Abstract
We have recently isolated a rhesus macaque cytotoxic T cell line, 2N5.1, that specifically recognizes an N-myristoylated 5-mer peptide (C14-Gly-Gly-Ala-Ile-Ser [C14nef5]) derived from the simian immunodeficiency virus (SIV) Nef protein. Such C14nef5-specific T cells expand in the circulation of SIV-infected monkeys, underscoring the capacity of T cells to recognize viral lipopeptides; however, the molecular basis for the lipopeptide antigen presentation remains to be elucidated. Here, functional studies indicated that the putative antigen-presenting molecule for 2N5.1 was likely to have two separate antigen-binding sites, one for interaction with a C14-saturated acyl chain and the other for anchorage of the C-terminal serine residue. Mutants with alanine substitutions for the second glycine residue and the fourth isoleucine residue were not recognized by 2N5.1 but interfered with the presentation of C14nef5 to 2N5.1, indicating that these structural analogues retained the ability to interact with the antigen-presenting molecules. In contrast to the highly specific recognition of C14nef5 by 2N5.1, an additional cytotoxic T cell line, SN45, established independently from a C14nef5-stimulated T cell culture, showed superb reactivity to both C14nef5 and an N-myristoylated Nef 4-mer peptide, and therefore, the C-terminal serine residue was dispensable for the recognition of lipopeptides by the SN45 T cells. Furthermore, the mutants with alanine substitutions were indeed recognized by the SN45 T cells. Given that N-myristoylation of the Nef protein occurs in the conserved motifs and is critical for viral pathogenesis, these observations predict that the lipopeptide-specific T cell response is difficult for viruses to avoid by simply introducing amino acid mutations.
INTRODUCTION
Modern immunology has established a central paradigm for antigen (Ag) presentation that major histocompatibility complex (MHC) class I and class II molecules bind peptide Ags and present them to CD8+ and CD4+ T cells, respectively (1). The important role of MHC-restricted T cells in various aspects of acquired immunity has been noted, and effective protein vaccines have been developed to control many infectious diseases. Subsequently, the repertoire of Ags recognized by T cells has been expanded to include not only proteins, but also lipidic molecules. Human group 1 CD1 molecules (CD1a, -b, and -c) are capable of binding glycolipids and presenting them to T cells. Such glycolipid-specific group 1 CD1-restricted T cells have been shown to expand significantly in response to mycobacterial infections, and a role for them in controlling intracellular microbes has been suggested (2–5).
Unlike bacteria, viruses do not possess their own lipids, and thus, lipid-specific adaptive immunity may not function efficiently against viral infections. However, viruses can indeed biosynthesize their own lipopeptides by utilizing the host cellular machinery. Human (HIV) and simian (SIV) immunodeficiency viruses borrow the host-derived N-myristoyl-transferase and its substrate, myristoyl-coenzyme A (CoA), for coupling a saturated C14 fatty acid (myristic acid) to the N-terminal glycine residue of the Nef protein (6). This lipidation reaction, referred to as N-myristoylation, is a key modification for anchoring the Nef protein to the plasma membrane, thereby assisting its immunosuppressive activity (7). Interestingly, our previous study indicated that the host-acquired immunity was equipped with cytotoxic T cells capable of monitoring the N-myristoylation of the Nef protein (8). A rhesus macaque CD8+ T cell line, 2N5.1, specifically recognized an N-myristoylated, but not unmodified, 5-mer peptide of the SIV Nef protein. Furthermore, the number of N-myristoylated Nef peptide-specific T cells was increased significantly in the circulation of SIV-infected monkeys, and the plasma viral load in infected monkeys was found to correlate reciprocally with the number of lipopeptide-specific T cells (8). Taken together, these results point to an intriguing possibility that, in addition to peptides and lipids, viral lipopeptides may comprise a new repertoire of Ags recognized by host T cells.
To gain insight into the molecular basis for lipopeptide Ag presentation, we established an additional CD8+ T cell line, SN45, independent of 2N5.1, that recognized the same N-myristoylated 5-mer peptide. A comparative study of the two T cell lines detected different molecular patterns for the recognition of lipopeptide Ags. Strikingly, the mutant with a C-terminal serine deletion and the mutants with alanine substitutions of the N-myristoylated 5-mer peptide were recognized by the SN45 T cells, suggesting that pathogenic viruses may find difficulties in escaping from the lipopeptide-specific T cell responses by simply introducing amino acid mutations.
MATERIALS AND METHODS
Synthesis of lipopeptide Ags.
Chemical reagents were purchased from Nacalai Tesque (Kyoto, Japan) unless otherwise indicated. The lipopeptide Ags listed in Fig. 1 were synthesized as described previously (8). Briefly, peptides were synthesized by a manual 9-fluorenylmethoxy carbonyl (Fmoc) solid-phase peptide synthesis technique using Wang resin precoupled with a relevant C-terminal amino acid (EMD Chemicals, Gibbstown, NJ). Acylation was carried out by reacting the N-terminal amino acid group with acid anhydrides prepared with N,N′-diisopropylcarbodiimide, followed by the release of the acylated peptides in 95% trifluoroacetic acid. Purification of the crude samples was performed by high-performance liquid chromatography (HPLC) with a gradient elution based on water and methanol with 0.1% trifluoroacetic acid. After freeze-drying, the purified samples were subjected to liquid chromatography (LC)-mass spectrometry, using a C18 column (GL Sciences, Torrance, CA) with a solvent system of water and methanol with 0.1% formic acid. The observed m/z of the [M + H]+ for each purified sample was consistent with the monoisotopic mass (Fig. 1), thus confirming the identity of the synthesized lipopeptides.
Fig 1.
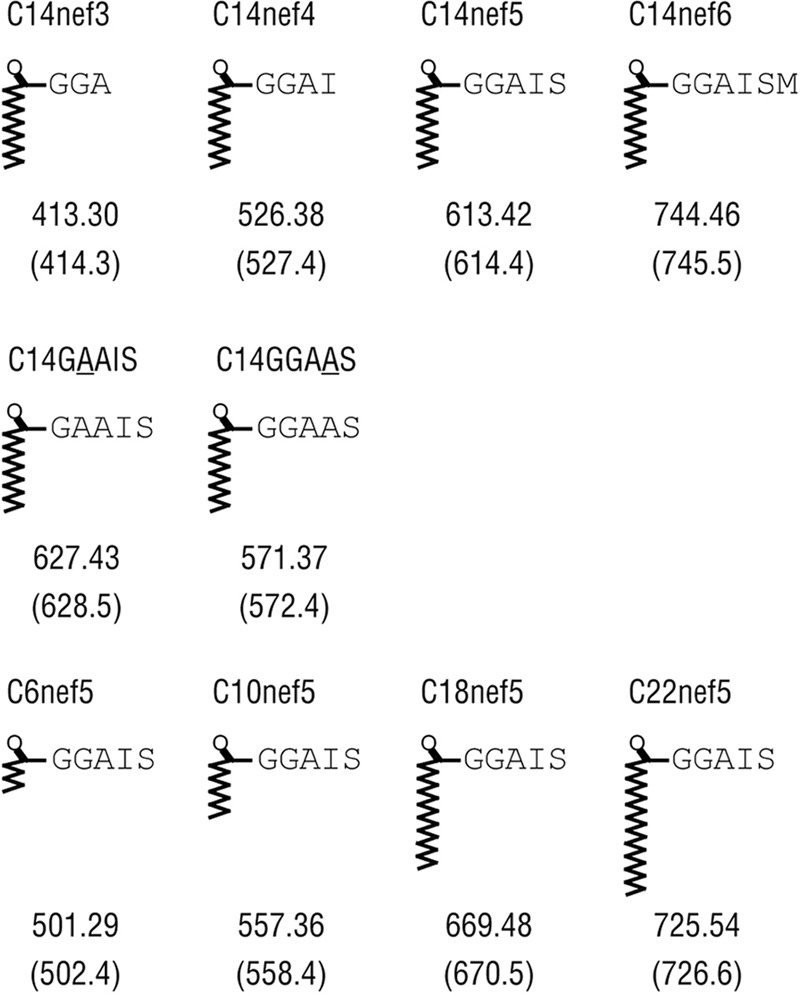
Synthetic lipopeptides used in this study. The names and the chemical structures are shown. The monoisotopic mass of each compound, as well as the observed m/z of the [M + H]+ (in parentheses), are also shown.
Establishment of lipopeptide-specific rhesus macaque T cell lines and flow cytometric analysis.
The C14-Gly-Gly-Ala-Ile-Ser (C14nef5)-specific T cell line 2N5.1 was described previously (8). Another C14nef5-specific T cell line, SN45, was obtained independently from a SIV-infected monkey (MM521). Peripheral blood mononuclear cells (PBMCs) (1.2 × 107/well) were cultured with C14nef5 at a concentration of 5 μg/ml, and antigenic stimulation was repeated every 2 weeks in the presence of irradiated autologous PBMCs. Interleukin 2 (IL-2) was added at 0.3 nM after the second stimulation, and the concentration was gradually increased to 3 nM by the fourth stimulation. RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (HyClone, Logan, UT), 2-mercaptoethanol (Invitrogen), penicillin, and streptomycin was used for T cell culture. The expression of T cell markers on the T cell line was analyzed by flow cytometry, as described previously (8).
T cell assays.
T cells (5 × 104/well) were incubated with each synthetic lipopeptide (5 μg/ml) in the presence of irradiated autologous or allogeneic PBMCs (3 × 105/well), using 96-well flat-bottom microtiter plates. In some experiments, irradiated allogeneic PBMCs (2 × 105/well) were preincubated for 30 min with test competitors (0.5 μg/ml or 5 μg/ml), and then, responder T cells (5 × 104/well) and C14nef5 (50 ng/ml) were added. After 24 h, aliquots of the culture supernatants were collected, and the amount of either gamma interferon (IFN-γ) or granulocyte-macrophage colony-stimulating factor (GM-CSF) released into the medium was measured using Mabtech ELISA kits (Nacka Strand, Sweden). To examine if the T cell response might be mediated by MHC or CD1 molecules, PBMCs (3 × 105/well) were incubated with saturating amounts (5 μg/ml) of monoclonal antibodies (Abs) to CD1a (10H3), CD1b (b3.1), CD1c (M241), MHC class I (W6/32), and MHC class II (L243) or negative-control Ab (P3) for 20 min before the addition of responder T cells (5 × 104/well) and the C14nef5 Ag (5 μg/ml). Alternatively, the LLC-MK2 rhesus macaque kidney epithelial cell line was transiently transfected with rhesus macaque group 1 CD1 genes (CD1A, CD1B, and CD1C) (9) or MM521-derived MHC class I genes (Mamu-A1*02, Mamu-A1*110, and Mamu-B*56) and used as Ag-presenting cells (2.5 × 104/well) in the T cell assays described above.
TCR cloning.
T-cell receptor (TCR) cloning was performed by the inverse-PCR method (10, 11). Briefly, total RNA was extracted from 1 × 106 T cells, and oligo(dT)-primed double-stranded cDNA was synthesized from 0.25 μg of the total RNA using PrimeScript reverse transcriptase (TaKaRa Bio, Inc., Otsu, Japan), RNase H (New England BioLabs, Inc., Ipswich, MA), Escherichia coli DNA polymerase I (New England BioLabs, Inc.), and E. coli DNA ligase (New England BioLabs, Inc.), followed by treatment with T4 DNA polymerase (New England BioLabs, Inc.) for blunt-end formation. The blunt-ended DNA was then circularized with T4 DNA ligase (New England BioLabs, Inc.) and used as a template for inverse PCR with a pair of Cα- or Cβ-specific primers oriented in opposite directions. The primers used were as follows: TCRα forward, 5′-GGG TCG ACG ACC TCA TGT CTA GCA CAG T-3′; TCRα reverse, 5′-GCA TGC GGC CGC CCT GCT ATG CTG TGT ATC-3′; TCRβ forward, 5′-GGG TCG ACA CAG CGA CCT TGG GTG GG-3′; TCRβ reverse, 5′-GCA TGC GGC CGC GGT CAA GAG AAA GGA TTC-3′. The amplified TCR genes were cloned into pBlueScript II (Stratagene, La Jolla, CA). More than 10 clones were sequenced, using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA).
Animals.
The rhesus macaques (Macaca mulatta) used in this study were treated humanely in accordance with institutional regulations, and the experimental protocols were approved by the Committee for Experimental Use of Non-Human Primates at the Institute for Virus Research, Kyoto University. For infection, SIVmac239 (12) was inoculated intravenously at a dose of 2,000 50% tissue culture-infective doses (TCID50).
Nucleotide sequence accession numbers.
Sequences were deposited in the DDBJ/GenBank/EMBL databases under the following accession numbers: AB701289 (2N5.1 α chain), AB701290 (2N5.1 β chain), AB701291 (SN45 α chain), and AB701292 (SN45 β chain).
RESULTS
Establishment of an additional C14nef-specific T cell line, SN45.
We had previously isolated a rhesus monkey T cell line, 2N5.1, that specifically recognized the N-myristoylated 5-mer lipopeptide (C14nef5) derived from the SIV Nef protein (8). Another C14nef5-specific T cell line, termed SN45, was obtained independently by repeated stimulation of rhesus macaque PBMCs with C14nef5. As for 2N5.1 (8), the SN45 T cells were CD4− and CD8α+ (Fig. 2A), and produced IFN-γ in response to C14nef5, but no response was observed when myristic acid and the 5-mer peptide were added as a free form (Fig. 2B). Therefore, the SN45 T cells specifically recognized the 5-mer peptide that was conjugated covalently with myristic acid.
Fig 2.
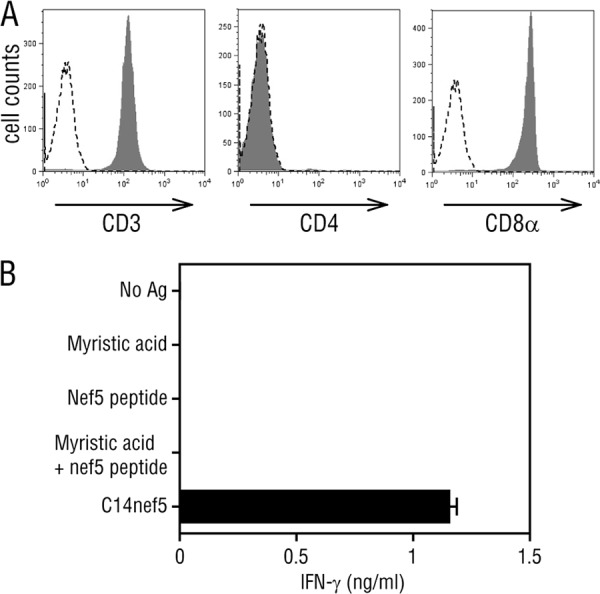
Specific recognition of C14nef5 by SN45. (A) The surface expression of T cell markers was analyzed for SN45 by flow cytometry (filled histograms). A dashed line in each panel indicates a negative-control histogram. (B) SN45 T cells (5 × 104/well) were stimulated with the indicated Ags (5 μg/ml) in the presence of irradiated autologous PBMCs (3 × 105/well), and the amount of IFN-γ released into the culture medium was measured. Assays were performed in triplicate, and mean values and standard deviations (SD) are shown.
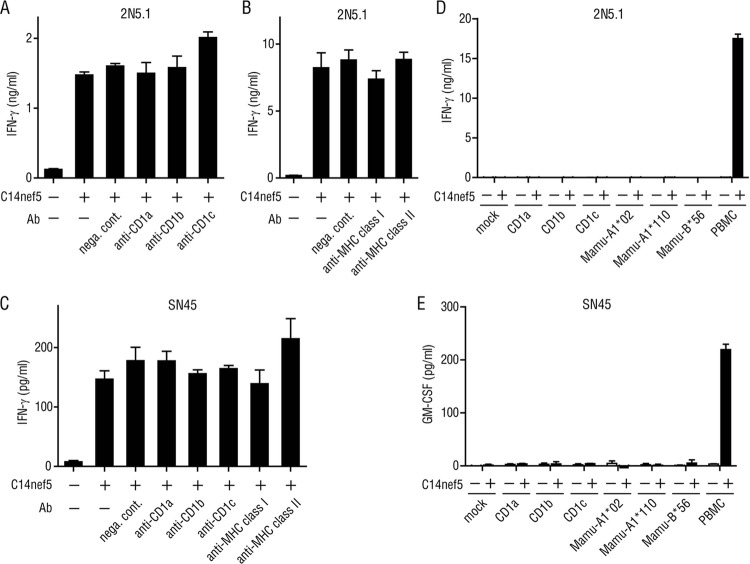
The TCR usage of 2N5.1 and SN45 was determined by inverse PCR, in which the TCR genes were randomly cloned and sequenced. For both T cell lines, a single pair of TCR α and β chains was detected, suggesting that the cell lines were clonal. Both T cell lines expressed distinct Vα and Vβ families and exhibited clonotypic variations in the junctional region, except for the SN45 TCRα chain, which was germ line encoded without N additions (Table 1). Therefore, the recognition of the C14nef5 lipopeptide by 2N5.1 is unlikely to be mediated by invariant-type TCRs. It remains to be addressed whether SN45 may represent a new subset of semi-invariant TCR-expressing cells, but obviously, the cells are distinct from the CD1d-restricted, Vα24+ natural killer (NK) T cells (13). Ab-blocking experiments suggested that none of the classical MHC molecules and group 1 CD1 molecules could mediate the lipopeptide Ag presentation to 2N5.1 (Fig. 3A and B) and SN45 (Fig. 3C). Furthermore, rhesus macaque cell transfectants expressing rhesus macaque group 1 CD1 molecules (CD1a, CD1b, and CD1c) and those expressing MM521-derived MHC class I molecules (Mamu-A1*02, Mamu-A1*110, and Mamu-B*56) failed to present C14nef5 to the T cell lines (Fig. 3D and E). Therefore, the molecular identity of the Ag-presenting molecules for the C14nef5 lipopeptide has not yet been determined, but as shown below, functional studies predicted that two discernible molecules exist in rhesus macaques that are capable of presenting N-myristoylated peptides to T cells.
Table 1.
TCR usage of 2N5.1 and SN45
| T cell line | Genes | Sequencea |
||||
|---|---|---|---|---|---|---|
| Vα | Vβ | Junction | Jα | Jβ | ||
| TCRα | ||||||
| 2N5.1 | TRAV35–TRAJ54 | GTYFCAG | QNW | GAQKLVFG | ||
| SN45 | TRAV4–TRAJ6 | VYYCLVG | GGGYVLTFG | |||
| TCRβ | ||||||
| 2N5.1 | TRBV27–TRBJ27 | YLCASSY | SGQA | YEQYFGP | ||
| SN45 | TRBV3–TRBJ27 | YFCASSQ | DLGAGEV | YEQYFGP | ||
The TCR usage of the two T cell lines was determined by inverse PCR, and the deduced amino acid sequences of the junctional regions are shown.
Fig 3.
Involvement of MHC and CD1 molecules in the recognition of C14nef5 by 2N5.1 and SN45. Autologous PBMCs (3 × 105/well) were preincubated with a saturating amount (5 μg/ml) of the indicated Abs and cultured with 2N5.1 (A and B) and SN45 (C) T cells (5 × 104/well) in the presence (+) or absence (−) of C14nef5 (5 μg/ml). Note that these Abs were used to block relevant human T cell responses specifically and are known to recognize the corresponding monkey molecules efficiently (9, 23–26). nega. cont., negative control. (D and E) The LLC-MK2 rhesus macaque cell line was transiently transfected by lipofection with rhesus macaque CD1 genes or MM521-derived MHC class I genes (Mamu-A1*02, Mamu-A1*110, and Mamu-B*56) and tested for the ability to present C14nef5 to 2N5.1 (D) and SN45 (E). The transfection efficiency was approximately 50%, as determined by flow cytometric analysis of CD1-transfected cells labeled with relevant anti-CD1 Abs. The error bars indicate standard deviation (SD).
2N5.1, but not SN45, was stimulated by all the donors tested.
While the presentation of peptide Ags to T cells is mediated by highly polymorphic MHC molecules, the activation of glycolipid-specific T cells depends on non-MHC-encoded molecules of the CD1 family that are virtually monomorphic. To gain insight into the yet unidentified Ag-presenting molecules for the C14nef5 lipopeptide, we wished to determine if the restriction elements for the two lipopeptide-specific T cell lines might be shared functionally among individuals. Allogeneic PBMCs derived from all 9 donor rhesus macaques tested could present the C14nef5 lipopeptide Ag to 2N5.1 (Fig. 4A). In sharp contrast, only a single donor (MM460), and not the other 2 donors (MM450 and MM499), was capable of presenting C14nef5 to SN45 (Fig. 4B). The superb capacity of MM450- and MM499-derived PBMCs to present Ag to T cells was confirmed by demonstrating that the two donors were able to present C14nef5 to 2N5.1 (Fig. 4A). Studies of 8 additional monkeys revealed that two donors (MM1774 and MM1795), but not the other 6, could present the Ag to SN45 (Fig. 4C), indicating that the capacity to activate SN45 was not shared broadly among the subjects. Thus, these results suggested that the Ag-presenting molecules for 2N5.1 and SN45 were different.
Fig 4.
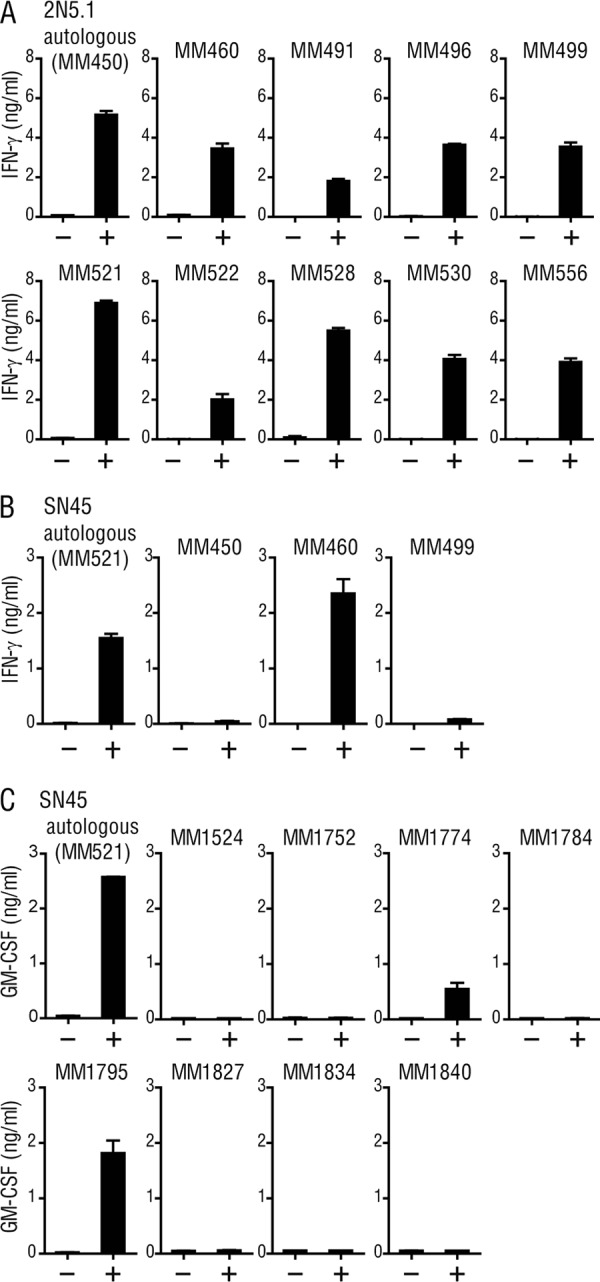
Responses of 2N5.1 and SN45 to C14nef5 in the presence of either autologous or allogeneic PBMCs. MM450-derived 2N5.1 T cells (A) and MM521-derived SN45 T cells (B) were stimulated with C14nef5 (+) or unstimulated (−) in the presence of irradiated autologous or allogeneic PBMCs. The amount of IFN-γ released into the medium was measured as for Fig. 2B. (C) For SN45, additional studies were performed with 8 allogeneic donors, and the amount of GM-CSF released into the medium was measured. The error bars indicate SD.
Distinct patterns of Ag recognition by 2N5.1 and SN45.
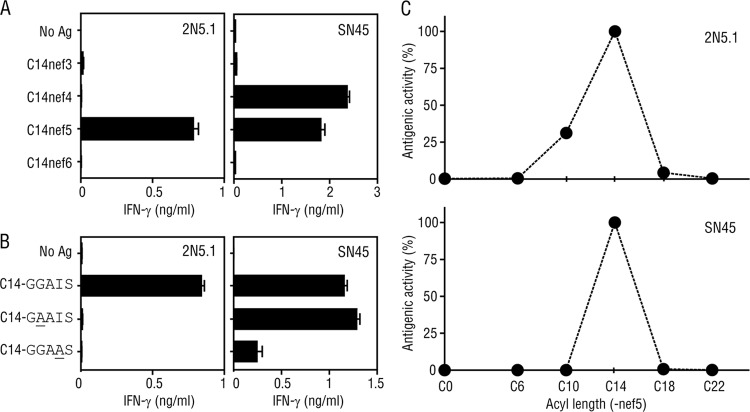
We then compared the two C14nef5-specific T cell lines in terms of their abilities to recognize an array of related compounds. We first examined whether the T cell lines might differentially recognize Ags with altered peptide lengths and amino acid compositions. Both cell lines were obtained by repeated stimulation with C14nef5 in an in vitro culture, and the 2N5.1 T cells faithfully recognized C14nef5, but not N-myristoylated 3-mer (C14nef3; C14-GGA), 4-mer (C14nef4; C14-GGAI), and 6-mer (C14nef6; C14-GGAISM) peptides of the Nef protein (Fig. 5A, left) (8). Furthermore, an alanine substitution (underlined) for either the second glycine residue (C14-GAAIS) or the isoleucine residue (C14-GGAAS) of C14nef5 resulted in total abrogation of the antigenic activity (Fig. 5B, left) (8). In sharp contrast, the SN45 T cells recognized C14nef4, as well as C14nef5 (Fig. 5A, right), and were capable of reacting to the mutated Ags, albeit less efficiently to C14-GGAAS (Fig. 5B, right).
Fig 5.
Responses of 2N5.1 and SN45 to an array of synthetic lipopeptides. (A) 2N5.1 (left) and SN45 (right) T cells were stimulated with either the N-myristoylated Nef 3-mer (C14nef3), 4-mer (C14nef4), 5-mer (C14nef5), or 6-mer (C14nef6) peptide, and the IFN-γ response of the T cells was measured. (B) T cells were stimulated with C14nef5 (C14-GGAIS) or each of the mutants with alanine substitutions (C14-GAAIS and C14-GGAAS), and the IFN-γ response of the T cells was measured. The error bars indicate SD. (C) 2N5.1 (top) and SN45 (bottom) T cells were stimulated with a Nef 5-mer peptide that was either unconjugated (C0) or conjugated with a saturated C6, C10, C14, C18, or C22 fatty acid, and the IFN-γ response of the T cells was assessed. The ratio of each response to the response to C14nef5 is shown as the antigenic activity.
We next addressed whether the length of the acyl chain impacts the efficiency of T cell activation. Pentamer Nef peptides conjugated with either shorter saturated fatty acids (C6nef5 and C10nef5) or longer saturated fatty acids (C18nef5 and C22nef5) were synthesized and tested for the ability to stimulate the T cell lines. As shown in Fig. 5C, both 2N5.1 (top) and SN45 (bottom) exhibited the highest reactivity to the authentic Ag with a saturated C14 fatty acid. It was also noted that, whereas SN45 failed to respond to any of the altered Ags tested (Fig. 5C, bottom), 2N5.1 showed moderate reactivity to C10nef5 (Fig. 5C, top).
A role for the C14nef5 serine residue in lipopeptide Ag presentation.
SN45 recognized both C14nef4 and C14nef5 (Fig. 5A), suggesting that the C-terminal serine residue of C14nef5 was dispensable for the activation of the T cells. On the other hand, 2N5.1 recognized C14nef5, but not C14nef4 (Fig. 5A), which pointed to a critical role for the serine residue either as an anchoring residue, as part of the T cell epitope, or both. We favored the hypothesis that the serine residue of C14nef5 functions as an anchoring residue because it was shared among many N-myristoylated proteins. We reasoned that if this was the case, even an excess amount of C14nef4 could not replace C14nef5 at the Ag-binding site. To address this directly, Ag-presenting cells were preincubated with excess amounts of C14nef4, and then the 2N5.1 T cells and the authentic C14nef5 Ag were added to the culture. As predicted, C14nef4 (C14-GGAI) failed to interfere with the 2N5.1 T cell response to C14nef5 (Fig. 6A). In sharp contrast, preincubation with excess amounts of the mutants with alanine substitutions (underlined) (C14-GAAIS and C14-GGAAS) resulted in dose-dependent inhibition of the 2N5.1 cell response to C14nef5 (Fig. 6B and C, respectively). Therefore, the C-terminal serine residue played a critical role in the activation of 2N5.1 cells and likely mediated an anchoring function. Furthermore, we found that excess amounts of the 5-mer peptide with a short acyl chain (C6-GGAIS) failed to block the response of 2N5.1 and SN45 T cells to C14nef5 (Fig. 6D and data not shown). Taken together, these results, obtained from inhibition experiments with an array of blockers, indicated that, whereas the attached myristic acid was important for the activation of both T cell lines, the C-terminal serine residue played a different role.
Fig 6.
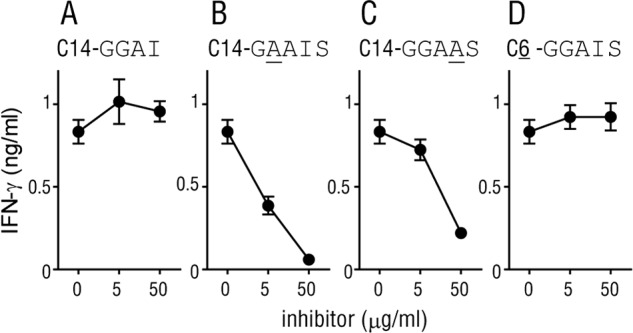
Inhibition of the response of 2N5.1 to C14nef5 by competitors. Irradiated autologous PBMCs were preincubated with excess amounts of the indicated blockers, for which mutated residues are underlined, and then the 2N5.1 responder cells and the C14nef5 Ag were added to the culture as described in Materials and Methods. After 24 h, the culture supernatants were collected, and the amount of IFN-γ released into the medium was measured. The error bars indicate SD.
DISCUSSION
The analysis of the two CD8+ T cell lines, 2N5.1 and SN45, that recognized the same lipopeptide Ag, C14nef5, revealed their shared and unshared properties, allowing us to grasp the molecular basis for lipopeptide Ag presentation and T cell activation (Fig. 7). One of the most remarkable similarities is that the optimal length of the attached acyl chain is C14 (Fig. 5C). Both T cell lines failed to recognize Ags with a longer saturated acyl chain (C18nef5 and C22nef5), suggesting that the putative Ag-presenting molecules, tentatively termed LP1 for 2N5.1 and LP2 for SN45, may form a hydrophobic pocket with a depth suitable for accommodating the attached myristic acid. It should also be noted that the Ag with a C10 acyl chain (C10nef5) was able to stimulate 2N5.1, but not SN45 (Fig. 5C), pointing to the possibility that the reduced hydrophobic interaction of the short acyl chain with the LP1 Ag-presenting molecules might be compensated for by an additional interaction, such as that mediated by an anchoring amino acid residue of the peptide, as discussed below. Such additional modes of interaction may be absent or only weak for LP2, resulting in a failure of SN45 to recognize C10nef5.
Fig 7.
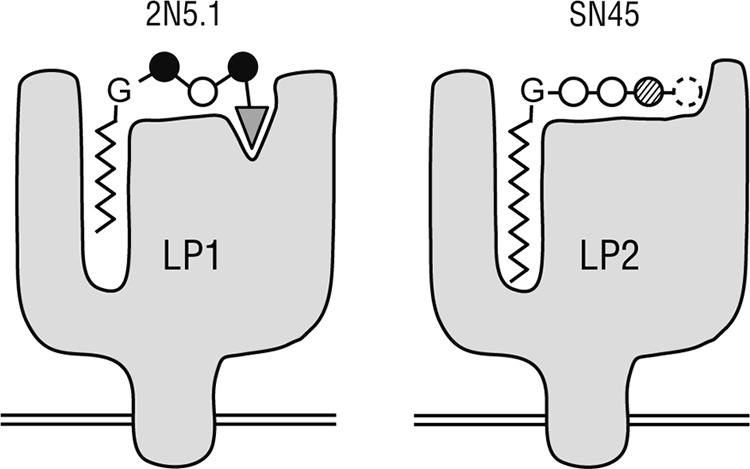
Schematic model of the putative Ag-presenting molecules for 2N5.1 (LP1) and SN45 (LP2). LP1 (left) has two separate Ag-binding sites, one for the acyl chain and the other for the anchoring serine residue (triangle) of C14nef5. The second (glycine) and fourth (isoleucine) residues (solid circles) are positioned outward for preferential interaction with the 2N5.1 TCR. Note that even the Nef 5-mer peptide with a saturated C10 acyl chain (C10nef5) can bind to LP1 for recognition by 2N5.1, although its affinity for LP1 is lower than that of C14nef5. LP2 (right) also has an acyl-chain-binding pocket, but the serine residue critical for binding to LP1 is dispensable while the isoleucine residue (dashed circle) is essential. The myristic acid only fits into the hydrophobic pocket of LP2, and Ags with a longer or shorter acyl chain fail to be presented to the SN45 cells.
The present study also detected substantial differences in Ag recognition between the two cell lines. The C-terminal serine residue of C14nef5, a crucial element for the N-myristoylation motif (6), was an absolute requirement for recognition by 2N5.1, as the T cell line recognized C14nef5, but not C14nef4 with a C-terminal serine deletion (Fig. 5A). The excess amount of C14nef4 failed to inhibit the 2N5.1 reactivity to C14nef5, favoring the idea that this residue functions as an anchor for the stable binding of LP1 (Fig. 7, left, triangle). As discussed above, this interaction may play a significant role in capturing C10nef5, which has only weak affinity for the acyl-chain-accommodating pocket. Furthermore, the mutants with alanine substitutions (underlined) (C14-GAAIS and C14-GGAAS) were not recognized by 2N5.1 but were able to inhibit its recognition of C14nef5, indicating that the mutants indeed bound to LP1. Therefore, it would be reasonable to predict that the second glycine and the isoleucine residues (Fig. 7, left, solid circles) would be positioned preferentially for T cell recognition.
The putative Ag-presenting molecule, LP2, which mediates Ag presentation to SN45, appears much less stringent in terms of Ag binding. The C14nef4 Ag lacking the C-terminal serine residue could be recognized by SN45 as efficiently as or even more efficiently than C14nef5 (Fig. 5A), immediately excluding the anchoring model proposed for LP1 (Fig. 7, right, dashed circle). As C14nef3 failed to stimulate SN45, the fourth amino acid residue, isoleucine, would be particularly important for either binding to LP2, T cell recognition, or both. As described above, Ag binding to LP2 is likely to depend heavily on the full range of C14 acyl chain interactions with the hydrophobic pocket.
Our study indicates that the T cell Ag repertoire of N-myristoylated peptides includes those with 4-mer and 5-mer peptides. Typically, N-myristoylation occurs for proteins with the N-terminal motif Gly-X-X-X-Ser/Thr (in which X is any amino acid) (6), and thus, the Ag diversity that can be generated as a result of amino acid alterations would be greatly limited compared with the 8- to 10-mer peptides presented by polymorphic MHC class I molecules. This indicates that, although introducing amino acid mutations in the target proteins is an efficient strategy that pathogenic viruses, such as HIV, have evolved to escape from cytotoxic T cell attack, the short stretch of the N-terminal amino acid residues of the Nef protein that contains N-myristoylation signal is hard to mutate without affecting the function of the protein. This also points to the possibility that the discrimination of foreign lipopeptides from self by the immune system may not be safely and strictly enforced. Viral infections are often associated with or followed by manifestations of autoimmune disorders (14–18), which could possibly be accounted for by the development of viral lipopeptide-specific T cells that may cross-react with self lipopeptides. On the other hand, products of a fraction of oncogenes, such as c-src, are N-myristoylated to function, and previous studies detected highly upregulated expression of N-myristoyl-transferase in cancer cells (19–22). Therefore, the aberrant or dysregulated expression of N-myristoylated cancer-associated proteins may result in activation of lipopeptide-specific T cells capable of recognizing abnormal cells derived from the self. The present study can potentially shed light on a new aspect of viral immunity, cancer immunity, and autoimmunity that has never been appreciated previously.
ACKNOWLEDGMENTS
We thank Mariko Horiike for assistance in performing this study.
This work was supported by grants from the Japan Society for the Promotion of Science (grant numbers 22659188 and 24659481) and from the Senshin Medical Research Foundation (to M.S.). It was also supported by the Cooperation Research Program of the Primate Research Institute, Kyoto University. D.M. is a Research Fellow of the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Germain RN, Margulies DH. 1993. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 11:403–450 [DOI] [PubMed] [Google Scholar]
- 2. Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, Leon L, Brenner M, Wilson IA, Altman JD, Moody DB. 2011. CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J. Exp. Med. 208:1741–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komori T, Nakamura T, Matsunaga I, Morita D, Hattori Y, Kuwata H, Fujiwara N, Hiromatsu K, Harashima H, Sugita M. 2011. A microbial glycolipid functions as a new class of target antigen for delayed-type hypersensitivity. J. Biol. Chem. 286:16800–16806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, McMurray DN, LeClair KP, Porcelli SA, Brenner MB. 2003. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int. Immunol. 15:915–925 [DOI] [PubMed] [Google Scholar]
- 5. Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, Porcelli SA. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404:884–888 [DOI] [PubMed] [Google Scholar]
- 6. Boutin JA. 1997. Myristoylation. Cell Signal. 9:15–35 [DOI] [PubMed] [Google Scholar]
- 7. Aldrovandi GM, Gao L, Bristol G, Zack JA. 1998. Regions of human immunodeficiency virus type 1 nef required for function in vivo. J. Virol. 72:7032–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morita D, Igarashi T, Horiike M, Mori N, Sugita M. 2011. Cutting edge: T cells monitor N-myristoylation of the Nef protein in simian immunodeficiency virus-infected monkeys. J. Immunol. 187:608–612 [DOI] [PubMed] [Google Scholar]
- 9. Morita D, Katoh K, Harada T, Nakagawa Y, Matsunaga I, Miura T, Adachi A, Igarashi T, Sugita M. 2008. Trans-species activation of human T cells by rhesus macaque CD1b molecules. Biochem. Biophys. Res. Commun. 377:889–893 [DOI] [PubMed] [Google Scholar]
- 10. DerSimonian H, Sugita M, Glass DN, Maier AL, Weinblatt ME, Reme T, Brenner MB. 1993. Clonal V alpha 12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J. Exp. Med. 177:1623–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uematsu Y, Wege H, Straus A, Ott M, Bannwarth W, Lanchbury J, Panayi G, Steinmetz M. 1991. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc. Natl. Acad. Sci. U. S. A. 88:8534–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kestler HW, III, Li Y, Naidu YM, Butler CV, Ochs MF, Jaenel G, King NW, Daniel MD, Desrosiers RC. 1988. Comparison of simian immunodeficiency virus isolates. Nature 331:619–622 [DOI] [PubMed] [Google Scholar]
- 13. Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. 2002. Homeostasis of V alpha 14i NKT cells. Nat. Immunol. 3:966–974 [DOI] [PubMed] [Google Scholar]
- 14. Fitzpatrick EA, Avdiushko M, Kaplan AM, Cohen DA. 1999. Role of virus replication in a murine model of AIDS-associated interstitial pneumonitis. Exp. Lung Res. 25:647–661 [DOI] [PubMed] [Google Scholar]
- 15. Kang JA, Mohindru M, Kang BS, Park SH, Kim BS. 2000. Clonal expansion of infiltrating T cells in the spinal cords of SJL/J mice infected with Theiler's virus. J. Immunol. 165:583–590 [DOI] [PubMed] [Google Scholar]
- 16. Mokhtarian F, Shi Y, Zhu PF, Grob D. 1994. Immune responses, and autoimmune outcome, during virus infection of the central nervous system. Cell. Immunol. 157:195–210 [DOI] [PubMed] [Google Scholar]
- 17. Ohyama Y, Carroll VA, Deshmukh U, Gaskin F, Brown MG, Fu SM. 2006. Severe focal sialadenitis and dacryoadenitis in NZM2328 mice induced by MCMV: a novel model for human Sjogren's syndrome. J. Immunol. 177:7391–7397 [DOI] [PubMed] [Google Scholar]
- 18. Watanabe R, Wege H, ter Meulen V. 1983. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature 305:150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boutin JA, Ferry G, Ernould AP, Maes P, Remond G, Vincent M. 1993. Myristoyl-CoA:protein N-myristoyltransferase activity in cancer cells. Purification and characterization of a cytosolic isoform from the murine leukemia cell line L1210. Eur. J. Biochem. 214:853–867 [DOI] [PubMed] [Google Scholar]
- 20. Patwardhan P, Resh MD. 2010. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol. Cell. Biol. 30:4094–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajala RV, Datla RS, Moyana TN, Kakkar R, Carlsen SA, Sharma RK. 2000. N-myristoyltransferase. Mol. Cell. Biochem. 204:135–155 [DOI] [PubMed] [Google Scholar]
- 22. Shoji S, Kurosawa T, Inoue H, Funakoshi T, Kubota Y. 1990. Human cellular src gene product: identification of the myristoylated pp60c-src and blockage of its myristoyl acylation with N-fatty acyl compounds resulted in the suppression of colony formation. Biochem. Biophys. Res. Commun. 173:894–901 [DOI] [PubMed] [Google Scholar]
- 23. Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, Porcelli SA, Brenner MB. 1999. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 189:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manning CH, Heise ER. 1991. Biochemical analysis of class I and class II MHC antigens in cynomolgus macaques by one-dimensional isoelectric focusing. Tissue Antigens 37:56–65 [DOI] [PubMed] [Google Scholar]
- 25. Parham P, Ploegh HL. 1980. Molecular characterization of HLA-A, B homologues in owl monkeys and other nonhuman primates. Immunogenetics 11:131–143 [DOI] [PubMed] [Google Scholar]
- 26. Sugita M, Kumagai S, Ota M, Inoko H, Tsuji K, Imura H. 1992. Demonstration of the requirement for self antigen in the activation of autoreactive T cells. Int. Immunol. 4:119–124 [DOI] [PubMed] [Google Scholar]