of VZV gH and gL immunoprecipitated by rec-RC IgG
| Protein | Predicted mass (kDa) | Observed mass (kDa) | No. of assigned spectra | Mascot ion score | Protein coverage (%) |
|---|---|---|---|---|---|
| ORF 37 (gH) | 93.6 | 94–122 | 1,024–1,215 | 30–154 | 48–60 |
| ORF 60 (gL) | 17.6 | 16–18 | 88–114 | 31–91 | 23–28 |
rec-RC IgG detects the gH/gL complex but not gH or gL in isolation.
To analyze rec-RC IgG binding to VZV envelope glycoproteins, HEK293 cells were transfected with a plasmid expressing VZV gH, a plasmid expressing VZV gL, or both plasmids, followed by immunostaining with rec-RC IgG. No rec-RC IgG staining was seen in cells transfected with either gH or gL, whereas positive staining was seen in cells transfected with both plasmids (Fig. 5). Thus, rec-RC IgG binds only to a complex formed by the two glycoproteins at the cell surface.
Fig 5.
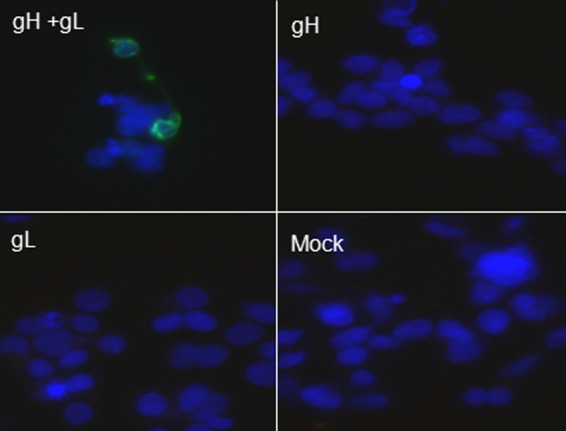
rec-RC IgG detects the gH/gL complex. HEK293 cells were transfected with plasmids expressing VZV gH and gL and stained with rec-RC IgG. Note the positive staining in cells transfected with both plasmids (top left) but not in cells transfected with a plasmid expressing gH only (top right) or gL only (bottom left) or in cells where plasmids were omitted (bottom right). Blue, DAPI; green, rec-RC IgG. Magnification, ×360.
DISCUSSION
Here we report the construction of a human recombinant bivalent anti-VZV antibody, rec-RC IgG, generated from circulating plasma blasts of a healthy individual 7 days after immunization with a zoster vaccine. Zostavax is an attenuated live virus vaccine that effectively boosts immunity to VZV and reduces the 3-year incidence of zoster by more than 51.3% (34). Although little is known concerning virus epitopes that generate an immune response after zoster, VZV gE, gB, and IE62 are immunodominant targets of CD4 and CD8 T cells (35). Our rec-RC IgG antibody neutralized VZV, and mass spectrometry analysis of cell membrane proteins immunoprecipitated by rec-RC IgG from VZV-infected HFL cells identified only VZV gH and gL. In VZV-infected formaldehyde-fixed cells, rec-RC IgG localized gH/gL to the cell membrane and cytoplasmic vacuole-like structures, whereas in unfixed cells, the gH/gL complex was restricted to the cell membrane. In both instances, the gH/gL complex colocalized with VZV gE, similar to the colocalization of gH with gE seen when monoclonal anti-gH and anti-gE antibodies were used to stain VZV-infected human embryonic lung cells (36).
The detection of VZV gH/gL in cells after transfection with vectors expressing both gH and gL, but not after transfection with a vector expressing only gH or only gL, indicates that rec-RC IgG recognizes a conformation-dependent epitope in the protein complex, a notion supported by a lack of gH or gL detection on Western blots or after immunoprecipitation of detergent-treated proteins from virus-infected cells (data not shown). Monoclonal antibodies against conformation-dependent VZV gH epitopes have been shown to neutralize VZV (3, 11), as did our rec-RC IgG antibody. Importantly, gH and possibly gL are present in cell surface areas of “viral highways” and may be involved in cell-to-cell spread of VZV (5).
The gH/gL complexes of herpes simplex virus 1 (HSV-1) and cytomegalovirus (CMV) are involved in virus entry. Antibodies against the HSV-1 gH/gL protein complex prevent virus-cell fusion in part by activating NF-κB through Toll-like receptor 2 binding (37). Antibodies recognizing CMV gH and gL are major neutralizing components of CMV hyperimmune globulin (38). Our study adds VZV gH/gL to the list of herpesvirus glycoprotein complexes that function in virus spread and represent attractive therapeutic targets to inhibit virus infection. At present, prophylactic treatment of individuals at risk for VZV infection uses a polyclonal antibody prepared from pooled human sera. Because VZV gH/gL antibodies develop following zoster immunization, and possibly after primary VZV infection (22), antibodies directed against this glycoprotein complex should be considered in designing therapies involving passive immunity or subunit vaccines. Finally, our study of a single individual emphasizes a method of producing unlimited amounts of specific neutralizing human monoclonal anti-VZV antibody that can be used to study VZV pathogenesis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants NS32623 (R.J.C., R.M., and D.G.), AG032958 (R.J.C., R.M., and D.G.), and AG006127 (D.G.) from the National Institutes of Health.
We thank Jun Kim (Regis University, Denver, CO) and Richard P. Cohrs for technical assistance, Marina Hoffman for editorial review, and Lori DePriest for manuscript preparation.
Footnotes
Published ahead of print 17 October 2012
REFERENCES
- 1. Rodriguez JE, Moninger T, Grose C. 1993. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology 196:840–844 [DOI] [PubMed] [Google Scholar]
- 2. Vleck SE, Oliver SL, Reichelt M, Rajamani J, Zerboni L, Jones C, Zehnder J, Grose C, Arvin AM. 2010. Anti-glycoprotein H antibody impairs the pathogenicity of varicella-zoster virus in skin xenografts in the SCID mouse model. J. Virol. 84:141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vleck SE, Oliver SL, Brady JJ, Blau HM, Rajamani J, Sommer MH, Arvin AM. 2011. Structure-function analysis of varicella-zoster virus glycoprotein H identifies domain-specific roles for fusion and skin tropism. Proc. Natl. Acad. Sci. U. S. A. 108:18412–18417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Z, Selariu A, Warden C, Huang G, Huang Y, Zaccheus O, Cheng T, Xia N, Zhu H. 2010. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog. 6:e1000971 doi:10.1371/journal.ppat.1000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole NL, Grose C. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella-zoster virus. Rev. Med. Virol. 13:207–222 [DOI] [PubMed] [Google Scholar]
- 6. Dubey L, Steinberg SP, LaRussa P, Oh P, Gershon AA. 1988. Western blot analysis of antibody to varicella-zoster virus. J. Infect. Dis. 157:882–888 [DOI] [PubMed] [Google Scholar]
- 7. Giller RH, Winistorfer S, Grose C. 1989. Cellular and humoral immunity to varicella zoster virus glycoproteins in immune and susceptible human subjects. J. Infect. Dis. 160:919–928 [DOI] [PubMed] [Google Scholar]
- 8. Haumont M, Jurdan M, Kangro H, Jacquet A, Massaer M, Deleersnyder V, Garcia L, Bosseloir A, Bruck C, Bollen A, Jacobs P. 1997. Neutralizing antibody responses induced by varicella-zoster virus gE and gB glycoproteins following infection, reactivation or immunization. J. Med. Virol. 53:63–68 [DOI] [PubMed] [Google Scholar]
- 9. Watson B, Gupta R, Randall T, Starr S. 1994. Persistence of cell-mediated and humoral immune responses in healthy children immunized with live attenuated varicella vaccine. J. Infect. Dis. 169:197–199 [DOI] [PubMed] [Google Scholar]
- 10. Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schodel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ. 2009. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J. Infect. Dis. 200:1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akahori Y, Suzuki K, Daikoku T, Iwai M, Yoshida Y, Asano Y, Kurosawa Y, Shiraki K. 2009. Characterization of neutralizing epitopes of varicella-zoster virus glycoprotein H. J. Virol. 83:2020–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arvin AM, Kinney-Thomas E, Shriver K, Grose C, Koropchak CM, Scranton E, Wittek AE, Diaz PS. 1986. Immunity to varicella-zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. J. Immunol. 137:1346–1351 [PubMed] [Google Scholar]
- 13. Grose C, Edwards DP, Friedrichs WE, Weigle KA, McGuire WL. 1983. Monoclonal antibodies against three major glycoproteins of varicella-zoster virus. Infect. Immun. 40:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montalvo EA, Grose C. 1987. Assembly and processing of the disulfide-linked varicella-zoster virus glycoprotein gpII(140). J. Virol. 61:2877–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugano T, Tomiyama T, Matsumoto Y, Sasaki S, Kimura T, Forghani B, Masuho Y. 1991. A human monoclonal antibody against varicella-zoster virus glycoprotein III. J. Gen. Virol. 72:2065–2073 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki K, Akahori Y, Asano Y, Kurosawa Y, Shiraki K. 2007. Isolation of therapeutic human monoclonal antibodies for varicella-zoster virus and the effect of light chains on the neutralizing activity. J. Med. Virol. 79:852–862 [DOI] [PubMed] [Google Scholar]
- 17. Forghani B, Ni L, Grose C. 1994. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology 199:458–462 [DOI] [PubMed] [Google Scholar]
- 18. Grose C, Carpenter JE, Jackson W, Duus KM. 2010. Overview of varicella-zoster virus glycoproteins gC, gH and gL. Curr. Top. Microbiol. Immunol. 342:113–128 [DOI] [PubMed] [Google Scholar]
- 19. Maresova L, Kutinova L, Ludvikova V, Zak R, Mares M, Nemeckova S. 2000. Characterization of interaction of gH and gL glycoproteins of varicella-zoster virus: their processing and trafficking. J. Gen. Virol. 81:1545–1552 [DOI] [PubMed] [Google Scholar]
- 20. Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. U. S. A. 107:866–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foung SK, Perkins S, Koropchak C, Fishwild DM, Wittek AE, Engleman EG, Grumet FC, Arvin AM. 1985. Human monoclonal antibodies neutralizing varicella-zoster virus. J. Infect. Dis. 152:280–285 [DOI] [PubMed] [Google Scholar]
- 22. Kausmally L, Waalen K, Lobersli I, Hvattum E, Berntsen G, Michaelsen TE, Brekke OH. 2004. Neutralizing human antibodies to varicella-zoster virus (VZV) derived from a VZV patient recombinant antibody library. J. Gen. Virol. 85:3493–3500 [DOI] [PubMed] [Google Scholar]
- 23. Lloyd-Evans P, Gilmour JE. 2000. Expression of neutralizing recombinant human antibodies against varicella zoster virus for use as a potential prophylactic. Hybridoma 19:143–149 [DOI] [PubMed] [Google Scholar]
- 24. Sugano T, Matsumoto Y, Miyamoto C, Masuho Y. 1987. Hybridomas producing human monoclonal antibodies against varicella-zoster virus. Eur. J. Immunol. 17:359–364 [DOI] [PubMed] [Google Scholar]
- 25. Marasco WA, Sui J. 2007. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 25:1421–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yokoyama T, Ayabe S, Miyagi H, Sugano T, Otsu A, Sato H, Kageyama S, Fujii T, Shiraki K. 2001. Varicella-zoster virus gH:gL contains a structure reactive with the anti-human gamma chain of IgG near the glycosylation site. J. Gen. Virol. 82:331–334 [DOI] [PubMed] [Google Scholar]
- 27. Azarkh Y, Dolken L, Nagel M, Gilden D, Cohrs RJ. 2011. Synthesis and decay of varicella zoster virus transcripts. J. Neurovirol. 17:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith K, Garman L, Wrammert J, Zheng Capra N-YJD, Ahmed R, Wilson PC. 2009. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 4:372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Owens GP, Ritchie AM, Burgoon MP, Williamson RA, Corboy JR, Gilden DH. 2003. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J. Immunol. 171:2725–2733 [DOI] [PubMed] [Google Scholar]
- 30. Owens GP, Bennett JL, Lassmann H, O'Connor KC, Ritchie AM, Shearer A, Lam C, Yu X, Birlea M, DuPree C, Williamson RA, Hafler DA, Burgoon MP, Gilden D. 2009. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann. Neurol. 65:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahalingam R, Wellish M, Cohrs R, Debrus S, Piette J, Rentier B, Gilden DH. 1996. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. U. S. A. 93:2122–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Mata S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon R, Dalmau J. 2009. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann. Neurol. 65:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davison AJ, Scott JE. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759–1816 [DOI] [PubMed] [Google Scholar]
- 34. Kleemann P, Distler E, Wagner EM, Thomas S, Klobuch S, Aue S, Schnurer E, Schild H, Theobald M, Plachter B, Tenzer S, Meyer RG, Herr W. 2012. Varicella-zoster virus glycoproteins B and E are major targets of CD4+ and CD8+ T cells reconstituting during zoster after allogeneic transplantation. Haematologica 97:874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352:2271–2284 [DOI] [PubMed] [Google Scholar]
- 36. Shiraki K, Daikoku T, Takemoto M, Yoshida Y, Suzuki K, Akahori Y, Okuno T, Kurosawa Y, Asano Y. 2011. Neutralizing anti-gH antibody of varicella-zoster virus modulates distribution of gH and induces gene regulation, mimicking latency. J. Virol. 85:8172–8180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. 2012. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J. Virol. 86:6555–6562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. 2012. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J. Virol. 86:7444–7447 [DOI] [PMC free article] [PubMed] [Google Scholar]