Abstract
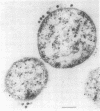
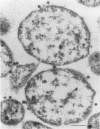
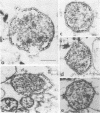
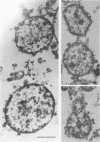
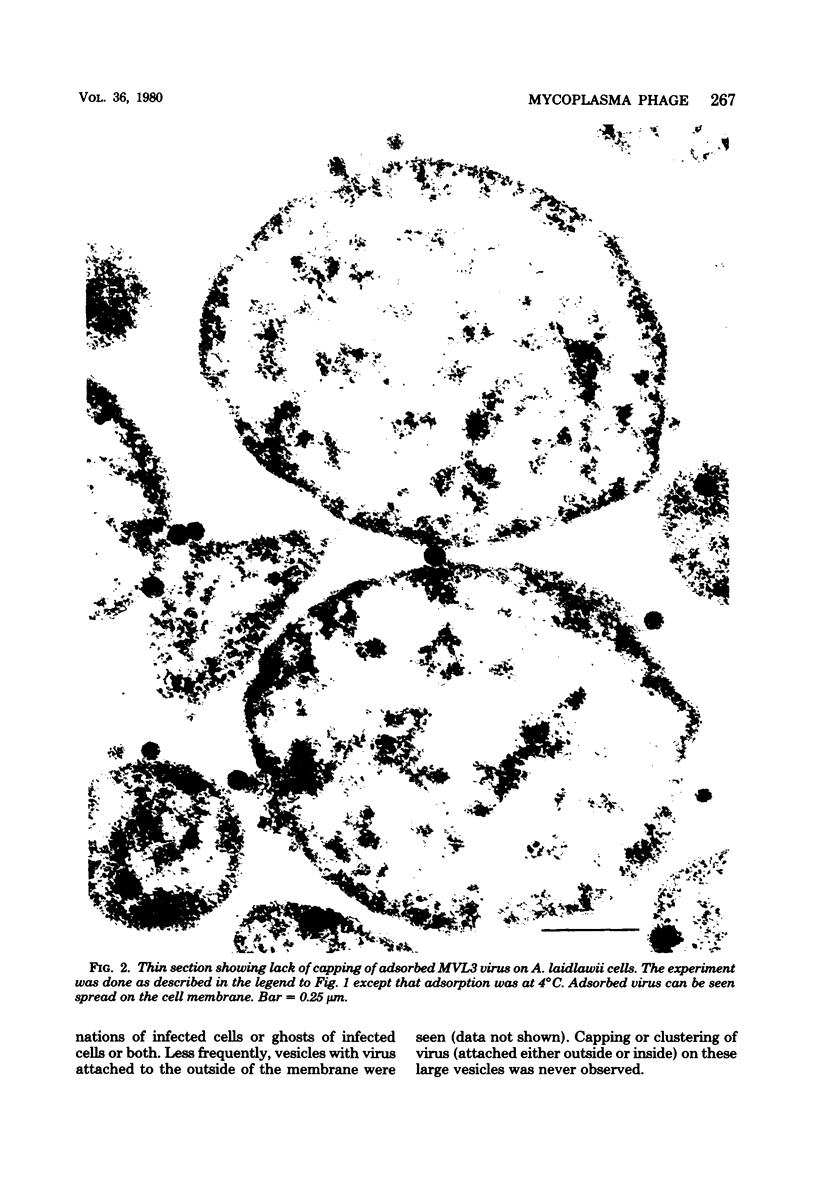
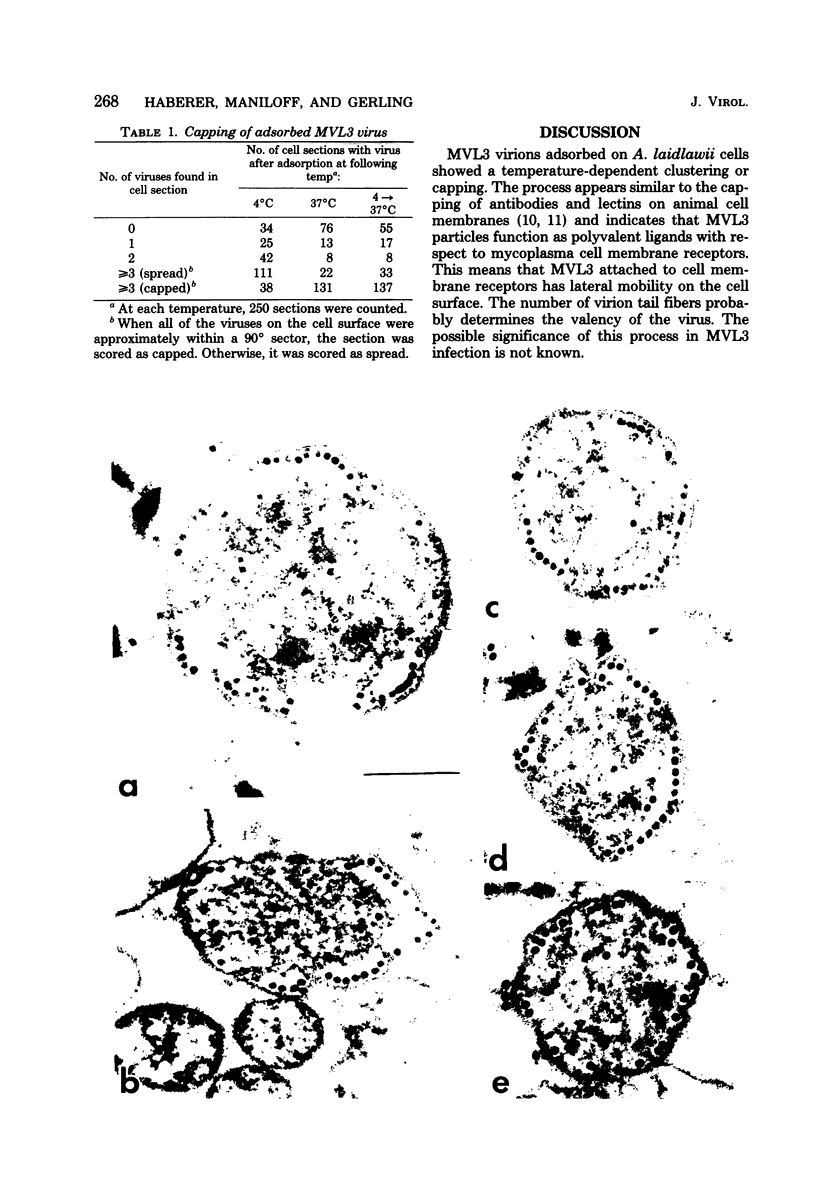
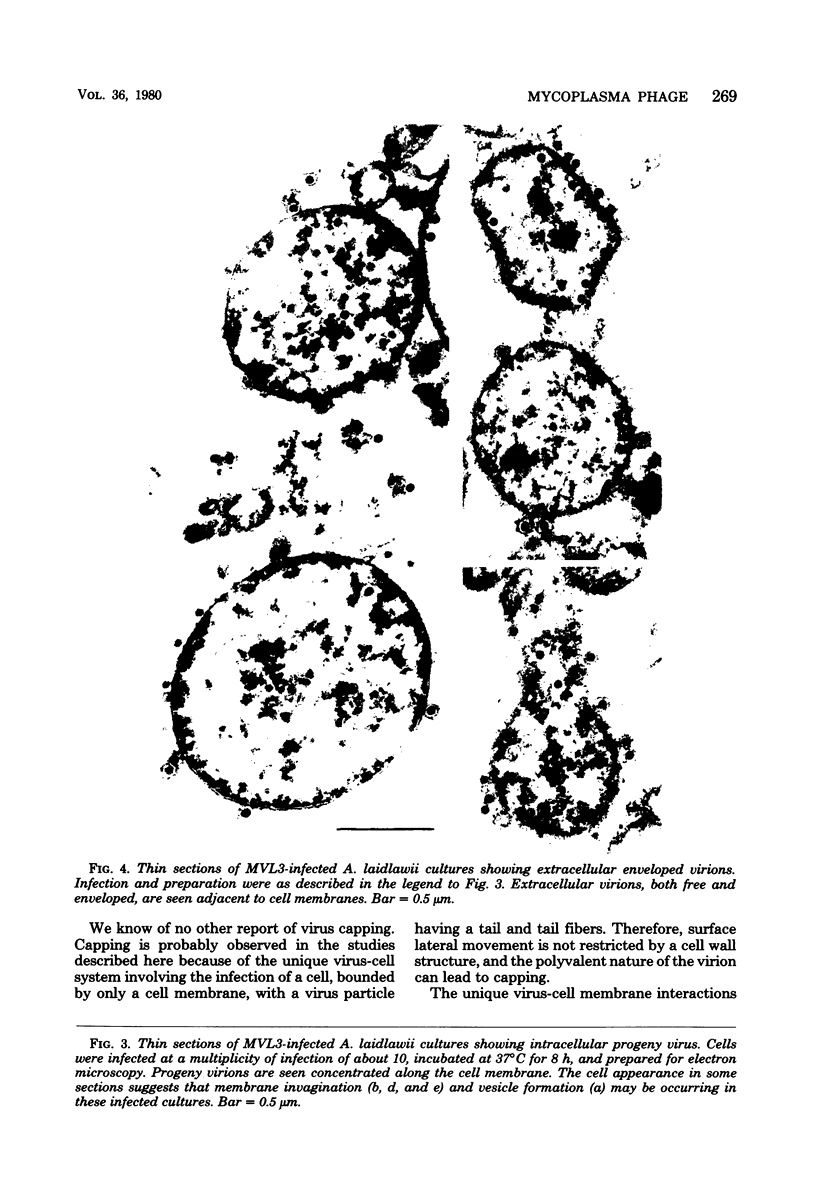
By electron microscopic studies, the adsorption and release of nonlytic, cytocidal mycoplasma virus MVL3, which infects ACholeplasma laidlawii cells, have been examined. The MVL3 virion has a polyhedral head, collar, short tail, and tail fibers and contains linear double-stranded DNA. Adsorbed MVL3 virus showed a temperature-dependent clustering or capping on the mycoplasma cell membrane. During infection, a number of virus-cell membrane-related structures were observed, suggesting a general model in which MVL3-infected cells release progeny virions in membrane vesicles. These vesicles must then break down to release MVL3 particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gourlay R. N., Wyld S. G., Bland A. P. Demonstration by electron microscopy of intracellular virus in Acholeplasma laidlawii infected with either MV-L3 or a similar but serologically distinct virus (BN1 virus). J Gen Virol. 1979 Feb;42(2):315–322. doi: 10.1099/0022-1317-42-2-315. [DOI] [PubMed] [Google Scholar]
- Haberer K., Klotz G., Maniloff J., Kleinschmidt A. K. Structural and biological properties of mycoplasmavirus MVL3: an unusual virus-procaryote interaction. J Virol. 1979 Oct;32(1):268–275. doi: 10.1128/jvi.32.1.268-275.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. Factors in fusion of cells by HVJ. Curr Top Microbiol Immunol. 1969;48:102–128. doi: 10.1007/978-3-642-46163-7_5. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. D. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. 3. Membrane-associated intracellular bacteriophages. Virology. 1969 Jun;38(2):285–296. doi: 10.1016/0042-6822(69)90370-5. [DOI] [PubMed] [Google Scholar]
- Séchaud J., Kellenberger E. Electron microscopy of DNA-containing plasms. IV. Glutaraldehyde-uranyl acetate fixation of virus-infected bacteria for thin sectioning. J Ultrastruct Res. 1972 Jun;39(5):598–607. doi: 10.1016/s0022-5320(72)90124-4. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]