Abstract
Simian hemorrhagic fever virus (SHFV) is an arterivirus that causes severe disease in captive macaques. We describe two new SHFV variants subclinically infecting wild African red-tailed guenons (Cercopithecus ascanius). Both variants are highly divergent from the prototype virus and variants infecting sympatric red colobus (Procolobus rufomitratus). All known SHFV variants are monophyletic and share three open reading frames not present in other arteriviruses. Our data suggest a need to modify the current arterivirus classification.
TEXT
Simian hemorrhagic fever virus (SHFV) is a member of the family Arteriviridae, together with equine arteritis virus (EAV), lactate dehydrogenase elevating virus (LDV), and porcine reproductive and respiratory syndrome virus (PRRSV) (1). SHFV was first isolated from captive macaques (Macaca sp.) in 1964 after nearly simultaneous outbreaks in Soviet and American primate centers (2–4), possibly having originated from subclinically infected wild-caught patas monkeys (Erythrocebus patas), green monkeys (Chlorocebus aethiops), or guinea baboons (Papio papio) (5, 6). Much of what is known about SHFV comes from prototype variants LVR 42-0/M6941 and Sukhumi, isolated during the original two outbreaks, and their derivatives (4, 7).
We recently discovered two novel SHFV variants infecting a male red colobus monkey (Procolobus rufomitratus) from Kibale National Park, Uganda (8). These viruses are highly divergent from each other and the prototype variants but, like the prototype variants, contain three unique open reading frames (ORFs) (2a, 2b, and 3) downstream from the replicase-encoding ORFs (8, 9). This genomic architecture may be characteristic of the SHFV taxon.
Here, we report the discovery of two novel SHFV variants in red-tailed guenons (Cercopithecus ascanius) from the same location where we previously reported SHFV in a red colobus (8). In 2010, we sampled 13 Kibale red-tailed guenons as part of a larger study of primate ecology, conservation, and health (10). Animals were anesthetized and samples were collected as previously described (8). Viral RNA was prepared from blood plasma for direct sequencing as previously described (8), with minor modifications for sequencing on an Illumina MiSeq instrument. De novo assembly of sequence reads yielded complete SHFV coding genomes from three individuals (two females, RT05 and RT11, and one male, RT10). A fourth nearly complete SHFV coding genome was recovered from another male individual, RT09, and small gaps were filled by PCR and 3′ rapid amplification of cDNA ends (RACE) followed by Sanger sequencing (8).
Viral genomes were annotated with CLC Genomics Workbench version 5.5 (CLC Bio, Aarhus, Denmark), and putative ORFs were confirmed by querying the NCBI GenBank database (11). Open reading frames were individually aligned with prototype variant LVR 42-0/M6941 and red colobus variants SHFV-krc1 and SHFV-krc2 using a codon-based version of the MAFFT algorithm (12) implemented in Translator X (13). Individual ORF alignments were then concatenated, nucleotide-level similarities of resulting full-length coding genomes were calculated using MEGA5 (14), and sliding-window plots of inferred amino acid similarity were created with SimPlot version 3.5.1 (15). Phylogenetic relationships within the family Arteriviridae were estimated from nucleotide sequences of homologous ORFs of representative full-length coding genomes, aligned as described above but with regions of ambiguous alignment removed using Gblocks (16). Bayesian trees were constructed from concatenated alignments using MrBayes version 3.2.1 (17), with a substitution model of the form GTR+I+Γ selected using jModelTest (Akaike information criterion [AIC], ΔAIC to second-best model GTR+Γ = 71.7) (18), model parameters estimated from the data under default priors, and Markov chains run for one million generations, with the first 25% of sampled trees discarded as burn-in.
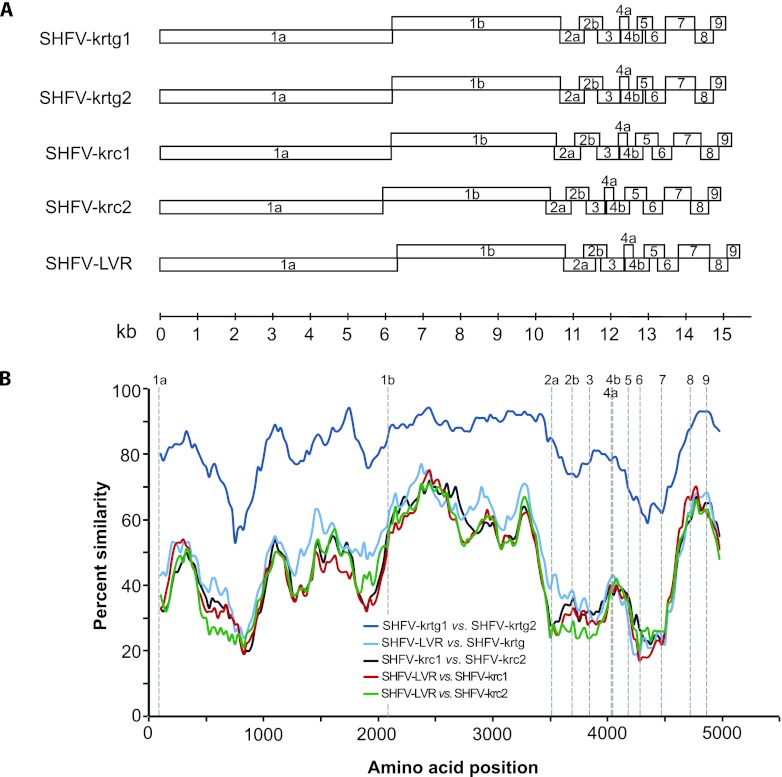
All four new viruses from red-tailed guenons contain two large replicase-encoding ORFs and several smaller downstream ORFs, each similar in size to homologous ORFs of the prototype variants and variants from red colobus (Fig. 1). As in other arteriviruses, ORF1a and ORF1b in the new SHFV variants contain a canonical heptanucleotide “slippery sequence” (UUUAAAC) and predicted downstream pseudoknot structure (19). The new viral genomes contain three ORFs, 2a, 2b, and 3, immediately downstream of the replicase, indicating conservation of this characteristic 3′ genomic architecture among all SHFV variants. Across the aligned coding genomes, the viruses from individuals RT05 and RT11 were similar to each other at the nucleotide level (94.3%), as were the viruses from individuals RT09 and RT10 (93.7%). However, these two variants were only 79.4% similar to each other at the nucleotide level and less similar still to prototype variant LVR 42-0/M6941 (54.1%) or variants from Kibale red colobus (50.1%), with variable amino acid conservation across the genomes (Fig. 1).
Fig 1.
(A) Genome organization of novel simian hemorrhagic fever viruses (SHFV) from Ugandan red-tailed guenons. The novel variants SHFV-krtg1 and SHFV-krtg2 are shown in comparison to the SHFV prototype variant LVR 42-0/M6941 and the recently discovered SHFV-krc1 and SHFV-krc2 variants from sympatric red colobus. Boxes represent open reading frames and are drawn to scale. (B) Sliding window similarity plots of percent amino acid identity among select SHFV variants across aligned coding genomes. The analysis was performed with a window size of 200 and a step size of 25. Dashed vertical lines indicate start positions of inferred viral proteins.
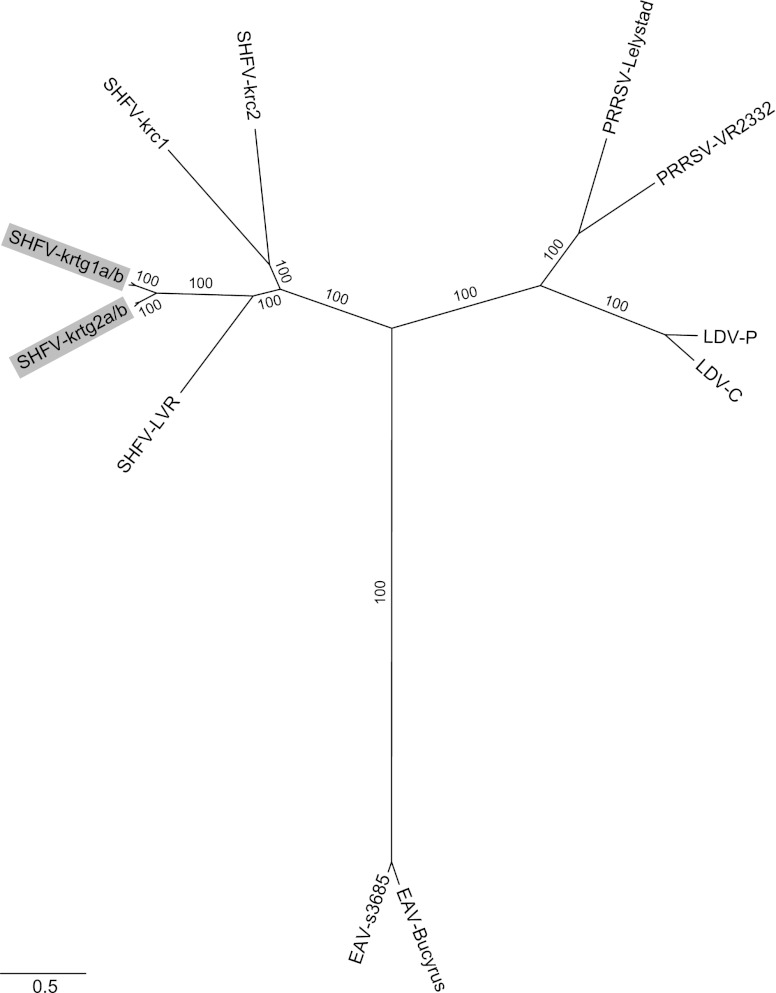
Our Bayesian phylogeny is consistent with established relationships of arteriviruses (8, 20, 21), supporting the monophyly of SHFV variants, the sister taxon relationship of LDV and PRRSV, and the divergence of EAV (Fig. 2). Within the SHFV clade, the new variants are highly divergent from the prototype variant and from SHFV variants found in sympatric red colobus. Based on their phylogenetic positions (Fig. 2), we designate the new viruses SHFV-krtg1 and SHFV-krtg2, to indicate their origins in Kibale red-tailed guenons and to reflect nomenclature previously used to describe simian immunodeficiency virus (SIV) and SHFV variants infecting Kibale red colobus (8, 22).
Fig 2.
Bayesian phylogenetic tree of newly discovered simian hemorrhagic fever viruses (SHFV) from red-tailed guenons and other arteriviruses, based on a 9,891 nucleotide alignment of homologous ORFs (1a, 1b, 2a, 2b, 3, 4, 5, 6, and 7, with reference to the EAV genome). New SHFV variants SHFV-krtg1a and -krtg1b (SHFV-krtg1a/b) (from individuals RT05 and RT11) and SHFV-krtg2a/b (from individuals RT09 and RT10) are highlighted (GenBank accession numbers JX473847 to JX473850). Other viruses represent the known diversity within each viral species based on available full-genome sequences, as follows (GenBank accession numbers in parentheses): SHFV-LVR, the SHFV prototype variant LVR 42-0/M6941 (NC_003092); SHFV-krc1 and SHFV-krc2 from Kibale red colobus (HQ845737 and HQ845738, respectively); PRRSV-Lelystad, the European (type 1) type strain (M96262); PRRSV-VR2332, the North American (type 2) type strain (U87392); EAV-Bucyrus (NC_002532); EAV-s3685 (GQ903794); LDV-P, the Plagemann strain (U15146); and LDV-C, the neurovirulent type C strain (L13298). Posterior clade probabilities are shown on branches; scale bar indicates nucleotide substitutions per site.
In Kibale, red-tailed guenons frequently form multispecies social groups with red colobus, in which occasional direct contact occurs (23, 24). Nevertheless, the phylogenetic divergence between SHFV from red-tailed guenons and SHFV from sympatric red colobus is approximately equivalent to that between PRRSV and LDV, which are currently assigned to different viral species. This observation strongly suggests that virus-host coevolution, rather than geography or ecological overlap, shapes the phylogeny of SHFV. Primates of the subfamilies Cercopithecinae and Colobinae diverged approximately 18 million years ago (25); cocirculating, divergent SHFV variants in both red-tailed guenons and red colobus may indicate ancient diversification of SHFV within sympatric host species or, possibly, more recent admixture of viruses due to transmission from other as-yet-unidentified hosts.
The unique 3′ genomic architecture of SHFV is conserved even across highly divergent SHFV variants. Furthermore, all SHFV variants described to date are monophyletic, even though SHFV ORFs 2a, 2b, and 3 have no homologs in the other arteriviruses and could not be included in our phylogenetic analyses of the Arteriviridae. Currently, the family Arteriviridae includes a single genus, Arterivirus (26). Given the unique and characteristic genomic architecture of all SHFV variants described to date, the monophyly of SHFV within the family Arteriviridae, and the association of SHFV with simian hosts, we suggest the reclassification of SHFV into a new genus: Simartevirus. The discovery of additional arteriviruses will help clarify the appropriateness of this proposed taxonomy, as well as any other taxonomic subdivisions within the family Arteriviridae that may be justified.
ACKNOWLEDGMENTS
We gratefully acknowledge the Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and Makerere University Biological Field Station for granting permission to conduct this research, J. Byaruhanga, P. Katurama, A. Nyamwija, J. Rusoke, A. Mbabazi, and P. Omeja for assistance in the field, and L. Kilby for assistance with permitting and logistics.
This work was funded by NIH grant TW009237 as part of the joint NIH-NSF Ecology of Infectious Disease program and the UK Economic and Social Research Council, and by the University of Wisconsin School of Medicine and Public Health from The Wisconsin Partnership Program through the Wisconsin Center for Infectious Disease (WisCID). J.H.K. performed this work as an employee of Tunnell Consulting, Inc., a subcontractor to Battelle Memorial Institute under its prime contract with NIAID, under contract no. HHSN272200200016I.
The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services or of the institutions and companies affiliated with the authors. Use of trade names is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 17 October 2012
REFERENCES
- 1. Cavanagh D, Brian DA, Brinton MA, Enjuanes L, Holmes KV, Horzinek MC, Lai MMC, Laude H, Plagemann PGW, Siddell SG, Spaan WJM, Taguchi F, Talbot PJ. 1994. Revision of the taxonomy of the Coronavirus, Torovirus, and Arterivirus genera. Arch. Virol. 135:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmer AE, Allen AM, Tauraso NM, Shelokov A. 1968. Simian hemorrhagic fever. I. Clinical and epizootiologic aspects of an outbreak among quarantined monkeys. Am. J. Trop. Med. Hyg. 17:404–412 [PubMed] [Google Scholar]
- 3. Ševcova ZV. 1967. Studies on the etiology of hemorrhagic fever in monkeys. Vopr. Virusol. 12:47–51 (In Russian.) [PubMed] [Google Scholar]
- 4. Tauraso NM, Shelokov A, Palmer AE, Allen AM. 1968. Simian hemorrhagic fever. III. Isolation and characterization of a viral agent. Am. J. Trop. Med. Hyg. 17:422–431 [PubMed] [Google Scholar]
- 5. Gravell M, London WT, Leon ME, Palmer AE, Hamilton RS. 1986. Differences among isolates of simian hemorrhagic fever (SHF) virus. Proc. Soc. Exp. Biol. Med. 181:112–119 [DOI] [PubMed] [Google Scholar]
- 6. London WT. 1977. Epizootiology, transmission and approach to prevention of fatal simian haemorrhagic fever in rhesus monkeys. Nature 268:344–345 [DOI] [PubMed] [Google Scholar]
- 7. Lapin BA, Shevtsova ZV. 1971. On the identity of two simian hemorrhagic fever virus strains (Sukhumi and NIH). Z. Versuchstierkd. 13:21–23 [PubMed] [Google Scholar]
- 8. Lauck M, Hyeroba D, Tumukunde A, Weny G, Lank SM, Chapman CA, O'Connor DH, Friedrich TC, Goldberg TL. 2011. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS One 6:e19056 doi:10.1371/journal.pone.0019056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Godeny EK, de Vries AA, Wang XC, Smith SL, de Groot RJ. 1998. Identification of the leader-body junctions for the viral subgenomic mRNAs and organization of the simian hemorrhagic fever virus genome: evidence for gene duplication during arterivirus evolution. J. Virol. 72:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldberg TL, Paige SB, Chapman CA. 2012. The Kibale EcoHealth Project: exploring connections among human health, animal health, and landscape dynamics in western Uganda, p 452–465 In Aguirre AA, Daszak P, Ostfeld RS. (ed), New directions in conservation medicine: applied cases of ecological health. Oxford University Press, New York, NY [Google Scholar]
- 11. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 12. Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30:3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abascal F, Zardoya R, Telford MJ. 2010. TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 38(Suppl):W7–W13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540–552 [DOI] [PubMed] [Google Scholar]
- 17. Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- 18. Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25:1253–1256 [DOI] [PubMed] [Google Scholar]
- 19. Snijder EJ, Meulenberg JJ. 1998. The molecular biology of arteriviruses. J. Gen. Virol. 79(Part 5):961–979 [DOI] [PubMed] [Google Scholar]
- 20. Godeny EK, Chen L, Kumar SN, Methven SL, Koonin EV, Brinton MA. 1993. Complete genomic sequence and phylogenetic analysis of the lactate dehydrogenase-elevating virus (LDV). Virology 194:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanada K, Suzuki Y, Nakane T, Hirose O, Gojobori T. 2005. The origin and evolution of porcine reproductive and respiratory syndrome viruses. Mol. Biol. Evol. 22:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldberg TL, Sintasath DM, Chapman CA, Cameron KM, Karesh WB, Tang S, Wolfe ND, Rwego IB, Ting N, Switzer WM. 2009. Coinfection of Ugandan red colobus (Procolobus [Piliocolobus] rufomitratus tephrosceles) with novel, divergent delta-, lenti-, and spumaretroviruses. J. Virol. 83:11318–11329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapman CA, Chapman LJ. 2000. Interdemic variation in mixed-species association patterns: common diurnal primates of Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 47:129–139 [Google Scholar]
- 24. Struhsaker TT. 1981. Polyspecific associations among tropical rain-forest primates. J. Comp. Ethol. 57:268–304 [Google Scholar]
- 25. Perelman P, Johnson WE, Roos C, Seuanez HN, Horvath JE, Moreira MA, Kessing B, Pontius J, Roelke M, Rumpler Y, Schneider MP, Silva A, O'Brien SJ, Pecon-Slattery J. 2011. A molecular phylogeny of living primates. PLoS Genet. 7:e1001342 doi:10.1371/journal.pgen.1001342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faaberg KS, Balasuriya UB, Brinton MA, Gorbalenya AE, Leung ECC, Nauwynck H, Snijder EJ, Stadejek T, Yang H, Yoo D. 2011. Family Arteriviridae, p 796–805 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus Taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]