Abstract
Macrophages (Mϕ) are first targets during human cytomegalovirus (HCMV) infection and are thought to be crucial for viral persistence and dissemination. However, since Mϕ are also a first line of defense and key modulators of the immune response, these cells are at the crossroad between protection and viral pathogenesis. To date, the Mϕ-specific contribution to the immune response against HCMV is still poorly understood. In view of the opposite roles of M1 and M2 Mϕ during initiation and resolution of the immune response, we characterized the effects of HCMV infection on classically activated M1 Mϕ and alternatively activated M2 Mϕ. Although HCMV susceptibility was higher in M2 Mϕ, HCMV established a productive and persistent infection in both types of Mϕ. Upon HCMV encounter, both types of Mϕ acquired similar features of classical activation and secreted high levels of proinflammatory cytokines and chemokines. As a functional consequence, conditioned media obtained from HCMV-infected M1 and M2 Mϕ potently activated freshly isolated monocytes. Finally, compared to HCMV-infected monocyte-derived dendritic cells, infected M1 and M2 Mϕ were more efficient in stimulating proliferation of autologous T cells from HCMV-seropositive donors at early times (24 h) postinfection, while the Mϕ immunostimulatory properties were reduced, but not abrogated, at later times (72 h postinfection). In summary, our findings indicate that Mϕ preserve proper antigen presentation capacity upon HCMV infection while enhancing inflammation, thus suggesting that Mϕ play a role in the maintenance of the large HCMV-specific T-cell repertoire in seropositive individuals.
INTRODUCTION
Human cytomegalovirus (HCMV) (1) is a herpesvirus that persistently infects the majority of the human population. After primary infection, HCMV remains lifelong in its host, being able to avoid clearance from the immune system. Whether HCMV persists in a truly latent state (defined as persistence in the absence of detectable infectious virus particles) or in a continuous low-level replication state is not clear (2, 3). However, the observation that around 10% of CD8+ and CD4+ T cells in the peripheral blood of healthy seropositive persons are committed to anti-HCMV responses (4) argues for continuous restimulation of T cells with antigens produced during phases of viral reactivation or low-grade active replication. Antigen recognition and T-cell activation are defined by the tightly regulated interaction between the T-cell receptor (TCR) and antigenic peptides that are presented in the context of class I or class II major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APC). A number of studies have shown that the most potent APC, i.e., dendritic cells (DC), are severely impaired by HCMV in their antigen presentation, migration, and T-cell activation capabilities (reviewed in reference 5). How APC that are altered in their function can trigger and maintain a massive HCMV-specific T-cell repertoire is difficult to explain. Due to their dual nature of being permissive to HCMV infection (6–9) and being professional APC (10), macrophages (Mϕ) would represent the ideal site for antigen production, processing, and presentation to the adaptive branch of the immune system during HCMV infection.
We and others have shown that Mϕ are highly susceptible to HCMV infection in vitro and that these cells produce viral progeny (11–15). Nevertheless, the majority of previous studies did not take into account that, in the context of immunity and inflammation, Mϕ acquire different activation states. For the sake of simplicity, Mϕ have been classified along what could be viewed as a linear scale, in which M1 Mϕ represent one extreme and M2 Mϕ the other (16). In this classification, the M1 designation refers to classically activated Mϕ, namely, cells that are capable of sustaining the immune response to pathogens through release of proinflammatory factors as well as efficient antigen presentation and T-cell stimulation. The M2 designation refers to alternatively activated Mϕ, namely, a very heterogeneous group of cells contributing to resolution of inflammation, tissue repair, extracellular matrix remodeling, and pathogen scavenging. Recent evidence indicates different susceptibilities of M1 and M2 Mϕ to HCMV infection (17, 18). Nevertheless, the course of HCMV infection in these two types of Mϕ as well as the Mϕ-specific contribution to the adaptive immune response against HCMV still remains elusive.
In this study, we addressed how Mϕ polarization defines HCMV susceptibility and how HCMV infection modifies Mϕ activation. We also determined the capability of HCMV-infected Mϕ to present antigen to T cells by setting up an autologous mixed leukocyte reaction assay.
MATERIALS AND METHODS
Ethics statement.
All buffy coats used in this study were purchased from the Transfusion Center of the Ulm University Hospital (IRB granted to Institut für Klinische Transfusionsmedizin und Immungenetik Ulm GmbH, Ulm, Germany) and were obtained from anonymized healthy blood donors. All blood donors gave written informed consent to approve and authorize the use of their blood for medical, pharmaceutical, and research purposes.
Cell cultures.
Peripheral blood mononuclear cells (PBMC) from HCMV-seronegative and HCMV-seropositive donors (tested by Vidas CMV IgG [bioMérieux, France]) were isolated from buffy coats (Institut für Klinische Transfusionsmedizin und Immungenetik Ulm GmbH, Ulm, Germany) by centrifugation over Lymphoprep (PAA Laboratories, Germany) according to standard protocols. A portion of the PBMC were resuspended in RPMI containing 40% fetal bovine serum (FBS) (Sigma Chemical Co., St. Louis, MO) and 10% dimethyl sulfoxide (Sigma-Aldrich Chemie, Munich, Germany) and stored at −80°C for subsequent use in T-cell proliferation assays, while the remaining PBMC were used for monocyte purification. Monocytes were isolated by negative selection with magnetic microbeads according to the manufacturer's instructions (monocyte isolation kit II; Miltenyi Biotec, Bergisch Gladbach, Germany). M1 and M2 Mϕ were obtained by culturing 3 × 106 monocytes/ml in hydrophobic Lumox dishes (Sarstedt, Nümbrecht, Germany) in standard medium (RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin) (Biochrom KG, Berlin, Germany) containing 100 ng/ml recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) or rhM-CSF (R&D Systems, Minneapolis, MN), respectively. Cells were incubated for 7 days in a 5% CO2 incubator at 37°C. At day 3, half of the medium was changed and the growth factors replenished. Monocyte-derived DC were obtained by culturing 3 × 106 monocytes/ml in standard medium containing 100 ng/ml rhGM-CSF and 25 ng/ml interleukin-4 (IL-4) (R&D Systems). At day 6 of culture, maturation was induced by 24 h stimulation with 100 ng/ml of lipopolysaccharide (LPS) (Sigma-Aldrich). Prior to infection, Mϕ and mature DC (mDC) were counted, resuspended in standard medium without growth factors, and inoculated with cell-free viral stocks.
Preparation of viral stocks and infection of Mϕ cultures.
The HCMV endotheliotropic strains TB40E (kindly provided by C. Sinzger, University of Ulm, Germany) and TB4-IE2-EYFP (19) were produced by infected human foreskin fibroblasts (HFF) cultivated in minimal essential medium (MEM) with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. Infectious supernatants were recovered at maximum cytopathic effect and cleared of cellular debris by centrifugation (7,000 × g for 10 min). Virus was pelleted by ultracentrifugation at 95,000 × g (Beckmann model SW28 unit) for 60 min, resuspended in sucrose phosphate buffer, frozen at −80°C, and thawed before single use. Virus stocks were negative for contamination with mycoplasmas, as determined with MicoAlert (Cambrex, Rockland, MD). Virus titers (PFU/ml) were determined on HFF by plaque assay as previously described (20). For viral growth curves, 3 h after TB40E infection with a multiplicity of infection (MOI) of 5, Mϕ were washed with citrate buffer (40 nM Na citrate, 10 mM KCl, 135 mM NaCl, pH 3.0) in order to inactivate unabsorbed virus (21), the standard medium was replenished, and at the indicated time points after infection, supernatants were collected and stored at −80°C for subsequent determination of the infectious titers.
SEM and TEM.
Electron microscopy investigations of Mϕ were performed as described previously (22). Briefly, Mϕ were seeded overnight on carbon-coated sapphire discs with a finder grid pattern (3-mm diameter; Engineering Office, M. Wohlwend GmbH, Sennwald, Switzerland) prior to HCMV infection or other stimulations. For scanning electron microscopy (SEM), Mϕ were either left untreated, infected with the wild-type TB40E or the fluorescent recombinant virus TB4-IE2-EYFP (both at an MOI of 5), or stimulated with 100 ng/ml LPS (Sigma-Aldrich) and 20 ng/ml gamma interferon (IFN-γ) (R&D Systems). At 24 h p.i., Mϕ were labeled with 10 μg/ml Hoechst 33342 (Molecular Probes Inc., Eugene, OR) for 10 min at 37°C, and then IE2-positive (green and blue fluorescence) and IE2-negative (blue fluorescence alone) Mϕ were photographed using the letters imprinted on the discs as a coordinate system. Sapphire discs were then fixed with 2.5% glutaraldehyde and 1% sucrose in 0.1 M phosphate buffer at pH 7.3 and imaged in a Hitachi S-5200 scanning electron microscope at an accelerating voltage of 10 kV (22). The correlation of fluorescence and SEM signals was achieved by reconciling for each cell the fluorescence signal with the signal from the electron microscope. For transmission electron microscopy (TEM), mock- and TB40E-infected (MOI of 5) Mϕ were cultivated for 3, 5, or 7 days and then high-pressure frozen, freeze-substituted, and finally embedded in plastic as previously described (22). The samples were imaged with a Zeiss transmission electron microscope at an acceleration voltage of 80 kV.
Flow cytometry.
Samples were acquired using a FACSCalibur (Becton, Dickinson, San Jose, CA) equipped with Cell Quest software (BD Immunocytometry Systems). Fluorescence-activated cell sorter (FACS) staining was performed according to conventional protocols at 4°C in the presence of 0.01% NaN3. Nonspecific binding sites on monocytes, Mϕ, and mDC were blocked with phosphate-buffered saline (PBS) containing 10% human immunoglobulins (Flebogamma; Grifols Deutschland GmbH, Langen, Germany) and 3% FBS before the addition of either primary antibodies or matching isotypic controls. For the immunophenotype, fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD1a, -CD14, -CD16, -CD36, -CD80, -CD83, -CD86, -HLA-A,B,C, -HLA-DR, -CD163, and -CD206 and matching isotypic controls (BD Pharmingen, San Diego, CA) were used. For chemokine receptor staining, unlabeled anti-CCR1 and matching isotypic controls (Dako, Eching, Germany) were used in combination with PE-conjugated rabbit anti-mouse immunoglobulins (Dako).
Secretome analysis.
Mϕ were seeded in standard medium at a concentration of 1 × 106 cells/ml and either left untreated, infected with TB40E by using an MOI of 5, or stimulated with 100 ng/ml LPS (Sigma-Aldrich) and 20 ng/ml IFN-γ (R&D System). After 24 h, cell-free supernatants were collected and stored at −80°C. A panel of 27 cytokines was analyzed using the Bio-Plex suspension assay (Bio-Rad Laboratories, Munich, Germany) and a Luminex 200 system, according to the manufacturer's procedure. Assayed cytokines were IL-1β, IL-1rα, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, G-CSF, GM-CSF, fibroblast growth factor (FGF), IFN-γ, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein 1 (MCP-1)/CCL2, MIP-1α/CCL3, MIP-1β/CCL4, RANTES/CCL5, eotaxin/CCL24, and IP-10/CXCL10.
Immunofluorescence analysis.
Monoclonal antibodies (MAb) reactive against the immediate-early proteins IE72 and IE86 (MAb E13; Argene-Biosoft, Varilhes, France), the early/late protein pp65 (MAb CINApool; Argene-Biosoft) and the late protein pp150 (MAb XP1; Dade Behring, Schwalbach, Germany) were chosen for their capacity to detect viral proteins characteristic of the three phases of the HCMV replication cycle. Mϕ were seeded in μ-Slide 8 wells (Ibidi, Martinsried, Germany), mock or TB40E infected (MOIs of 0.5, 1, 5, and 10), fixed with 4% formaldehyde, permeabilized with 0.2% Triton X-100, and probed with MAbs against viral antigens, followed by incubation with Alexa 488-conjugated goat anti-mouse Ig (ICN Biomedical, Eschwege, Germany). Nuclei and cytoplasm were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) and Evans blue, respectively. Staining was detected using a Zeiss Axioskop2 fluorescence microscope (Zeiss, Oberkochen, Germany).
Autologous T-cell proliferation assay.
M1 and M2 Mϕ as well as mDC were left untreated, incubated with an MOI of 5 of either the replication-competent or UV-inactivated TB40E, or stimulated with 50 μg/ml tetanus toxoid. Depending on the time point of interest, the cells were collected at 1 or 3 days. After recovering, cells were extensively washed, irradiated (3,000 cGy), and plated in decreasing numbers with previously cryopreserved autologous PBMC. Autologous PBMC were thawed, washed, resuspended in RPMI containing 5% human AB serum (Institut für Klinische Transfusionsmedizin und Immungenetik Ulm GmbH), and plated in triplicate at 1 × 105 cells per well in a 96-well-U-bottom plate (Corning, NY). Different ratios of Mϕ or mDC (defined as stimulators) and PBMC (defined as responders) (stimulator/responder ratios of 1:1 to 1:2,048) were cocultured for 5 days and subsequently pulsed with 1 μCi/well [3H]thymidine for 18 h. Proliferation was determined by measuring the [3H]thymidine incorporation in a β-counter (Wallac MicroBeta TriLux; PerkinElmer, Rodgau, Germany) and calculating the stimulation index (SI) as follows: SI = cpm for PBMC plus stimulators/cpm for PBMC alone.
Statistical analysis.
Statistical analysis of the results was performed using an unpaired, two-tailed Student t test. Differences with a P value of <0.05 were considered significant.
RESULTS
Generation of M1 and M2 Mϕ in vitro by stimulation of monocytes with GM-CSF or M-CSF.
M1 and M2 Mϕ were obtained by stimulating human circulating monocytes ex vivo with 100 ng/ml of either GM-CSF or M-CSF (23, 24). Maturation of monocytes into Mϕ was accompanied by an increased size and acquisition of a polarized cellular shape. Regardless of the growth factor that was employed, Mϕ attached to the substrate through foot-like extensions of the plasma membrane and showed a very complex cell surface with small hollows, pit indentations, and irregularly shaped humps (Fig. 1A). The immunophenotypic analysis confirmed that M1 Mϕ expressed higher levels of the molecules involved in antigen presentation, such as CD1a, CD80, and HLA-DR, and lower levels of the scavenging receptor CD163, the Fc receptor CD16, and the chemokine receptor CCR1 than M2 Mϕ (Fig. 1B). Further secretome analysis showed that while M1 Mϕ secreted large amounts of the proinflammatory mediators IL-1β, IL-12, TNF-α, IL-6, IL-8, MIP-1α/CCL3, and RANTES/CCL5, M2 Mϕ released larger amounts of the anti-inflammatory mediators IL-1rα and IL-10 and of the angiogenic factor VEGF (Fig. 1C). Altogether, the phenotypic and secretory features of the two subsets of monocyte-derived Mϕ satisfied the expected properties of M1 and M2 Mϕ.
Fig 1.

Differentiation of human monocytes into morphologically, phenotypically, and functionally distinct Mϕ subsets. (A) Monocytes were purified from buffy coats with a negative immunomagnetic selection and then incubated for 7 days in the presence of 100 ng/ml of either GM-CSF or M-CSF. The representative scanning electron microscopy pictures show the morphological features of monocytes immediately after isolation and monocyte-derived Mϕ at day 7 of in vitro culture. Bars, 10 μm. (B) Monocytes or M1 or M2 Mϕ were harvested, stained for the indicated markers and examined by flow cytometry. Bars depict mean values ± standard deviations (SD) for five blood donors. *, P < 0.05. (C) M1 or M2 Mϕ were seeded in fresh medium (1 × 106 cells/ml) and either left untreated (n.s.) or stimulated for 24 h with LPS (100 ng/ml) and IFN-γ (20 ng/ml). The concentrations of the indicated cytokines/chemokines were evaluated by Bio-Plex technology. Each symbol represents cells obtained from one blood donor. Horizontal lines represent mean values ± standard errors of the means (SEM). *, P < 0.05.
HCMV establishes a productive and persistent infection in M1 and M2 Mϕ.
In order to quantify the percentage of cells initiating the viral cycle, M1 and M2 Mϕ were challenged with the same MOI of the endotheliotropic HCMV strain TB40E. At 24 h p.i., nuclei of Mϕ expressing immediate-early proteins 1 and 2 (IE1-2) were detected by indirect immunofluorescence. As shown in Fig. 2A, even though the susceptibility of Mϕ to HCMV changed in a virus dose (MOI)-dependent manner, the percentages of HCMV-infected M1 Mϕ were under all conditions lower than those of M2 Mϕ. Despite the fact that immediate-early (IE1-2), early/late (pp65), and late (pp150) viral proteins were expressed earlier and more abundantly in M2 than in M1 Mϕ (Fig. 2B), HCMV successfully established a productive infection in both types of Mϕ (Fig. 2C). The release of viral progeny started at day 3 p.i. and reached the maximum levels at between days 4 and 8. During this time frame, M2 Mϕ released roughly 10 times more virus than M1 Mϕ. Notably, in the two types of Mϕ, low but steady levels of infectious virus were detected until day 21, thus indicating the establishment of a persistent infection.
Fig 2.
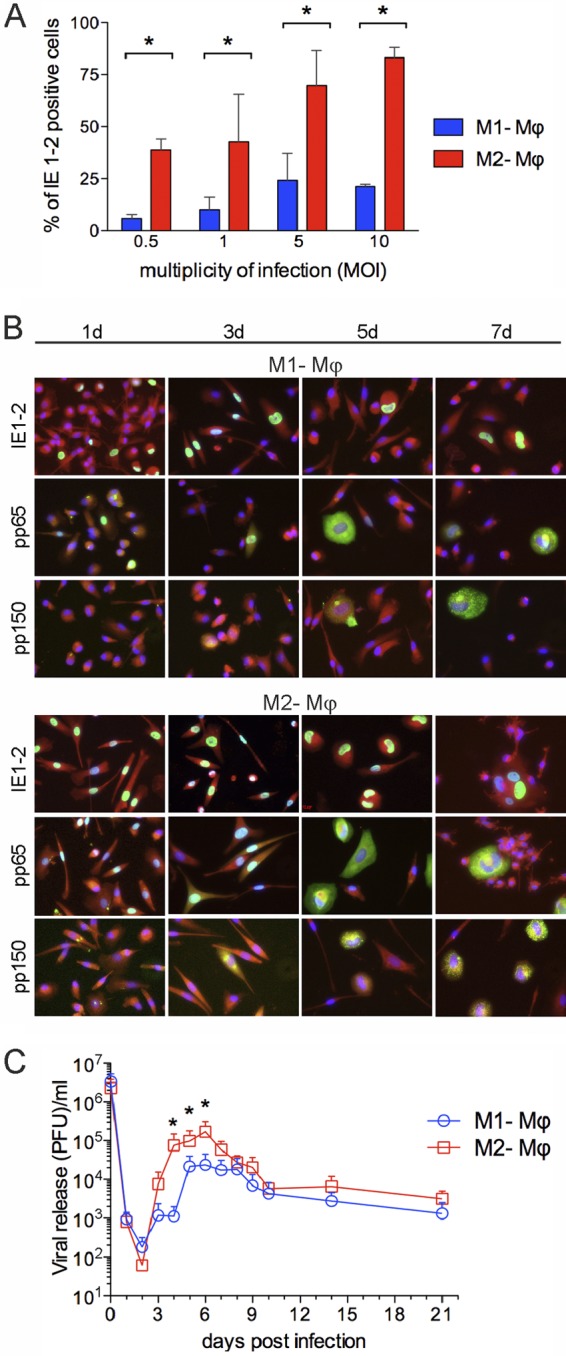
HCMV establishes a persistent infection in M1 and M2 Mϕ. (A) M1 and M2 Mϕ were seeded in μ-Slides and inoculated with the indicated multiplicity of infection (MOI) of TB40E, and at 24 h postinfection, the viral immediate-early (IE1-2) proteins were detected by indirect immunofluorescence. The percentages of HCMV-infected Mϕ were calculated by counting and correlating DAPI- and IE1-2-positive nuclei in five randomly selected microscopic fields for each experiment. Bars depict mean values ± standard deviations (SD) for 10 different blood donors. *, P < 0.05. (B) M1 and M2 Mϕ were infected with TB40E (MOI of 5), and at the indicated time points cells were stained with MAbs (green staining) specific for the immediate-early proteins IE1-2, early protein pp65, and late phosphoprotein pp150. Cell nuclei and cytoplasm were counterstained with DAPI (blue) and Evans blue (red), respectively. All photographs (original magnification, ×40) are from one representative donor of 10. (C) M1 and M2 Mϕ were inoculated with TB40E (MOI, 5) for 3 h, washed with acid buffer in order to inactivate unabsorbed input virus, and replenished with fresh medium. Samples of the supernatants were taken at the indicated time points after infection and titrated on human fibroblasts. Values are means ± SD from four independent experiments. *, P < 0.05 between M1 and M2 Mϕ.
Ultrastructural analysis of HCMV morphogenesis in Mϕ.
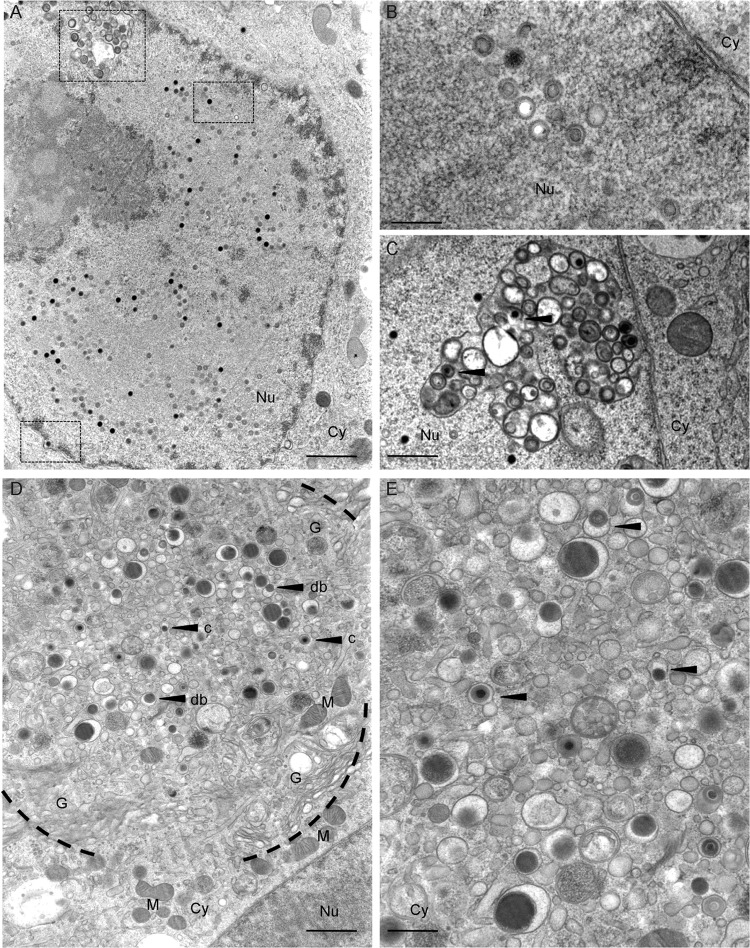
Next, we investigated by electron microscopy the production of viral progeny in M1 and M2 Mϕ. Due to their higher susceptibility, the ultrastructural analysis of the viral replication cycle resulted in clearer results when analyzing M2 Mϕ (Fig. 3). Starting at day 5 p.i., abundant viral capsids were visible in the Mϕ nuclei, accompanied by morphological changes such as nuclear enlargement, widening of the perinuclear space, and margination of heterochromatin (Fig. 3A). All three types of HCMV capsids (A capsids [lacking a scaffold or DNA core], B capsids [containing a scaffold], and C capsids [containing a DNA core]) were seen scattered throughout the nuclear matrix (Fig. 3B) or associated with complex membranous infoldings in the process of primary envelopment (Fig. 3C). At day 7 p.i., the assembly compartment (25) became clearly visible in the Mϕ cytoplasm (Fig. 3D) as a region characterized by a circular disposition of hypertrophic Golgi cisternae and enlarged endoplasmic reticulum, surrounding a central accumulation of maturing virus particles and dense bodies (Fig. 3E).
Fig 3.
Ultrastructural analysis of HCMV morphogenesis in human primary Mϕ. M2 Mϕ were infected with TB40E (MOI, 5). At 5 days postinfection, cells were fixed by high-pressure freezing, freeze-substituted, plastic embedded, and analyzed by electron microscopy after sectioning. (A) Detail of an infected nucleus showing, emphasized by the scattered squares, a cross-sectioned infolding, several scattered capsids, and a capsid budding into the perinuclear space (bar, 1 μm). (B) Magnified cross-section of the nucleus showing A (white), B (gray), and C (black) capsids (bar, 250 nm). (C) Magnified cross-section through an infolding with intermediate stages of primary envelopment visible (bar, 500 nm). (D) Overview image of an assembly compartment showing the typical rearrangement of cellular organelles and vesicles together with the accumulation of capsids (arrow c) and dense bodies (arrow db) (bar, 1 μm). (E) Magnified cross-section through the assembly compartment showing the intermediate stages of secondary envelopment (bar, 500 nm). Black arrowheads show capsids and dense bodies in close association with membranous structures. Cy, cytoplasm; Nu, nucleus; G, Golgi complex; M, mitochondria.
HCMV induces morphological and functional markers of classical activation in both types of Mϕ.
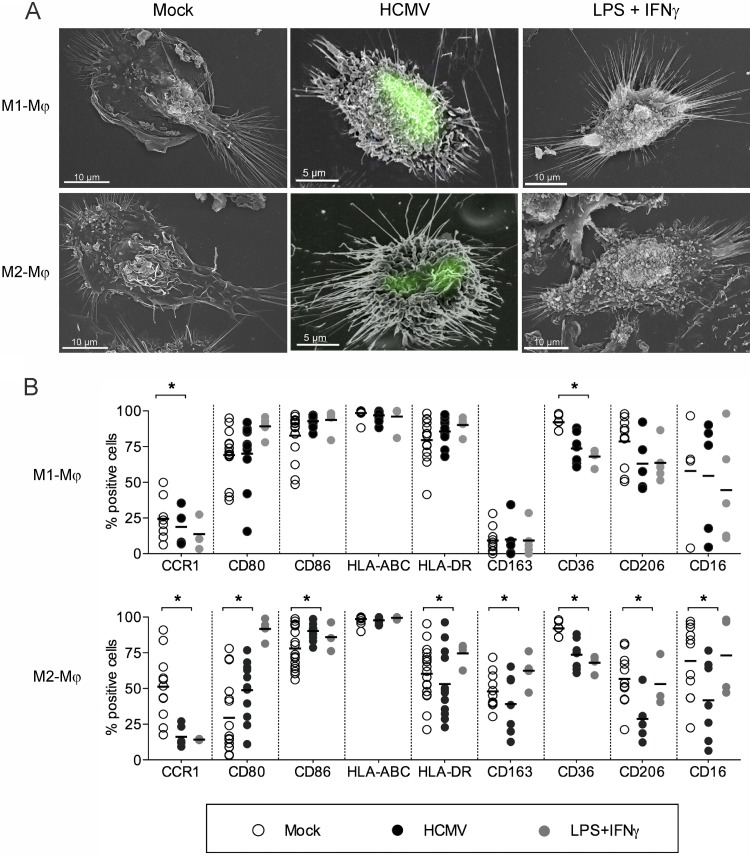
The proinflammatory potential of HCMV was investigated by comparing the morphology and immunophenotype acquired by Mϕ upon 24 h stimulation with HCMV or LPS plus IFN-γ. By employing SEM, we observed that upon HCMV encounter, both M1 and M2 Mϕ cultures appeared frilly due to the presence of several filiform extensions. Similar protrusions were observed in Mϕ activated by LPS plus IFN-γ but were lacking in mock-infected Mϕ cultures (Fig. 4A). To examine whether HCMV-infected Mϕ showed morphological signs of activation, we used the recombinant fluorescent TB4-IE2-EYFP (19) and correlated the fluorescence and SEM signals (26). As shown in Fig. 4A, IE2-positive M1 and M2 Mϕ appeared to be activated and exhibited long and tubular protrusions.
Fig 4.
HCMV induces morphological and immunophenotypic features of classical activation. M1 and M2 Mϕ were left untreated (mock), infected with either TB40E or TB4-IE2-EYFP (both at an MOI of 5), or stimulated with LPS plus IFN-γ (100 ng/ml and 20 ng/ml, respectively) for 24 h. (A) Scanning electron microscopy pictures show similar morphological features in Mϕ infected by TB4-IE2-EYFP (green fluorescent nuclei) and Mϕ stimulated by LPS plus IFN-γ. Pictures are representative of one out of three blood donors. (B) Mϕ were harvested, stained for the indicated markers, and examined by flow cytometry. Each symbol represents cells obtained from one blood donor. Horizontal lines represent the mean values of the percentages of positive cells. *, P < 0.05.
Concomitantly, the immunophenotype of HCMV-infected Mϕ cultures resembled that of LPS-IFN-γ-stimulated Mϕ (Fig. 4B). Compared to mock-infected cultures, HCMV and LPS-IFN-γ stimulations induced increased expression (measured as the percentage of positive cells and/or mean fluorescence intensity [MFI]) of costimulatory and MHC class I molecules and lower expression of scavenger receptors (CD163 and CD36), mannose receptor (CD206), and Fc receptor CD16 (Fig. 4B and data not shown). Interestingly, while the expression of HLA-DR was upregulated in M1 Mϕ upon HCMV infection (MFI of 140 ± 110 versus 200 ± 142 for mock- versus HCMV-infected cells, respectively; P = 0.0189), the expression levels of this marker were downregulated in HCMV-infected M2 Mϕ cultures (MFI of 50 ± 12 versus 34 ± 15 for mock- versus HCMV-infected cells, respectively; P = 0.0032).
HCMV-dependent proinflammatory activation of Mϕ is mediated by a paracrine mechanism.
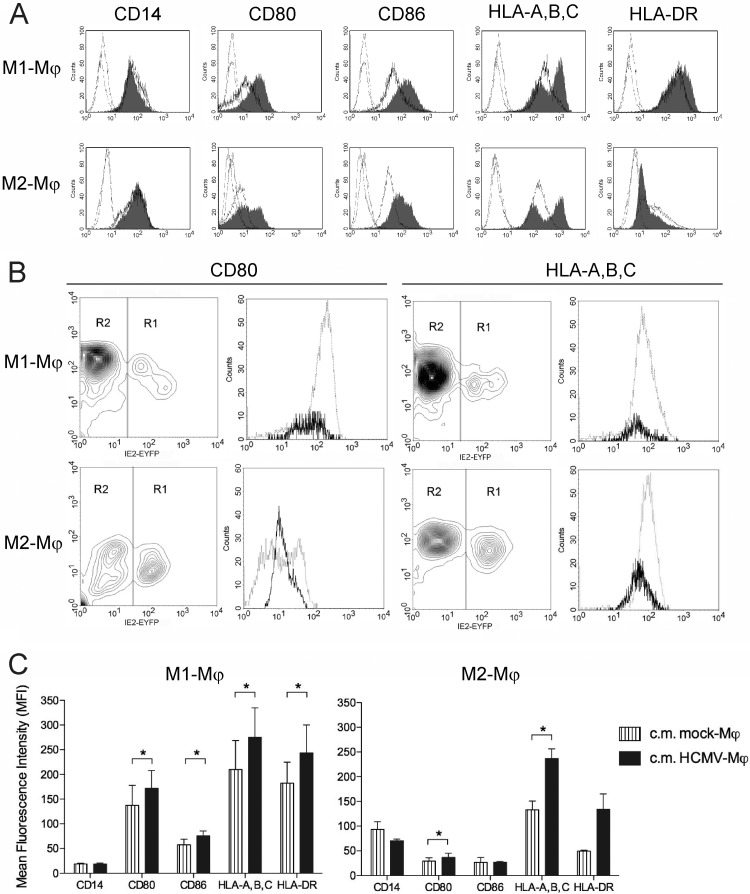
Since at the multiplicity of infection and the time point of infection used in the immunophenotypic analysis roughly 20% of M1 Mϕ were IE1-2 positive compared to roughly 70% of M2 Mϕ, we wanted to differentiate the direct effects caused by HCMV infection from bystander effects. As shown in Fig. 5A, double-peak histograms, indicating the existence of two populations with either high or low expression levels, were observed for CD80 and HLA-A,B,C in infected M2 and in infected M1 and M2 cultures, respectively. In contrast, the upregulation of CD86 and HLA-DR in M1 Mϕ as well as the downregulation of HLA-DR in M2 Mϕ were observed in all cells present in the HCMV-infected cultures. By infecting M1 and M2 Mϕ with the fluorescent virus TB4-IE2-EYFP, we could differentially analyze infected and bystander Mϕ within the same infected cultures, and as shown in Fig. 5B, we observed that HLA-A,B,C was lower in IE2-positive M1 and M2 Mϕ than in bystander IE2-negative cells. Similarly, lower expression of CD80 was observed in IE2-positive M2 Mϕ than in bystander cells, but such an effect was visible in only a few preparations of infected M1 Mϕ. Conversely, the expression levels of CD86 and HLA-DR were similar in IE2-positive and IE2-negative cells in both M1 and M2 Mϕ cultures (data not shown). To address whether soluble factors released in HCMV-infected Mϕ cultures could account for the observed changes in the Mϕ immunophenotype, freshly prepared Mϕ were incubated with virus-free conditioned media (c.m.) obtained from HCMV-infected M1 and M2 Mϕ cultures. As shown in Fig. 5C, the c.m. obtained from HCMV-infected Mϕ induced higher levels of expression of CD80, CD86, HLA-A,B,C, and HLA-DR than c.m. obtained from mock-infected cultures, indicating that soluble mediators could classically activate bystander cells by a paracrine mechanism. Altogether these data reveal that while the main direct effect exerted by HCMV infection in both M1 and M2 Mϕ subsets is the downregulation of HLA-A,B,C (and possibly the downregulation of CD80), soluble factors released in the Mϕ supernatants upon HCMV encounter are responsible for the activation of bystander cells.
Fig 5.
Direct and bystander effects exerted by HCMV on M1 and M2 Mϕ. (A) At 1 day postinfection, mock- and HCMV-infected Mϕ (TB40E, MOI of 5) were harvested, stained for the indicated markers, and examined by flow cytometry. Cell surface expression of the indicated molecules was investigated in mock- and HCMV-infected Mϕ (thick-line histograms and gray-filled histograms, respectively). Staining with isotype-matched control antibodies (thin-line histograms) in mock- and HCMV-infected Mϕ is shown. Representative data from one of 10 blood donors are shown. (B) M1 and M2 Mϕ were infected with TB4-IE2-EYFP (MOI 5) for 24 h and then stained with PE-conjugated anti-CD80 and anti-HLA-A,B,C antibodies (the fluorescence intensity for these markers is shown on the y axes). On the basis of the IE2 green fluorescence (shown on the x axes), cells were gated into either region 1 (R1, IE2 antigen positive) or region 2 (R2, IE2 antigen negative). Thick-line histograms depict cells gated in R1, while thin-line histograms depict cells gated in R2. Representative data from one of five experiments (five blood donors) are shown. (C) M1 and M2 Mϕ (obtained from four different donors) were seeded in fresh medium (1 × 106 cells/ml) and either left untreated (mock-Mϕ) or infected with TB40E at an MOI of 5 (HCMV-Mϕ,); 24 h later, the conditioned media (c.m.) were collected, centrifuged, filtered in order to remove cell debris and viral particles, and pooled together. Freshly prepared M1 and M2 Mϕ were incubated in the conditioned media for 24 h before the expression of the indicated molecules was measured as mean fluorescence intensity. Results are means ± standard errors of the means (SEM) from three experiments (three blood donors). *, P < 0.05.
HCMV induces a proinflammatory secretome in both M1 and M2 Mϕ.
In order to characterize how HCMV infection affects the secretory function of Mϕ, we performed a comprehensive analysis of the factors released by M1 and M2 Mϕ during the first 24 h of HCMV or LPS-IFN-γ stimulation. As shown in Fig. 6A, both subtypes of Mϕ responded to HCMV infection by secreting a plethora of proinflammatory factors. Compared to mock-infected Mϕ, significantly increased amounts of inflammatory cytokines (IL-1β, IL-2, IL-6, IL-8, IL-12, IL-15, TNF-α, and IFN-γ) and chemokines (MCP-1/CCL2, MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5) were detected in the supernatants of HCMV-infected M1 and M2 Mϕ. Concomitantly (Fig. 6B), we observed that HCMV infection induced in both types of Mϕ the secretion of vascular endothelial growth factor (VEGF) and only in M2 Mϕ the release of IL-1rα, IL-4, and IL-10. Out of 27 soluble factors, only 5 were shown to be differentially secreted by HCMV-infected M1 or M2 Mϕ, and while HCMV-infected M1 cultures contained larger amounts of IL-6, TNF-α, IL-12, and IFN-γ, HCMV-infected M2 cultures secreted significantly larger amounts of IL-10 (data not shown). Interestingly, the secretome of HCMV-infected Mϕ was strongly skewed toward a proinflammatory profile and closely resembled the secretome of Mϕ stimulated with LPS plus IFN-γ.
Fig 6.
The secretome of HCMV-infected M1 and M2 Mϕ is skewed toward a proinflammatory profile. M1 and M2 Mϕ obtained from four different donors were seeded in fresh medium (1 × 106 cells/ml) and either left untreated (mock), stimulated with LPS plus IFN-γ (100 ng/ml and 20 ng/ml), or infected with TB40E at an MOI of 5 (HCMV); 24 h later, the concentrations of proinflammatory (A) and anti-inflammatory (B) soluble factors were evaluated by Bio-Plex human cytokine assay. Mean values ± standard errors of the means (SEM) are reported. *, P < 0.05 between mock- and HCMV-infected Mϕ.
HCMV-infected Mϕ sustain inflammation.
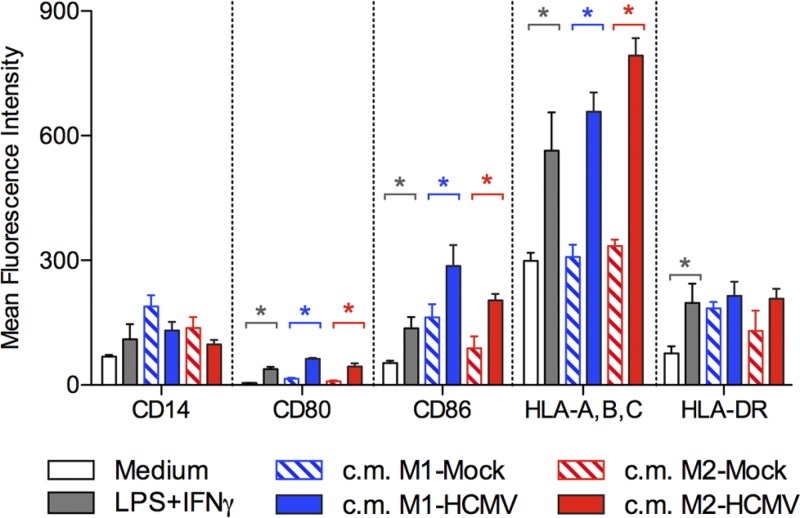
The release of inflammatory factors upon HCMV infection suggests that infected Mϕ might be involved in the amplification of the inflammatory response. In order to demonstrate this function, we analyzed the activation status of monocytes incubated with virus-free supernatants obtained from HCMV-infected Mϕ. As a positive control, monocytes were directly stimulated with LPS plus IFN-γ. As shown in Fig. 7, conditioned supernatants obtained from HCMV-infected M1 and M2 cultures induced monocyte activation as shown by upregulation of costimulatory molecules (CD80 and CD86) and MHC class I molecules. Interestingly, the monocyte activation driven by factors released by HCMV-infected Mϕ was comparable to or even stronger than the LPS-IFN-γ-induced activation.
Fig 7.
HCMV-infected M1 and M2 Mϕ release factors that activate monocytes. M1 and M2 Mϕ conditioned media (c.m.) were obtained from mock- and HCMV-infected Mϕ as described in the legend to Fig. 5. Freshly prepared monocytes were incubated overnight in M1 or M2 Mϕ conditioned media and then analyzed for the expression of the indicated markers. As negative and positive controls, monocytes were incubated in either medium alone (RPMI–10% FBS [medium]) or medium containing LPS plus IFN-γ (100 ng/ml and 20 ng/ml, respectively). Bars represent the mean fluorescence intensity for the indicated markers in four independent experiments ± standard deviation (SD). *, P < 0.05 between the indicated conditions.
HCMV-infected Mϕ efficiently stimulate proliferation of autologous T cells at 24 h p.i.
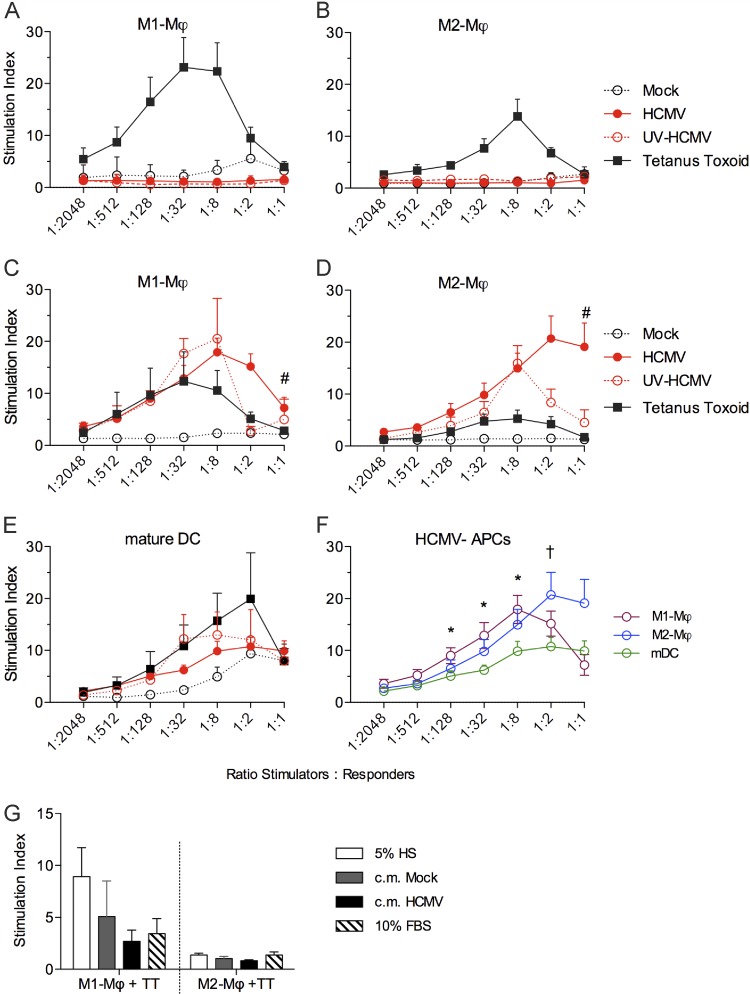
Next, we evaluated whether HCMV-infected M1 and M2 Mϕ were capable of efficiently presenting antigens to T cells by employing autologous T-cell proliferation assays. In order to reproduce the condition of naïve or memory T-cell populations, Mϕ and responder T cells were obtained from HCMV-seronegative and -seropositive donors, respectively. First, we excluded that the serological status of the blood donor could influence HCMV susceptibility because we measured similar infection rates in Mϕ obtained from seropositive and seronegative donors (data not shown). By using the recall antigen tetanus toxoid (Fig. 8A to D), we demonstrated that both types of Mϕ could present antigens and stimulate proliferation of autologous T cells. The classically activated M1 Mϕ presented the tetanus toxoid antigens more efficiently and induced higher T-cell proliferation than M2 Mϕ. When Mϕ were obtained from HCMV-seronegative donors (Fig. 8A and B), neither M1 nor M2 Mϕ were able to present HCMV antigens and stimulate autologous T-cell proliferation, thus confirming that Mϕ are poor stimulators of naïve T lymphocytes. The lack of T-cell stimulation (Fig. 8A and B) was independent of viral gene expression, and both types of Mϕ inoculated with the replication-competent HCMV or with the UV-inactivated HCMV could not stimulate T-cell proliferation. Interestingly, when cells were obtained from HCMV-seropositive donors (Fig. 8C and D), both M1 and M2 Mϕ were capable of presenting HCMV antigens and inducing proliferation of autologous T cells. The extents of T-cell stimulation induced by HCMV-infected M1 and M2 Mϕ cultures were similar, and only at the Mϕ/PBMC ratio of 1:1 did we observe that HCMV-infected M2 Mϕ induced a stronger stimulation than M1 Mϕ.
Fig 8.
HCMV-infected M1 and M2 Mϕ efficiently stimulate proliferation of autologous T cells at 24 h p.i. M1 and M2 Mϕ as well as mature monocyte-derived DC (mDC) were left untreated (mock), incubated with an MOI of 5 of either replication-competent (HCMV) or UV-inactivated TB40E (UV-HCMV), or stimulated with 50 μg/ml tetanus toxoid for 1 day. Mϕ or mDC were then collected, irradiated, and used as stimulators for autologous PBMC (responders) at various Mϕ/PBMC ratios (depicted on the x axes). Cells were cocultured for 6 days, and the stimulation index was determined after [3H]thymidine incorporation as described in Materials and Methods. (A and B) Mϕ and PBMC were obtained from 10 HCMV-seronegative blood donors. Data are mean values ± standard errors of the means (SEM). (C and D) Mϕ and PBMC were obtained from 10 HCMV-seropositive blood donors. Data are mean values ± SEM. #, P < 0.05 between M1 and M2 Mϕ infected with HCMV. (E) mDC and PBMC were obtained from eight HCMV-seropositive donors. Data are mean values ± SEM. (F) HCMV-infected M1 Mϕ, M2 Mϕ, and mDC cultures were compared for their T-cell stimulatory potential (the stimulation index is reported on the y axis). *, P < 0.05 between mDC and M1 Mϕ; †, P < 0.05 between mDC and M2 Mϕ. (G) M1 and M2 Mϕ were stimulated with 50 μg/ml tetanus toxoid (TT) for 1 day prior to coculture with autologous PBMC at a Mϕ/PBMC ratio of 1:8. Cocultures were performed in either RPMI plus 5% human serum (5% HS), RPMI plus 10% FBS (10% FBS), or virus-free conditioned media (c.m.) from mock- (c.m. Mock) or HCMV-infected (c.m. HCMV) M1 and M2 Mϕ cultures (obtained as described for Fig. 5).
To compare the immunostimulatory abilities of HCMV-infected M1 and M2 Mϕ with those of infected monocyte-derived DC, we performed similar T-cell proliferation assays using as stimulators mDC obtained from the same seropositive donors as M1 and M2 Mϕ. Consistent with their mature state, mDC efficiently presented the tetanus toxoid antigen to autologous T cells and potently induced T-cell proliferation (Fig. 8E). In agreement with previous studies (27), mDC were not highly susceptible to HCMV infection (rate of IE1-2 positive cells, ≤15%) and maintained expression levels of MHC and costimulatory molecules similar to those in mock-infected cells (Table 1). Even though equipped for efficient T-cell stimulation, mDC infected with the replication-competent HCMV or inoculated with the UV-inactivated HCMV stimulated T-cell proliferation less efficiently than HCMV-Mϕ (Fig. 8E and F).
Table 1.
FACS analysis of the expression levels of immunomodulatory molecules in mock- or HCMV-infected mature monocyte-derived dendritic cells
| Molecule | Expression level (MFI)a in: |
|||||
|---|---|---|---|---|---|---|
| Mock-infected mDC |
HCMV-infected mDC |
|||||
| Donor 1 | Donor 2 | Donor 3 | Donor 1 | Donor 2 | Donor 3 | |
| CD14 | 12.37 | 6.54 | 8.14 | 12.31 | 6.79 | 10.09 |
| CD1a | 115.82 | 926.80 | 280.28 | 101.42 | 876.22 | 332.11 |
| CD80 | 224.38 | 335.33 | 269.16 | 258.84 | 377.19 | 298.41 |
| CD86 | 542.74 | 168.42 | 189.86 | 656.93 | 178.31 | 200.06 |
| CD83 | 13.12 | 61.21 | 62.28 | 19.41 | 67.70 | 60.20 |
| HLA-A,B,C | 265.94 | 440.51 | 648.41 | 267.81 | 463.15 | 706.62 |
| HLA-DR | 288.58 | 486.04 | 265.87 | 262.40 | 500.77 | 294.85 |
MOI of 5, at 24 h postinfection.
To exclude that T-cell proliferation induced by HCMV-infected Mϕ could be caused by soluble factors secreted in the HCMV-infected Mϕ cultures, we employed virus-free c.m. from mock- and HCMV-infected Mϕ as incubation medium in an autologous mixed-leukocyte reaction (MLR) and assessed their impact on the T-cell proliferation induced by tetanus toxoid-loaded Mϕ. As shown in Fig. 8G, T-cell proliferation in response to Mϕ that were loaded with tetanus toxoid was not increased by the c.m. obtained from HCMV-infected Mϕ cultures, thus excluding the presence of soluble activators of T cells in Mϕ supernatants upon HCMV infection. Altogether, our results suggest that Mϕ can efficiently and specifically present HCMV-antigens to T cells and induce their proliferation.
HCMV reduces but does not abrogate Mϕ immunostimulatory ability at 72 h p.i.
Since many immune evasion genes, specifically the US2 to -11 genes (28–32), are known to be more effective later during infection (48 to 72 h p.i.), we addressed the arsenal for antigen presentation and the ability to stimulate autologous T cells of HCMV-infected Mϕ at 72 h p.i. As shown in Fig. 9, HCMV-infected M1 Mϕ cultures exhibited increased levels of costimulatory and MHC class I molecules compared to mock-infected cells (Fig. 9A), while HCMV-infected M2 Mϕ cultures showed a significant reduction of MHC class II expression compared to mock-infected cultures (Fig. 9B). As shown in Fig. 9C, when HCMV-Mϕ were employed as stimulators in an autologous MLR, both types of Mϕ cultures at 72 h p.i. could still induce T-cell proliferation but to a lesser extent than at 24 h p.i. Thus, HCMV can reduce but not abrogate the Mϕ immunostimulatory abilities at later times p.i.
Fig 9.
HCMV reduces but does not abrogate the immunostimulatory potential of Mϕ at 72 h postinfection. (A and B) At 3 days postinfection, mock- and HCMV-infected (TB40E, MOI of 5) M1 Mϕ (A) and M2 Mϕ (B) were harvested, stained for the indicated markers, and examined by flow cytometry. Each symbol represents cells obtained from one blood donor, and horizontal lines represent the mean values. *, P < 0.05 between mock- and HCMV-infected Mϕ cultures. (C) M1 and M2 Mϕ were mock or HCMV infected (TB40E, MOI of 5). Cells were harvested at 1 or 3 days postinfection, irradiated, and used as stimulators for autologous PBMC at various Mϕ/PBMC ratios (depicted on the x axis). Cells were cocultured for 6 days, and the stimulation index was determined after [3H]thymidine incorporation as described in Materials and Methods. Data are mean values ± standard errors of the means (SEM) obtained from 5 different blood donors. *, P < 0.05 between 1 day p.i. and 3 days p.i.
DISCUSSION
Several studies have proven the biological relevance of Mϕ during natural HCMV infection, revealing that Mϕ and their monocytic precursors are tightly involved in the regulation of HCMV latency and reactivation (6, 7). While monocytes are not fully permissive to HCMV and represent latency reservoirs and vehicles for viral dissemination (33), Mϕ support HCMV reactivation, completion of the viral cycle, and production of viral progeny (7, 8). Mϕ are present in all tissues as immune sentinels, and their proper activation in response to the cellular microenvironment ensures an effective immune response against pathogens. While it is clear that HCMV encodes an arsenal of proteins that alter or hijack the host immune response (1), the immunological functions of HCMV-infected Mϕ have been poorly investigated. In the past, the heterogeneity of Mϕ preparations and the use of different HCMV strains have generated divergent results. As an example, the immunophenotypic analysis of HCMV-infected Mϕ has demonstrated both upregulation (15, 34, 35) and downregulation (36, 37) of MHC and costimulatory molecules. In the last few years, new methods for the production of M1 and M2 polarized Mϕ have fueled the growth of the entire field (38), thus leading to significant advances in the creation of in vitro models of Mϕ cultures. Two different approaches have been mainly applied: (i) Mϕ are differentiated from monocytes using M-CSF followed by polarizing stimulants such as LPS plus IFN-γ or IL-4, or (ii) two antithetic growth factors (GM-CSF and M-CSF) (39) are employed to induce Mϕ differentiation and polarization (24, 40–43). Since IFN-γ possesses antiviral properties that could intrinsically inhibit in vitro infection with HCMV, we chose to employ GM-CSF and M-CSF to induce monocyte differentiation to Mϕ. Our data showed that the resulting Mϕ satisfied the morphological, immunophenotypic, and secretory requirements necessary for classification into M1 and M2 Mϕ.
In line with previous studies, we found that M2 Mϕ are more susceptible to HCMV infection than M1 Mϕ (17, 18). Additionally, we observed a more efficient expression of all three classes of viral proteins as well as a 10-times-higher release of viral progenies in M2 compared to M1 Mϕ. The ultrastructural analysis of the course of infection revealed that the viral morphogenetic events occurring in primary Mϕ were comparable to those previously observed in fibroblasts (44, 45). Importantly, we observed that HCMV established a persistent infection in both types of Mϕ, maintaining a low-level productive infection for long time (21 days of in vitro culture), highlighting the possibility that Mϕ are a persistent source of viral antigens for the stimulation of the immune system.
Further, we demonstrated that 24 h after HCMV infection, both types of Mϕ exhibited immunophenotypic features of classical activation and resembled Mϕ stimulated by LPS and IFN-γ. Interestingly, by correlating scanning electron microscopy and fluorescence microscopy, we demonstrated that morphological signs of activation were present in IE-positive cells in the Mϕ cultures. Opposite to the inhibitory effect exerted by HCMV on DC (27, 46–49), the immunophenotype of HCMV-infected Mϕ cultures resembled that of LPS-IFN-γ stimulated Mϕ, with increased expression of costimulatory and MHC class I molecules and lower expression of scavenger, mannose, and Fc receptors. By using the recombinant fluorescent TB40-IE2-EYFP (19), we performed a differential analysis of the infected and bystander cells present in Mϕ cultures inoculated with HCMV and observed that only MHC class I molecules (in both types of Mϕ) and CD80 (in M2 Mϕ) were downmodulated by HCMV infection, being expressed at lower levels in IE2-positive than in IE-negative cells. On the other hand, the proinflammatory activation acquired by M1 and M2 Mϕ upon HCMV stimulation was mediated by a paracrine mechanism exerted by soluble factors released in the conditioned media of HCMV-infected Mϕ cultures.
The morphological and immunophenotypic activation of infected Mϕ cultures was paralleled by a functional skew toward a proinflammatory profile, and HCMV-infected M1 and M2 Mϕ cultures secreted increased amounts of inflammatory cytokines (IL-1β, IL-2, IL-6, IL-8, IL-12, IL-15, TNF-α, and IFN-γ) and chemokines (MCP-1/CCL2, MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5). In agreement with Romo and colleagues (18), our data highlight the proinflammatory potential of HCMV and its capacity to classically activate both types of Mϕ. Even though increased amounts of the anti-inflammatory factors IL-1rα, IL-4, and IL-10 were secreted by infected Mϕ compared to mock-infected cells—a situation that could lead to a mixed M1 and M2 phenotype (50, 51)—the proinflammatory potential induced by HCMV in Mϕ appeared to be predominant, as demonstrated by the fact that conditioned media obtained from HCMV-infected Mϕ cultures induced a strong activation of newly generated monocytes. As monocytes are among the first cells recruited to the site of infection, our finding may be relevant to explain how HCMV is able to maintain inflammation (and potentially infection, by viral reactivation in infiltrating monocytes [52]) in infected tissues. Altogether these findings suggest that HCMV acts as a powerful proinflammatory stimulus and that HCMV infection drives both types of Mϕ toward an M1 profile in an attempt to mount an efficient Th1 response. Since a balanced polarization of Mϕ into M1 and M2 cells is critical in mediating an effective and not deleterious immune response, HCMV-driven M1 polarization of Mϕ could contribute to chronic inflammation and tissue damage (53–55).
Finally, although they are generally considered professional APC, the potential of different types of Mϕ to stimulate T-cell proliferation has been poorly investigated. Here, we showed that M1 and M2 Mϕ were capable of stimulating proliferation of T cells in a tetanus toxoid recall assay; as expected (24, 56), the classically activated M1 Mϕ presented the tetanus toxoid antigens more efficiently and induced higher T-cell proliferation than M2 Mϕ. Notably, despite the reduced expression levels of MHC class I (on IE-positive M1 and M2 Mϕ) and of MHC class II (on infected M2 Mϕ) exerted by the virus, HCMV-infected M1 and M2 Mϕ properly stimulated proliferation of autologous T cells obtained from HCMV-seropositive but not -seronegative donors at early time points (24 h) p.i. This finding suggests that upon HCMV infection, Mϕ activate specific memory but not naïve T cells. Conversely, the immunostimulatory abilities of infected Mϕ were reduced, but not abrogated, at later time points (72 h) p.i. Finally, HCMV-infected Mϕ exhibited better immunostimulatory abilities than mDC obtained from the same donors and treated with the same amount of virus. Thus, similarly to their murine counterpart (57), human Mϕ are productively infected by HCMV but preserve immunological functionality. We propose that during active HCMV infection, the persistent viral replication observed in Mϕ paired with maintained antigen presentation capacities could provide the necessary antigenic boost to maintain large amounts of CD8+ and CD4+ cells committed to HCMV in the peripheral blood of healthy virus carriers (4).
In summary, HCMV-infected Mϕ preserve immunological functionality while enhancing inflammation. Our data provide new insights into the immunological functions of Mϕ and suggest that HCMV infection of Mϕ may have a profound influence on the adaptive antiviral immune response in vivo.
ACKNOWLEDGMENTS
We thank Anke Lüske for excellent viral stock preparations, Marlies Just for testing the HCMV serostatus of healthy blood donors, and Max Bastian (Paul-Ehrlich-Institut, Langen, Germany) for helpful discussion about T-cell in vitro stimulation.
This study was supported by Deutsche Forschungsgemeinschaft (DFG) project SPP 1175 Me 1740/1 (to T.M.), Bundesministerium für Bildung und Forschung (BMBF) project C (to T.M.), Carl Zeiss Stiftung project “Infektionsbiologie humaner Makrophagen” (to G.F.), Ludovisi Blanceflor Stiftung (to G.F.), RFO2009 from the University of Bologna, Italy (to S.V.), and the Swedish Research Council, the Swedish Society of Medicine, the Tobias Foundation, the Swedish Children Cancer Research Foundation, the Heart and Lung Foundation, and the Stockholm County Council, Sweden (to C.S.-N.).
The funders were not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
G.F. designed the research, performed the experiments, analyzed the results, produced the figures, and wrote the manuscript; S.V. designed the research, analyzed the results, and wrote the manuscript; C.B., L.W., S.Z., and S.S. performed the in vitro experiments and analyzed the results; P.W. and M.B. analyzed the results; and C.S.-N. and T.M. designed the research, analyzed the results, and wrote the manuscript.
We declare no competing financial interests.
Footnotes
Published ahead of print 10 October 2012
REFERENCES
- 1. Mocarski ES, Jr, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2701–2772 In Knipe DM, Howley PM, Griffin BE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Sinclair J, Sissons P. 2006. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 87:1763–1779 [DOI] [PubMed] [Google Scholar]
- 3. Soderberg-Naucler C. 2008. HCMV microinfections in inflammatory diseases and cancer. J. Clin. Virol. 41:218–223 [DOI] [PubMed] [Google Scholar]
- 4. Crough T, Khanna R. 2009. Immunobiology of human cytomegalovirus: from bench to bedside. Clin. Microbiol. Rev. 22:76–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varani S, Frascaroli G, Landini MP, Soderberg-Naucler C. 2009. Human cytomegalovirus targets different subsets of antigen-presenting cells with pathological consequences for host immunity: implications for immunosuppression, chronic inflammation and autoimmunity. Rev. Med. Virol. 19:131–145 [DOI] [PubMed] [Google Scholar]
- 6. Michelson S. 1997. Interaction of human cytomegalovirus with monocytes/macrophages: a love-hate relationship. Pathol. Biol. 45:146–158 [PubMed] [Google Scholar]
- 7. Sinclair J. 2008. Human cytomegalovirus: latency and reactivation in the myeloid lineage. J. Clin. Virol. 41:180–185 [DOI] [PubMed] [Google Scholar]
- 8. Söderberg-Nauclér C, Streblow DN, Fish K, Allan-Yorke J, Smith PP, Nelson JA. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543–7554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor-Wiedeman J, Sissons P, Sinclair J. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hume DA. 2008. Macrophages as APC and the dendritic cell myth. J. Immunol. 181:5829–5835 [DOI] [PubMed] [Google Scholar]
- 11. Chevillotte M, Landwehr S, Linta L, Frascaroli G, Luske A, Buser C, Mertens T, von Einem J. 2009. Major tegument protein pp65 of human cytomegalovirus is required for the incorporation of pUL69 and pUL97 into the virus particle and for viral growth in macrophages. J. Virol. 83:2480–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fish KN, Depto AS, Moses AV, Britt W, Nelson JA. 1995. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J. Virol. 69:3737–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frascaroli G, Varani S, Blankenhorn N, Pretsch R, Bacher M, Leng L, Bucala R, Landini MP, Mertens T. 2009. Human cytomegalovirus paralyzes macrophage motility through down-regulation of chemokine receptors, reorganization of the cytoskeleton, and release of macrophage migration inhibitory factor. J. Immunol. 182:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan KA, Coaquette A, Davrinche C, Herbein G. 2009. Bcl-3-regulated transcription from major immediate-early promoter of human cytomegalovirus in monocyte-derived macrophages. J. Immunol. 182:7784–7794 [DOI] [PubMed] [Google Scholar]
- 15. Sinzger C, Eberhardt K, Cavignac Y, Weinstock C, Kessler T, Jahn G, Davignon JL. 2006. Macrophage cultures are susceptible to lytic productive infection by endothelial-cell-propagated human cytomegalovirus strains and present viral IE1 protein to CD4+ T cells despite late downregulation of MHC class II molecules. J. Gen. Virol. 87:1853–1862 [DOI] [PubMed] [Google Scholar]
- 16. Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poglitsch M, Weichhart T, Hecking M, Werzowa J, Katholnig K, Antlanger M, Krmpotic A, Jonjic S, Horl WH, Zlabinger GJ, Puchhammer E, Saemann MD. 2012. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am. J. Transplant. 12:1458–1468 [DOI] [PubMed] [Google Scholar]
- 18. Romo N, Magri G, Muntasell A, Heredia G, Baia D, Angulo A, Guma M, López-Botet M. 2011. Natural killer cell-mediated response to human cytomegalovirus-infected macrophages is modulated by their functional polarization. J. Leukoc. Biol. 90:717–726 [DOI] [PubMed] [Google Scholar]
- 19. Straschewski S, Warmer M, Frascaroli G, Hohenberg H, Mertens T, Winkler M. 2010. Human cytomegaloviruses expressing yellow fluorescent fusion proteins—characterization and use in antiviral screening. PLoS One 5:e9174 doi:10.1371/journal.pone.0009174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wentworth BB, French L. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253–258 [DOI] [PubMed] [Google Scholar]
- 21. Goodrum F, Jordan CT, Terhune SS, High K, Shenk T. 2004. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood 104:687–695 [DOI] [PubMed] [Google Scholar]
- 22. Walther P, Wang L, Liessem S, Frascaroli G. 2010. Viral infection of cells in culture—approaches for electron microscopy. Methods Cell Biol. 96:603–618 [DOI] [PubMed] [Google Scholar]
- 23. Akagawa KS. 2002. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int. J. Hematol. 76:27–34 [DOI] [PubMed] [Google Scholar]
- 24. Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. 2004. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. U. S. A. 101:4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tandon R, Mocarski ES. 2011. Cytomegalovirus pUL96 is critical for the stability of pp150-associated nucleocapsids. J. Virol. 85:7129–7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giepmans BN. 2008. Bridging fluorescence microscopy and electron microscopy. Histochem. Cell Biol. 130:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senechal B, Boruchov AM, Reagan JL, Hart DN, Young JW. 2004. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood 103:4207–4215 [DOI] [PubMed] [Google Scholar]
- 28. Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, Peterson PA, Yang Y, Fruh K. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613–621 [DOI] [PubMed] [Google Scholar]
- 29. Jones TR, Sun L. 1997. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. U. S. A. 93:11327–11333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lehner PJ, Karttunen JT, Wilkinson GW, Cresswell P. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. U. S. A. 94:6904–6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769–779 [DOI] [PubMed] [Google Scholar]
- 33. Bolovan-Fritts CA, Mocarski ES, Wiedeman JA. 1999. Peripheral blood CD14(+) cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood 93:394–398 [PubMed] [Google Scholar]
- 34. Maciejewski JP, Bruening EE, Donahue RE, Sellers SE, Carter C, Young NS, St. Jeor S. 1993. Infection of mononucleated phagocytes with human cytomegalovirus. Virology 195:327–336 [DOI] [PubMed] [Google Scholar]
- 35. Onno M, Pangault C, Le Friec G, Guilloux V, Andre P, Fauchet R. 2000. Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J. Immunol. 164:6426–6434 [DOI] [PubMed] [Google Scholar]
- 36. Odeberg J, Cerboni C, Browne H, Karre K, Moller E, Carbone E, Soderberg-Naucler C. 2002. Human cytomegalovirus (HCMV)-infected endothelial cells and macrophages are less susceptible to natural killer lysis independent of the downregulation of classical HLA class I molecules or expression of the HCMV class I homologue, UL18. Scand. J. Immunol. 55:149–161 [DOI] [PubMed] [Google Scholar]
- 37. Odeberg J, Soderberg-Naucler C. 2001. Reduced expression of HLA class II molecules and interleukin-10- and transforming growth factor beta1-independent suppression of T-cell proliferation in human cytomegalovirus-infected macrophage cultures. J. Virol. 75:5174–5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mosser DM. 2003. The many faces of macrophage activation. J. Leukoc. Biol. 73:209–212 [DOI] [PubMed] [Google Scholar]
- 39. Hamilton JA. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533–544 [DOI] [PubMed] [Google Scholar]
- 40. Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. 2007. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178:5245–5252 [DOI] [PubMed] [Google Scholar]
- 41. Martinez FO, Gordon S, Locati M, Mantovani A. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177:7303–7311 [DOI] [PubMed] [Google Scholar]
- 42. van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff TH, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH. 2010. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 185:1442–1449 [DOI] [PubMed] [Google Scholar]
- 43. Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. 2006. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J. Leukoc. Biol. 79:285–293 [DOI] [PubMed] [Google Scholar]
- 44. Buser C, Fleischer F, Mertens T, Michel D, Schmidt V, Walther P. 2007. Quantitative investigation of murine cytomegalovirus nucleocapsid interaction. J. Microsc. 228:78–87 [DOI] [PubMed] [Google Scholar]
- 45. Buser C, Walther P, Mertens T, Michel D. 2007. Cytomegalovirus primary envelopment occurs at large infoldings of the inner nuclear membrane. J. Virol. 81:3042–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beck K, Meyer-Konig U, Weidmann M, Nern C, Hufert FT. 2003. Human cytomegalovirus impairs dendritic cell function: a novel mechanism of human cytomegalovirus immune escape. Eur. J. Immunol. 33:1528–1538 [DOI] [PubMed] [Google Scholar]
- 47. Grigoleit U, Riegler S, Einsele H, Laib SK, Jahn G, Hebart H, Brossart P, Frank F, Sinzger C. 2002. Human cytomegalovirus induces a direct inhibitory effect on antigen presentation by monocyte-derived immature dendritic cells. Br. J. Haematol. 119:189–198 [DOI] [PubMed] [Google Scholar]
- 48. Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997–1009 [DOI] [PubMed] [Google Scholar]
- 49. Schempp S, Topp M, Kessler T, Sampaio KL, Dennehy KM, Einsele H, Hahn G, Grigoleit GU, Jahn G. 2011. Deletion mutant of human cytomegalovirus lacking US2-US6 and US11 maintains MHC class I expression and antigen presentation by infected dendritic cells. Virus Res. 155:446–454 [DOI] [PubMed] [Google Scholar]
- 50. Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD. 2008. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J. Immunol. 181:698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. 2009. NF-kappaB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res. 144:329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soderberg-Naucler C, Fish KN, Nelson JA. 1997. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Invest. 100:3154–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marashi SM, Raeiszadeh M, Workman S, Rahbar A, Soderberg-Naucler C, Klenerman P, Chee R, Webster AD, Milne RS, Emery VC. 2011. Inflammation in common variable immunodeficiency is associated with a distinct CD8(+) response to cytomegalovirus. J. Allergy Clin. Immunol. 127:1385–1393 e1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Soderberg-Naucler C. 2006. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Intern. Med. 259:219–246 [DOI] [PubMed] [Google Scholar]
- 55. Varani S, Landini M-P. 2011. Cytomegalovirus-induces immunopathology and its clinical consequences. Herpesviridae 2:6–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mantovani A, Sica A, Locati M. 2005. Macrophage polarization comes of age. Immunity 23:344–346 [DOI] [PubMed] [Google Scholar]
- 57. Hengel H, Reusch U, Geginat G, Holtappels R, Ruppert T, Hellebrand E, Koszinowski UH. 2000. Macrophages escape inhibition of major histocompatibility complex class I-dependent antigen presentation by cytomegalovirus. J. Virol. 74:7861–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]