Abstract
Japanese macaque rhadinovirus (JMRV) is a novel gamma-2 herpesvirus that was isolated from a Japanese macaque (JM) with an inflammatory demyelinating encephalomyelitis referred to as Japanese macaque encephalomyelitis, a disease that possesses clinical and histopathological features resembling multiple sclerosis in humans. Genomic DNA sequence analysis reveals that JMRV is a gammaherpesvirus closely related to rhesus macaque rhadinovirus (RRV) and human herpesvirus 8. We describe here the complete nucleotide sequence and structure of the JMRV genome, as well as the sequence of two plaque isolates of this virus. Analysis of the JMRV genome not only demonstrates that this virus shares a number of genes with RRV that may be involved in pathogenesis but also indicates the presence of unique JMRV genes that could potentially contribute to disease development. The knowledge of the genomic sequence of JMRV, and the ability to easily propagate the virus in vitro, make JMRV infection of JM an attractive model for examining the potential role of an infectious viral agent in the development of demyelinating encephalomyelitis disease in vivo.
INTRODUCTION
We recently described the occurrence of a spontaneous demyelinating disease that possesses clinical and histopathological features resembling multiple sclerosis (MS) in a colony of Japanese macaques (JM; Macaca fuscata) housed at the Oregon National Primate Research Center (1). This disease, referred to as Japanese macaque encephalomyelitis (JME), presents with clinical features such as ataxia and paralysis and, in some cases, is associated with episodes of recovery and relapse. Magnetic resonance imaging of several JME cases revealed multiple gadolinium-enhancing T1-weighted hyperintensities in the white matter of the cerebral hemispheres, cerebella, brainstems, and cervical spinal cords of animals. Histopathological similarities with MS were also observed and were characterized by the presence of multifocal plaque-like demyelinated lesions accompanied with inflammatory cell infiltrates and loss of oligodendrocytes and axons. Of particular interest was the isolation of a novel gammaherpesvirus, referred to as Japanese macaque rhadinovirus (JMRV), from a central nervous system (CNS) lesion of a JM that had developed JME (1). JMRV was subsequently found in other JME lesions but not in normal white matter tissue of affected animals, suggesting the virus is a passenger within the inflammatory cells infiltrating the lesion or may be associated with the development of JME. We report here the complete sequence and structure of the JMRV genome, as well as two plaque isolates of this virus, and demonstrate that JMRV is closely related to other primate gammaherpesviruses, while also possessing unique features. The expression pattern of several JMRV-unique open reading frames (ORFs) is also examined.
MATERIALS AND METHODS
Viral genomic DNA isolation.
The identification and preliminary characterization of JMRV strain 17792 (JMRV17792) was previously described (1). JMRV17792 is referred to throughout the present study as JMRV. To isolate purified viral genomic DNA for sequence analysis, primary rhesus fibroblasts were seeded in 850-cm2 roller bottles, infected at a multiplicity of infection (MOI) of 0.01, and incubated until the appearance of full cytopathic effect. Next, the cells and supernatants were collected and subjected to centrifugation at 1,000 × g for 10 min. The clarified supernatants were then collected, the cell pellet was sonicated and spun at 1,000 × g for 10 min, and all clarified supernatants were pooled. The virus was then pelleted from supernatants by centrifugation at 12,500 × g for 1 h at 4°C, and the resulting pellet was resuspended in 1 ml of 1 mM Tris-HCl (pH 8.0)-1 mM EDTA (TE) and added to the top of a six-step sorbitol gradient, ranging from 20 to 70%. The gradients were spun in a Beckman SW41 rotor for 2 h at 18,000 rpm at 4°C. The virus-containing band at the 50 to 60% interface was collected and diluted with 15 ml of cold 1 mM Tris-HCl and then pelleted by centrifugation in the SW41 rotor for 50 min at 18,000 rpm at 4°C. The washed virus pellet was resuspended in 9.2 ml of TE (pH 8.0), and particles were digested at 37°C overnight in 0.6 ml of 10% sodium dodecyl sulfate and 0.2 ml of proteinase K (10 mg/ml) to release the viral DNA. Finally, the viral DNA was purified by CsCl2 gradient centrifugation in a Beckman Ti75 rotor at 38,400 rpm for 72 h, and the collected fractions were dialyzed against TE (pH 8.0).
Sequence analysis of the JMRV genome.
To facilitate DNA sequencing of the JMRV genome, a shotgun subclone library was generated, and the DNA sequence of the viral genome was determined essentially as described previously for rhesus cytomegalovirus (2). Using this approach, the entire genome was sequenced with a 6-fold redundancy. The complete sequence for JMRV is available in GenBank under accession number AY528864.
Analysis of JMRV ORFs.
ORFs were identified with MacVector software (MacVector, Inc., Cary, NC), and the target search criterion for an ORF was DNA sequence encoding a protein of at least 80 amino acids (aa). The ORFs that were identified in this manner were translated and analyzed using the BLASTP tool from the NCBI using default parameters to identify other known proteins with homology.
Sequence alignments and phylogenetic analysis.
Sequence alignments were performed with CLUSTAL W, and phylogenetic analysis was performed by bootstrap analysis with the neighbor-joining method, using MacVector software. Herpesvirus DNA polymerase protein sequences utilized in phylogenetic analysis were obtained from GenBank (herpesvirus saimiri [HVS], CAA45632; human herpesvirus 8 [HHV-8], AAC57086; Epstein-Barr virus [EBV] YP_401712; rhesus lymphocryptovirus, YP068007; murine herpesvirus 68 [MHV-68], AAB66388; rhesus macaque rhadinovirus [RRV], AAD21336; JMRV, AAS99991).
Terminal repeat (TR) identification.
Restriction digest analysis was performed by digesting 2 μg of viral DNA with enzyme overnight at 37°C and analyzing the products on a 1% agarose gel containing ethidium bromide. An ∼1.6-kb HindIII restriction fragment was identified and excised from the gel, purified using a Zymoclean gel DNA recovery kit (Zymo Research, Irvine, CA), and cloned into vector pSP73 (Promega, Madison, WI) digested with the same enzyme. A clone containing the insert was identified, and plasmid DNA was then isolated and sequenced.
Analysis of unique JMRV ORF expression.
Primary rhesus fibroblasts were infected with JMRV at an MOI of 5, and individual cultures were then treated with 75 μg of cycloheximide (CHX)/ml to inhibit protein synthesis, 7.5 μM ganciclovir (GCV) to inhibit DNA replication, or left untreated to allow the definition of gene expression as immediate-early, early, or late, respectively. The time points for collection of RNA were 24 h (+CHX), 48 h (+GCV), and 72 h (untreated), since these three classes of genes are expressed at these approximate time points after JMRV infection in vitro. RNA from mock-infected cells collected at 72 h served as a negative control for all reactions. RNA was isolated from infected cells using TRI Reagent (Sigma-Aldrich, St. Louis, MO). Reverse transcription-PCR (RT-PCR) was performed with a Superscript III One-Step RT-PCR System (Life Technologies, Grand Island, NY) and gene-specific primers designed to amplify the precise length of each predicted ORF, and the resulting reactions were run on a 1.7% agarose gel. Analysis of secretion signals for each predicted protein were performed with the SignalP 4.0 server (3) and the Secretome 2.0 Server (4; http://www.cbs.dtu.dk/services).
Sequence analysis of JMRV plaque isolates.
Plaque-purified isolates of JMRV were obtained by dilution of the original stock of virus on primary rhesus fibroblasts and picking single plaques. After two further rounds of plaque purification, two isolates (denoted 3A1 and 12E2) were identified and selected for propagation and analysis. Stocks of each virus were grown in primary rhesus fibroblasts, and viral DNA was isolated using procedures described above. Next, short read sequence analysis of the purified viral DNA was performed by the OHSU Massively Parallel Sequencing Shared Resource using an Illumina GA IIx sequencer, and the resulting reads were computationally assembled into a consensus sequence for each viral genome using the program Velvet. Using this method, an estimated 300-fold depth of coverage was achieved for each nucleotide in the viral genome. Any apparent gaps in genomic sequences introduced as a result of errors in the assembly process were confirmed by PCR, using purified viral DNA as a template and unique primers flanking each region. PCR products resulting from these analyses were purified and directly sequenced, and the genomic sequences were then manually edited to reflect the correct sequence at these locations.
RESULTS
Sequence analysis of the JMRV genome.
To determine the complete sequence of JMRV, a shotgun subclone library spanning the entire genome was generated from purified viral genomic DNA using methods described previously (5). Next, individual clones were sequenced, and the resulting reads were assembled into a consensus sequence representing the entire JMRV genome. The sequencing redundancy achieved with this approach was ∼6-fold. As sequenced, the JMRV genome is 131,217 bp in length, and restriction digestion of purified viral DNA confirmed the overall organization and structure of the determined genomic sequence (Table 1 and Fig. 1). In addition, CLUSTAL W alignment of the complete genomic sequences of JMRV and RRV17577 indicates that these viruses share 89.5% identity at the nucleotide level. The complete genomic sequence of JMRV is available in GenBank under accession number AY528864.
Table 1.
Restriction fragments of the JMRV genome
| Restriction fragment | Size (bp)a | Nucleotide positions |
|---|---|---|
| HindIII | 28,873 | 82234–111106 |
| 23,773 | 137–23909 | |
| 18,781 | 111107–129887 | |
| 9,867 | 39334–49200 | |
| 9,511 | 49201–58711 | |
| 6,912 | 74047–80958 | |
| 6,348 | 32133–38480 | |
| 4,883 | 69164–74046 | |
| 4,750 | 23910–28659 | |
| 4,624 | 58712–63335 | |
| 3,473 | 28660–32132 | |
| 2,552 | 66201–68752 | |
| 2,463 | 63738–66200 | |
| 1,275 | 80959–82233 | |
| 853 | 38481–39333 | |
| 755 | 129888–130642 | |
| 575* | 130643–131217 | |
| 411 | 68753–69163 | |
| 402 | 63336–63737 | |
| 136* | 1–136 | |
| BamHI | 22,949 | 20762–43710 |
| 15,460 | 96218–111677 | |
| 11,194 | 9568–20761 | |
| 10,544 | 85477–96020 | |
| 9,986* | 121232–131217 | |
| 9,748 | 43711–53458 | |
| 9,567* | 1–9567 | |
| 7,320 | 76430–83749 | |
| 7,137 | 53459–60595 | |
| 5,836 | 63607–69442 | |
| 5,136 | 71294–76429 | |
| 4,466 | 115052–119517 | |
| 3,374 | 111678–115051 | |
| 3,011 | 60596–63606 | |
| 1,851 | 69443–71293 | |
| 1,727 | 83750–85476 | |
| 1,627 | 119518–121144 | |
| 197 | 96021–96217 | |
| 87 | 121145–121231 |
*, Fragments linked to a variable number of terminal repeat sequences.
Fig 1.
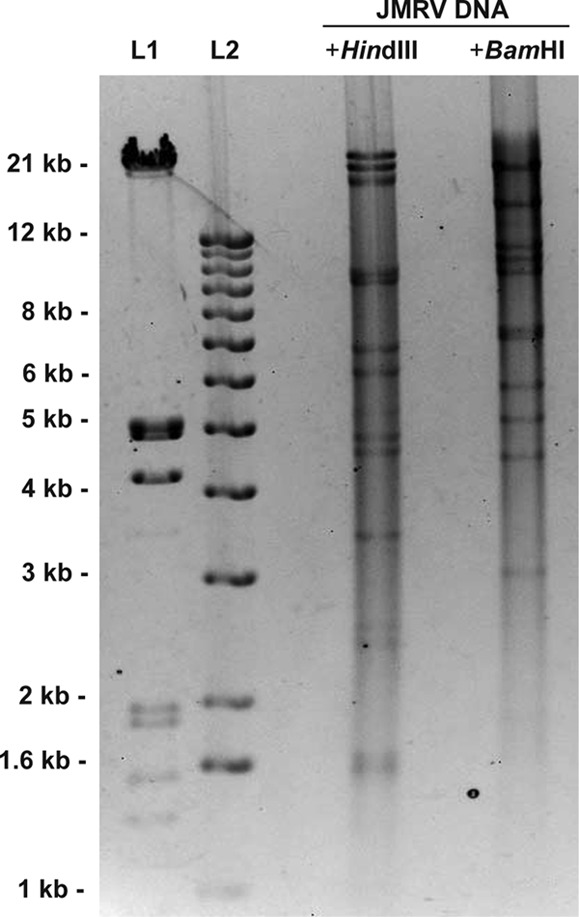
Restriction digest analysis of JMRV DNA. A 2.5-μg portion of purified viral genomic DNA was digested overnight with HindIII or BamHI and run on a 0.7% agarose gel. The patterns obtained for both digestions correlate with the predicted fragment sizes for the JMRV genomic sequence, as listed in Table 1. L1 and L2 represent DNA ladders run as size standards.
Structurally, the JMRV genome is similar to that of other gammaherpesviruses, possessing a linear double-stranded DNA genome comprised of a single long unique region (LUR) flanked by terminal repeat (TR) sequences at the genome termini (Fig. 2). Partial TR sequences were initially identified at both ends of the genome based on the presence of a series of identical repetitive tandem repeat sequences near the genome termini. The sequence of the complete TR was also determined and is described in more detail below. When excluding the partial TR sequences, the LUR spans nucleotides (nt) 907 to 129986 of the JMRV sequence. The overall G+C content of the JMRV LUR is 51.6%, which is less than the 52.2% G+C content of the RRV LUR and the 53.5% G+C content of the HHV-8 LUR (6).
Fig 2.
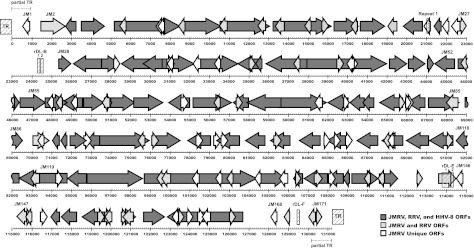
Map of the JMRV genome. All predicted JMRV ORFs are depicted by arrows, and the scale (in base pairs) indicates their approximate genomic location. ORFs are numbered from left to right as described in Table 3, with only selected ORFs labeled for purposes of clarity. JMRV ORFs that are homologous to RRV ORFs are shaded light gray, those homologous to both RRV and HHV-8 ORFs are shaded dark gray, and those unique to JMRV are white. Internal repeat regions are indicated by hashed boxes and are labeled with the corresponding repeat name above their location. Hashed boxes at the ends of the genome depict the location of TRs, and dashed lines at the genome termini indicate the approximate location of the partial TR sequences.
Internal repeat sequences were identified in the LUR of JMRV, with a majority being similar in size, structure, and location to the repeat in divergent loci (rDL) that were previously identified in RRV17577. The repeat regions identified in JMRV that possess similarity to those in RRV were thus designated with similar nomenclature as rDL-B 1 and 2, rDL-E 1 and 2, and rDL-F. Only one internal repeat was identified in JMRV (designated Repeat 1) that does not appear to have a counterpart in RRV and consists of four repeat elements in tandem from nt 21026 to 21121. The sequences, sizes, and locations of all of the identified internal JMRV repeats are presented in Table 2. As is the case in other herpesviruses, the actual number of repeat elements present within each repeat region is also likely somewhat variable within individual viral genomes, reflecting the fluctuating nature of the size of these regions during replication.
Table 2.
JMRV repeat sequencesa
| JMRV repeat | Genomic position (nt) | Element size (no. of nt) | Sequence | No. of copies | RRV counterpart | %G+C |
|---|---|---|---|---|---|---|
| Repeat 1 | 21026–21121 | 24 | GGCGTCTCCCCCGGAGTCTCCCCC | 4 | None | 79.2 |
| rDL-B 1 | 24250–24483 | 26 | TAGCTCCTAATGTTTGCCTTGCCGCC | 9 | rDL-B 1 | 53.8 |
| rDL-B 2 | 24649–25098 | 25 | CGTTCCCCGAGGGTCCCGGTCTCCC | 18 | rDL-B 2 | 78.0 |
| rDL-E 1 | 113629–114236 | 19 | GTGCAGGTCCCCCGGTGGG | 32 | rDL-E 1 | 79.0 |
| rDL-E 2 | 114237–114320 | 28 | GCTCCGGGTGGCTCCGGGTGGGGTGGCG | 3 | rDL-E 2 | 82.1 |
| rDL-F | 129459–129590 | 22 | AGCTAGGGTGAGGGCTGGGGTG | 6 | rDL-F | 68.2 |
nt, nucleotides.
Identification of JMRV TRs.
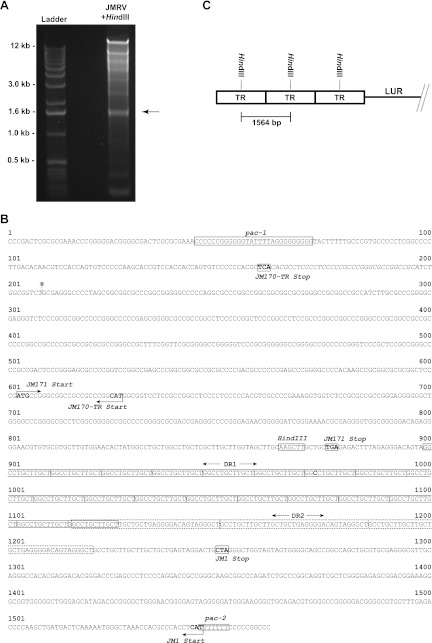
The observation of identical repeated sequences at the termini of the JMRV genome suggested an apparent duplication in these regions that were both likely to represent at least a portion of a TR unit. Further, the presence of a unique HindIII site within the repeated sequence at both ends of the genome indicated that it might be possible to isolate and identify the complete TR unit via restriction digestion analysis with this enzyme. Indeed, when viral DNA was subjected to digestion with HindIII, the resulting pattern indicated the presence of an ∼1.6-kb restriction fragment (Fig. 3A), which is not a fragment size predicted to be generated by digestion with this enzyme (Table 1). Importantly, the intensity of this band was also stronger than other low-molecular-weight bands of similar size produced by HindIII digestion, suggesting that this sequence is likely overrepresented in the viral genome, and thus, may be derived from multiple copies of the TR unit present in the viral genome. This observation is similar to that made during the identification of the HHV-8 TR (7).
Fig 3.
Identification of the TR sequence. (A) Purified viral DNA was digested with HindIII and run on a 1% agarose gel, resulting in the identification of an ∼1.6-kb digestion product corresponding to the terminal repeat (TR) unit of JMRV. This fragment was purified, cloned, and sequenced. (B) The TR unit of JMRV is 1,564 bp in length and possesses defining features of a herpesvirus TR, including the presence of direct repeats (DR1 and DR2) and packaging motifs (pac-1 and pac-2). The TR also possesses three potential ORFs: JM1, JM171, and JM170-TR. JM170-TR is a variant of JM170 that possesses an alternate 49 nt at its 3′ end. The alternate 49-nt sequence of JM170-TR is denoted in the TR sequence by italicized lettering, and the location of the junction of the LUR and partial TR located at the right end of the genome is marked by an asterisk. (C) Diagram depicting the predicted layout of TR units at the termini of the JMRV genome. The size marker indicates the fragment produced by HindIII digestion of viral DNA, in relation to the orientation of the TR units. The exact number of copies of the TR at the ends of the genome is unknown and varies between individual viral genomes.
To determine the sequence of the 1.6-kb HindIII fragment, the corresponding band was isolated from an agarose gel, purified, cloned into vector pSP73, and a clone containing the fragment was then isolated and subjected to sequence analysis. The digestion product was identified as a 1,564-bp HindIII fragment with a G+C content of 75%, and structural features similar to those found in other herpesvirus TR sequences (8). The precise order of the sequence of the TR unit was further defined based on the partial TR sequences present at both ends of the genome (Fig. 3B). Importantly, the TR of JMRV possesses sequences that have homology to herpesvirus pac-1 (Cn-Gn-T motif-Gn) and pac-2 (Nn-Tn-Nn) motifs, which are conserved sequences involved in directing the cleavage and packaging of head-to-tail viral genome concatemers (9). As in other herpesviruses, the TR sequence is bordered on the left side by a pac-1 motif, and the right side by a pac-2 motif. Tandem direct repeat (DR) sequences also exist within the TR, with the first repeat unit, direct repeat 1 (DR1), being composed of 12mer (GGCCTGCTTGCT). The observed number of DR1 varies in number from 24 (left partial TR) to 26 (right partial TR) in the genomic sequence and is present in 25 copies in the cloned TR fragment. The DR1 sequences are followed by a 35mer repeat unit (GCCTGCTTGCTTGCTGCTGAGGGGACAGTAGGGCT), termed direct repeat 2 (DR2), with the first repeat unit overlapping with the last DR1 unit present in the TR sequence. Three tandem copies of the DR2 unit were observed in both partial TRs in the genomic sequence, as well as the cloned TR sequence.
Based on the complete TR unit sequence and on the partial TR sequences present at the genome termini, the true LUR of JMRV was determined to span nt 907 to 129985 of the genomic sequence, with partial TR sequences located from nt 1 to 906 at the left end of the genome and from nt 129986 to 131217 at the right end of the genome. The partial TR identified at the left end of the genome consists of the last 906 bp of a complete TR unit and thus may simply represent an incompletely sequenced but fully intact TR, whereas the partial TR at the right end of the genome actually lacks the first 208 bp of the TR unit sequence and therefore represents an incomplete TR directly adjacent to the LUR. Although the genome termini as sequenced only possess partial fragments of the TR at their ends, they are likely to extend further into the TR unit sequence and also be flanked by an unknown number of multiple copies of the TR in tandem (Fig. 3C). It is unclear at this point exactly how many tandem copies of the 1,564-bp TR unit are typically present at the ends of the viral genome, although based on predictions made for gammaherpesviruses HVS and HHV-8 (7, 10), JMRV is likely to possess a relatively fixed overall number of ∼35 total TRs per genome, with the exact number of TRs located at each end of the JMRV genome varying between different molecules.
JMRV ORF analysis.
The target search criterion for identification of ORFs in the viral genome were set for sequences predicted to encode proteins of 80 aa or more. Putative ORFs identified in this fashion were translated, and homologous proteins were identified using BLASTP with standard settings. Genes are numbered from left to right starting with the first predicted ORF in the genomic sequence, and the JM prefix precedes each gene number. The arrangement of JMRV genes is shown in Fig. 2.
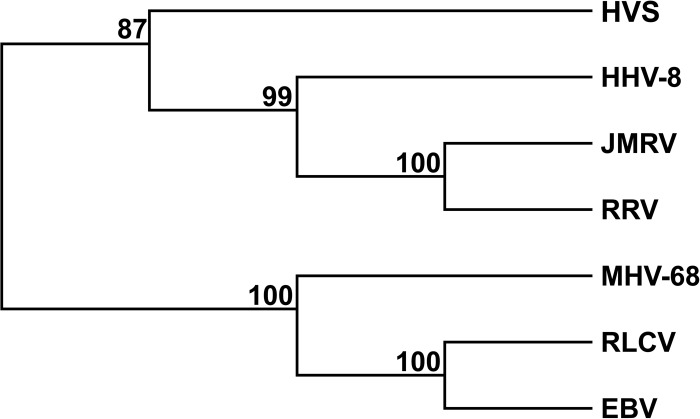
After identification of the ORFs in JMRV, a phylogenetic analysis was performed by bootstrap analysis using protein sequences for the DNA polymerase from JMRV and several gammaherpesviruses, including HHV-8, RRV, HVS, rhesus lymphocryptovirus, MHV-68, and EBV. The results of this analysis confirm that JMRV is a gammaherpesvirus most closely related to HHV-8 and RRV (Fig. 4). Indeed, the ORFs shared between these viruses are arranged collinearly (6, 11–13), and of the 171 predicted ORFs in JMRV, 88 encode proteins that are homologous to known or predicted proteins that were previously identified in RRV (6, 14). The highest level of identity observed between JMRV and RRV proteins is 99% (RRV ORF25, ORF26, ORF39, ORF49, ORF55, and ORF62), while the lowest is 48% (RU4-R) (Table 3).
Fig 4.
Phylogenetic tree of selected gammaherpesviruses. The protein sequence of DNA polymerase from JMRV, RRV, HHV-8, HVS, RLCV, MHV-68, and EBV were subjected to bootstrap analysis (1,000 replicates) using the neighbor joining method. Numbers represent the percentage of bootstrap trees that contain the same branch point.
Table 3.
Predicted JMRV ORFsa
| JMRV ORF | First nt | Last nt | Coding strandb | Size (aa) | Putative functionc | RRV ORF homologue (nt position)d | Homologue size (no. of aa) | % Identity of overlape |
|---|---|---|---|---|---|---|---|---|
| JM001 | 890 | 594 | – | 98 | Unknown | None | ||
| JM002 | 1444 | 2721 | + | 425 | Signal transduction, transformation | R1 | 423 | 85 |
| JM003 | 3359 | 2778 | – | 193 | Dihydrofolate reductase | ORF2 | 188 | 95 |
| JM004 | 3526 | 4713 | + | 395 | Complement regulatory protein | ORF4 | 645 | 56 |
| JM005 | 5146 | 8544 | + | 1132 | ssDNA binding protein | ORF6 | 1,132 | 98 |
| JM006 | 5895 | 5581 | – | 104 | Unknown | Unnamed (6480–6794) | 104 | 83 |
| JM007 | 7676 | 7410 | – | 88 | Unknown | Unnamed (8309–8575) | 88 | 94 |
| JM008 | 7722 | 7462 | – | 86 | Unknown | None | ||
| JM009 | 8792 | 8541 | – | 83 | Unknown | Unnamed (9440–9691) | 83 | 91 |
| JM010 | 8569 | 10629 | + | 686 | DNA packaging protein | ORF7 | 686 | 97 |
| JM011 | 10929 | 10606 | – | 107 | Unknown | Unnamed (11436–11828) | 130 | 94 |
| JM012 | 10616 | 13102 | + | 828 | Glycoprotein B | ORF8 | 829 | 96 |
| JM013 | 12858 | 12565 | – | 97 | Unknown | Unnamed (13467–13760) | 97 | 97 |
| JM014 | 13219 | 16257 | + | 1012 | DNA polymerase catalytic subunit | ORF9 | 1014 | 97 |
| JM015 | 13827 | 13267 | – | 186 | Unknown | Unnamed (14170–14736) | 188 | 85 |
| JM016 | 15117 | 14809 | – | 102 | Unknown | Unnamed (15646–16026) | 126 | 92 |
| JM017 | 15726 | 16031 | + | 101 | Unknown | Unnamed (16635–16940) | 101 | 88 |
| JM018 | 16351 | 17601 | + | 416 | Unknown | ORF10 | 384 | 95 |
| JM019 | 17501 | 17226 | – | 91 | Unknown | Unnamed (18136–18411) | 91 | 94 |
| JM020 | 17610 | 18839 | + | 409 | Unknown | ORF11 | 409 | 98 |
| JM021 | 19641 | 19018 | – | 207 | Viral IL-6 | R2 | 207 | 91 |
| JM022 | 20862 | 19861 | – | 333 | Thymidylate synthase | ORF70 | 333 | 93 |
| JM023 | 21235 | 20969 | – | 88 | Unknown | None | ||
| JM024 | 21748 | 21386 | – | 120 | Viral macrophage inflammatory protein | R3 | 115 | 74 |
| JM025 | 22120 | 21845 | – | 91 | Unknown | None | ||
| JM026 | 22701 | 22363 | – | 112 | Unknown | None | ||
| JM027 | 22700 | 23008 | + | 102 | Unknown | RU1-R | 102 | 74 |
| JM028 | 25392 | 25955 | + | 187 | viral Bcl-2 | ORF16 | 187 | 96 |
| JM029 | 27672 | 26062 | – | 536 | Capsid protein | ORF17 | 536 | 94 |
| JM030 | 27545 | 28444 | + | 299 | Unknown | ORF18 | 299 | 97 |
| JM031 | 28138 | 28431 | + | 97 | Unknown | None | ||
| JM032 | 30095 | 28452 | – | 547 | Tegument protein | ORF19 | 547 | 97 |
| JM033 | 30642 | 29590 | – | 350 | Unknown | ORF20 | 350 | 91 |
| JM034 | 30641 | 32311 | + | 556 | Thymidine kinase | ORF21 | 557 | 97 |
| JM035 | 30825 | 31181 | + | 118 | Unknown | Unnamed (32278–32637) | 119 | 87 |
| JM036 | 31111 | 30863 | – | 82 | Unknown | None | ||
| JM037 | 32608 | 32279 | – | 109 | Unknown | None | ||
| JM038 | 32298 | 34478 | + | 726 | Glycoprotein H | ORF22 | 704 | 75 |
| JM039 | 33553 | 33254 | – | 99 | Unknown | None | ||
| JM040 | 35038 | 34475 | – | 187 | Unknown | ORF23 | 402 | 96 |
| JM041 | 34974 | 35285 | + | 103 | Unknown | Unnamed (36427–36828) | 134 | 89 |
| JM042 | 35687 | 35301 | – | 128 | Unknown | ORF23 | 402 | 95 |
| JM043 | 37938 | 35737 | – | 733 | Unknown | ORF24 | 732 | 97 |
| JM044 | 37612 | 37346 | – | 88 | Unknown | Unnamed (38732–38998) | 88 | 88 |
| JM045 | 37937 | 42073 | + | 1378 | Major capsid protein | ORF25 | 1,378 | 99 |
| JM046 | 39498 | 39821 | + | 107 | Unknown | Unnamed (40884–41207) | 107 | 87 |
| JM047 | 40282 | 39842 | – | 146 | Unknown | None | ||
| JM048 | 40188 | 40439 | + | 83 | Unknown | None | ||
| JM049 | 42105 | 43022 | + | 305 | Capsid protein | ORF26 | 305 | 99 |
| JM050 | 43047 | 43871 | + | 274 | Unknown | ORF27 | 269 | 91 |
| JM051 | 44035 | 44310 | + | 91 | Unknown | ORF28 | 91 | 91 |
| JM052 | 45406 | 44360 | – | 348 | DNA packaging protein | ORF29b | 348 | 98 |
| JM053 | 45720 | 46373 | + | 217 | Unknown | ORF31 | 217 | 96 |
| JM054 | 46195 | 45836 | – | 119 | Unknown | Unnamed (47209–47568) | 119 | 93 |
| JM055 | 46310 | 47695 | + | 461 | DNA packaging protein | ORF32 | 464 | 95 |
| JM056 | 47676 | 48686 | + | 336 | Tegument protein | ORF33 | 336 | 91 |
| JM057 | 48136 | 47768 | – | 122 | Unknown | None | ||
| JM058 | 48607 | 48218 | – | 129 | Unknown | Unnamed (49066–50040) | 324 | 87 |
| JM059 | 49587 | 48604 | – | 327 | DNA packaging protein | ORF29a | 327 | 97 |
| JM060 | 49276 | 49010 | – | 88 | Unknown | None | ||
| JM061 | 49586 | 50572 | + | 328 | Unknown | ORF34 | 327 | 95 |
| JM062 | 50950 | 50504 | – | 148 | Unknown | Unnamed (51874–52413) | 179 | 94 |
| JM063 | 50553 | 51002 | + | 149 | Unknown | ORF35 | 149 | 95 |
| JM064 | 50908 | 52215 | + | 435 | Serine/threonine protein kinase | ORF36 | 435 | 96 |
| JM065 | 52196 | 53638 | + | 480 | Alkaline exonuclease | ORF37 | 480 | 97 |
| JM066 | 55018 | 53882 | – | 378 | Glycoprotein M | ORF39 | 378 | 99 |
| JM067 | 54536 | 54850 | + | 104 | Unknown | None | ||
| JM068 | 55153 | 57156 | + | 667 | DNA helicase/primase complex | ORF40 | 468 | 96 |
| JM069 | 56474 | 56124 | – | 116 | Unknown | Unnamed (57497–57847) | 116 | 96 |
| JM070 | 57968 | 57153 | – | 271 | Unknown | ORF42 | 272 | 95 |
| JM071 | 57325 | 57573 | + | 82 | Unknown | Unnamed (58700–58948) | 82 | 97 |
| JM072 | 59652 | 57907 | – | 581 | Capsid protein | ORF43 | 576 | 98 |
| JM073 | 59591 | 61963 | + | 790 | Helicase/primase complex | ORF44 | 790 | 98 |
| JM074 | 61662 | 61249 | – | 137 | Unknown | None | ||
| JM075 | 63065 | 62004 | – | 353 | Immediate-early phase viral replication | ORF45 | 352 | 90 |
| JM076 | 62823 | 63086 | + | 87 | Unknown | None | ||
| JM077 | 63874 | 63107 | – | 255 | Uracil-DNA glycosylase | ORF46 | 255 | 91 |
| JM078 | 64341 | 63850 | – | 163 | Glycoprotein L | ORF47 | 169 | 55 |
| JM079 | 65769 | 64600 | – | 389 | Unknown | ORF48 | 389 | 92 |
| JM080 | 65629 | 65880 | + | 83 | Unknown | Unnamed (67028–67279) | 83 | 96 |
| JM081 | 65963 | 66208 | + | 81 | Unknown | None | ||
| JM082 | 66905 | 66000 | – | 301 | Unknown | ORF49 | 301 | 99 |
| JM083 | 66058 | 66315 | + | 85 | Unknown | Unnamed (67456–67713) | 85 | 97 |
| JM084 | 67096 | 68640 | + | 514 | Replication and transcription activator | ORF50 | 514 | 95 |
| JM085 | 68594 | 67920 | – | 224 | Unknown | Unnamed (69312–69956) | 214 | 88 |
| JM086 | 68957 | 69469 | + | 170 | bZIP transcription factor | ORF51 | 161 | 83 |
| JM087 | 70026 | 70760 | + | 244 | Glycoprotein | Glycoprotein R8.1 | 230 | 90 |
| JM088 | 70606 | 70328 | – | 92 | Unknown | Unnamed (71728–71970) | 80 | 89 |
| JM089 | 71420 | 71001 | – | 139 | Unknown | ORF52 | 139 | 96 |
| JM090 | 71797 | 71483 | – | 104 | Envelope glycoprotein | ORF53 | 104 | 98 |
| JM091 | 71873 | 72745 | + | 290 | dUTPase | ORF54 | 290 | 98 |
| JM092 | 72520 | 72942 | + | 140 | Unknown | None | ||
| JM093 | 73438 | 72806 | – | 210 | Unknown | ORF55 | 210 | 99 |
| JM094 | 73420 | 75936 | + | 838 | Helicase/primase complex | ORF56 | 828 | 97 |
| JM095 | 75722 | 75447 | – | 91 | Unknown | Unnamed (76848–77123) | 91 | 96 |
| JM096 | 76035 | 76304 | + | 89 | Unknown | Unnamed (77451–77753) | 100 | 80 |
| JM097 | 76162 | 77484 | + | 440 | Immediate-early phosphoprotein | ORF57 | 442 | 93 |
| JM098 | 79098 | 77857 | – | 413 | vIRF | R6 | 415 | 87 |
| JM099 | 78411 | 78656 | + | 81 | Unknown | None | ||
| JM100 | 79163 | 79579 | + | 138 | Unknown | None | ||
| JM101 | 80510 | 79269 | – | 413 | vIRF | R7 | 415 | 91 |
| JM102 | 79521 | 79796 | + | 91 | Unknown | None | ||
| JM103 | 81891 | 80836 | – | 351 | vIRF | R8 | 351 | 93 |
| JM104 | 83153 | 82068 | – | 361 | vIRF | R9 | 253 | 95 |
| JM105 | 82206 | 82613 | + | 135 | Unknown | Unnamed (83629–84111) | 160 | 82 |
| JM106 | 84785 | 83628 | – | 385 | vIRF | R10 | 385 | 86 |
| JM107 | 84837 | 85082 | + | 81 | Unknown | None | ||
| JM108 | 86104 | 84932 | – | 390 | vIRF | R11 | 390 | 83 |
| JM109 | 85290 | 85550 | + | 86 | Unknown | None | ||
| JM110 | 85968 | 86243 | + | 91 | Unknown | None | ||
| JM111 | 87546 | 86479 | – | 355 | vIRF | R12 | 355 | 88 |
| JM112 | 88801 | 87707 | – | 364 | vIRF | R13 | 364 | 87 |
| JM113 | 88023 | 87763 | – | 86 | Unknown | Unnamed (89178–89438) | 86 | 76 |
| JM114 | 88395 | 88087 | – | 102 | Unknown | None | ||
| JM115 | 90129 | 89047 | – | 360 | Unknown | ORF58 | 360 | 96 |
| JM116 | 91324 | 90140 | – | 394 | DNA replication protein | ORF59 | 394 | 97 |
| JM117 | 91017 | 91259 | + | 80 | Unknown | Unnamed (92432–92674) | 80 | 97 |
| JM118 | 92399 | 91455 | – | 314 | Ribonucleotide reductase small subunit | ORF60 | 314 | 98 |
| JM119 | 94747 | 92381 | – | 788 | Ribonucleotide reductase large subunit | ORF61 | 788 | 97 |
| JM120 | 93345 | 92929 | – | 138 | Unknown | Unnamed (94342–94758) | 138 | 92 |
| JM121 | 94175 | 94468 | + | 97 | Unknown | None | ||
| JM122 | 95746 | 94751 | – | 331 | Assembly/DNA maturation protein | ORF62 | 331 | 99 |
| JM123 | 95745 | 98564 | + | 939 | Tegument protein | ORF63 | 939 | 96 |
| JM124 | 98568 | 104468 | + | 1966 | Tegument protein | ORF64 | 2,548 | 96 |
| JM125 | 99753 | 99388 | – | 121 | Unknown | Unnamed (100644–101165) | 173 | 93 |
| JM126 | 100054 | 99611 | – | 147 | Unknown | Unnamed (101023–101466) | 147 | 95 |
| JM127 | 101428 | 101108 | – | 106 | Unknown | None | ||
| JM128 | 102568 | 102290 | – | 92 | Unknown | None | ||
| JM129 | 103150 | 102857 | – | 97 | Unknown | Unnamed (104269–104562) | 97 | 91 |
| JM130 | 104643 | 104302 | – | 113 | Unknown | None | ||
| JM131 | 104555 | 105856 | + | 433 | Tegument protein | ORF64 | 2,548 | 88 |
| JM132 | 106003 | 105581 | – | 140 | Unknown | None | ||
| JM133 | 106729 | 106220 | – | 169 | Capsid protein | ORF65 | 169 | 98 |
| JM134 | 106299 | 106559 | + | 86 | Unknown | Unnamed (107716–107976) | 86 | 95 |
| JM135 | 108079 | 106733 | – | 448 | Unknown | ORF66 | 448 | 92 |
| JM136 | 107289 | 107696 | + | 135 | Unknown | Unnamed (108708–109040) | 110 | 84 |
| JM137 | 108780 | 107974 | – | 268 | Tegument protein | ORF67 | 224 | 98 |
| JM138 | 108002 | 108316 | + | 104 | Unknown | None | ||
| JM139 | 109056 | 108796 | – | 86 | DNA packaging protein | ORF67.5 | 86 | 95 |
| JM140 | 109190 | 110563 | + | 457 | Glycoprotein | ORF68 | 457 | 95 |
| JM141 | 110166 | 109783 | – | 127 | Unknown | Unnamed (111202–111585) | 127 | 90 |
| JM142 | 110904 | 110578 | – | 108 | Unknown | Unnamed (111979–112323) | 114 | 95 |
| JM143 | 110585 | 111478 | + | 297 | Capsid maturation protein | ORF69 | 297 | 97 |
| JM144 | 112447 | 112698 | + | 83 | Unknown | RU3-R | 101 | 69 |
| JM145 | 113610 | 114479 | + | 289 | Unknown | RU4-R | 486 | 48 |
| JM146 | 114753 | 114427 | – | 108 | Unknown | None | ||
| JM147 | 115918 | 115214 | – | 234 | Unknown | RU13-L | 151 | 65 |
| JM148 | 115671 | 115928 | + | 85 | Unknown | None | ||
| JM149 | 115983 | 115717 | – | 88 | Unknown | RU13-L | 151 | 92 |
| JM150 | 116366 | 116650 | + | 94 | Unknown | None | ||
| JM151 | 117442 | 116918 | – | 174 | v-FLIP | ORF71 | 174 | 91 |
| JM152 | 118265 | 117501 | – | 254 | Cyclin D | ORF72 | 254 | 93 |
| JM153 | 119918 | 118608 | – | 436 | Latency-associated nuclear antigen | ORF73 | 448 | 86 |
| JM154 | 119237 | 119617 | + | 126 | Unknown | Unnamed (121540–121920) | 126 | 87 |
| JM155 | 119614 | 119940 | + | 108 | Unknown | Unnamed (121917–122234) | 105 | 73 |
| JM156 | 120075 | 119824 | – | 83 | Unknown | Unnamed (122118–122369) | 83 | 96 |
| JM157 | 120484 | 120744 | + | 86 | Unknown | Unnamed (122778–123038) | 86 | 91 |
| JM158 | 120572 | 121333 | + | 253 | CD200 homologue | R15 | 253 | 97 |
| JM159 | 121186 | 120689 | – | 165 | Unknown | Unnamed (122983–123480) | 165 | 90 |
| JM160 | 121628 | 122656 | + | 342 | G protein-coupled receptor | ORF74 | 342 | 96 |
| JM161 | 126658 | 122762 | – | 1298 | FGARAT/tegument protein | ORF75 | 1,298 | 95 |
| JM162 | 123398 | 123126 | – | 90 | Unknown | None | ||
| JM163 | 123423 | 123677 | + | 84 | Unknown | Unnamed (125718–125972) | 84 | 88 |
| JM164 | 123810 | 124133 | + | 107 | Unknown | None | ||
| JM165 | 124395 | 124790 | + | 131 | Unknown | None | ||
| JM166 | 125165 | 124683 | – | 160 | Unknown | None | ||
| JM167 | 126063 | 126371 | + | 102 | Unknown | Unnamed (128358–128666) | 102 | 90 |
| JM168 | 128493 | 128122 | – | 123 | Unknown | None | ||
| JM169 | 129046 | 128789 | – | 85 | Unknown | RK15 exon 1 | 81 | 42 |
| JM170 | 130404 | 129970 | – | 144 | Unknown | Unnamed (132318–132731) | 137 | 90 |
| JM171 | 130380 | 130655 | + | 91 | Unknown | Unnamed (132707–133009) | 100 | 76 |
The data are highlighted as follows: JMRV ORFs with homology to previously predicted RRV ORFs (unshaded, normal typeface), JMRVORFs with homology to previously unidentified and unnamed RRV ORFs (gray shaded), and JMRV ORFs with no identifiable homolog in RRV (unshaded, boldface type). nt, nucleotide(s); aa, amino acid(s).
+, Top strand; –, bottom strand.
Based on the known and predicted functions of homologous proteins.
Based on sequence homology to RRV ORFs.
That is, the percent identity of overlapping regions of viral proteins.
Eighty-three small ORFs that encode putative proteins of ≥80 aa were also identified in JMRV. Predicted proteins of similar size were not previously identified in RRV, since this is less than the cutoff level that was utilized during the initial characterization of RRV (6). Thus, to determine whether any of these ORFs are specific to JMRV, the RRV genome was reanalyzed to include ORFs that would encode proteins of ≥80 aa. From this analysis it was determined that 44 of the small JMRV ORFs are also predicted to be present in the RRV genome (denoted in Table 3 as unnamed ORFs based on the nucleotide position in RRV), while 39 are unique to JMRV. The function of the proteins predicted to be encoded by the 39 JMRV-specific ORFs remains unknown, and BLAST analysis does not suggest any readily apparent homology to any known proteins. However, due to the presence of these unique ORFs in JMRV, and their absence in RRV, it is possible that some of these ORFs may encode proteins that confer unique pathogenic qualities to JMRV. These ORFs are being examined in further detail to determine their potential functions.
Interestingly, several ORFs are predicted to be present in locations of the genome containing repeat sequences. For example, JM145 spans the region containing rDL-E 1 and 2, while Repeat 1 is located within JM23. If these regions are in fact capable of producing functional transcripts and supporting protein expression, these ORFs could produce variable proteins depending on the exact structure of the repeats within a given virus. Further, examination of the TR unit sequence indicates the presence of two complete predicted ORFs that are also contained within the partial TR sequences at the left and right end of the sequenced genome (JM1 and JM171, respectively), as well as a variant of JM170.
As the last predicted ORF at the right end of the genome, JM170 actually overlaps the junction of the LUR and the partial TR sequence located at the genome terminus. Thus, while the form of JM170 found within the complete TR unit contains a nearly full duplication of JM170 sequence, it varies in that the last 16 bp of the 435-bp JM170 ORF are replaced with a different 49-bp sequence. Due to this difference, this ORF has been denoted as JM170-TR. If JM170-TR is indeed transcribed and expressed from the TR, it would result in the production of a protein in which the C-terminal 4 aa of the predicted 144-aa JM170 protein are replaced with a different 15-aa sequence. Therefore, JM170-TR may represent a variant of JM170 that is strictly expressed from the TR unit.
The apparent duplication of portions of the viral genome as parts of the TR results in the possibility that viral genes contained within this sequence may actually be present in multiple copies in the virus due to amplification of the TR. This suggests that viral genes present within the TR of JMRV that are capable of being transcribed and expressed might be present in abundance in infected cells. A similar observation has been made in MHV-68, which also contains predicted ORFs within the TR (15). However, the putative functions of all three proteins encoded by the ORFs located in the JMRV TR are currently unknown and, based on database searches, none appear to share strong sequence homology to any known proteins. Regardless, it will be important to determine whether or not these genes are highly expressed during infection and, if so, what impact(s) they may potentially have on viral disease.
Expression of JMRV-unique genes during infection.
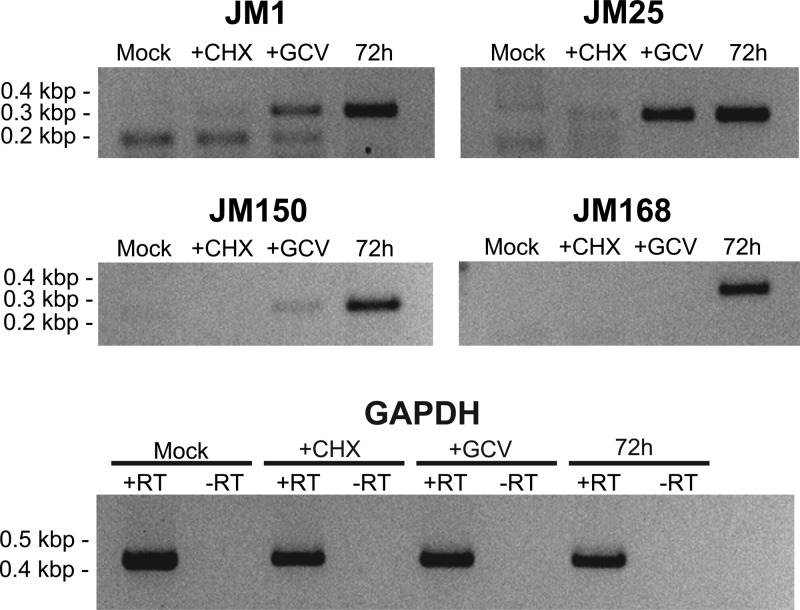
To address whether any predicted unique JMRV ORFs are actually expressed during JMRV infection, analysis was undertaken to identify transcripts produced by a selection of these ORFs. Specifically, four JMRV-unique ORFs were examined; JM1, JM25, JM150, and JM168, which are predicted to encode small proteins of 10, 9.8, 10.2, and 14 kDa, respectively. These ORFs were chosen since they are located in distinct regions of the genome and are do not overlap with any other predicted JMRV ORFs. In addition, one of these ORFs, JM1, is located within the TR unit of JMRV and thus represents a gene with the potential to be present in multiple copies within infected cells. To determine whether these genes are actually expressed during JMRV infection and also to attempt to define their kinetic class, transcriptional analysis was undertaken using RNA isolated from JMRV-infected primary rhesus fibroblasts. Briefly, cells were infected with JMRV and treated with cycloheximide (CHX) to inhibit protein synthesis, ganciclovir (GCV) to inhibit DNA replication, or left untreated to define transcripts as immediate-early, early, or late, respectively. The time points for collection of RNA were 24 h (+CHX), 48 h (+GCV), and 72 h (untreated). RNA from mock-infected cells collected at 72 h served as a negative control for all reactions. The isolated RNA was then used in RT-PCR with primers specific to each ORF, and the results from this analysis demonstrated that transcripts associated with all four predicted ORFs are produced in JMRV-infected cells (Fig. 5). In regard to the kinetics of their expression, JM1 is expressed as an early gene, JM25 is an early gene with some leaky immediate-early expression, JM150 is a late gene with some leaky early expression, and JM168 is a strict late gene.
Fig 5.
Analysis of JMRV-unique gene expression. RNA was purified from primary rhesus fibroblasts infected with JMRV and subjected to RT-PCR using primers specific for JMRV-unique ORFs. Cells were infected at an MOI of 5, treated with CHX, GCV, or left untreated, and collected at 24, 48, and 72 h, respectively. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) reactions with RT (+RT) serve as controls for the presence of RNA, and GAPDH reactions without RT (−RT) demonstrate absence of DNA in each sample.
Despite the fact that the predicted proteins encoded by these ORFs do not appear to possess significant homology to any known proteins, and their potential functions currently remain unknown, given their small sizes it is possible that they represent secreted proteins that are somehow involved in pathogenesis. Although analysis of the protein sequences encoded by these ORFs for possible secretory motifs does not reveal the presence of classical signal peptides, nonclassical secretion signals are predicted to exist for JM1, JM25, and JM150 (data not shown). Further characterization of these genes is ongoing to determine their exact functions and assess what roles they may play in the development of JMRV-associated disease.
Deep sequencing analysis of JMRV plaque isolates.
In addition to the original stock of JMRV, which likely represents a swarm of strain variants, two plaque-purified isolates of JMRV were also obtained. These viruses were designated isolates 3A1 and 12E2. Both plaque isolates were examined by in vitro growth analysis and found to replicate with similar kinetics to the parental virus (data not shown). To compare the genomic sequence of these plaque isolates, genomic DNA was purified from each virus, and deep sequencing analysis of the complete genome of each isolate was performed. After assembly of the individual reads from the deep sequencing analysis into contiguous sequences, a consensus sequence for each virus was generated representing the complete genomic sequence of each isolate. Using this approach, a sequencing redundancy of ∼300-fold was obtained for both genomes.
Upon alignment of all of the JMRV genome sequences, several regions in both of the plaque isolate genomes were initially found to have small gaps in sequence compared to the original parental JMRV genome. Specifically, nine gaps were identified in the LUR of the consensus sequence of each plaque isolate (Table 4), most of which were determined to be located in regions corresponding to internal repeat sequences, including rDL-B 1 and 2, rDL-E 1 and 2, and rDL-F. These gaps all represent regions incompletely assembled during the generation of a consensus sequence for each isolate, due to the size of the short sequence reads generated by this method of sequencing and the repetitive nature of sequences in these regions. In addition to the gaps in LUR sequence, the presence of the highly repetitive DR1 and DR2 units in the TR resulted in smaller portions of the partial TR sequences in the isolate genomes being assembled. Specifically, the partial TR sequence at the left and right end of each genome extends only as far as the beginning of DR2 and DR1, respectively.
Table 4.
Characterization of sequencing gapsa
| Sequencing gap | Genomic location (nt)b |
Gap size (bp)c |
Edited genomic sequence (nt)d |
Associated JMRV repeat | |||
|---|---|---|---|---|---|---|---|
| 3A1 | 12E2 | 3A1 | 12E2 | 3A1 | 12E2 | ||
| 1 | 20493 | 20493 | 45 | 45 | 20493–20537 | 20493–20537 | Repeat 1 |
| 2 | 23721 | 23721 | 170 | 196 | 23721–23890 | 23721–23916 | rDL-B 1 |
| 3 | 24093 | 24119 | ND | ND | NA | NA | rDL-B 2 |
| 4 | 26910 | 26936 | 35 | 35 | 26911–26945 | 26937–26971 | |
| 5 | 54754 | 54780 | 181 | 190 | 54755–54935 | 54781–54970 | |
| 6 | 82834 | 82860 | 110 | 110 | 82835–82944 | 82861–82970 | |
| 7 | 110918 | 110944 | 30 | 30 | 110919–110948 | 110945–110974 | |
| 8 | 112670 | 112696 | ND | ND | NA | NA | rDL-E 1 and 2 |
| 9 | 127842 | 127868 | 92 | 92 | 127843–127934 | 127869–127960 | rDL-F |
ND, not determined; NA, not applicable.
That is, the first missing nucleotide in the gap.
That is, the determined size of the missing sequence based on PCR analysis.
That is, the location of the sequence confirmed by PCR and manually edited.
To confirm the identity of the gaps in LUR sequence of the plaque isolate genomes, PCR was performed using purified viral DNA from each respective plaque isolate and primer sets designed to amplify across these regions, followed by direct sequencing of the resulting products (Table 4). Only gaps 3 and 8 in the LUR of the plaque isolates, corresponding to rDL-B 2 and rDL-E 1/2, respectively, proved to be recalcitrant to complete PCR and sequencing across the entire region due to a combination of their highly repetitive nature and high GC content. The remaining gaps in the LUR sequences of the plaque isolates were all completely analyzed and confirmed to contain identical sequences to those identified in the parental JMRV genome, with only a few variances in repeat unit numbers detected in some locations. The nucleotide sequences of the plaque isolates were manually edited at locations confirmed by PCR and sequencing to reflect the presence of the sequences excluded from the original assemblies (Table 4). The sequences of both isolates are available in GenBank under accession numbers JN885136 (isolate 3A1) and JN885137 (isolate 12E2).
Based on nucleotide alignments of the complete genomic sequence of each virus (including the partial TR sequences), isolate 3A1 and 12E2 were found to be 100% identical to each other and 98.4% identical to the genomic sequence of the parental JMRV. Further, when only the LUR sequences of each genome were compared, isolates 3A1 and 12E2 were both found to be 99.2% identical to the parental JMRV genome sequence, with all of the observed differences between the parental and plaque isolate genomes being attributed to the lack of complete sequences across repeat regions rDL-B 2, rDL-E 1, and rDL-E 2 in the plaque isolates, as well as the presence of one ambiguous residue in isolate 3A1 and two ambiguous residues in isolate 12E2. Thus, both plaque isolates of JMRV represent clonal viruses derived from the parental JMRV strain, with which they share a high level of genetic identity.
DISCUSSION
JMRV was recently isolated from a JM that developed JME, a disease that possesses clinical and histopathological features that resemble MS in humans (1). As reported here, we have fully sequenced the complete genome of JMRV, as well as two plaque isolates of this virus, the results of which provide further evidence that JMRV is a gammaherpesvirus highly similar to other human and primate gammaherpesviruses. Specifically, JMRV is most closely related to RRV, a rhesus macaque herpesvirus that is associated with the development of lymphoproliferative disorders in simian immunodeficiency virus-infected rhesus macaques, as well as HHV-8, an oncogenic human herpesvirus (16, 17). Interestingly, JMRV is less closely related to EBV, a nearly ubiquitous human gammaherpesvirus that has been suggested to be associated in the development of MS (18). Despite these findings, it is possible that the genetic similarity of JMRV to human viruses such as EBV and HHV-8 is less important and may also suggest that an as-yet-unidentified gammaherpesvirus more closely related to JMRV, and potentially associated with MS, could also exist in humans.
JMRV possesses numerous unique ORFs not found even in closely related RRV, which may suggest these genes are important for the induction of virus-mediated disease in JMRV-infected animals. Examination of a selection of these predicted unique genes (JM1, JM25, JM150, and JM168) indicates that they are in fact expressed during JMRV infection in vitro and thus represent a potential source of novel viral protein products. One of these genes, JM1, is located within the TR unit and therefore likely to be present in multiple copies within the viral genome. Overrepresentation of JM1 in the viral genome could result in a high level of protein production from this gene during infection. Further analysis of these genes and their possible functions is ongoing.
In addition to unique genes, JMRV does share several ORFs with RRV that are of potential interest due to their predicted roles in immunoregulation and possible connections with inflammatory demyelinating disease development. These ORFs include JM21, JM24, and JM158, the homologues of RRV R2, R3, and R15, respectively. Although as yet functionally analyzed, JM21 encodes a viral homologue of interleukin-6, a cytokine that has been suggested to be linked to the development of MS (19); JM24 encodes a viral macrophage inflammatory protein (vMIP) that may be capable of affecting the infiltration of macrophages, a cell type which has been suggested to have a direct role in the development of MS lesions (20); and JM158 encodes a viral homologue of cellular CD200, a protein hypothesized to be critical for maintaining immune suppression in the brain that may also be dysregulated in MS patients (2, 21). Thus, it is possible that the expression of these viral genes in infected animals could induce immune alterations that ultimately result in the induction of JME.
Taken together, JMRV represents a novel and highly relevant model system for analyzing the possible role of an infectious agent in the development of inflammatory demyelinating disease. Due to the ability to easily propagate JMRV in vitro and the availability of the complete genomic sequence, identifying viral determinants of pathogenesis via molecular approaches may help shed significant light onto the role of specific viral genes in disease development.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants 8P51OD011092-53, CA075922 (S.W.W.), and CA132638 (S.W.W.) and U.S. Department of Defense grant W81XWH-09-1-0276 (S.W.W.).
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Axthelm MK, Bourdette DN, Marracci GH, Su W, Mullaney ET, Manoharan M, Kohama SG, Pollaro J, Witkowski E, Wang P, Rooney WD, Sherman LS, Wong SW. 2011. Japanese macaque encephalomyelitis: a spontaneous multiple sclerosis-like disease in a nonhuman primate. Ann. Neurol. 70:362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meuth SG, Simon OJ, Grimm A, Melzer N, Herrmann AM, Spitzer P, Landgraf P, Wiendl H. 2008. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J. Neuroimmunol. 194:62–69 [DOI] [PubMed] [Google Scholar]
- 3. Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8:785–786 [DOI] [PubMed] [Google Scholar]
- 4. Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. 2004. Feature-based prediction of non-classical and leaderless protein secretion. PEDS 17:349–356 [DOI] [PubMed] [Google Scholar]
- 5. Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. 2003. Complete sequence and genomic analysis of rhesus cytomegalovirus. J. Virol. 77:6620–6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Searles RP, Bergquam EP, Axthelm MK, Wong SW. 1999. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lagunoff M, Ganem D. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236:147–154 [DOI] [PubMed] [Google Scholar]
- 8. Vink C, Beuken E, Bruggeman CA. 1996. Structure of the rat cytomegalovirus genome termini. J. Virol. 70:5221–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deiss LP, Chou J, Frenkel N. 1986. Functional domains within the a sequence involved in the cleavage-packaging of herpes simplex virus DNA. J. Virol. 59:605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bankier AT, Dietrich W, Baer R, Barrell BG, Colbere-Garapin F, Fleckenstein B, Bodemer W. 1985. Terminal repetitive sequences in herpesvirus saimiri virion DNA. J. Virol. 55:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 12. Moore PS, Gao SJ, Dominguez G, Cesarman E, Lungu O, Knowles DM, Garber R, Pellett PE, McGeoch DJ, Chang Y. 1996. Primary characterization of a herpesvirus agent associated with Kaposi's sarcomae. J. Virol. 70:549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. U. S. A. 93:14862–14867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexander L, Denekamp L, Knapp A, Auerbach MR, Damania B, Desrosiers RC. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Virgin HWt, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orzechowska BU, Powers MF, Sprague J, Li H, Yen B, Searles RP, Axthelm MK, Wong SW. 2008. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood 112:4227–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong SW, Bergquam EP, Swanson RM, Lee FW, Shiigi SM, Avery NA, Fanton JW, Axthelm MK. 1999. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 190:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ascherio A, Munger KL. 2010. Epstein-Barr virus infection and multiple sclerosis: a review. J. Neuroimmune Pharmacol. 5:271–277 [DOI] [PubMed] [Google Scholar]
- 19. Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. 2011. Interleukin-6, a mental cytokine. Brain Res. Rev. 67:157–183 [DOI] [PubMed] [Google Scholar]
- 20. Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. 2005. Macrophages and neurodegeneration. Brain Res. Rev. 48:185–195 [DOI] [PubMed] [Google Scholar]
- 21. Koning N, Bo L, Hoek RM, Huitinga I. 2007. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann. Neurol. 62:504–514 [DOI] [PubMed] [Google Scholar]