Abstract
Successful viruses have evolved superior strategies to escape host defenses or exploit host biological pathways. Most of the viral immediate-early (ie) genes are essential for viral infection and depend solely on host proteins; however, the molecular mechanisms are poorly understood. In this study, we focused on the modification of viral IE proteins by the crayfish small ubiquitin-related modifier (SUMO) and investigated the role of SUMOylation during the viral life cycle. SUMO and SUMO ubiquitin-conjugating enzyme 9 (UBC9) involved in SUMOylation were identified in red swamp crayfish (Procambarus clarkii). Both SUMO and UBC9 were upregulated in crayfish challenged with white spot syndrome virus (WSSV). Replication of WSSV genes increased in crayfish injected with recombinant SUMO or UBC9, but injection of mutant SUMO or UBC9 protein had no effect. Subsequently, we analyzed the mechanism by which crayfish SUMOylation facilitates WSSV replication. Crayfish UBC9 bound to all three WSSV IE proteins tested, and one of these IE proteins (WSV051) was covalently modified by SUMO in vitro. The expression of viral ie genes was affected and that of late genes was significantly inhibited in UBC9-silenced or SUMO-silenced crayfish, and the inhibition effect was rescued by injection of recombinant SUMO or UBC9. The results of this study demonstrate that viral IE proteins can be modified by crayfish SUMOylation, prompt the expression of viral genes, and ultimately benefit WSSV replication. Understanding of the mechanisms by which viruses exploit host components will greatly improve our knowledge of the virus-host “arms race” and contribute to the development of novel methods against virulent viruses.
INTRODUCTION
SUMOylation, a mechanism of posttranslational control by which multiple proteins are covalently modified, plays a vital role in the “fine-tuning” regulation of protein function. In plants, SUMOylation participates in the regulation of blooming (1), hormone transduction (2, 3), and antiviral defense (4). SUMOylation influences several cellular processes in Drosophila and higher animals, including subcellular localization (5), substrate stability (6), and the regulation of transcriptional activity (7). Small ubiquitin-related modifiers (SUMOs) are approximately 11- to 12-kDa proteins that comprise a protein family highly conserved among different species (8). SUMO possesses a distinct positive-negative charge area and a flexible N-terminal intrusion rich in glycine (Gly) and proline residues, which provides the structural basis for protein-protein interactions (9). SUMO has been reported to covalently bind many important cellular proteins, such as the androgen receptor (10), c-jun (11), and p53 (12), to mediate their protein-protein interactions. SUMO is produced as an inactive precursor that is activated by hydrolysis at the C terminus which exposes double-Gly residues (13).
In contrast to ubiquitination, SUMOylation exclusively utilizes the E2 conjugating enzyme, namely, SUMO ubiquitin-conjugating enzyme 9 (UBC9). UBC9 is highly conserved from yeast to humans and contains a core ubiquitin-conjugating catalytic (UBCc) domain, which contains a conserved cysteine residue (Cys93). During SUMOylation, SUMO is transferred onto the active Cys93 of UBC9 by the activating enzyme E1 (14). UBC9 then transports SUMO onto the substrate, and an isopeptide bond forms between the double-Gly residues of SUMO and the Å-amino group of a substrate lysine residue (15). In vitro assays have confirmed that E1 activating enzyme and E2 conjugating enzyme are sufficient for substrate SUMOylation (14, 15).
Numerous recent studies have focused on the interactions between host SUMOylation and mammalian viruses. The immediate-early (IE) proteins of human cytomegalovirus (16) and herpesvirus 6 (17) interact with UBC9 and can be covalently modified by SUMO. Association of UBC9 and SUMO is essential for perinuclear localization of the Hantaan virus nucleocapsid protein (NP) (18). Ebola Zaire virus blocks production of type I interferon by exploiting host SUMOylation machinery (19). Moreover, human UBC9 plays an important role in the production of infectious human immunodeficiency virus (HIV-1) virions by SUMOylation of the major HIV-1 structural protein Gag (20). However, studies of the interactions between SUMOylation and viruses which infect invertebrates are rare.
White spot syndrome virus (WSSV) is a rod-shaped, enveloped virus and is the only member of the genus Whispovirus within the Nimaviridae family (21). With a broad host range among crustaceans, it is the most virulent virus to infect shrimp and leads to serious economic losses in the shrimp industry; however, no effective control methods or vaccine currently exists. Nevertheless, an effective method for quantitating infectious WSSV has been developed by using real-time PCR, which facilitates the virus detection (22). WSSV has one of the largest genomes (290 kb; double-stranded DNA [dsDNA]) of all known animal viruses (23). The transcription of viral genes begins with the immediate-early (ie) genes, followed by the early genes and late genes. ie genes generally encode regulatory proteins that are critical for viral replication. The expression of IE proteins forms the basis of viral lytic infection and relies solely on host proteins, whereas the transcription and translation of early and late genes depends on IE proteins (24). Moreover, ie genes encode proteins involved in altering the function of host proteins (25) and in weakening the host antiviral defense (26). Therefore, the functional analysis of IEs is of great significance in understanding the mechanisms of viral infection.
In this study, we discovered that injection of recombinant UBC9 or SUMO increased the expression of WSSV ie genes in red swamp crayfish (Procambarus clarkii); however, the injection of mutant UBC9 (mUBC9) or mutant SUMO (mSUMO) had no effect. Interestingly, all three WSSV IE proteins tested interacted with UBC9 and one of these IE proteins (WSV051) could be modified by SUMO in vitro. Pulldown assays and RNA interference (RNAi) indicated that crayfish UBC9 and SUMOylation were utilized by viral IE proteins to facilitate the expression of early and late viral genes, which ultimately benefited the replication of WSSV.
MATERIALS AND METHODS
Crayfish challenge, tissue collection, and cloning of UBC9 and SUMO cDNA.
Red swamp crayfish (P. clarkia; 10 to 20 g each) were purchased from the Jinan seafood market (Shandong Province, China) and cultured in water tanks. For the immune challenge, WSSV (China isolate; 106 copies) was injected into the abdominal segment of each crayfish using a microliter syringe. The control group crayfish were injected with an equal volume of phosphate-buffered saline (PBS; 140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4). At 0, 6, 12, 24, 48, and 72 h postchallenge, the hemolymph was extracted from the ventral sinus using a syringe with a 1/10 volume of 10% sodium citrate as an anticoagulant buffer and then centrifuged immediately at 800 × g for 15 min at 4°C to collect the hemocytes. Subsequently, other tissues (heart, hepatopancreas, gills, stomach, and intestine) were obtained from both groups by dissection (three crayfish per group). Total RNA was extracted from the hemocytes, heart, hepatopancreas, gills, stomach, and intestine of control and WSSV-challenged crayfish using Unizol reagent (Biostar, Shanghai, China). And all RNAs were digested by DNase I (Fermentas, Canada) at 37°C for 30 min to obtain DNA-free RNA for in vitro reverse transcription (RT).
RNA (5 μg) was reverse transcribed to first-strand cDNA using a Smart cDNA kit (Clontech, Santa Clara, CA) and the primers Oligo-anchor R and Smart F. The hepatopancreas cDNA was diluted 10-fold and used as a template for PCR. Specific primers were designed to clone full-length crayfish UBC9 and SUMO based on the cDNA sequences obtained from a P. clarkii hemocyte cDNA library. All of the primer sequences used in this study are listed in Table 1.
Table 1.
Oligonucleotide primers used in this study
| Primer name | Sequence (5′→3′)a |
|---|---|
| Gene cloning | |
| SMART F | TACGGCTGCGAGAAGACGACAGAAGGG |
| Oligo-anchor R | GACCACGCGTATCGATGTCGACT16(A/C/G) |
| UBC9 F | ATGTCTGGAATTGCAATTGC |
| SUMO F | ATGTCCGATAACGCTGACGCCAA |
| Semiquantitative PCR | |
| 18SRTF | TCTTCTTAGAGGGATTAGCGG |
| 18SRTF | AAGGGGATTGAACGGGTTA |
| UBC9RTF | CAGCCATTACCATCAAACAGA |
| UBC9RTR | TTCCTCCCACTAACTTCACTT |
| SUMORTF | ATACTCCTAAGTGGATGGTGC |
| SUMORTR | ATGAGAGGTTATCTATCGGCT |
| UBC9ReF | GCTAACAACTGGCACCGAAGA |
| UBC9ReR | CATGACTGAGAATGGAATGAAAGG |
| SUMOReF | CGGTAGCATCACTCCGCTTTCT |
| SUMOReR | TAGCCTTAGTCCTACACTCGTGGG |
| Recombinant expression | |
| UBC9ExF | TACTCACATATGATGTCTGGAATTGCAATTGCTCG |
| UBC9ExR | TACTCACTCGAGCTCGAATGGTGCCGACATTGCTT |
| mUBC9F | TCAGGAACTGTGTCCTTGTCACTATTAGAC |
| mUBC9R | ATAGTGACAAGGACACAGTTCCTGATGGGT |
| SUMOExF | TACTCAGGATCCATGTCCGATAACGCTGACGCCAA |
| SUMOExR | TACTCACTCGAGATGGCCTCCAGTTTGTTCCTGGT |
| SUMO-GGExR | TACTCACTCGAGTCAGCCTCCAGTTTGTTCCTGGTA |
| mSUMOExR | TACTCACTCGAGTCAAGTTTGTTCCTGGTAAACTTC |
| WSV051F | TACTCAGAATTCATGGACGTTTCTTCCTATAAG |
| WSV051R | TACTCAAAGCTTCGTCGCAACAACTTTAGCTAC |
| WSV069F | TACTCAGAATTCATGGCCTTTAATTTTGAAGAC |
| WSV069R | TACTCACTCGAGTTATACAAAGAATCCAGAAAT |
| WSV187F | TACTCAGAATTCATGGGGCGGGATACAAAACGG |
| WSV187R | TACTCACTCGAGTCAGCCGCATGAGTATAAATA |
| RNAi | |
| GFPiF | TACTCAGCGGCCGCATGAGCAAGGGCGAGGAACTG |
| GFPiR | TACTCACTCGAGCTTGTACAGCTCGTCCATGCC |
| UBC9iF | TACTCAGCGGCCGCATGTCTGGAATTGCAATTGCTCG |
| UBC9iR | TACTCACTCGAGCTCGAATGGTGCCGACATTGCTT |
| SUMOiF | TACTCAGCGGCCGCATGTCCGATAACGCTGACGCCAA |
| SUMOiR | TACTCACTCGAGATGGCCTCCAGTTTGTTCCTGGT |
Restriction enzyme cutting sites are underlined.
Phylogenetic and sequence analysis.
The cDNA sequence similarity analysis was performed using BLAST (http://www.ncbi.nlm.nih.gov/). The deduced amino acid sequences and predicted protein motifs were generated using ExPASy (http://au.expasy.org/) and SMART (http://smart.embl-heidelberg.de/index2.cgi). Sequence alignments were performed using Genedoc and ClustalW (http://www.psc.edu/biomed/genedoc). MEGA 4.0 (27) was used to construct phylogenetic trees.
Semiquantitative RT-PCR and real-time RT-PCR.
Semiquantitative RT-PCR was used to determine the distribution of SUMO and UBC9 in PBS-injected crayfish tissues using specific primer pairs UBC9RTF-UBC9RTR and SUMORTF-SUMORTR, respectively. The control group was added with sterile ultrapure water instead of tissue cDNA. 18S rRNA (EU920952.1) was amplified as an internal control using the specific primers 18SRTF and 18SRTR. The RT-PCR conditions were as follows: 1 cycle at 94°C for 3 min, 26 cycles at 94°C for 30 s, 55°C for 45 s, and 72°C for 30 s, and 1 cycle at 72°C for 10 min. Real-time quantitative RT-PCR (qRT-PCR) was employed to quantify the expression of UBC9 and SUMO after WSSV challenge using specific primer pairs UBC9ReF-UBC9ReR and SUMOReF-SUMOReR, with 18S rRNA as an internal control. The qRT-PCR reaction mixture consisted of 5 μl of SYBR Premix Ex Taq (TaKaRa, Tokyo, Japan), 2 μl of each primer (1 μM), and 1 μl of cDNA (diluted 1:100). The PCR was performed as follows: 94°C for 5 min, 40 cycles at 94°C for 15 s and 60°C for 50 s, and then melting from 60°C to 95°C performed with a real-time thermal cycler (Bio-Rad). Each experiment was repeated three times. The expression levels were analyzed by using the comparative threshold cycle (CT) method and the formula 2−ΔΔCT (ΔΔCT = ΔCTgene − ΔCT18S rRNA). Standard deviation and statistical analyses by unpaired sample t test were also employed; the significant difference was P < 0.05.
Expression and purification of recombinant proteins and generation of antiserum.
To study the function of crayfish UBC9 and SUMO, the proteins were recombinantly expressed in Escherichia coli. Primers incorporating NdeI and XhoI restriction sites (UBC9ExF and UBC9ExR) were used to generate the UBC9 open reading frame (ORF), which was subcloned into the plasmid pET-30a (+). Mutant UBC9 (mUBC9) was amplified using specific primer pairs UBC9ExF-mUBC9R and mUBC9F-UBC9ExR to introduce a Cys93-to-serine point mutation. The two cDNA fragments were mixed and amplified for 10 cycles using Pfu DNA polymerase (TransGen, Beijing, China), and the PCR product was used as the template to produce the full-length mUBC9 with primers UBC9ExF and UBC9ExR. After validating the mutation by sequencing, the cDNA fragment was digested by NdeI and XhoI and ligated to pET-30a (+). Both kinds of recombinant plasmids [pET-30a (+)-UBC9 and pET-30a (+)-mUBC9] were sequenced and transformed into E. coli BL21(DE3) cells. Recombinant protein was induced using 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and purified from the supernatant using a nickel-nitrilotriacetic acid (Ni-NTA) affinity column (GE Amersham Biosciences) according to the manufacturer's instructions.
As SUMO is initially produced as an inactive precursor with an additional residue following two double-Gly residues forming the active site, three different forms of SUMO were recombinantly expressed. The active form, SUMO-GG, had an exposed reaction center formed by deletion of the C-terminal residue to expose the double-Gly residues. Mutant SUMO (mSUMO) was expressed as a truncated protein lacking the C-terminal double-Gly residues. The cDNA fragments encoding SUMO, SUMO-GG, and mSUMO were generated by primers incorporating BamHI and XhoI restriction sites (Table 1), subcloned into pGEX-4T-1 plasmid, transformed into E. coli BL21 cells, and expressed as soluble proteins by induction with 0.5 mM IPTG. Recombinant SUMO, SUMO-GG, and mSUMO were purified by glutathione Sepharose 4B chromatography (GE Amersham Biosciences) according to the manufacturer's instructions.
To investigate whether UBC9 and SUMO interacted with WSSV IE proteins, three reported WSSV IE proteins were recombinantly expressed in E. coli: IE1/WSV069 (GenBank accession no. AAL33073.1) (24, 28, 29), WSV051 (GenBank accession no. AAL33055.1) (24), and WSV187 (GenBank accession no. AAL33191.1) (29). Using the WSSV genome as a template, the target genes were amplified and digested using EcoRI and XhoI. The recombinant vectors pET-30a (+)-wsv051 and pET-30a (+)-wsv069 were constructed and transformed into E. coli BL21(DE3), while recombinant pGEX-4T-1-wsv187 was transformed into BL21 cells. WSV051 and WSV069 were induced and purified as described for UBC9; WSV187 was induced and purified as described for SUMO. Purified UBC9, SUMO, WSV051, WSV069, and WSV187 were used as individual antigens to produce polyclonal antiserum in New Zealand rabbits, as previously described (30).
Western blotting of protein expression in crayfish challenged with WSSV.
Crayfish challenge and hemocyte collection were performed as described above. Additionally, the hepatopancreas was dissected at 0, 6, 12, 24, 48, and 72 h postchallenge. Both tissues were homogenized thoroughly in buffer (50 mM Tris-HCl [pH 7.5], 1% protein inhibitor cocktail) and centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant was collected. The protein concentration of each sample was assayed using a spectrophotometer (GE Amersham Biosciences), the samples were mixed with Laemmli buffer (60 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue), and Western blotting was performed as previously described (31).
Injection of recombinant proteins and WSSV infection.
To further investigate the effect of SUMOylation in crayfish, the SUMOylation level was increased in vivo by injection of 50 μg UBC9, mUBC9, SUMO, SUMO-GG, or mSUMO. WSSV (106 copies per crayfish) was injected 30 min later. Hemocyte genomic DNA was extracted from all groups 48 h later using a DNA extraction kit (TOYOBO, Osaka, Japan) and used as the template for semiquantitative RT-PCR to detect the replication of several WSSV genes (including the ie genes wsv051, wsv069, and wsv187 and the late genes vp28, vp26, vp24, vp19, and vp15) using the specific primers listed in Table 1; 18S rRNA was used as an internal control.
In addition, using hemocyte genomic DNA, real-time quantitative PCR (qRT-PCR) was performed with virus-specific primers to quantify infectious WSSV in samples. Likewise, 18S rRNA was used as an internal control. The PCR and procedure were similar to those described above. Each experiment was repeated three times. Standard deviation and statistical analyses by unpaired sample t test were also employed; the significant difference was P < 0.05. At the same time, hemocyte proteins were extracted from each group and subjected to Western blotting for VP28, a vital structural protein expressed in the late stage of the WSSV life cycle.
UBC9 and WSSV-IE protein pulldown assays.
In order to analyze the interaction of UBC9 with WSSV-IE proteins, the in vitro pulldown assays were performed. WSV069 and WSV051 (200 μg/ml) were digested with thrombin overnight at 22°C to release the His tag (to generate ΔWSV069 and ΔWSV051). Protein mixtures (total, 200 μg) containing His-tagged UBC9 and WSSV-IE (ΔWSV069, glutathione S-transferase [GST]–WSV187, and ΔWSV051) were incubated at 4°C for 30 min and then mixed with Ni-NTA affinity resins. Binding buffer (20 mM Tris-HCl [pH 7.9], 0.5 mM NaCl, 5 mM imidazole) was added, the resin was thoroughly washed with wash buffer (20 mM Tris-HCl [pH 7.9], 0.5 M NaCl, 60 mM imidazole), and then the proteins were eluted using elution buffer (20 mM Tris-HCl [pH 7.9], 0.5 mM NaCl, 1 M imidazole) and analyzed by 15% SDS-PAGE to investigate the protein interactions.
In vitro SUMOylation assay.
To determine whether WSSV-IE proteins were SUMOylation substrates, an in vitro SUMOylation assay was performed as previously described (32) with some modifications. Briefly, the SUMOylation assay reaction mixture included activated SUMO, E1, E2, and substrate, with ATP as an energy supply. SUMO-activating E1 was purchased commercially, as a heterodimeric enzyme of SAE1-SAE2 (Boston Biochem, Cambridge, MA). The recombinant proteins involved in the experiment included SUMO, SUMO-GG, or mSUMO, UBC9 or mUBC9 as E2, and WSV051, WSV069, or WSV187 as potential substrates. GST-tagged SUMO, SUMO-GG, and mSUMO were digested with thrombin at 22°C overnight and loaded onto a glutathione Sepharose 4B column to obtain untagged proteins. The SUMOylation system reaction mixture (20 μl) consisted of 10× SUMOylation buffer (200 mM HEPES [pH 7.5], 50 mM MgCl2, 100 mM ATP), 100 nM E1, 5 μM UBC9 or mUBC9, 9 μM SUMO or SUMO-GG or mSUMO, and 2 μM substrate (WSV051, WSV069, or WSV187). The reaction mixtures were incubated at 37°C for 3 h and analyzed by SDS-PAGE and Western blotting using antibodies against the substrates.
RNA interference and WSSV infection.
RNAi was performed by feeding the crayfish E. coli expressing specific dsRNAs. The ORFs of UBC9, SUMO, or the green fluorescent protein gene (GFP) (as a control) were inserted between double-T7 promoters in the plasmid L4440 (pPD129.36). The recombinant plasmids were transformed into competent E. coli HT115 (DE3) cells, and single colonies were cultured and expression of dsRNA was induced using 0.5 mM IPTG. Expression of dsRNA was confirmed 4 h after the addition of IPTG, and then cultures (300 ml) were collected, washed with 30 ml PBS (0.01 mM), and resuspended in 3 ml sterile PBS. The bacterial cell suspension (100 μl) was administered to individual crayfish every 24 h for 4 days. Hemocyte RNA was extracted from the negative-control group (bacteria expressing empty plasmid), dsGFP group, dsUBC9 group, and dsSUMO group and reverse transcribed to cDNA, and semiquantitative RT-PCR was performed to confirm the effect of target gene interference using 18S rRNA as an internal control. The primer sequences used are included in Table 1.
After the E. coli expressing dsRNA were administered to crayfish on the fourth day, 106 copies of WSSV were injected into the abdominal segment of the crayfish. Four treatment groups were prepared: WSSV infected, dsGFP RNAi and WSSV infected, dsUBC9 RNAi and WSSV infected, and dsSUMO RNAi and WSSV infected. Hemocyte genomic DNA was extracted from the five groups (with untreated crayfish as the negative control) 48 h postinjection to detect the abundance of viral ie genes (wsv051, wsv069, and wsv187) and viral late genes (vp28, vp26, vp24, vp19, and vp15) using semiquantitative PCR.
Furthermore, to confirm the function of the recombinant proteins in vivo, the crayfish were fed bacteria for 4 days to knock down UBC9 or SUMO, infected with WSSV, and then injected with 50 μg of the corresponding protein. Namely, UBC9 or mUBC9 was injected into the UBC9-silenced shrimp; SUMO, SUMO-GG, or mSUMO was injected into the SUMO-silenced group 30 min later. Hemocyte proteins were extracted 48 h post-protein injection and subjected to Western blotting to quantify the expression of VP28.
RESULTS
Crayfish UBC9 and SUMO were cloned and identified.
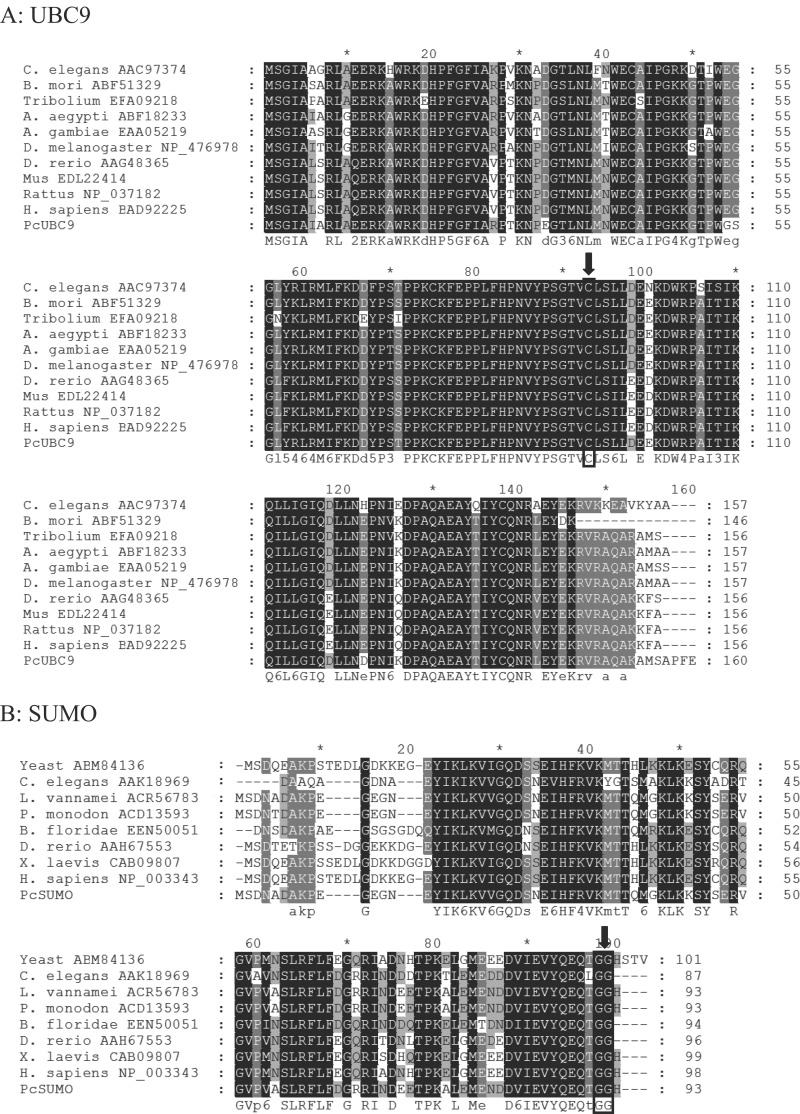
The cDNA and deduced amino acid sequences of UBC9 and SUMO are presented in Fig. 1. Full-length UBC9 cDNA (GenBank accession number JX177492) is 912 bp with an ORF of 483 bp, a 5′ untranslated region (5′-UTR) of 72 bp, and a 3′-UTR of 357 bp (Fig. 1A). The deduced protein contains 160 amino acids, with residues 7 to 157 forming an UBCc domain (Fig. 1A, gray shading). The active Cys93 residue (Fig. 1A, black ellipse), which is indispensable for binding SUMO, was also identified in the UBCc domain. The theoretical molecular mass of PcUBC9 is 18.26 kDa, with an isoelectric point of 8.87.
Fig 1.
Complete cDNA sequences and deduced amino acid sequences of UBC9 (GenBank accession number JX177492) and SUMO (GenBank accession number JX177493) of crayfish. The UBCc domain of UBC9 and UBQ domain of SUMO are shown with gray highlighting. The conserved active sites (Cys93 in UBC9 and double Gly in SUMO) are also indicated in black ellipses.
Full-length SUMO cDNA (GenBank accession number JX177493) is 960 bp, with an ORF of 282 bp, 5′-UTR of 59 bp, and 3′-UTR of 619 bp (Fig. 1B). The deduced protein contains 93 amino acids, with residues 15 to 92 forming an ubiquitin homologue (UBQ) domain (Fig. 1B, gray shading). The active site (Gly-92) was also identified in the deduced amino acid sequence (Fig. 1B, black ellipse). The theoretical molecular mass of SUMO is 10.55 kDa, with an isoelectric point of 4.99.
UBC9 and SUMO proteins are highly conserved.
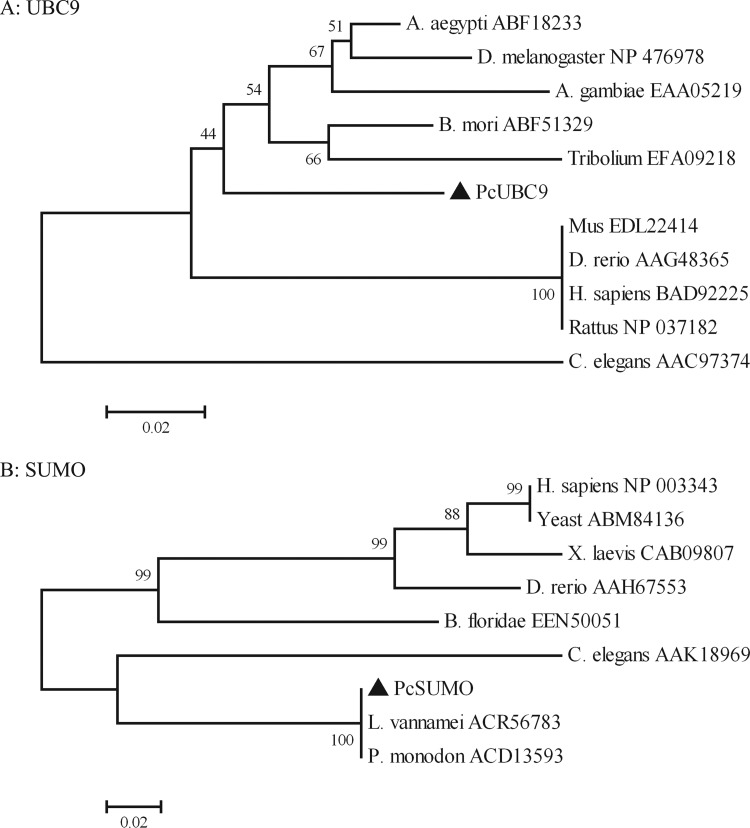
The amino acid sequences of UBC9 from crayfish and other species were aligned and used to produce a phylogenetic tree. The alignment analysis indicated that UBC9, especially the UBCc domain and Cys93 residue, is highly conserved (Fig. 2A; black frame under arrow). In the phylogenetic tree, crayfish UBC9 clustered with UBC9 from other arthropods to a degree. The vertebrate UBC9s were placed closely together and formed another clade. The C. elegans UBC9 formed a distinct clade (Fig. 3A).
Fig 2.
Alignment of UBC9 and SUMO of crayfish with those from other species. The alignment was performed by Genedoc and ClustalW. Both UBC9s and SUMOs are quite conserved in evolution. The catalytic site of UBC9 (Cys93) is labeled in the black box under the arrow in all species. In the lower panel, the specific double-Gly residues are boxed in the SUMO sequences from all species. Gray characters on black background, identity = 100%; gray characters on gray background, identity ≥ 75%; gray characters on white background, identity ≥ 50%. C. elegans, Caenorhabditis elegans; B. mori, Bombyx mori; Tribolium, Tribolium castaneum; A. aegypti, Aedes aegypti; A. gambiae, Anopheles gambiae; D. melanogaster, Drosophila melanogaster; D. rerio, Danio rerio; Mus, Mus musculus; Rattus, Rattus norvegicus; H. sapiens, Homo sapiens; Yeast, Saccharomyces cerevisiae; L. vannamei, Litopenaeus vannamei; P. monodon, Penaeus monodon; B. floridae, Branchiostomafloridae; X. laevis, Xenopus laevis.
Fig 3.
Phylogenetic analysis of UBC9 and SUMO. The neighbor-joining (N-J) tree was produced by MEGA 4.0, using bootstraps of 1,000 to test the reproducibility. UBC9 and SUMO of crayfish are labeled with a black triangle. The GenBank accession number of each selection is shown in the figure.
Alignment and phylogenetic analysis of SUMO proteins were also performed. SUMO is strikingly conserved from yeast to humans (Fig. 2B), indicating that SUMO proteins have been functionally indispensable throughout evolution. Moreover, crayfish SUMO (Fig. 3B, black triangle) was fairly close to SUMO from two other shrimp, Litopenaeus vannamei and Penaeus monodon. All SUMO sequences analyzed contained the C-terminal double-Gly motif, which supports the function of the covalent binding to the substrate (Fig. 2B, black frame under arrow).
UBC9 and SUMO were upregulated by WSSV infection.
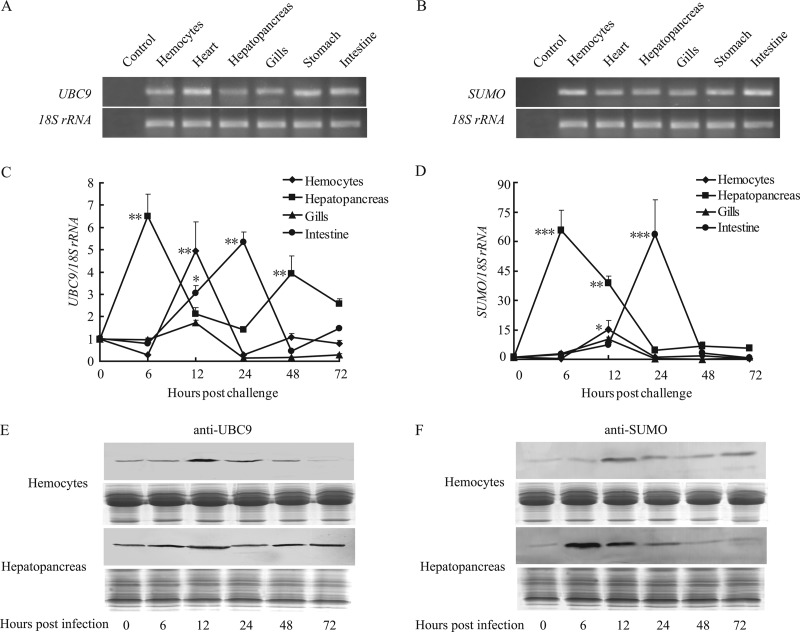
UBC9 and SUMO were both expressed ubiquitously in the crayfish hemocytes, heart, hepatopancreas, gills, stomach, and intestine (Fig. 4A and B). Four tissues (hemocytes, hepatopancreas, gills, and intestine) were selected for a WSSV infection time course assay. The mRNA levels of both genes simultaneously peaked at 6 h and 24 h post-WSSV infection in the hepatopancreas and intestine (Fig. 4C and D), indicating that UBC9 and SUMO responded to WSSV infection. UBC9 expression was also induced 12 h post-WSSV challenge in hemocytes, increasing by approximately 4-fold (Fig. 4C). Western blotting was performed to confirm the induction of UBC9 and SUMO protein expression after WSSV infection. Expression of UBC9 and SUMO was induced and peaked 12 h post-WSSV infection in hemocytes, consistent with the changes in the mRNA level (Fig. 4E and F). UBC9 was upregulated to a degree in the WSSV-challenged hepatopancreas (Fig. 4E), whereas SUMO expression increased sharply between 6 h and 24 h (Fig. 4F). In summary, the mRNA and protein expression patterns revealed that crayfish UBC9 and SUMO responded rapidly to WSSV infection, suggesting that these proteins may be involved in viral replication.
Fig 4.
UBC9 and SUMO were uniformly expressed in crayfish and were induced by WSSV challenge. (A and B) Semiquantitative PCR demonstrating that UBC9 (A) and SUMO (B) could be detected in all six of the crayfish tissues tested. (C and D) Quantitative real-time PCR demonstrating that UBC9 (C) and SUMO (D) were upregulated by WSSV challenge in the selected tissues (hemocytes, hepatopancreas, gills, and intestine). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to 0 h postinfection). (E and F) Western blotting confirmed that expression of UBC9 (E) and SUMO (F) was induced in crayfish hemocytes and hepatopancreas after WSSV infection.
Proteins involved in the study were recombinantly expressed and purified.
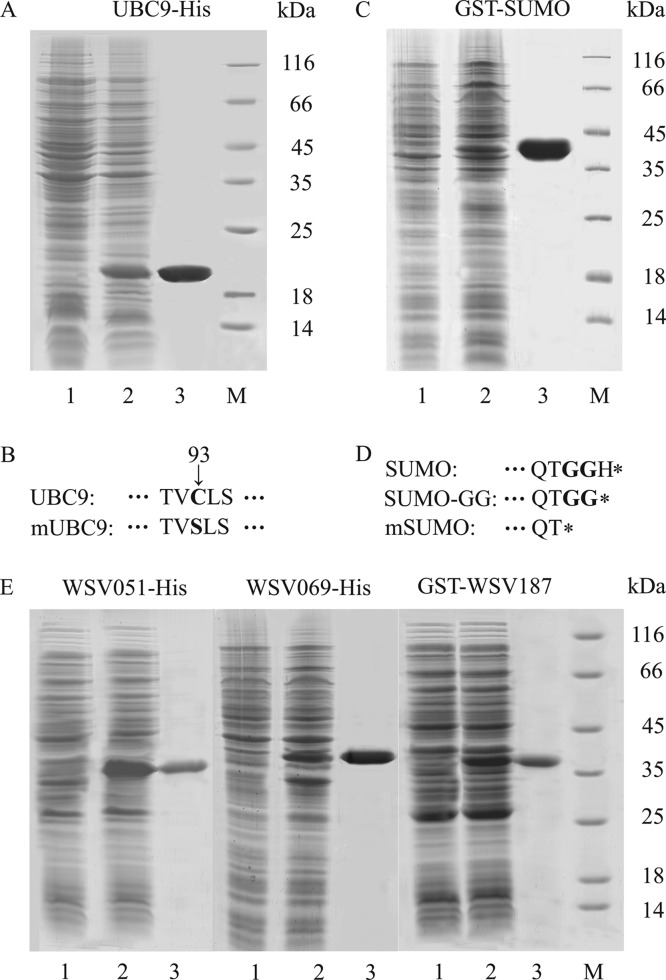
To further characterize the function of UBC9 and SUMO, the wild-type and mutant proteins were successfully expressed and purified in E. coli (Fig. 5A and C). As UBC9 E2 contains a conserved cysteine active site (Fig. 2A, black arrow), mutant UBC9 (mUBC9; Cys93 to Ser) was constructed and expressed (represented in Fig. 5B, in bold). SUMO is initially produced in vivo as an inactive precursor and then hydrolyzed by a specific protease to the active form (represented in Fig. 5D, in bold), SUMO-GG; therefore, recombinant SUMO-GG was expressed and purified. Moreover, mutant SUMO (mSUMO) lacking the double-Gly residues was also expressed as a truncated protein (represented in Fig. 5D). mUBC9, SUMO-GG, and mSUMO were also expressed and purified in the same way as UBC9 and SUMO (data not shown).
Fig 5.
Proteins in the study were recombinantly expressed and purified. (A, C, and E) Expression and purification of UBC9-His (A), GST-SUMO (C), and three viral IE proteins (WSV051-His, WSV069-His, and GST-WSV187) (E). Lanes 1, E. coli lysate without induction; lanes 2, E. coli lysate induced with 0.5 mM IPTG; lanes 3, purified protein; lanes M, standard protein markers. (B) Schematic diagram of the Cys93-to-Ser point mutation in mUBC9 (in bold). (D) Schematic diagrams of the C-terminal region amino acid (in bold) sequences of the inactive precursor (SUMO) and the active (SUMO-GG) and truncated (mSUMO) forms of SUMO.
In order to investigate the relations between viral IE proteins and host SUMOylation, three WSSV IE proteins (WSV051, WSV069, and WSV187) were recombinantly expressed. His-tagged WSV051 (Fig. 5E, left panel) and WSV069 (Fig. 5E, middle panel) were expressed as inclusion bodies, and GST-tagged WSV187 was expressed as a soluble protein (Fig. 5E, right panel). The IEs have predicted molecular masses of 34.22 kDa, 36.67 kDa, and 38.66 kDa, which were in agreement with the apparent masses of recombinant WSV051, WSV069, and WSV187 on SDS-PAGE.
Expression of WSSV late genes and proteins increased after injection of recombinant UBC9, SUMO, and SUMO-GG.
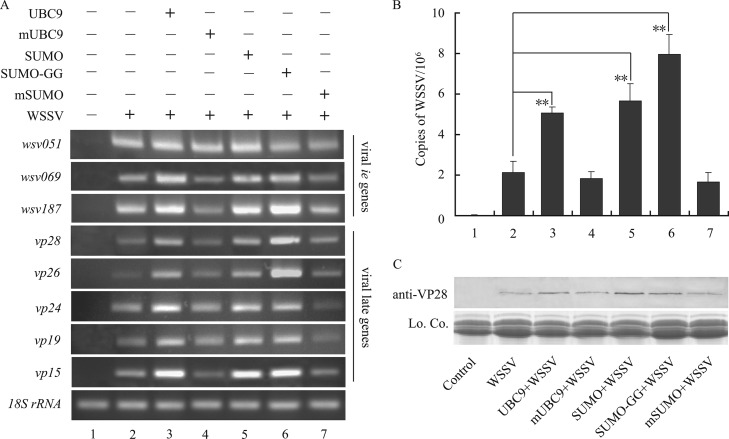
To investigate whether SUMOylation influences viral replication in vivo, the recombinant proteins (UBC9, mUBC9, SUMO, SUMO-GG, or mSUMO) were injected into WSSV-infected crayfish to increase the SUMOylation level. The expression levels of two of the three tested WSSV ie genes (wsv069 and wsv187) increased after injection of UBC9 or SUMO. However, the mutant proteins (mUBC9 or mSUMO) did not increase the expression of ie genes (Fig. 6A, lanes 4 and 7). Additionally, the expression of five late genes (vp28, vp26, vp24, vp19, and vp15) also increased in an obvious manner after the injection of UBC9 or SUMO (Fig. 6A, lanes 3 and 6). The viral late genes vp28, vp26, and vp15 also evidently responded to injection of SUMO-GG (Fig. 6A, lane 6). In addition, the results of qRT-PCR (Fig. 6B) were in accordance with the semiquantitative PCR results reported above. The number of copies of WSSV in crayfish injected with UBC9, SUMO, or SUMO-GG was 2- or 3-fold greater than that in crayfish infected with WSSV alone. In the same time, the mutant forms of UBC9 and SUMO (mUBC9 and mSUMO) lost the ability to enhance viral replication. These data indicated that increasing the level of SUMOylation by injecting UBC9, SUMO, or SUMO-GG promoted the replication of viral ie genes and the transcription of viral late genes, which would benefit WSSV replication.
Fig 6.
Viral gene replication and protein expression were increased by injection of recombinant UBC9, SUMO, and SUMO-GG. Crayfish were injected with WSSV 30 min after injection of the recombinant proteins, and hemocyte genomic DNA and proteins were extracted 48 h later. Semiquantitative PCR (A) and real-time PCR (B) were performed using hemocyte genomic DNA, and Western blotting (C) was performed with hemocyte proteins. The results revealed that injection of recombinant UBC9 (lanes 3), SUMO (lanes 5), or SUMO-GG (lanes 6) increased the transcription of WSSV ie and late genes (A), viral replication (B), and late protein expression (C). However, injection of the mutant protein mUBC9 (lanes 4) or mSUMO (lanes 7) did not enhance viral replication. Lo. Co., loading control. **, P < 0.01.
To confirm these results, hemocyte proteins were extracted and expression of VP28, the protein encoded by the late gene vp28, was quantified. Translation of VP28 was enhanced in crayfish injected with UBC9 (Fig. 6C, lane 3) or SUMO (Fig. 6C, lane 5) compared to crayfish injected with mUBC9 (Fig. 6C, lane 4) or mSUMO (Fig. 6C, lane 7). Additionally, crayfish injected with SUMO-GG, the active form of SUMO, also demonstrated a higher translation level of the viral late protein VP28 (Fig. 6C, lane 6). Therefore, increasing the level of SUMOylation enhanced the transcription and expression of viral late genes, which depend on IE proteins.
Crayfish UBC9 bound to all three viral IE proteins.
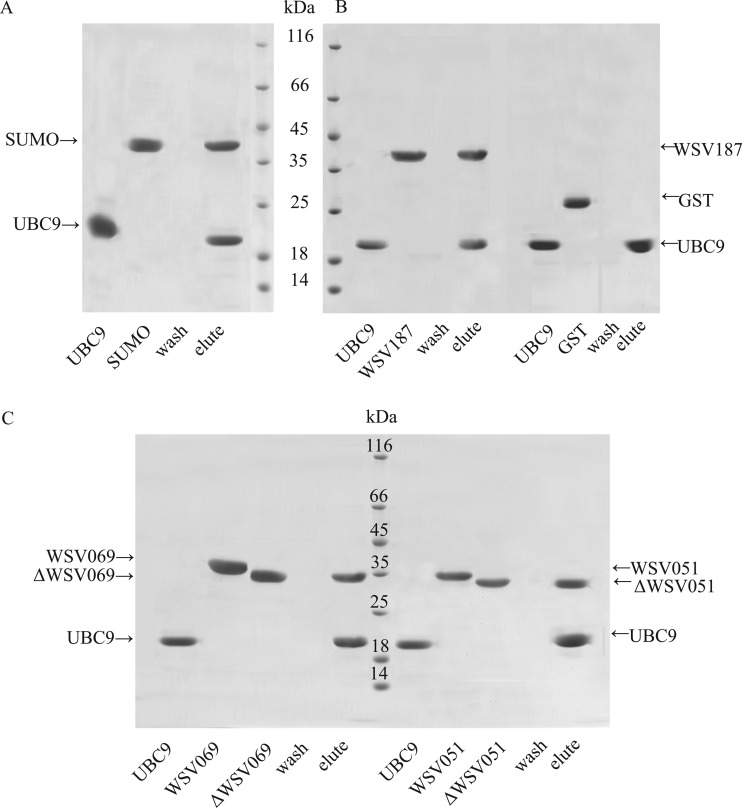
On the basis of the results above, we presumed that SUMOylation of viral IE proteins might play a role in WSSV replication. Therefore, the interactions between host UBC9 and SUMO and viral IE proteins were investigated. In vitro pulldown assays were performed to analyze the interactions between the recombinant proteins. First, binding of crayfish UBC9 and SUMO was demonstrated (Fig. 7A). WSV187 showed binding activity with UBC9 (Fig. 7B, left five lanes) but did not bind to the negative-control GST protein (Fig. 7B, right four lanes). His-tagged WSV051 and WSV069 were digested by thrombin to release the His tag (to generate the “Δ” forms). As shown in Fig. 7C, crayfish UBC9 could bind to both ΔWSV069 (Fig. 7C, left panel) and ΔWSV051 (Fig. 7C, right panel). However, no interaction was detected between SUMO and any IE protein (data not shown). In summary, crayfish UBC9 can bind directly to SUMO and three WSSV IE proteins examined in the study, WSV051, WSV069, and WSV187.
Fig 7.
UBC9 interacted with SUMO and WSSV IEs in vitro. Protein mixtures (200 μg total) containing 20% UBC9 and 80% GST-tagged SUMO, GST-tagged WSV187, ΔWSV051, ΔWSV069, or GST were incubated at 4°C for 30 min and purified using Ni-NTA affinity resin. (A) Crayfish UBC9 and SUMO interacted. (B) Host UBC9 bound to GST-WSV187 but not to the GST tag. (C) WSV069 and WSV051 were digested to release the His tags, and both ΔWSV069 (left panel) and ΔWSV051 (right panel) interacted with crayfish UBC9.
WSV051 was covalently modified by SUMO in vitro.
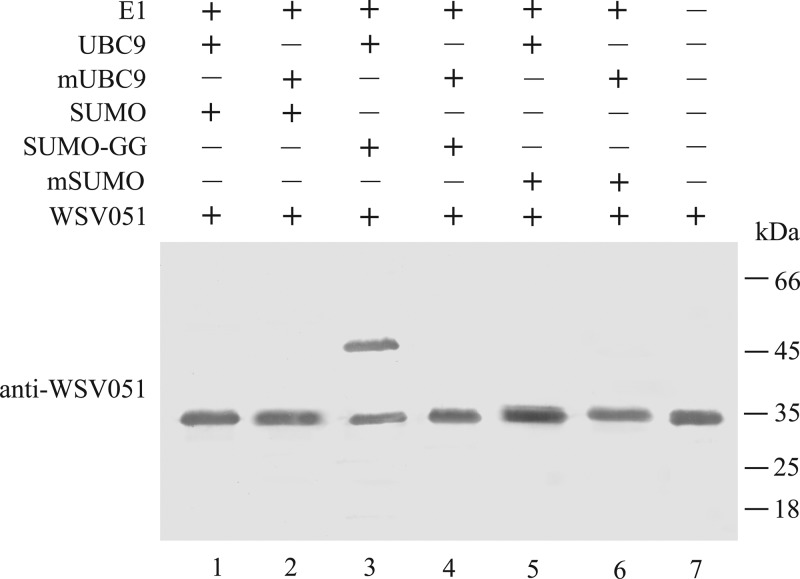
As all three IE proteins could bind UBC9 in vitro, we confirmed whether the IEs were SUMOylation substrates in vitro. Two forms of E2 (UBC9 and mUBC9) and three forms of SUMO (precursor SUMO, the active form SUMO-GG, and mSUMO) were tested. Only WSV051 could be modified by SUMOylation in vivo (Fig. 8); WSV069 and WSV187 were not modified (data not shown). Notably, only the active forms of E2 and SUMO (UBC9 and SUMO-GG) could modify WSV051 (Fig. 8, lane 3). The molecular mass of SUMO-modified WSV051 increased to approximately 46 kDa (Fig. 8, lane 3), which was consistent with the predicted increase in molecular mass (crayfish SUMO contains 93 amino acid residues and is approximately 11 kDa). Therefore, we concluded that the activated forms of UBC9 and SUMO-GG can catalyze SUMOylation and also that one viral IE protein, WSV051, was a potential SUMOylation substrate in vitro.
Fig 8.
The viral IE protein WSV051 was a potential SUMOylation substrate. WSV051 was SUMOylated only by UBC9 and the active form of SUMO, SUMO-GG. The 20-μl reaction system contained 20 mM HEPES (pH 7.5), 5 mM MgCl2, 10 mM ATP, 50 nM E1, 5 μM E2, 9 μM SUMO/SUMO-GG/mSUMO, and 2 μM WSV051. The reactions were stopped by the addition of SDS-PAGE sample buffer, and the reaction mixtures were boiled for 5 min and then analyzed by Western blotting using antibodies against the potential substrates.
UBC9 and SUMO were knocked down in crayfish hemocytes and gills by oral delivery of E. coli expressing dsRNA.
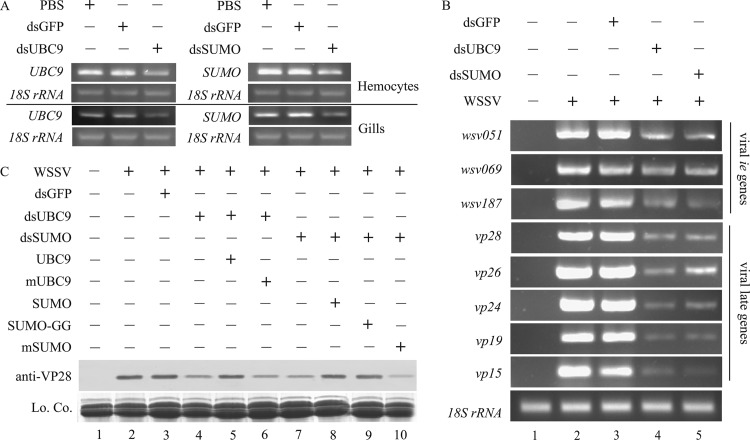
RT-PCR was performed using hemocyte and gill cDNA to confirm whether the delivery of bacteria expressing dsRNA was an effective RNAi method in crayfish. UBC9 and SUMO were significantly knocked down in the hemocytes and gills of crayfish fed E. coli expressing UBC9 dsRNA or SUMO dsRNA (Fig. 9A; approximately 60% reduction). Thus, the delivery of bacteria expressing dsRNA effectively interfered with target gene expression and worked well in the crayfish system.
Fig 9.
WSSV late gene and protein expression was suppressed by knockdown of UBC9 and SUMO and rescued by injection of recombinant UBC9 and SUMO. (A) UBC9 and SUMO were knocked down in crayfish by RNA interference via oral delivery of E. coli expressing dsRNA. The crayfish were fed PBS or bacteria expressing dsRNA against GFP, UBC9, or SUMO every 24 h for 4 days. The efficiency of RNAi was confirmed in crayfish hemocytes and gills by RT-PCR using 18S rRNA as an internal control. (B) Viral gene expression, especially the expression of late genes, was inhibited when host UBC9 or SUMO was knocked down. Crayfish were infected with WSSV after the delivery of E. coli expressing dsRNA for 4 days, and hemocyte genomic DNA was extracted 48 h postinfection. Semiquantitative PCR was performed to quantify the expression of viral ie genes (wsv051, wsv069, and wsv187) and late genes (vp28, vp26, vp24, vp19, and vp15) using 18S rRNA as the control. (C) Reduced viral VP28 protein after knockdown of UBC9 or SUMO in crayfish was rescued by the injection of 50 μg recombinant UBC9 (lane 5), SUMO (lane 8), or SUMO-GG (lane 10) 0.5 h postinfection but not by the injection of mUBC9 (lane 6) and mSUMO (lane 10). Hemocyte proteins were extracted 48 h later and subjected to Western blotting for VP28. Lo. Co., loading control.
The expression of viral ie genes declined and the expression of late genes was significantly inhibited in UBC9-silenced or SUMO-silenced crayfish.
RNA interference was performed to explore the effect of SUMOylation on WSSV replication. WSSV was injected into crayfish in which UBC9 or SUMO had been knocked down, and then the expression of viral genes was quantified in the hemocytes. Compared with the results seen with the two positive-control groups (Fig. 9B, lanes 2 and 3), the replication of all ie genes (wsv051, wsv069, and wsv187) and late genes (vp28, vp26, vp24, vp19, and vp15) was inhibited when either UBC9 or SUMO was knocked down (Fig. 9B, lanes 4 and 5). Moreover, expression of the late genes was repressed more than that of the ie genes, due to the fact that the transcription of late genes relies on viral IEs.
Expression of viral structural protein VP28 in UBC9-silenced or SUMO-silenced crayfish was rescued by injection of recombinant UBC9, SUMO, or SUMO-GG.
We previously demonstrated that injection of recombinant UBC9, SUMO, or SUMO-GG increased the expression level of viral late genes (Fig. 6). Therefore, we performed a “rescue” assay to investigate whether injection of two forms of UBC9 and three forms of SUMO could restore the expression of viral late protein in UBC9- or SUMO-silenced crayfish. Consistent with our hypothesis, the expression of viral VP28 was restored when recombinant UBC9 (Fig. 9C, lane 5 compared with lane 4) or SUMO (Fig. 9C, lane 8 compared with lane 7) was injected after knockdown of UBC9 or SUMO. Notably, injection of SUMO-GG also restored the expression of VP28 to normal levels in SUMO-silenced crayfish (Fig. 9C, lane 9 compared with lane 7). Nevertheless, mUBC9 (Fig. 9C, lane 6) and mSUMO (Fig. 9C, lane 10) lacking their active residues failed to regain the viral expression. In summary, when UBC9 or SUMO was knocked down, WSSV could not take full advantage of the impaired host SUMOylation system to modify viral IE proteins, which consequently attenuated viral protein expression and viral replication. Furthermore, the weakened viral replication could be rescued when host SUMOylation was recovered by injection of recombinant UBC9 or SUMO or SUMO-GG.
DISCUSSION
In this study, we identified and characterized P. clarkii UBC9 and SUMO and investigated their function during viral infection. In similarity to E2 enzymes in the ubiquitin proteasome pathway, crayfish UBC9 contains a core ubiquitin-conjugating catalytic domain (UBCc) with a conserved Cys93 (33). However, SUMOylation solely utilizes the E2 UBC9, whereas the ubiquitination pathway uses diverse E2s.
WSSV gene expression in crayfish was promoted by injection of recombinant UBC9 or SUMO and inhibited when host UBC9 or SUMO was knocked down. The expression of UBC9 or SUMO increased when recombinant UBC9 or SUMO was injected, which is likely to have enhanced the level of SUMOylation in crayfish cells. However, this raises the question of how recombinant proteins reach the target organ. As we know that the shrimp has an open circulation system, the proteins injected into hemolymph theoretically could spread throughout the whole body. When the exogenous proteins enter the hemocytes, they could participate in intracellular reactions. In our previous study, injected recombinant proteins could be detected in prawn hemocytes by both Western blotting and immunocytochemistry (31). Similarly, fluorescein isothiocyanate (FITC)-labeled recombinant immulectin-3 (IML-3) of Manduca sexta injected into larvae presented as large particles in hemocytes, suggesting that it enters cells through an endocytic pathway (34). Moreover, increasing numbers of different peptide-based vectors have been designed to take advantage of this principle for efficient delivery in vivo and in vitro (35).
The potential molecular mechanism and effects of UBC9 and SUMO during WSSV infection were analyzed further. As possible targets of SUMOylation, three IE proteins were recombinantly expressed and their interactions with host UBC9 and SUMO were tested using pulldown assays. Contrary to expectations, interactions between crayfish SUMO and viral IEs could not be detected; however, crayfish UBC9 could bind viral IEs (Fig. 7). These results can be explained from the perspective of the SUMOylation process. UBC9 transports SUMO to the substrate by catalyzing the formation of an isopeptide bond between the Gly of SUMO and substrate lysine residues. Therefore, UBC9 may directly and spatially interact with the substrate; however, covalent bonds are formed between SUMO and its substrates only in the presence of UBC9. In agreement with our results, nuclear protein RAP80 bound UBC9 of HEK293 cells and was previously reported as a novel SUMOylation target (36). Additionally, human Pellino-1, an adaptor of the Toll-like receptor, bound UBC9 in GST pulldown assays and is a SUMOylation substrate (37).
As interaction between UBC9 and viral IEs was verified in vitro, we tested whether the viral IEs were SUMOylation substrates. Although all three viral IEs could bind UBC9, only SUMO-modified WSV051 was detected using substrate-specific antibodies (Fig. 8). The WSV051, WSV069, and WSV187 protein sequences were analyzed using SUMOsp 2.0 (16, 17) to predict potential SUMOylation sites (38). At the medium threshold, two SUMO-modified sites were predicted in WSV051; however, no sites were predicted in WSV069 or WSV187. The SUMOylation sites in WSV051 (K40 and K180) were retained even at a high threshold, and both have the classical “ΨKXE” SUMOylation site sequence (Ψ is a hydrophobic amino acid; K is lysine; X is any amino acid residue; and E is glutamate) (39). This information supports our observation that only WSV051 could be SUMOylated in vitro.
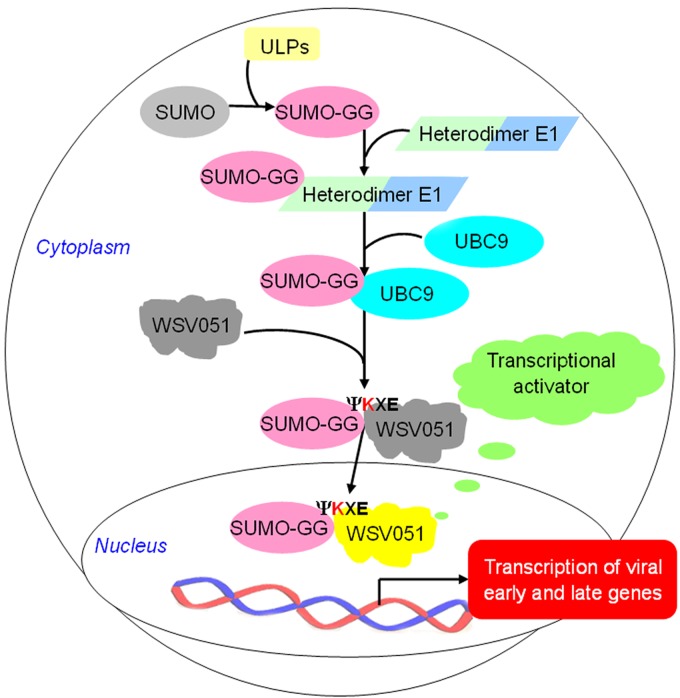
SUMOylation participates in multiple biological processes and plays a significant role in transcriptional regulation (9, 14). The most well-known SUMOylation substrate is p53, which is modified by SUMO at the C terminus of its DNA binding region (40) to activate transcriptional activity and eventually lead to cell apoptosis (12). Moreover, it has been reported that cellular SUMO competitively binds to the lysine residues of ubiquitination substrates, thus inhibiting the degradation of the substrates via the ubiquitin proteasome pathway (41–43). We presume that WSSV exploits host SUMOylation to modify viral IE proteins, and a proposed mechanism of viral IE protein SUMOylation is illustrated in Fig. 10. Host UBC9, located close to the nuclear pore complex (44), catalyzes the binding of active SUMO-GG to viral IEs, such as WSV051. In particular, the known viral IE, WSV051, not only exhibited transcriptional activity but also showed similarity to the BadM/Rrf2 family transcription factor (24). The SUMO-modified viral IE is then likely to be protected from the host ubiquitination pathway, allowing the protein to efficiently function in activation of the cascade of viral gene expression and ultimately facilitate viral replication.
Fig 10.
Schematic representation of the novel function of SUMOylation in WSSV-infected crayfish. Viral IE proteins such as WSV051 exploit the host SUMOylation pathway to activate the viral transcription cascade and the expression of early and late genes. As indicated in the figure, the conserved lysine (K of ΨKXE) of WSV051 could be modified by activated SUMO-GG. SUMO-modified viral IEs could enter the host nucleus and function as transcriptional activators to initiate the expression of viral early and late genes. ΨKXE: Ψ, bulky hydrophobic residue; K, lysine; X, any amino acid; E, glutamate. ULPs, ubiquitin-like protein-specific protease to activate SUMO molecules.
ACKNOWLEDGMENTS
We thank Shou-Wei Ding, University of California, Riverside, for kindly providing the RNAi vectors.
This work was financially supported by the National Natural Science Foundation of China (no. 31130056), National Basic Research Program of China (973 Program, no. 2012CB114405), the Ph.D. Programs Foundation of the Ministry of Education of China (no. 20110131130003), and Provincial Natural Science Foundation of Shandong, China (no. ZR2011CM014). The funders had no role in the study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Jin JB, Jin YH, Lee J, Miura K, Yoo CY, Kim WY, Van Oosten M, Hyun Y, Somers DE, Lee I, Yun DJ, Bressan RA, Hasegawa PM. 2008. The SUMO E3 ligase, AtSIZ1, regulates flowering by controlling a salicylic acid-mediated floral promotion pathway and through affects on FLC chromatin structure. Plant J. 53:530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chauchereau A, Amazit L, Quesne M, Guiochon-Mantel A, Milgrom E. 2003. Sumoylation of the progesterone receptor and of the steroid receptor coactivator SRC-1. J. Biol. Chem. 278:12335–12343 [DOI] [PubMed] [Google Scholar]
- 3. Kurepa J, Walker JM, Smalle J, Gosink MM, Davis SJ, Durham TL, Sung DY, Vierstra RD. 2003. The small ubiquitin-like modifier (SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and -2 conjugates is increased by stress. J. Biol. Chem. 278:6862–6872 [DOI] [PubMed] [Google Scholar]
- 4. Lee J, Nam J, Park HC, Na G, Miura K, Jin JB, Yoo CY, Baek D, Kim DH, Jeong JC, Kim D, Lee SY, Salt DE, Mengiste T, Gong Q, Ma S, Bohnert HJ, Kwak SS, Bressan RA, Hasegawa PM, Yun DJ. 2007. Salicylic acid-mediated innate immunity in Arabidopsis is regulated by SIZ1 SUMO E3 ligase. Plant J. 49:79–90 [DOI] [PubMed] [Google Scholar]
- 5. Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. 2003. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc. Natl. Acad. Sci. U. S. A. 100:15758–15763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohsako S, Takamatsu Y. 1999. Identification and characterization of a Drosophila homologue of the yeast UBC9 and hus5 genes. J. Biochem. 125:230–235 [DOI] [PubMed] [Google Scholar]
- 7. Schmidt D, Muller S. 2003. PIAS/SUMO: new partners in transcriptional regulation. Cell. Mol. Life Sci. 60:2561–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim ET, Kim YE, Huh YH, Ahn JH. 2010. Role of noncovalent SUMO binding by the human cytomegalovirus IE2 transactivator in lytic growth. J. Virol. 84:8111–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Müller S, Hoege C, Pyrowolakis G, Jentsch S. 2001. SUMO, ubiquitin's mysterious cousin. Nat. Rev. 2:202–213 [DOI] [PubMed] [Google Scholar]
- 10. Yang Y, Tse AK, Li P, Ma Q, Xiang S, Nicosia SV, Seto E, Zhang X, Bai W. 2011. Inhibition of androgen receptor activity by histone deacetylase 4 through receptor SUMOylation. Oncogene 30:2207–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rui HL, Fan E, Zhou HM, Xu Z, Zhang Y, Lin SC. 2002. SUMO-1 modification of the C-terminal KVEKVD of Axin is required for JNK activation but has no effect on Wnt signaling. J. Biol. Chem. 277:42981–42986 [DOI] [PubMed] [Google Scholar]
- 12. Kannan K, Kaminski N, Rechavi G, Jakob-Hirsch J, Amariglio N, Givol D. 2001. DNA microarray analysis of genes involved in p53 mediated apoptosis: activation of Apaf-1. Oncogene 20:3449–3455 [DOI] [PubMed] [Google Scholar]
- 13. Xu Z, Au SW. 2005. Mapping residues of SUMO precursors essential in differential maturation by SUMO-specific protease, SENP1. Biochem. J. 386:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hay RT. 2001. Protein modification by SUMO. Trends Biochem. Sci. 26:332–333 [DOI] [PubMed] [Google Scholar]
- 15. Desterro JM, Rodriguez MS, Kemp GD, Hay RT. 1999. Identification of the enzyme required for activation of the small ubiquitin-like protein SUMO-1. J. Biol. Chem. 274:10618–10624 [DOI] [PubMed] [Google Scholar]
- 16. Ahn JH, Xu Y, Jang WJ, Matunis MJ, Hayward GS. 2001. Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9. J. Virol. 75:3859–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomoiu A, Gravel A, Tanguay RM, Flamand L. 2006. Functional interaction between human herpesvirus 6 immediate-early 2 protein and ubiquitin-conjugating enzyme 9 in the absence of sumoylation. J. Virol. 80:10218–10228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maeda A, Lee BH, Yoshimatsu K, Saijo M, Kurane I, Arikawa J, Morikawa S. 2003. The intracellular association of the nucleocapsid protein (NP) of hantaan virus (HTNV) with small ubiquitin-like modifier-1 (SUMO-1) conjugating enzyme 9 (Ubc9). Virology 305:288–297 [DOI] [PubMed] [Google Scholar]
- 19. Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. 2009. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 5:e1000493 doi:10.1371/journal.ppat.1000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaber T, Bohl CR, Lewis GL, Wood C, West JT, Jr, Weldon RA., Jr 2009. Human Ubc9 contributes to production of fully infectious human immunodeficiency virus type 1 virions. J. Virol. 83:10448–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Q, Xu L, Li H, Qi YP, Yang F. 2009. Four major envelope proteins of white spot syndrome virus bind to form a complex. J. Virol. 83:4709–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durand SV, Lightner DV. 2002. Quantitative real-time PCR for the measurement of white spot syndrome virus in shrimp. J. Fish Dis. 25:381–389 [Google Scholar]
- 23. van Hulten MC, Witteveldt J, Peters S, Kloosterboer N, Tarchini R, Fiers M, Sandbrink H, Lankhorst RK, Vlak JM. 2001. The white spot syndrome virus DNA genome sequence. Virology 286:7–22 [DOI] [PubMed] [Google Scholar]
- 24. Li F, Li M, Ke W, Ji Y, Bian X, Yan X. 2009. Identification of the immediate-early genes of white spot syndrome virus. Virology 385:267–274 [DOI] [PubMed] [Google Scholar]
- 25. Cayrol C, Flemington EK. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748–2759 [PMC free article] [PubMed] [Google Scholar]
- 26. Duerst RJ, Morrison LA. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16:475–490 [DOI] [PubMed] [Google Scholar]
- 27. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 28. Lin F, Huang H, Xu L, Li F, Yang F. 2011. Identification of three immediate-early genes of white spot syndrome virus. Arch. Virol. 156:1611–1614 [DOI] [PubMed] [Google Scholar]
- 29. Liu WJ, Chang YS, Wang CH, Kou GH, Lo CF. 2005. Microarray and RT-PCR screening for white spot syndrome virus immediate-early genes in cycloheximide-treated shrimp. Virology 334:327–341 [DOI] [PubMed] [Google Scholar]
- 30. Du XJ, Wang JX, Liu N, Zhao XF, Li FH, Xiang JH. 2006. Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol. Immunol. 43:1633–1644 [DOI] [PubMed] [Google Scholar]
- 31. Chen AJ, Wang S, Zhao XF, Yu XQ, Wang JX. 2011. Enzyme E2 from Chinese white shrimp inhibits replication of white spot syndrome virus and ubiquitinates its RING domain proteins. J. Virol. 85:8069–8079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Werner A, Moutty MC, Moller U, Melchior F. 2009. Performing in vitro sumoylation reactions using recombinant enzymes. Methods Mol. Biol. 497:187–199 [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Taherbhoy AM, Hunt HW, Seyedin SN, Miller DW, Miller DJ, Huang DT, Schulman BA. 2010. Crystal structure of UBA2(ufd)-Ubc9: insights into E1-E2 interactions in Sumo pathways. PLoS One 5:e15805 doi:10.1371/journal.pone.0015805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu XQ, Tracy ME, Ling E, Scholz FR, Trenczek T. 2005. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem. Mol. Biol. 35:285–295 [DOI] [PubMed] [Google Scholar]
- 35. Andaloussi SE, Lehto T, Mager I, Rosenthal-Aizman K, Oprea II, Simonson OE, Sork H, Ezzat K, Copolovici DM, Kurrikoff K, Viola JR, Zaghloul EM, Sillard R, Johansson HJ, Said Hassane F, Guterstam P, Suhorutsenko J, Moreno PM, Oskolkov N, Halldin J, Tedebark U, Metspalu A, Lebleu B, Lehtio J, Smith CI, Langel U. 2011. Design of a peptide-based vector, PepFect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic acids Res. 39:3972–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan J, Yang XP, Kim YS, Joo JH, Jetten AM. 2007. RAP80 interacts with the SUMO-conjugating enzyme UBC9 and is a novel target for sumoylation. Biochem. Biophys. Res. Commun. 362:132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim JH, Sung KS, Jung SM, Lee YS, Kwon JY, Choi CY, Park SH. 2011. Pellino-1, an adaptor protein of interleukin-1 receptor/toll-like receptor signaling, is sumoylated by Ubc9. Mol. Cells 31:85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ren J, Gao X, Jin C, Zhu M, Wang X, Shaw A, Wen L, Yao X, Xue Y. 2009. Systematic study of protein sumoylation: development of a site-specific predictor of SUMOsp 2.0. Proteomics 9:3409–3412 [DOI] [PubMed] [Google Scholar]
- 39. Spengler ML, Kurapatwinski K, Black AR, Azizkhan-Clifford J. 2002. SUMO-1 modification of human cytomegalovirus IE1/IE72. J. Virol. 76:2990–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melchior F, Hengst L. 2002. SUMO-1 and p53. Cell Cycle 1:245–249 [PubMed] [Google Scholar]
- 41. Lin X, Liang M, Liang YY, Brunicardi FC, Feng XH. 2003. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J. Biol. Chem. 278:31043–31048 [DOI] [PubMed] [Google Scholar]
- 42. Smet-Nocca C, Wieruszeski JM, Leger H, Eilebrecht S, Benecke A. 2011. SUMO-1 regulates the conformational dynamics of thymine-DNA glycosylase regulatory domain and competes with its DNA binding activity. BMC Biochem. 12:4 doi:10.1186/1471-2091-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. 2004. SUMO modification of Huntingtin and Huntington's disease pathology. Science 304:100–104 [DOI] [PubMed] [Google Scholar]
- 44. Zhu S, Zhang H, Matunis MJ. 2006. SUMO modification through rapamycin-mediated heterodimerization reveals a dual role for Ubc9 in targeting RanGAP1 to nuclear pore complexes. Exp. Cell Res. 312:1042–1049 [DOI] [PubMed] [Google Scholar]