Abstract
The term synanthropic describes organisms that thrive in human-altered habitats. Where synanthropic nonhuman primates (NHP) share an ecological niche with humans, cross-species transmission of infectious agents can occur. In Bangladesh, synanthropic NHP are found in villages, densely populated cities, religious sites, and protected forest areas. NHP are also kept as performing monkeys and pets. To investigate possible transmission of enteric picornaviruses between humans and NHP, we collected fecal specimens from five NHP taxa at16 locations in Bangladesh during five field sessions, from January 2007 to June 2008. Specimens were screened using real-time PCR assays for the genera Enterovirus, Parechovirus, and Sapelovirus; PCR-positive samples were typed by VP1 sequencing. To compare picornavirus diversity between humans and NHP, the same assays were applied to 211 human stool specimens collected in Bangladesh in 2007 to 2008 for acute flaccid paralysis surveillance. Picornaviruses were detected in 78 of 677 (11.5%) NHP fecal samples. Twenty distinct human enterovirus (EV) serotypes, two bovine EV types, six human parechovirus serotypes, and one virus related to Ljungan virus were identified. Twenty-five additional enteroviruses and eight parechoviruses could not be typed. Comparison of the picornavirus serotypes detected in NHP specimens with those detected in human specimens revealed considerable overlap. Strikingly, no known simian enteroviruses were detected among these NHP populations. In conclusion, enteroviruses and parechoviruses may be transmitted between humans and synanthropic NHP in Bangladesh, but the directionality of transmission is unknown. These findings may have important implications for the health of both human and NHP populations.
INTRODUCTION
Cross-species transmission of infectious agents occurs between humans and several species of nonhuman primates (NHP) in a variety of contexts and in diverse geographic areas (1–5). The potential for cross-species transmission exists wherever humans and NHP come into contact. Human-NHP interaction is common in Asia, particularly in Bangladesh, where humans and NHP have lived sympatrically for centuries (6–9). The contexts of contact between humans and NHP in Bangladesh are a microcosm of what is seen in much of South and Southeast Asia. The rhesus macaque, Macaca mulatta, is one of the most successful primates on the planet. In Bangladesh, free-ranging rhesus macaques are found in a broad range of habitats, from protected forests and nature preserves, where there is little overlap with humans, to rural villages, gardens, and religious sites and to densely populated cities. This synanthropic monkey particularly thrives in human-altered habitats. In the state of Uttar Pradesh in Northern India, it is estimated that more than 80% of the rhesus population has become “urbanized” in the past 2 decades (10). Rhesus macaques are also commonly kept as pets and are transported around the country as performing monkeys. Another synanthropic NHP, the Hanuman langur, is found in association with humans in Bangladesh but is not nearly as successful, in terms of population size and distribution, as the rhesus macaque.
Simian picornaviruses were first isolated in the 1950s from macaques used in biomedical research and from primary monkey cell cultures used in vaccine production (11–15). Most of these picornaviruses are classified within the genus Enterovirus (EV), with the rest in the genus Sapelovirus (16–19). In humans, EV may cause illness ranging from undifferentiated fever and the common cold to rashes, meningitis, encephalitis, acute flaccid paralysis, and neonatal sepsis (20). There is serological evidence for cross-species transmission of enteroviruses between humans and NHP (21, 22), and human enteroviruses have occasionally been isolated from NHP (23–25), but there are few data on enteroviral disease in NHP. We recently identified an “unusual” group of human EVs (EV76, EV89, EV90, and EV91), isolated from stool specimens of children with acute flaccid paralysis in Bangladesh (26). These viruses were more closely related to simian EV in the species Enterovirus A than to other human viruses in the species, suggesting that cross-species transmission of EVs may occur between humans and NHP in Bangladesh.
Several other enteric picornaviruses either have been detected previously in NHP or could be expected to infect NHP based on their relatedness to known simian and human picornaviruses. Parechoviruses (PeVs) (genus Parechovirus) cause disease in humans, with a spectrum of illness similar to that of the human enteroviruses (27). The single report of NHP infection with human parechoviruses (28) did not firmly link infection with disease. Similarly, one report suggested that infection with sapeloviruses (genus Sapelovirus) may be associated with neurologic disease in NHP, but the data were not conclusive (29). There are no published data on the factors that influence parechovirus transmission from humans to NHP or within NHP populations.
The objectives of this study were to determine the plausibility of human-NHP cross-species picornavirus transmission by detecting and analyzing the picornavirus genome in NHP feces to determine whether Bangladesh NHP are infected with EV76, EV89, EV90, or EV91 and to determine the most likely context(s) of human-NHP interaction to be involved in cross-species transmission.
MATERIALS AND METHODS
Study sites and sample collection.
The study protocol was approved by the University of Washington Institutional Animal Care and Use Committee (approval number 4233-01). A total of 678 fecal specimens, representing five NHP taxa from a variety of contexts, were collected from 16 different locations in Bangladesh during January 2007, August 2007, November 2007, January 2008, and June 2008 (Fig. 1 and Tables 1 and 2). Species, context of human contact, and global positioning system (GPS) coordinates were recorded for each specimen. Fecal material was collected only from freshly deposited stools. January to March is typically the low season for enterovirus circulation among humans in Bangladesh, while May to October is the high season (30). Human stool specimens were collected in 2007 and 2008 during routine acute flaccid paralysis surveillance activities, according to World Health Organization methods (31). Poliovirus-positive stools were excluded from this convenience sample. All specimens were kept cold and immediately frozen upon return from the field. Samples were stored at −80°C until shipped on dry ice to Atlanta, GA, for analysis.
Fig 1.
Distribution of sampling sites and picornavirus detection in fecal samples collected from NHP in Bangladesh, 2007 to 2008.
Table 1.
Ecological context and sample size of feces collected from NHP species
| Species | Urban | Shrine | Tea garden | Wild | Performing | Pet | Total no. of samples | No. of picornavirus-positive fecal samples (%) |
|---|---|---|---|---|---|---|---|---|
| Capped langur (Trachypithecus pileatus) | 0 | 0 | 6 | 13 | 0 | 0 | 19 | 1/19 (5.3%) |
| Hanuman langur (Semnopithecus entellus) | 37 | 0 | 0 | 0 | 0 | 0 | 37 | 6/37 (16.2%) |
| Hoolock gibbon (Hylobates hoolock) | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0/2 |
| Pig-tailed macaque (Macaca leonina) | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0/1 |
| Rhesus macaque (M. mulatta) | 394 | 196 | 10 | 5 | 11 | 2 | 618 | 71/618 (11.5%) |
| Total | 431 | 196 | 16 | 21 | 11 | 2 | 677 | 78/677 (11.5%) |
Table 2.
Characteristics of sampling locations, ecological contexts, season, species present, and the presence and prevalence of picornaviruses among NHP in Bangladesh
| Sampling site | Picornaviruses detected during dry season | Picornaviruses detected in wet season | No. of fecal samples in dry/wet seasons (total number of fecal samples with picornavirus infections) | Ecological context | Species present | Habitat overlap between humans and NHP | No. of fecal samples picornavirus positive by ecological context (%) |
|---|---|---|---|---|---|---|---|
| Dhamrai | EV75, EV90, EV97, HPeV(2),a New LVb | CVB3(2), E33, New HEV-B, HPeV1 | 45, 35 (10)c | Urban | Rhesus macaque | ++++ | 35/431 (8.1%) |
| Old Dhaka City | EVf(2) | 10, 36 (2) | Urban | Rhesus macaque | ++++ | ||
| Bormi | CVA2, EV99 | CVB1, EV76(2) | 24, 26 (5) | Urban | Rhesus macaque | ++++ | |
| Narayanganj | ND | 8, 0 (0) | Urban | Rhesus macaque | ++++ | ||
| Shariatpur | E11, HPeV1, HPeV4 | ND | 10, 0 (2)e | Urban | Rhesus macaque | +++ | |
| Old Chandpur Bazar | EVf | ND | 50, 0 (1) | Urban | Rhesus macaque | +++ | |
| Charmuguria | HPeV14 (3), HPeV(3)a | 61, 50 (6) | Urban | Rhesus macaque | +++ | ||
| Rampur | HPeV(2)a | BEV1 | 34, 5 (3) | Urban | Rhesus macaque | ++ | |
| Keshabpur | ND | CVA19(2), CVB6(4) | 0, 37 (6) | Urban | Hanuman langur, rhesus macaque | ++ | |
| Sylhet | E11, E19(3), E20(2), E21, E29, EV74, EV75, CVA5, EV(3),f EV(18),g HPeV1(3), HPeV5, HPeV12, HPeV15 | 160, 36 (37)d | Shrine | Rhesus macaque | +++ | 37/196 (18.9%) | |
| Malnichara | ND | 16, 0 (0) | Tea garden | Rhesus macaque, capped langur | ++ | 0/16 (0%) | |
| Lawachara | BEV2 | ND | 15, 0 (1) | Wild | Rhesus macaque, capped langur, pigtail macaque | + | 3/21 (14.3%) |
| Sundarbans | ND | 0, 3 (0) | Wild | Rhesus macaque | + | ||
| Satchari | EV,f LV-likeh | ND | 3, 0 (2) | Wild | Rhesus macaque, pigtail macaque, hoolock gibbon, capped langur | + | |
| Near Dhamrai | CVA24 | 6, 5 (1) | Performing monkey | Rhesus macaque | Captive | 3/13 (23.1%) | |
| Karamjal | ND | CVA24(2) | 0, 2 (2) | Pet | Rhesus macaque | Captive |
Parechovirus was detected by real-time RT-PCR but VP1 RT-PCR failed. 5′ NTR sequence consistent with HPeV.
New Ljungan virus detected with real-time RT-PCR. VP1 partial sequence most closely related to LV.
One fecal sample contained a mixture of EV75 and an untyped PeV.
One fecal sample contained a mixture of HPeV1 and an untyped EV.
One fecal sample contained a mixture of E11 and HPeV4.
EV was detected by real-time RT-PCR but VP1 RT-PCR failed. 5′ NTR sequence consistent with genus EV.
EV was detected by real-time RT-PCR, but VP1 RT-PCR failed. 5′ NTR sequence consistent with genus EV, but two nucleotide mismatches in the probe-target sequence.
Parechovirus was detected by real-time RT-PCR but VP1 RT-PCR failed. 5′ NTR sequence consistent with LV.
Laboratory testing.
RNA was extracted directly from 10% (wt/vol) stool suspensions, as described previously (32), and tested for enterovirus (EV), parechovirus (PeV), and sapelovirus by genus-specific TaqMan real-time reverse transcriptase PCR (RT-PCR) assays, targeting the 5′ nontranslated region (5′ NTR) (Tables 3 to 5) (18, 33, 35). The reaction conditions for3 the sapelovirus 5′ NTR real-time primers (AN626 and AN628) and probe (AN627) were as described for the parechovirus assay (37), except the thermocycler annealing and detection temperature was set to 58°C rather than 60°C. In some cases, the 5′ NTR real-time PCR amplicon was sequenced and analyzed by a BLASTn query of GenBank to eliminate the possibility of false-positive results and to confirm the identity of the targeted picornavirus genus.
Table 3.
Enterovirus primers and probes used in the study
| Primer | Sequence | Amino acid motif | Gene | Locationa | Reference |
|---|---|---|---|---|---|
| AN350 | GGCCCCTGAATGCGGCTAATCC | None | 5′ NTR | 449–470 | 33, 34 |
| AN351 | GCGATTGTCACCATWAGCAGYCA | None | 5′ NTR | 599–577 | 33, 34 |
| AN234 | FAM-CCGACTACTTTGGGWGTCCGTGT-BHQ1 | None | 5′ NTR | 537–559 | 33, 34 |
| AN32 | GTYTGCCA | WQT | VP1 | 3009–3002 | 32 |
| AN33 | GAYTGCCA | WQS | VP1 | 3009–3002 | 32 |
| AN34 | CCRTCRTA | YDG | VP1 | 3111–3104 | 32 |
| AN35 | RCTYTGCCA | WQS | VP1 | 3010–3002 | 32 |
| 224 | GCIATGYTIGGIACICAYRT | AMLGTH(I/L/M) | VP3 | 1977–1996 | 32 |
| 222 | CICCIGGIGGIAYRWACAT | M(F/Y)(I/V)PPG(A/G) | VP1 | 2969–2951 | 32 |
| AN89 | CCAGCACTGACAGCAGYNGARAYNGG | PALTA(A/V)E(I/T)G | VP1 | 2602–2627 | 32 |
| AN88 | TACTGGACCACCTGGNGGNAYRWACAT | M(F/Y)(I/V)PPGGPV | VP1 | 2977–2951 | 32 |
Location relative to the genome of PV1-Mahoney (GenBank accession number V01149).
Table 5.
Sapelovirus 5′ NTR real-time (TaqMan) RT-PCR primers and probe used in the study
| Primer | Sequence | Amino acid motif | Gene | Locationa | Source or reference |
|---|---|---|---|---|---|
| AN626 | TGGCGCATGCTCWTGGCATTAC | None | 5′NTR | 567–588 | 18 |
| AN628 | AGCAWTCCATGGGGGRTT | None | 5′NTR | 703–686 | 18 |
| AN627 | FAM-CCAGCCGCGRCCCTRTCAGGYAG-BHQ1 | None | 5′NTR | 658–636 | This work |
Location relative to the genome of simian sapelovirus 1, strain 2383 (GenBank accession number AY064708).
Virus identifications of genus-specific real-time RT-PCR-positive specimens were determined by nested or seminested RT-PCR targeting a portion of the genome region encoding the VP1 capsid protein (Tables 3 and 4), followed by amplicon sequencing (32, 36). An additional human PeV (HPeV) partial VP1 assay (does not amplify Ljungan virus species) was used on a small number of specimens that yielded an unreadable sequence with the complete VP1 assay (Tables 3 and 4). The cDNA reactions for these specimens were performed as described previously for the parechovirus complete VP1 assay (36). The PCR1 reaction differed slightly from the previously described parechovirus complete VP1 PCR1 assay in that the HPeV primers (AN486 and AN488) were used at 0.5 μM in the final reaction volume of 50 μl. Similarly, the HPeV PCR2 primers (AN268 and AN489) were used at 0.4 μM in the final reaction volume of 50 μl. Thermocycler profiles for the HPeV partial VP1 assay were as described for the EV partial VP1 assay (36). Virus type identity was determined by comparison of the VP1 amplicon nucleotide and deduced amino acid sequences with the VP1 sequences of all the reference strains for each virus genus by script-driven sequential pairwise comparison using the program Gap (Wisconsin Sequence Analysis Package, version 11.0; Accelrys, Inc., San Diego, CA) as described previously (32). For viruses which appeared to represent unique virus types (<75% nucleotide identity to all known types), complete VP1 sequences were determined and analyzed as described previously (38). If the complete VP1 sequences were still distinct from all known types, the sequence was forwarded to the Picornaviridae Study Group of the International Committee for the Taxonomy of Viruses for registration of a new type.
Table 4.
Parechovirus primers and probes used in the study
| Primer | Sequence | Amino acid motif | Gene | Locationa | Source or reference |
|---|---|---|---|---|---|
| AN344 | GGCCCCWGRTCAGATCCAYAGT | None | 5′ NTR | 616–595 | 35 |
| AN345 | GTAACASWWGCCTCTGGGSCCAAAAG | None | 5′ NTR | 422–447 | 35 |
| AN257 | HEX-CCTRYGGGTACCTYCWGGGCATCCTTC-BHQ1 | None | 5′ NTR | 583–557 | 35 |
| AN273 | AARTAGTC | DYF | 2A | 3236–3243 | 36 |
| AN274 | AARTAATC | DYF | 2A | 3236–3243 | 36 |
| AN275 | TCRCAGTT | NCE | 2A | 3309–3302 | 36 |
| AN276 | TCRCAATT | NCE | 2A | 3309–3302 | 36 |
| AN277 | ATRAATTT | KFI | 2B | 3564–3557 | 36 |
| AN278 | ATRAACTT | KFI | 2B | 3564–3557 | 36 |
| AN353 | GACAATAGTTTTGAAATNACNATHCCNTA | TIPY | VP3 | 2126–2154 | 36 |
| AN355 | CTCCAACTATAATGCCATARTGYTTRTARAANCC | GFYKH | 2A | 3119–3086 | 36 |
| AN357 | GAATAAAATGGTACTGANARNGTCATYTGYTC | EQM(S/T)(F/L) | VP1 | 2829–2798 | 36 |
| AN358 | AACTATAATGCCATARTGYTTRTARAANCC | GFYKH | 2A | 3115–3086 | 36 |
| AN369 | ACCAAGGTTGACAACATTTTYGGNMGNGC | FGRA | VP1 | 2531–2559 | 36 |
| AN486 | GGIACIGGRAARAAIARRTTNGG | PN(L/F)FFP | VP1 | 2945–2967 | This work |
| AN488 | ACIASIGCICARGAYGAYGGNCC | TSAQDDGP | VP1 | 2435–2457 | This work |
| AN489 | GGTACTGACAGTGTCATCTGYTCNCCNGCNGG | PAGEQM | VP1 | 2789–2820 | This work |
| AN268 | ACCAAGGTAGACAACCTATTTGGNMGNGCNTGG | GRAW | VP1 | 2529–2563 | This work |
Location relative to the genome of HPeV1-Harris (GenBank accession number S45208).
VP1 nucleic acid sequences were aligned using the Pileup program (Wisconsin Package), and phylogenetic relationships were inferred by the neighbor-joining method implemented in MEGA, version 4.0 (39), using the Kimura two-parameter method for computing evolutionary distances (40). Regions containing alignment gaps were omitted from the analysis. Support for specific tree topologies was estimated by bootstrap analysis with 1,000 pseudoreplicate data sets. VP1 nucleotide sequence distances were computed for picornavirus serotypes with multiple detections in NHP (MegAlign program, DNAStar Lasergene 9 Core Suite, Madison, WI). Different picornavirus lineages within a serotype were arbitrarily defined as any two viruses (or virus groups) with <95% VP1 nucleotide identity to one another, as described previously for polioviruses (41, 42). Lineages are very closely related viruses with a recent common ancestor, while viruses with >5% nucleotide differences are considered to be genetically distant enough to be evolving independently.
Nucleotide sequence accession numbers.
All VP1 sequences produced in this study were deposited in GenBank under the accession numbers JX538033 to JX538226 (enteroviruses) and JX565593 to JX565644 (parechoviruses).
RESULTS
A total of 678 fecal specimens were obtained from NHP (Table 1). Rhesus macaques, the most widely distributed and most synanthropic NHP in Bangladesh, accounted for 618 (91.3%) of the specimens. Hanuman langurs, capped langurs, hoolock gibbons, and a pigtail macaque accounted for the remaining samples. Specimens were obtained in the following six ecological contexts: urban (n = 431), shrine (n = 196), a tea garden (n = 16), performing (n = 11), pet (n = 2), and wild (n = 21) (Table 1).
Genus-specific real-time PCR assays were used to directly screen RNA from fecal extracts for three human and simian enteric picornavirus genera (Enterovirus, Parechovirus, and Sapelovirus). A total of 78 specimens were positive for at least one picornavirus by real-time PCR; 57 contained an enterovirus only, 18 contained a parechovirus only, and three specimens contained both an enterovirus and a parechovirus (Table 6 and Fig. 1). Sapeloviruses were not detected in any specimens. Picornavirus was detected in 8.7% of rhesus macaque specimens, 16.2% of Hanuman langur specimens, and one capped langur and in every context sampled except the tea garden (Tables 1 and 2).
Table 6.
Picornavirus serotypes detected in NHP fecal samples
| Virus species | Serotype | No. of serotypes detected | NHP species | Context |
|---|---|---|---|---|
| Enterovirus types | ||||
| HEV-A | CVA2 | 1 | Rhesus macaque | Urban |
| HEV-A | CVA5 | 1 | Rhesus macaque | Shrine |
| HEV-A | EV76 | 2 | Rhesus macaque | Urban |
| HEV-A | EV90 | 1 | Rhesus macaque | Urban |
| HEV-B | CVB1 | 1 | Rhesus macaque | Urban |
| HEV-B | CVB3 | 2 | Rhesus macaque | Urban |
| HEV-B | CVB6 | 4 | Hanuman langur | Urban |
| HEV-B | E11 | 2a | Rhesus macaque | Urban, shrine |
| HEV-B | E19 | 3 | Rhesus macaque | Shrine |
| HEV-B | E20 | 2 | Rhesus macaque | Shrine |
| HEV-B | E21 | 1 | Rhesus macaque | Shrine |
| HEV-B | E29 | 1 | Rhesus macaque | Shrine |
| HEV-B | E33 | 1 | Rhesus macaque | Urban |
| HEV-B | EV74 | 1 | Rhesus macaque | Shrine |
| HEV-B | EV75 | 2b | Rhesus macaque | Urban, shrine |
| HEV-B | EV97 | 1 | Rhesus macaque | Urban |
| HEV-B | New-EV101 | 1 | Rhesus macaque | Urban |
| HEV-C | CVA19 | 2 | Hanuman langur | Urban |
| HEV-C | CVA24 | 3 | Rhesus macaque | Pet, performing |
| HEV-C | EV99 | 1 | Rhesus macaque | Urban |
| BEV | BEV1 | 1 | Rhesus macaque | Urban |
| BEV | BEV2 | 1 | Capped langur | Wild |
| EVa | Not typed | 7 | Rhesus macaque | Urban, shrine, wild |
| EV divergentb | Not typed | 18 | Rhesus macaque | Shrine |
| Parechovirus types | ||||
| HPeV | HPeV1 | 5c | Rhesus macaque | Urban, shrine |
| HPeV | HPeV4 | 1d | Rhesus macaque | Urban |
| HPeV | HPeV5 | 1 | Rhesus macaque | Shrine |
| HPeV | HPeV12 | 1 | Rhesus macaque | Shrine |
| HPeV | HPeV14 | 3 | Rhesus macaque | Urban |
| HPeV | HPeV15 | 1 | Rhesus macaque | Shrine |
| PeV | Not typed | 8e | Rhesus macaque | Urban, shrine, wild |
| Ljungan virus | Ljungan virus | 1 | Rhesus macaque | Urban |
EV was detected by real-time RT-PCR, but VP1 RT-PCR failed. 5′ NTR sequence was consistent with the genus Enterovirus.
EV was detected by real-time RT-PCR, but VP1 RT-PCR failed. 5′ NTR sequence was consistent with the genus Enterovirus, but two nucleotide mismatches in the probe target sequence.
One fecal sample contained a mixture of HPeV1 and an untyped EV.
One fecal sample contained a mixture of E11 and HPeV4.
One fecal sample contained a mixture of EV75 and an untyped PeV.
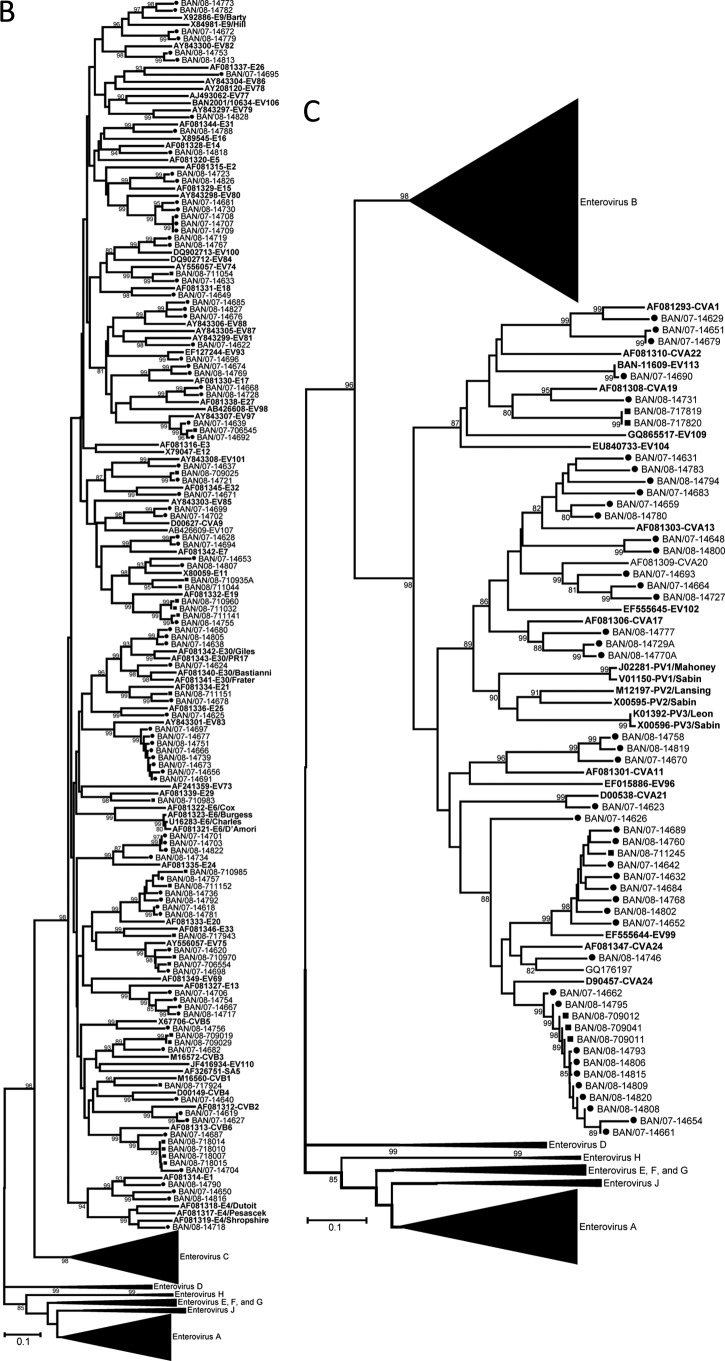
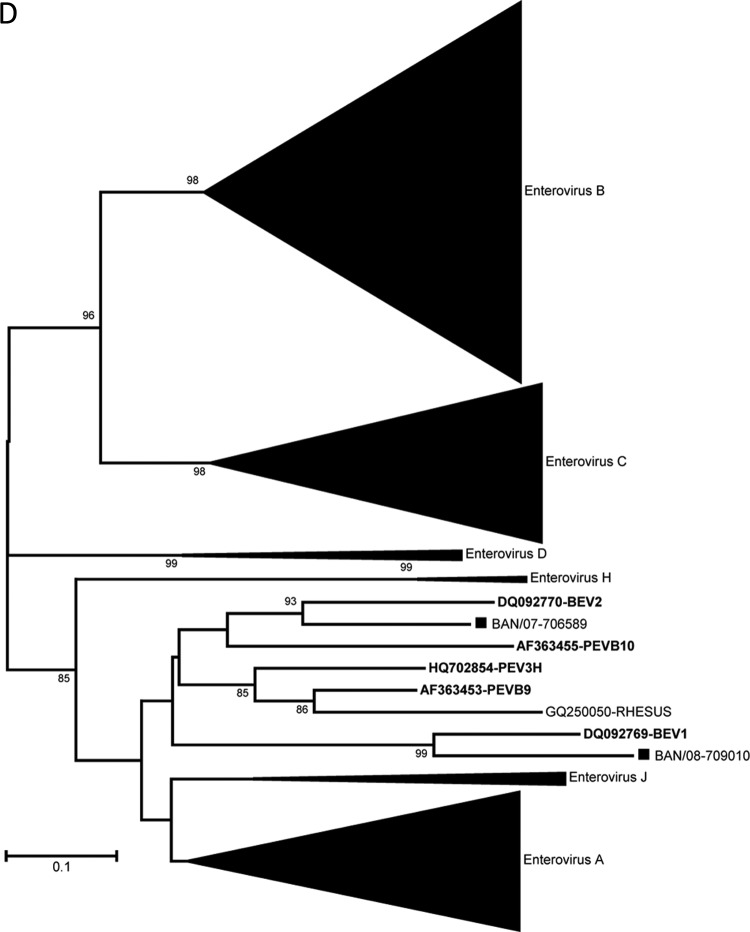
The virus types present in the NHP stools were identified by RT-PCR amplification and sequencing of a portion of the region encoding the VP1 capsid protein (Table 6). Twenty-two distinct EV serotypes were detected in the NHP specimens. Twenty types were from three of the four known human enterovirus species, represented by four types in the species Enterovirus A, 13 types in the species Enterovirus B, and three types in the species Enterovirus C (Table 6 and Fig. 2). For types detected in more than one specimen (i.e., EV76, coxsackievirus B3 (CVB3), CVB6, echovirus 11 (E11), E19, E20, EV75, CVA19, and CVA24), the partial VP1 sequences from the same type were closely related to one another when the viruses were from the same geographic location, setting, and sampling period (CVA24, CVB3, EV76, CVB6, and CVA19; range, 99.5 to 100% nucleotide identity) (Fig. 2). All the other multiple-detection EVs with differences in geographic location, setting, or sampling period showed greater VP1 diversity, with one exception. The pair of E20 specimens collected 1 year apart from Sylhet NHP were 99.1% identical. Of three shrine monkey E19 specimens collected from Sylhet, one pair collected at the same time were identical but differed by 6.1% nucleotide identity from the third sampled 1 year later. NHP E11 specimens, collected during the same sampling period, from Sylhet and Nandarshar and in different settings, differed by 13.3% nucleotide identity from each other and both differed by 24.3% nucleotide identity from the prototype E11 (GenBank accession number X80059). EV75 specimens from Sylhet and Dhamrai differed by 6.5% nucleotide identity. Two viruses were identified as bovine enteroviruses (BEV), BEV1 and BEV2 (Table 6 and Fig. 2D). Twenty-five samples were positive in the EV real-time RT-PCR assay but negative for VP1, so they could not be typed. The 5′ NTR real-time PCR amplicons were sequenced for these viruses. Searching the GenBank nonredundant nucleotide database using the BLASTn algorithm, the 25 5′ NTR sequences were split into two groups (data not shown). Seven specimens were clearly EV with the TaqMan probe target sequence fully conserved, while the other 18 nearly identical sequences were most consistent with EV and had two nucleotide differences in the 3′ end of the TaqMan probe target sequence. The latter group of 18 viruses (all shrine rhesus macaques, with 9 stool samples from November 2007 and 9 stool samples from January 2008) had divergent 5′ NTR sequences, which may indicate the presence of one or more novel EVs.
Fig 2.
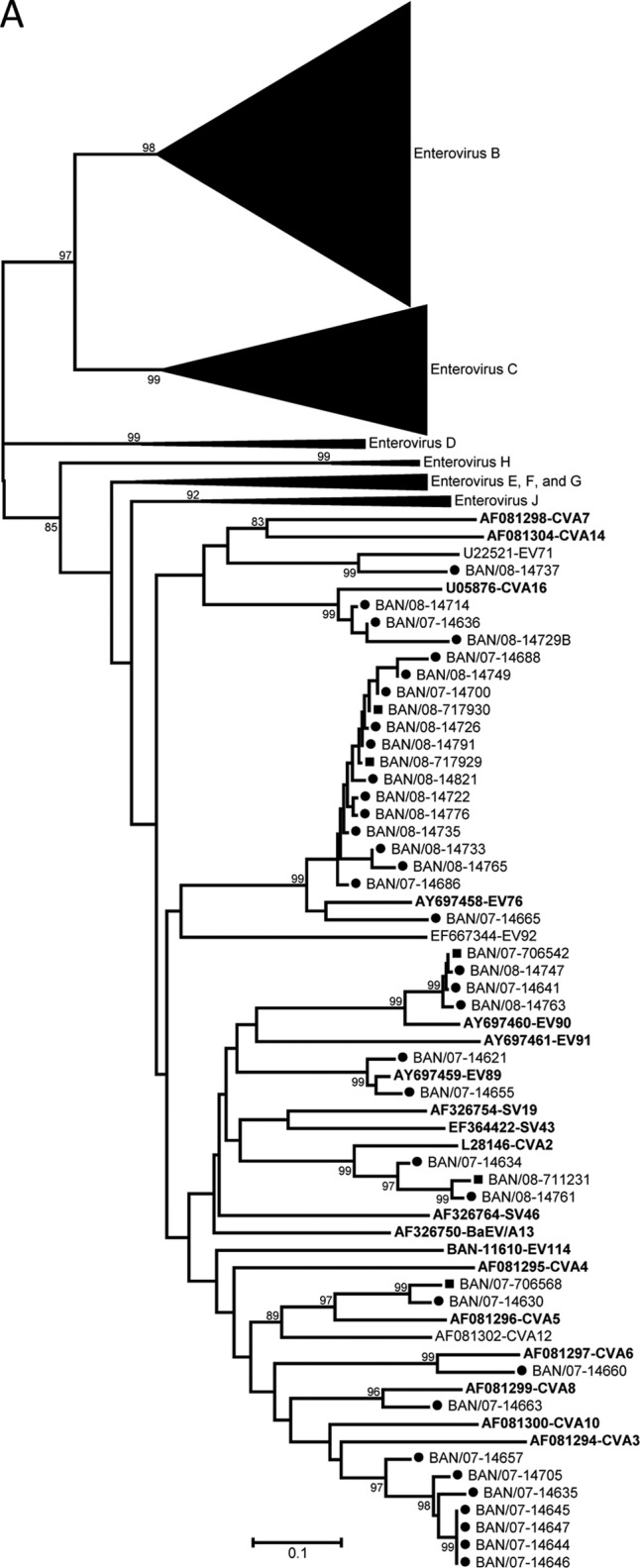
Phylogenetic relationships of NHP and human enteroviruses from Bangladesh based on an analysis of partial VP1 sequences. The trees were generated by the neighbor-joining algorithm using the Kimura two-parameter nucleotide substitution model (40) implemented in MEGA 4 (39). Bootstrap values ≥80% are shown. Filled squares, sequences from simian samples; filled circles, samples from human samples. Viruses in the species enterovirus A (A); viruses in the species enterovirus B (B); viruses in the species enterovirus C (C); viruses in the species enterovirus J (D).
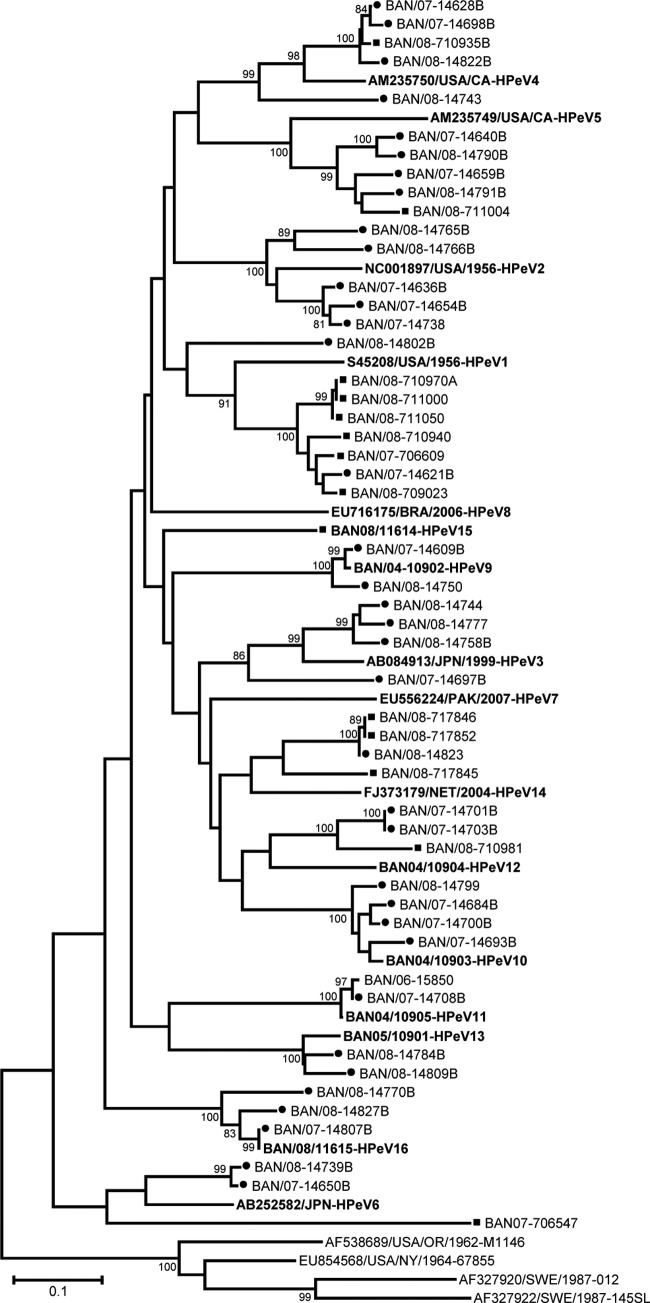
Twenty-one samples, all from rhesus macaques, were positive for parechovirus by real-time RT-PCR. VP1 RT-PCR and sequencing identified five HPeV1, one HPeV4 (a mixture of HPeV4 and E11), one HPeV5, one HPeV12, three HPeV14, and one HPeV15 (designated the prototype strain of this type) (Table 6 and Fig. 3). An additional eight samples were negative in the parechovirus VP1 typing assay, one of which was a mixture of PeV and EV75. Cycle threshold (CT) values for these eight specimens ranged from 39 to 42, at or near the limit of detection of the real-time and VP1 RT-PCR assays. Analyses of the 5′ NTR parechovirus real-time RT-PCR amplicon sequences by BLASTn searches of the GenBank nonredundant nucleotide database showed seven sequences most consistent with human parechoviruses and one sequence (from a wild rhesus macaque) most consistent with Ljungan virus, a rodent parechovirus. A single fecal sample from an urban rhesus macaque yielded a partial VP1 sequence that was most similar to those of Ljungan viruses (56.6 to 59.9% nucleotide identity; 59.5% to 62.7% amino acid identity), rather than to the HPeVs (45.5 to 51% nucleotide identity; 38.4% to 44.5% amino acid identity). The genetic distance of this strain from its nearest neighbor suggests that it is a new Ljungan virus type (Fig. 3), but we have so far been unable to generate additional capsid sequence to confirm this possibility. Strikingly, no known simian EVs were identified in any of the NHP fecal samples screened in this study (but see our accompanying paper for this project, which characterizes EVs among NHP at the Dhaka Zoo that were collected during the same sampling periods [43]).
Fig 3.
Phylogenetic relationships of NHP and human parechoviruses from Bangladesh based on an analysis of partial VP1 sequences. The trees were generated by the neighbor-joining algorithm using the Kimura two-parameter nucleotide substitution model (40) implemented in MEGA 4 (39). Filled squares, sequences from simian samples; filled circles, samples from human samples. Bootstrap values ≥80% are shown.
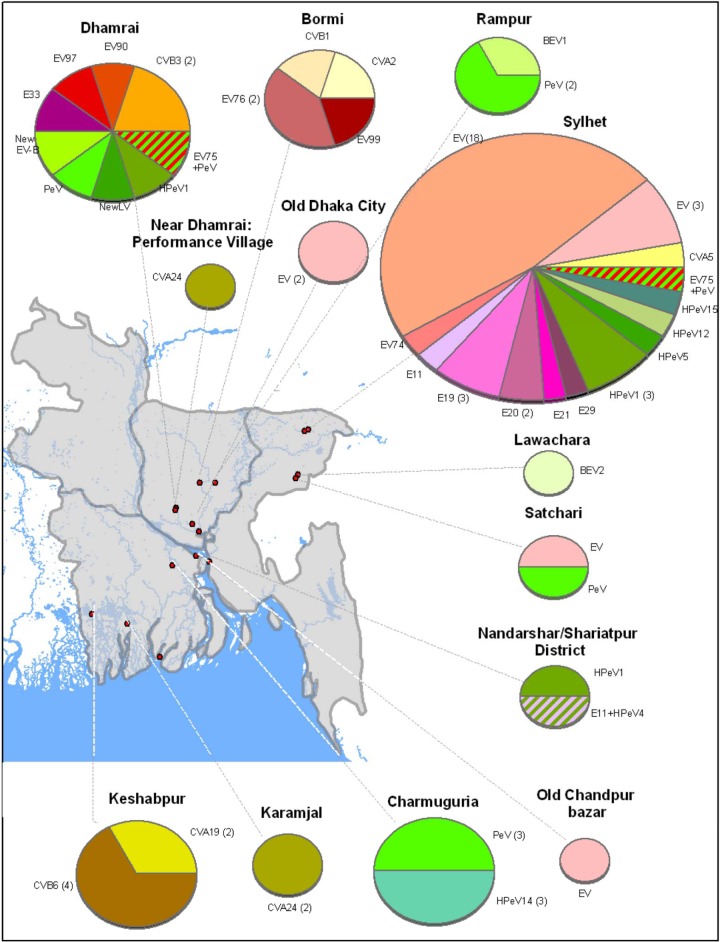
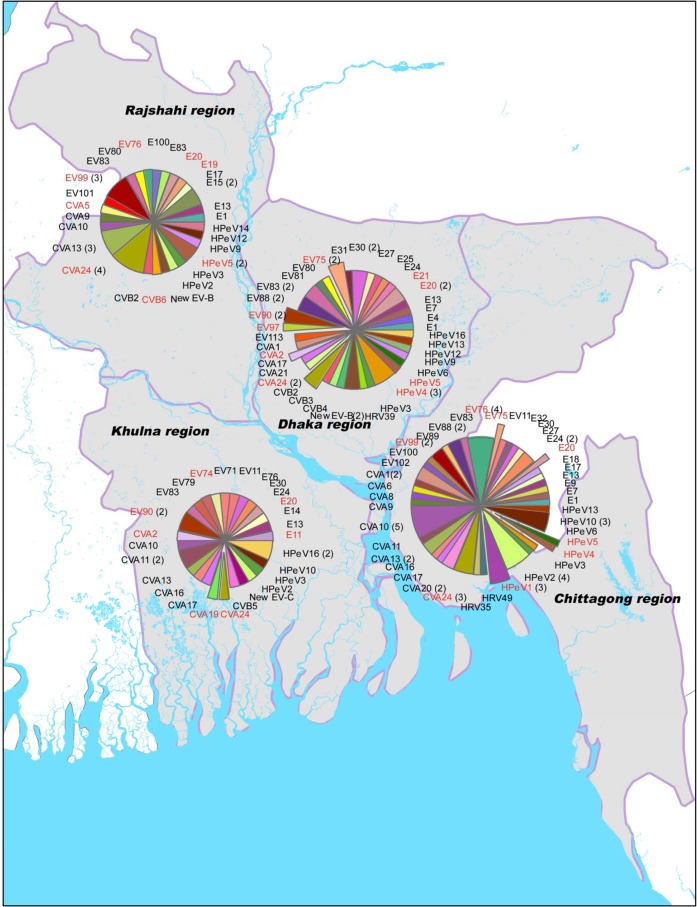
In order to compare the NHP picornavirus flora to that of surrounding human populations, we performed the same analyses on 156 fecal specimens collected from Bangladeshi children between 2007 and 2008 during routine surveillance for acute flaccid paralysis (Fig. 4). It should be noted that these human samples were not collected in the same areas as the NHP sampling sites. The types of EV detected in these human specimens were extraordinarily diverse, with 75 types represented among the 156 samples. Similarly, 14 of 16 types of HPeV were identified from the 36 parechovirus-positive human specimens (Fig. 3). Nearly 75% of the EV serotypes and 83% of the human parechovirus types detected in the NHP samples were also detected in human stool samples. The two largest combined NHP/human EV groups were EV76 (2 NHP and 13 human specimens) and CVA24 (3 NHP and 11 human specimens). The combined EV76 group consisted of three lineages, the largest of which contained a pair of NHP specimens and 10 human EV76 specimens (95 to 99.5% nucleotide identity) (Fig. 2A). The large EV76 lineage was obtained from widespread geographic locations and contained specimens from two sampling periods approximately 1 year apart. Similarly, the combined CVA24 group split into two lineages, the largest of which consisted of all 3 NHP specimens (human companion rhesus macaques, two pets and one performing) and 10 human CVA24 specimens (97.8 to 100% nucleotide identity) (Fig. 2C). The human CVA24-positive specimens were from widespread locations in Bangladesh and spanned two sampling periods about 1 year apart. At the opposite extreme, two NHP and two human E11 specimens from four different locations split into four distinct lineages. The NHP E11 pair sampled at the same time differed by 13.3% nucleotide identity, while the human E11 pair sampled 1 year apart differed by 16.2% nucleotide identity. The NHP and human E11 pairs differed by an average of 22.7% (Fig. 2B). Similarly, for EV99, one NHP virus and nine human viruses split into 10 lineages, differing by 6.6 to 11.6% nucleotide identity (Fig. 2C). Overall, 17 human EV types were detected in at least one NHP. Of these 17 EVs, 10 EV types (59%) had at least one NHP/human lineage (including EV90) (Fig. 2A to C). Of the 6 HPeV types that were detected in NHP specimens, 5 were also found in human specimens. NHP/human parechovirus lineages were observed in four of five (80%) instances (HPeV1, -4, -5, and -14) (Fig. 3). HPeV1 was the most common human virus found in NHP, with six detections, including one mixture that also contained an EV. Like the E11 and EV99 groups, the NHP/human HPeV5 group, consisting of one NHP and four human specimens, exhibited considerable genetic diversity, with four lineages (Fig. 3). Four human rhinoviruses (HRV) were detected and identified in human stool specimens (Fig. 4). No HRV were detected in NHP.
Fig 4.
Picornaviruses detected in stools of children identified during routine acute flaccid paralysis surveillance activities, 2007 to 2008. The data are grouped by four regions of the country. Picornaviruses detected in this human sample set, which were also detected in the current study's nonhuman primate (NHP) fecal samples, are highlighted in red. Exploded pie pieces indicate those picornaviruses that were detected in both human and NHP samples collected in the same region.
DISCUSSION
A growing body of research over the past decades has contributed to our understanding of picornavirus infection in NHP. Experimental infection has shown the capacity of Asian monkey species to sustain infection with human enteroviruses (44–46), and numerous studies have reported the presence of antibody to human EV in a variety of NHP species in natural settings (21, 22).
A caveat of many of the cited serologic studies, however, is the possibility that the observed reactivities could be attributed to cross-reactivity following previous exposure to related simian EV. Our study used molecular methods to conclusively document the presence of a wide range of human enteroviruses and parechoviruses in NHP. With a population estimated at 158.5 million, a growth rate of 1.6%, and an overall density of 1,101 persons per km2, Bangladesh is one of the most densely populated countries in the world. Bangladesh's infrastructure is relatively undeveloped and sanitation is poor in many areas (47). These conditions favor efficient fecal-oral transmission of enteroviruses and other enteric pathogens, resulting in a high rate of infection throughout most of the country (26, 30, 48).
The close association of NHP and human populations in Bangladesh provides conditions favorable for the interspecies transmission of infectious agents. Rhesus macaques, in particular, thrive in the human-altered habitats of villages, urban areas, or religious sites found throughout Bangladesh. Macaque population densities in these areas vary from a single troop of less than 20 animals to urban areas like Dhamrai and Bormi, where up to 200 monkeys may roam through the villages. Habitat destruction and anthropogenic changes of the environment have dramatically reduced the ability of macaques to naturally disperse. Rhesus macaques have a matrilineal social structure; subadult males are forced from their natal group and are expected to seek out a new home group. Most of the urban and village NHP populations that we sampled were effectively isolated by a lack of forested corridors that would allow the natural dispersion and movement of male macaques. Thus, man-made changes in the macaque habitat may have tended, over time, to increase the amount of human/NHP contact and hence the opportunity for cross-species disease transmission.
While the population density of NHP species is much lower than that of human populations, some of the same factors that influence the risk of enteric infections in humans also apply to NHP living in close association with humans. Macaques in Bangladesh, and elsewhere throughout Asia, are notorious for their boldness, particularly where there is competition over access to food and water. The urban monkey's motto could well be “what's mine is mine and what's yours is mine,” a proclivity that increases the likelihood that they come into contact with humans and the by-products of human settlements, including waste. Therefore, it is not surprising that we have detected human enteric viruses in a significant proportion of the NHP specimens sampled. In fact, all but two (both bovine viruses) of the enteric picornaviruses detected in NHP whose habitats overlapped with humans (animals in urban areas and religious sites and performing and pet monkeys) were serotypes that have been associated previously only with human infection and disease. These findings are consistent with previous studies, demonstrating that NHP living in close association with humans are at risk of infection with human pathogens (1, 2, 49). In contrast, of the 21 NHP living in protected forests in this study, only three were picornavirus positive. Of these, one virus was conclusively identified as a bovine EV, one virus was most related to Ljungan virus (rodent parechovirus), and the third virus was an unknown EV.
No known simian picornaviruses were detected in NHP fecal specimens tested for this study. We can offer a few possible explanations for this finding. First, it is possible that efficient transmission of simian picornaviruses requires correspondingly higher NHP population densities, as are found typically in zoos and research colonies (21, 37, 50, 51). Indeed, in our accompanying paper to this article, which analyzed fecal specimens from zoo NHP in Bangladesh, we found that over 90% of EV detected were similar to simian EV serotypes previously identified in NHP (43). Multiple-age caging and a high density within cages may create favorable conditions for persistent EV cycling and variation by interspecies transmission. All of these factors have been studied in the context of closed-system, intensive “factory” farming, especially for poultry and swine (52–55). Moreover, picornavirus serotypes detected in the zoo population tended to persist over time, in contrast to the fluctuation in detection of specific types in the free-ranging populations examined in the present paper. It is possible that simian picornaviruses may be better adapted to simian hosts, while human viruses are less efficient at infection and transmission in NHP and tend to be more efficiently eliminated.
The smaller numbers of human EV detections among zoo NHP could also be explained by their relative lack of contact with humans and human waste, compared to their free-ranging urban counterparts. Although the zoo is situated in the heart of a densely populated city, cages prevent the animals from having extensive physical contact with humans and the by-products of human settlement. In contrast, free-ranging urban NHP are immersed in the Bangladeshi high-population density human milieu. These NHP are partly dependent on humans for food through scavenging and drinking water from urban environmental sources. Due to poor sanitary conditions, the human environmental EV challenge is probably continuous and high. Conceivably, the detection of no known simian EVs in this study may have resulted from the supplanting of NHP viruses with human EVs in these isolated urban/shrine NHP populations. One implication of this scenario is that the direction of transmission in these contexts is mainly from human to NHP. This study does not provide data suitable for analyzing the efficiency of human EV horizontal transmission within the urban/shrine NHP population.
The human stool specimens analyzed in this study were collected during the same period, 2007 to 2008, in which our NHP data were acquired, but at different locations and at different time points: a convenience sample, we present these results for purposes of general comparison. Though the two sample sets were not collected in the same locations or at the same time points, they are similar in both the total prevalence and diversity within each set for EV and HPeV (75 EV types among 156 detections in humans versus 25 EV types among 61 detections in NHP and 14 HPeV types among 36 detections in humans versus 6 HPeV types among 13 detections in NHP) and also overlap significantly with respect to picornavirus serotypes (Table 6 and Fig. 2 to 4). Of the 25 distinct EV serotypes detected in the NHP specimens, 16 appeared in the human samples from the same period. Similarly, of the 6 HPeV types detected in the NHP specimens, 5 were identified in the human specimens from the same period. The very close genetic relationships (human/NHP lineages) between some of the EV and HPeV types identified in humans and NHP further support the idea that there are shared local picornavirus reservoirs in Bangladesh, from which both humans and NHP may become infected. The genetic diversity observed in some of the other NHP/human serotype groups (E11; four very divergent lineages) is expected in a tropical country with poor sanitation and is likely the result of widespread, year-round circulation of EVs and HPeVs in localized areas.
One of the original goals of the study was to seek the unusual enteroviruses, EV76, EV89, EV90, and EV91, in NHP to determine whether they were simian viruses transmitted to humans. EV76 and EV90 were detected in urban rhesus macaques but in very small numbers, with only three detections total (Table 6 and Fig. 1). EV76 was the most common picornavirus in the human sample set (n = 13) and contained a large lineage with 10 human specimens and both NHP EV76 specimens (95 to 99.5% nucleotide identity). The three human specimens and one NHP EV90 specimen also formed a single lineage of very closely related viruses (98.3 to 99.7% nucleotide identity). Two EV89-positive human stool samples were found, but EV89 was not detected in the NHP sample set. EV91 was not detected in either human or NHP specimens. The VP1 genetic data imply that EV76 and EV90 are circulating in humans and, at the very least, present in NHP. EV76 circulation in humans was widespread during this 2-year sampling period, based on the number of detections and the distant geographical locations around Bangladesh, where specimens were collected. Harvala et al. recently demonstrated a high EV76 seroprevalence among adults in Cameroon and Zimbabwe, as well as among African Old World monkeys and chimpanzees (21). However, neither study can definitively demonstrate that EV76, EV89, EV90, and EV91 are simian viruses that only recently infected humans.
Enterovirus seasonality is less pronounced in tropical and subtropical regions than in temperate climates, with peak human transmission and clinical disease generally corresponding to the wet season. Our detections in free-living NHP were generally consistent with this observation, with detection rates in a narrow range, from 6% during the dry season to 14% in August 2007, during the height of the wet season (2).
The range of CT values, from 22 to 45, for EV and HPeV detected by real-time PCR was similar to that observed when testing human fecal samples with the same assays, in both the present study and previous studies (34, 35). Although the assays were not designed to be strictly quantitative, this finding suggests that human enteroviruses and parechoviruses probably replicate to similar titers in the human and simian enteric tracts. Thus, one would expect humans and NHP to transmit enteric picornaviruses at similar rates. Given the poor sanitation conditions in Bangladesh, this finding lends further support to the concept that humans and free-ranging NHP constitute a single reservoir from which both humans and NHP can be infected. Although the comparison of our NHP data set with a convenience human sampling provided a significant amount of useful information, additional focused studies are needed to assess the impact of enteric picornavirus infection on NHP health and the potential for NHP to participate in picornavirus transmission or as a reservoir for human infection.
ACKNOWLEDGMENTS
We thank our student assistants in the wildlife branch of the Department of Zoology at Jahangirnagar University, as well as the inhabitants, both human and simian, at the sites where we collected these data. We also thank the Bangladesh Forest Department for their permission and constant support of our research program. Administrative and data support were provided by R. Liszanckie and J. Johnson. H. Engel, L. Engel, J. Heidrich, and L. Johnson remain devoted field assistants particularly adept at spotting monkey feces.
This work was partially supported by grants NIH-NIAID R03AI064865 and NIH-NCRR P51 RR 00166.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Jones-Engel L, Engel GA, Schillaci MA, Babo R, Froehlich J. 2001. Detection of antibodies to selected human pathogens among wild and pet macaques (Macaca tonkeana) in Sulawesi, Indonesia. Am. J. Primatol. 54:171–178 [DOI] [PubMed] [Google Scholar]
- 2. Jones-Engel L, Engel GA, Schillaci MA, Lee B, Heidrich J, Chalise M, Kyes RC. 2006. Considering human-primate transmission of measles virus through the prism of risk analysis. Am. J. Primatol. 68:868–879 [DOI] [PubMed] [Google Scholar]
- 3. Jones-Engel L, Engel GA, Schillaci MA, Rompis A, Putra A, Suaryana KG, Fuentes A, Beer B, Hicks S, White R, Wilson B, Allan JS. 2005. Primate-to-human retroviral transmission in Asia. Emerg. Infect. Dis. 11:1028–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones-Engel L, May CC, Engel GA, Steinkraus KA, Schillaci MA, Fuentes A, Rompis A, Chalise MK, Aggimarangsee N, Feeroz MM, Grant R, Allan JS, Putra A, Wandia IN, Watanabe R, Kuller L, Thongsawat S, Chaiwarith R, Kyes RC, Linial ML. 2008. Diverse contexts of zoonotic transmission of simian foamy viruses in Asia. Emerg. Infect. Dis. 14:1200–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schillaci MA, Jones-Engel L, Engel GA, Paramastri Y, Iskandar E, Wilson B, Allan JS, Kyes RC, Watanabe R, Grant R. 2005. Prevalence of enzootic simian viruses among urban performance monkeys in Indonesia. Trop. Med. Int. Health 10:1305–1314 [DOI] [PubMed] [Google Scholar]
- 6. Engel G, O'Hara TM, Cardona-Marek T, Heidrich J, Chalise MK, Kyes R, Jones-Engel L. 2010. Synanthropic primates in Asia: potential sentinels for environmental toxins. Am. J. Phys. Anthropol. 142:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuentes A, Kalchik S, Gettler L, Kwiatt A, Konecki M, Jones-Engel L. 2008. Characterizing human-macaque interactions in Singapore. Am. J. Primatol. 70:879–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones-Engel L, Engel GA, Heidrich J, Chalise M, Poudel N, Viscidi R, Barry PA, Allan JS, Grant R, Kyes R. 2006. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg. Infect. Dis. 12:900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sha JC, Gumert MD, Lee BP, Jones-Engel L, Chan S, Fuentes A. 2009. Macaque-human interactions and the societal perceptions of macaques in Singapore. Am. J. Primatol. 71:825–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Southwick C, Malik I, Siddiqi MF. 2005. Rhesus commensalism in India: problems and prospects, p 240–257 In Patterson J, Wallis J. (ed), Commensalism and conflict: human-primate interface. American Society of Primatologists, Norman, OK [Google Scholar]
- 11. Fuentes-Marins R, Rodriguez AR, Kalter SS, Hellman A, Crandell RA. 1963. Isolation of enteroviruses from the “normal” baboon (Papio doguera). J. Bacteriol. 85:1045–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heberling RL, Cheever FS. 1965. Some characteristics of the simian enteroviruses. Am. J. Epidemiol. 81:106–123 [DOI] [PubMed] [Google Scholar]
- 13. Hoffert WR, Bates ME, Cheever FS. 1958. Study of enteric viruses of simian origin. Am. J. Hyg. 68:15–30 [DOI] [PubMed] [Google Scholar]
- 14. Hull RN, Minner JR, Smith JW. 1956. New viral agents recovered from tissue cultures of monkey kidney cells. I. Origin and properties of cytopathogenic agents S.V.1, S.V.2, S.V.4, S.V.5, S.V.6, S.V.11, S.V.12, and S.V.15. Am. J. Hyg. 63:204–215 [DOI] [PubMed] [Google Scholar]
- 15. Malherbe H, Harwin R. 1963. The cytopathic effects of vervet monkey viruses. S. Afr. Med. J. 37:407–411 [PubMed] [Google Scholar]
- 16. Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg AM, Pallansch MA, Palmenberg AC, Skern T, Stanway G, Yamashita T, Zell R. 2011. Picornaviridae, p 855–880 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. Elsevier, San Diego, CA [Google Scholar]
- 17. Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg AM, Pallansch MA, Palmenberg AC, Skern T, Stanway G, Yamashita T, Zell R. 2010, posting date Picornaviridae Study Group. http://www.picornastudygroup.com/
- 18. Oberste MS, Maher K, Pallansch MA. 2003. Genomic evidence that simian virus 2 and six other simian picornaviruses represent a new genus in Picornaviridae. Virology 314:283–293 [DOI] [PubMed] [Google Scholar]
- 19. Oberste MS, Maher K, Pallansch MA. 2002. Molecular phylogeny and proposed classification of the simian picornaviruses. J. Virol. 76:1244–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pallansch MA, Oberste MS. 2004. Coxsackievirus, echovirus, and other enteroviruses, p 2047–2051 In Gorbach SL, Bartlett JG, Blacklow NR. (ed), Infectious diseases, 3rd ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 21. Harvala H, McIntyre C, Imai N, Clasper L, Djoko CF, LeBreton M, Vermeulen M, Saville A, Mutapi F, Tamoufé U, Kiyang J, Biblia TG, Midzi N, Mduluza T, Pépin J, Njoum R, Smura T, Fair JN, Wolfe NDRM, Simmonds P. 2012. High seroprevalence of enterovirus infections in apes and Old World monkeys. Emerg. Infect. Dis. 18:283–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalter SS, Heberling RL. 1971. Comparative virology of primates. Bacteriol. Rev. 35:310–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harvala H, Sharp CP, Ngole EM, Delaporte E, Peeters M, Simmonds P. 2011. Detection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in Cameroon: relationship with human and simian enteroviruses. J. Virol. 85:4480–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He W, Lu H, Song D, Cheng J, Gai X, Chen Q, Gao F. 2008. Isolation and identification of coxsackievirus in Sichuan golden monkey. Chinese J. Virol. 24:312–316 [PubMed] [Google Scholar]
- 25. Miyagi J, Tsuhako K, Kinjo T, Iwamasa T, Kamada Y, Kinju T, Koyanagi Y. 1999. Coxsackievirus B4 myocarditis in an orangutan. Vet. Pathol. 36:452–456 [DOI] [PubMed] [Google Scholar]
- 26. Oberste MS, Maher K, Michele SM, Belliot G, Uddin M, Pallansch MA. 2005. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus A. J. Gen. Virol. 86:445–451 [DOI] [PubMed] [Google Scholar]
- 27. Pallansch MA, Oberste MS. 2009. Enteroviruses and Parechoviruses, p 249–282 In Specter S, Hodinka RL, Young SA. (ed), Clinical virology manual. ASM Press, Washington, DC [Google Scholar]
- 28. Shan TL, Wang CM, Cui L, Delwart E, Yuan CL, Zhao W, Guo W, Dai XQ, Yu Y, Hua XG. 2010. Human parechovirus infections in monkeys with diarrhea, China. Emerg. Infect. Dis. 16:1168–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaufmann AF, Gary GW, Broderson JR, Perl DP, Quist KD, Kissling RE. 1973. Simian virus 16 associated with an epizootic of obscure neurologic disease. Lab. Anim. Sci. 23:812–818 [PubMed] [Google Scholar]
- 30. Oberste MS, Maher K, Williams AJ, Dybdahl-Sissoko N, Brown BA, Gookin MS, Penaranda S, Mishrik N, Uddin M, Pallansch MA. 2006. Species-specific RT-PCR amplification of human enteroviruses: a tool for rapid species identification of uncharacterized enteroviruses. J. Gen. Virol. 87:119–128 [DOI] [PubMed] [Google Scholar]
- 31. World Health Organization 2001. Manual for the virological investigation of polio (WHO/EPI/GEN/97.01). World Health Organization, Geneva, Switzerland [Google Scholar]
- 32. Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 44:2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kilpatrick DR, Yang C-F, Ching K, Vincent A, Iber J, Campagnoli R, Mandelbaum M, De L, Yang S-J, Nix A, Kew OM. 2009. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 47:1939–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oberste MS, Penaranda S, Rogers SL, Henderson E, Nix WA. 2010. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J. Clin. Virol. 49:73–74 [DOI] [PubMed] [Google Scholar]
- 35. Nix WA, Maher K, Niklasson B, Lindberg M, Johansson S, Pallansch MA, Oberste MS. 2008. Detection of all known parechoviruses by real time-PCR. J. Clin. Microbiol. 46:2519–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nix WA, Maher K, Pallansch MA, Oberste MS. 2010. Parechovirus typing in clinical specimens by nested or semi-nested VP1 PCR coupled with sequencing. J. Clin. Virol. 48:202–207 [DOI] [PubMed] [Google Scholar]
- 37. Nix WA, Jiang B, Maher K, Strobert E, Oberste MS. 2008. Identification of enteroviruses in naturally infected captive primates. J. Clin. Microbiol. 46:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oberste MS, Schnurr D, Maher K, al-Busaidy S, Pallansch MA. 2001. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. J. Gen. Virol. 82:409–416 [DOI] [PubMed] [Google Scholar]
- 39. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 40. Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120 [DOI] [PubMed] [Google Scholar]
- 41. Kew OM, Mulders MN, Lipskaya GY, da Silva EE, Pallansch MA. 1995. Molecular epidemiology of polioviruses. Semin. Virol. 6:401–414 [Google Scholar]
- 42. Shulman LM, Handsher R, Yang CF, Yang SJ, Manor J, Vonsover A, Grossman Z, Pallansch M, Mendelson E, Kew OM. 2000. Resolution of the pathways of poliovirus type 1 transmission during an outbreak. J. Clin. Microbiol. 38:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oberste MS, Feeroz MM, Maher K, Nix WA, Engel GA, Begum S, Hasan KM, Oh G, Pallansch MA, Jones-Engel L. 2013. Naturally acquired picornavirus infections in primates at the Dhaka Zoo. J. Virol. 87:572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lashkevich VA, Koroleva GA, Tereshkina NV, Lukashev AN, Grigor'eva LV, Titova IP. 1996. Superacute lethal liver necrosis in monkeys infected with highly pathogenic variants of enteroviruses (ECHO 11 and ECHO 19 viruses). Vopr. Virusol. 41:198–206 (In Russian.) [PubMed] [Google Scholar]
- 45. Schmidt NJ, Dennis J, Lennette EH, Ho HH, Shinomoto TT. 1965. Antibody responses of rhesus (Macaca mulatta) monkeys experimentally infected with coxsackieviruses of group B and group A, type 9. J. Immunol. 95:54–69 [PubMed] [Google Scholar]
- 46. Wenner HA, TeYong L, Kamitsuka PS. 1961. Experimental infections with coxsackie viruses. I. Studies on virulence and pathogenesis in cynomolgus monkeys. Arch. Gesamte Virusforsch. 10:426–450 [PubMed] [Google Scholar]
- 47. Central Intelligence Agency 2009. The world factbook. Central Intelligence Agency, Washington, DC [Google Scholar]
- 48. Tanaka G, Faruque AS, Luby SP, Malek MA, Glass RI, Parashar UD. 2007. Deaths from rotavirus disease in Bangladeshi children: estimates from hospital-based surveillance. Pediatr. Infect. Dis. J. 26:1014–1018 [DOI] [PubMed] [Google Scholar]
- 49. Köndgen S, Kühl H, N′Goran P, Walsh P, Schenk S, Ernst N, Biek R, Formenty P, Mätz-Rensing K, Schweiger B, Junglen S, Ellerbrok H, Nitsche A, Briese T, Lipkin I, Pauli G, Boesch C, Leendertz F. 2008. Pandemic human viruses cause decline of endangered great apes. Curr. Biol. 18:1–5 [DOI] [PubMed] [Google Scholar]
- 50. Kalter SS, Heberling RL, Field J. 1981. Isolation of an enterovirus (SV19) from baboons (Papio cynocephalus). Lab. Anim. Sci. 31:190–191 [PubMed] [Google Scholar]
- 51. Rodriguez AR, Kalter SS, Heberling RL, Helmke RJ, Guajardo JE. 1977. Viral infections of the captive Kenya baboon (Papio cynocephalus): a five-year epidemiologic study of an outdoor colony. Lab. Anim. Sci. 27:356–371 [PubMed] [Google Scholar]
- 52. Chew-Lim M, Ng CY. 1987. Recurrent viruses in a Singapore intensive pig farming estate. Ann. Acad. Med. Singapore 16:651–654 [PubMed] [Google Scholar]
- 53. Negovetich NJ, Feeroz MM, Jones-Engel L, Walker D, Alam SM, Hasan K, Seiler P, Ferguson A, Friedman K, Barman S, Franks J, Turner J, Krauss S, Webby RJ, Webster RG. 2011. Live bird markets of Bangladesh: H9N2 viruses and the near absence of highly pathogenic H5N1 influenza. PLoS One 6:e19311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trock SC, Gaeta M, Gonzalez A, Pederson JC, Senne DA. 2008. Evaluation of routine depopulation, cleaning, and disinfection procedures in the live bird markets, New York. Avian Dis. 52:160–162 [DOI] [PubMed] [Google Scholar]
- 55. Webster RG, Hulse DJ. 2004. Microbial adaptation and change: avian influenza. Rev. Sci. Tech. 23:453–465 [DOI] [PubMed] [Google Scholar]