Abstract
Monocyte-derived dendritic cells (MDDCs) play a key role in the regulation of the immune system and are the target of numerous gene therapy applications. The genetic modification of MDDCs is possible with human immunodeficiency virus type 1 (HIV-1)-derived lentiviral vectors (LVs) but requires high viral doses to bypass their natural resistance to viral infection, and this in turn affects their physiological properties. To date, a single viral protein is able to counter this restrictive phenotype, Vpx, a protein derived from members of the HIV-2/simian immunodeficiency virus SM lineage that counters at least two restriction factors present in myeloid cells. By tagging Vpx with a short heterologous membrane-targeting domain, we have obtained HIV-1 LVs incorporating high levels of this protein (HIV-1-Src-Vpx). These vectors efficiently transduce differentiated MDDCs and monocytes either as previously purified populations or as populations within unsorted peripheral blood mononuclear cells (PBMCs). In addition, these vectors can be efficiently pseudotyped with receptor-specific envelopes, further restricting their cellular tropism almost uniquely to MDDCs. Compared to conventional HIV-1 LVs, these novel vectors allow for an efficient genetic modification of MDDCs and, more importantly, do not cause their maturation or affect their survival, which are unwanted side effects of the transduction process. This study describes HIV-1-Src-Vpx LVs as a novel potent tool for the genetic modification of differentiated MDDCs and of circulating monocyte precursors with strong potential for a wide range of gene therapy applications.
INTRODUCTION
Monocyte-derived dendritic cells (MDDCs) are primary actors of the immune system and play a central role in its homeostasis (1). For these reasons, MDDCs are at the basis of a number of gene therapy strategies directed against tumors or viral infections (2).
The genetic modification of MDDCs has been achieved to various degrees with virus- and non-virus-based methods. Among the former, primate lentiviral vectors (LVs) derived primarily, but not exclusively, from human immunodeficiency virus type 1 (HIV-1) seem highly efficient, probably due to the natural tropism of this virus for cells of myeloid origin (3–8). However, even in the case of LVs, efficient transduction rates of MDDCs require high viral inputs, and multiplicities of infection (MOIs) ranging from 10 to 500 have been described (3–5, 7–10). Contrary to more permissive cells, MDDCs, like other human myeloid cells (monocytes and macrophages), display a strong resistance to HIV-1, a phenomenon that is particularly acute during the early phases of infection, i.e., those phases of interest for gene therapy purposes (11–13). This relative resistance can be bypassed in certain instances through the use of high doses of viral inputs (14–16), but this exposes target cells to modifications that can affect their maturation, their survival, or their functionality and that are largely due to the presence of large amounts of viral components (8–10).
We and others have previously determined that this restrictive phenotype could be relieved through the use of Vpx, a viral protein that is encoded by members of the simian immunodeficiency virus SM (SIVSM)/HIV-2 lineage and absent from HIV-1 (11, 17–23). Indeed, when provided in trans onto target MDDCs via noninfectious virion-like particles derived from SIVMAC (VLP-Vpx), Vpx increased the susceptibility of MDDCs to infection with a wide range of lentiviruses, particularly with HIV-1 (11, 18, 22, 24). Vpx counteracts at least two restriction factors that hamper lentiviral infection in myeloid cells at the reverse transcription step: the apolipoprotein B editing catalytic polypeptide 3A (APOBEC3A) and the sterile alpha motif hydrolase domain 1 (SAMHD1) proteins (20, 21, 25–30).
Several attempts have been carried out over the years to transfer the positive property of Vpx on lentiviral infection to HIV-1 LVs (16, 22, 24). Recently, this goal has been achieved by modifying the p6 domain of HIV-1 Gag through which Vpx is normally packaged into cognate viral particles (24). The particles so generated displayed an increased infectivity toward MDDCs; however, they induced a potent maturation of transduced cells accompanied by high interferon secretion (24), which may constitute a serious drawback for most therapeutic applications.
In the present study, we have achieved an efficient incorporation of Vpx into HIV-1 particles in a different manner. More specifically, we have engineered a modified version of Vpx by fusing it to the membrane-targeting domain of the c-Src protein. As a result of this fusion, Vpx is retargeted outside the nucleus onto membranes in target cells, where it is efficiently incorporated into viral particles (HIV-1-Src-Vpx). These vectors display an increased infectivity toward MDDCs and monocytes and exhibit a skewed cellular tropism toward cells of myeloid origins in unpurified peripheral blood mononuclear cells (PBMCs), providing the first example of how a nonstructural viral protein can be used to modify the natural tropism of lentiviruses. This preference for myeloid cells can be further restricted upon pseudotyping with envelope proteins targeting cellular receptors expressed in MDDCs, indicating that the combination between Src-Vpx and myeloid cell-specific envelopes is a particularly potent combination to achieve both selective and efficient transduction in these cells.
Lastly, we carefully examined the phenotype of MDDCs transduced with conventional HIV-1 LVs compared to that of HIV-1-Src-Vpx LVs and determined that, contrary to the former, HIV-1-Src-Vpx LVs did not induce MDDC maturation or affect their survival.
MATERIALS AND METHODS
Cells.
HEK293T and HeLa cells were maintained in Dulbecco's modified Eagle medium (DMEM) plus 10% of fetal bovine serum (FBS). Human PBMCs were obtained from healthy donors as described in reference 31. Briefly, monocytes at ≥95% purity were obtained from PBMCs after performing Ficoll and Percoll gradients and negative depletion of contaminating cells (monocyte isolation kit II human; Miltenyi Biotech). Primary blood lymphocytes (PBLs) were harvested after performing Ficoll and Percoll density gradients. Primary cells were maintained in RPMI 1640 medium and 10% FBS. When indicated, PBLs were activated with 1 μg/ml of phytohemagglutinin (PHA; Sigma) and 150 U/ml of interleukin-2 (IL-2; AIDS Research and Reference Reagents Program of the NIH) for 24 h prior to use. Immature MDDCs were obtained in the presence of 100 ng/ml of granulocyte-macrophage colony-stimulating factor and interleukin-4 (GM-CSF and IL-4, respectively; Abcys) for 4 days. When indicated, plasmacytoid dendritic cells (pDCs) where purified to an average of 95% from the blood of healthy donors after two rounds of positive selection based on the pDC marker BDCA-4 according to the manufacturer's instructions (Miltenyi). Cells were maintained in RPMI 1640 supplemented of 10% fetal calf serum and 1 ng/ml of interleukin 3 (R&D Systems).
Constructs, LV production, and cell transduction.
The HIV-1 LVs used here have been described before (Fig. 1A) (31). A Flag-tagged version of SIVMAC Vpx was cloned under the control of an EF-1α promoter (Invitrogen), and Src-Vpx was obtained by addition of the first 11 amino acids of c-Src corresponding to its membrane-targeting domain (MGSSKSKPKDP). To release Vpx from this membrane-targeting domain once the protein was incorporated into viral particles, the sequence KARVLAEA was added between the c-Src epitope and Flag-Vpx. This sequence is present between the p27/p29 domains of Gag and is normally recognized as the last processing event mediated by the viral protease during viral particle maturation. LV production was carried out upon transfection of HEK293T cells with Gag-Pol plus accessory proteins of HIV-1 (8.2), Env (the vesicular stomatitis virus G protein [VSVg]), and green fluorescent protein (GFP)-coding miniviral genome (pRRL with a self-inactivating long terminal repeat) coding vectors at a ratio of 8:4:8 (32). When indicated, GFP was replaced by human CD8, and LVs were pseudotyped with a mutated version of the G protein of the Sindbis virus (SVGmu) (41). Vpx-coding plasmids were added at 4, 8, and 16 μg in the transfection used to produce vectors. Forty-eight hours after transfection, supernatants were purified by ultracentrifugation through a two-step sucrose gradient (45 and 25% [wt/vol] sucrose) and then titrated by protein content (exogenous reverse transcriptase activity [exo-RT]) or by their infectious titers on HeLa cells.
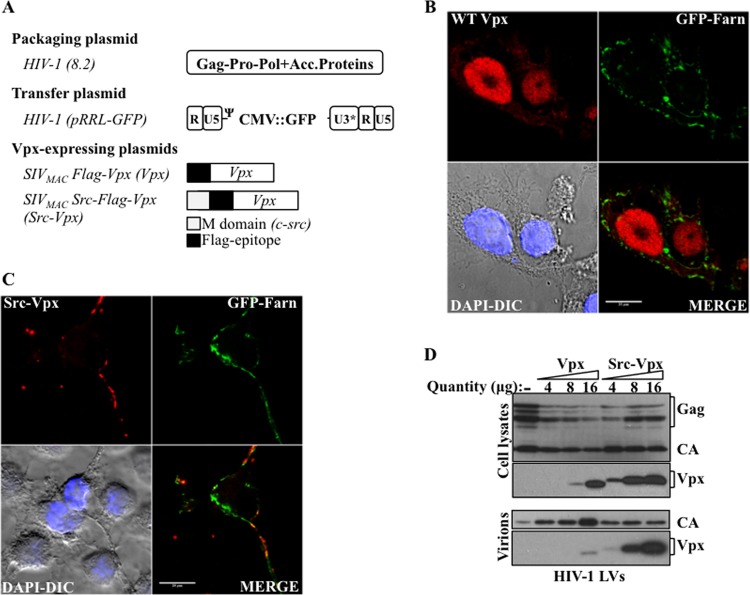
Fig 1.
Engineering of a modified version of SIVMAC Vpx that is retargeted into the cell cytoplasm and is efficiently incorporated into HIV-1 LVs. (A) Schematic representation of the constructs used here. Acc.Proteins, accessory proteins. (B and C) HEK293T cells were transfected with WT or Src-Vpx along with a plasma membrane-targeted version of GFP containing the farnesylation sequence of Ras (GFP-Farn), and then cells were analyzed by confocal microscopy to examine the localization of Vpx. The pictures present the representative staining observed for 100 cells examined per construct. DAPI-DIC, 4′,6-diamidino-2-phenylindole-diffential interference contrast image. (D) HIV-1 LVs were produced by cotransfection of HEK293T with packaging, transfer, and VSVg coding plasmids in the presence of different amounts of WT Vpx or of Src-Vpx. Virion particles were purified by ultracentrifugation, normalized by protein content (exogenous reverse transcriptase activity, or exo-RT), and analyzed by WB.
Transductions were carried out for 2 h on 105 cells (5 × 105 for PBMCs). The percentage of transduced GFP-positive cells was assessed 3 days posttransduction by flow cytometry (5 days for monocytes and PBMCs and 1 day for Western blot [WB] analysis of SAMHD1 levels). All plasmids were produced under endotoxin-free conditions (Macherey-Nagel). When indicated, MDDCs were matured with lipopolysaccharide (LPS; Sigma) for 24 h prior to analysis.
Western blotting, flow cytometry, apoptosis, and alpha interferon (IFN-α) secretion analysis.
Western blot analyses were carried out using standard methods. Monoclonal anti-Flag and anti-capsid (CA) antibodies were obtained from Sigma and the NIH AIDS Research and Reference Reagents Program, respectively. The anti-SAMHD1 monoclonal antibody was purchased from AbCam. Secondary antibodies were purchased from Dako.
For flow cytometry analysis, the following antibodies were used: anti-CD1a-eFluor450 (HI149) (eBiosciences); anti-CD14-allophycocyanin (APC) (M5E2), anti-CD86-fluorescein isothiocyanate (FITC) (2331), anti-CD80-phycoerythrin (PE) (L307.4), and anti-HLA-DR-PE (L243) (BD Pharmingen); anti-CD40-PE (mAb89) and anti-CD83-PE (HB15A) (Immunotech); and anti-HLA-ABC-PE (W6/32) (Dako).
Apoptosis was determined using the PE annexin V apoptosis detection kit I (BD Pharmingen) according to the manufacturer's instructions. To avoid staining interference caused by LV-mediated expression of GFP, LVs coding for a human CD8 reporter were used, and transduced cells were identified with an anti-CD8-APC-conjugated antibody (SK1; eBiosciences).
IFN-α secretion was quantified using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (AbCys) on the supernatant of MDDCs obtained 2 days after transduction. As a control, pDCs well known to secrete high levels of IFN-α were stimulated for 30 min with inactivated influenza virus provided at an MOI of 8 (kindly provided by Bruno Lina and Manuel Rosa Calatrava, Faculty of Medicine, Laennec, Lyon, France).
Confocal microscopy.
HEK293T cells were directly grown on coverslips and transiently transfected with DNAs coding for Flag-tagged wild-type (WT) Vpx and Src-Vpx derived from SIVMAC along with DNA coding for a GFP tagged with the farnesylation sequence derived from Ras that labels the plasma membrane (kind gift of Philippe Mangeot, Department of Virology, INSERM U758, Lyon, France). Cells were then fixed in 4% formalin and permeabilized with 0.2% Triton X-100. After immunostaining, images were acquired using a spectral Leica sp5 or LSM 710 microscope. Primary antibodies are described above, and secondary antibodies were purchased from Vector Laboratories.
RESULTS
Engineering of a modified version of SIVMAC Vpx that is efficiently incorporated into HIV-1-derived LVs.
To obtain HIV-1 vectors capable of incorporating high levels of Vpx, we used a strategy in which the Vpx protein derived from SIVMAC was fused at its N terminus to the membrane-targeting domain of c-Src, an 11-amino-acid epitope sufficient to drive membrane localization, and to a Flag epitope to facilitate its detection (Fig. 1A). This short membrane-targeting domain can functionally substitute for the corresponding domain of HIV-1 Gag during virion particle assembly and functionally targets heterologous proteins to cellular membranes (33–35), so that we surmised that it should drive Vpx at sites of virion assembly and force its incorporation into virion particles. Indeed, when the intracellular localizations of WT Vpx and Src-Vpx were analyzed by confocal microscopy after transient transfection of 293T cells, the addition of the Src tag was sufficient to redistribute Vpx outside the nucleus, where the majority of WT Vpx is localized (Fig. 1B and C; images are representative of more than 100 scored cells per condition). This distribution coincided with that of a plasma membrane farnesylated GFP (36).
To determine whether this relocalization was sufficient to drive efficient incorporation of Src-Vpx into HIV-1 vectors, HIV-1 LVs were produced by transient transfection of HEK293T cells with DNAs coding for Gag-Pol and nonstructural viral proteins, the pantropic envelope VSVg, a GFP-coding transfer vector, and increasing amounts of unmodified Flag-tagged Vpx or of Src-Flag-Vpx (as indicated). Virions were harvested, purified by ultracentrifugation, normalized by protein content, and analyzed by WB (Fig. 1D). Both unmodified Vpx and Src-Vpx were highly expressed in LV-producing cells, although the addition of the Src tag seemed to stabilize Vpx. In contrast to the poor packaging of Vpx, Src-Vpx was highly incorporated into HIV-1 LVs.
Examination of the infectivity of HIV-1-Src-Vpx vectors.
To determine the functional consequences of the incorporation of Vpx into HIV-1 LVs, exo-RT-normalized amounts of virions were used to challenge HeLa cells, MDDCs, monocytes, and PHA/IL-2-activated PBLs prior to flow cytometry analysis 3 days postchallenge (Fig. 2A and B). As expected, HeLa cells were highly susceptible to viral infection and Vpx did not modify infection rates. MDDCs were more resistant to viral transduction (10-fold higher doses of viral inputs were used for MDDCs than for HeLa cells), and Vpx affected transduction rates. Specifically, while the incorporation of unmodified Vpx did not augment HIV-1 infectivity in a statistically significant manner at any of the concentrations used, the incorporation of Src-Vpx increased it proportionally to the amount of the protein packaged into virion particles, so that on average around 80% of MDDCs were transduced with an MOI of 1 with the highest dose (16 μg of Src-Vpx). Given that this amount of Src-Vpx was the best one among those tested, all subsequent experiments presented in the manuscript were carried out with this amount of Src-Vpx. In addition, given that the incorporation of unmodified Vpx did not affect the behavior of HIV-1 LVs, likely due to a suboptimal incorporation of Vpx (i.e., the two were equivalent in terms of transduction rates, induction of maturation, and induction of cell death [not shown]), only Src-Vpx was used.
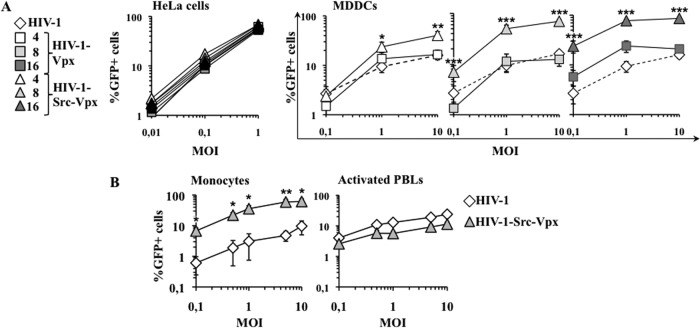
Fig 2.
HIV-1-Src-Vpx vectors display increased infectivity specifically in MDDCs and monocytes. (A) Normalized amounts of LVs (by determining their infectious titer in HeLa cells) were used at the indicated multiplicity of infection (MOI) on both HeLa cells and MDDCs. The percentage of transduced GFP-positive cells was determined 3 days later by flow cytometry in triplicate for HeLa cells and on cells from 9 donors for MDDCs. For clarity, the results obtained on MDDCs are presented on separate graphs. The results obtained upon transduction with conventional HIV-1 LVs are reported on all of the graphs for a direct comparison (dotted lines). (B) HIV-1-Src-Vpx LVs incorporating the optimal amount of Src-Vpx (16 μg) were used to transduce monocytes and PBLs that had been activated with PHA/IL-2 for 24 h prior to viral challenge. The graphs present averages and standard errors of the means (SEM) obtained with cells derived from 6 to 9 donors. Statistical analysis was performed according to an unpaired Student t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
When identical HeLa infectious units were used on purified monocytes or activated PBLs, HIV-1-Src-Vpx displayed an obvious advantage over conventional HIV-1 LVs on purified monocytes (Fig. 2B). However, the vectors behaved similarly in activated PBLs, in line with previous data indicating that Vpx exerts a major effect in cells of myeloid origin.
Overall, these results indicate that the forced incorporation of Src-Vpx into HIV-1 LVs results in a transduction process that is very efficient in cells of myeloid origin.
Transduction of MDDCs with HIV-1-Src-Vpx LVs leads to the degradation of endogenous SAMHD1.
A number of recent reports indicate that the cellular protein SAMHD1 specifies a major restriction during MDDC transduction by reducing the pool of intracellular nucleosides available for reverse transcription and indicate that Vpx relieves this restriction by degrading it (20, 21, 29, 37). To determine whether HIV-1-Src-Vpx LVs could similarly degrade SAMHD1, MDDCs were transduced with the same amounts of HIV-1 and HIV-1-Src-Vpx (MOI of 5), and cells were lysed 24 h afterwards for WB analysis (Fig. 3). Transduction with HIV-1-Src-Vpx LVs led to a robust degradation of endogenous SAMHD1, contrary to the case with conventional HIV-1 LVs, thus strongly correlating the ability to degrade this protein with the observed increase in viral infectivity.
Fig 3.
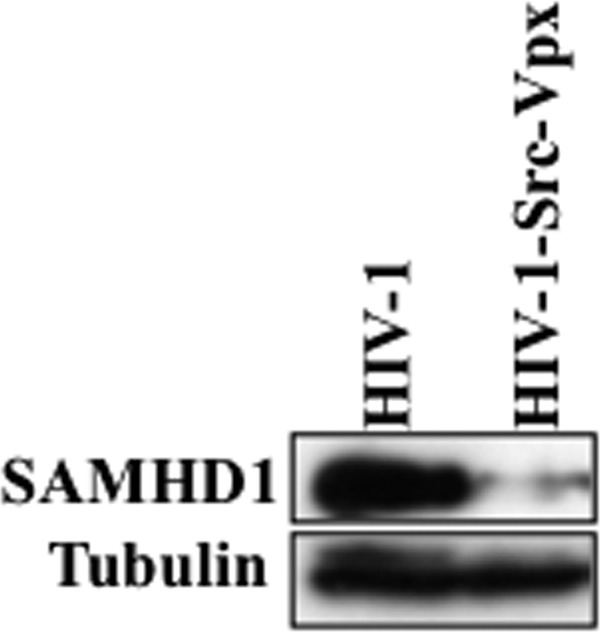
Transduction of MDDCs with HIV-1-Src-Vpx LVs induces SAMHD1 degradation. MDDCs were challenged with normalized amounts of HIV-1 and HIV-1-Src-Vpx LVs (MOI, 5) prior to cell lysis and WB analysis 24 h later. A representative result is shown here.
HIV-1-Src-Vpx LVs efficiently target nonstimulated monocytes within primary blood mononuclear cells.
The fact that the incorporation of Src-Vpx into HIV-1 LVs increased the infectivity of these vectors in myeloid cells indicated that Vpx could be used to skew the cellular tropism of HIV-1 LVs in cases in which multiple cell populations are simultaneously present. To determine whether this was the case, unsorted and nonstimulated PBMCs were challenged with HIV-1 and HIV-1-Src-Vpx vectors, and the extent of infection was determined 3 to 5 days posttransduction by flow cytometry along with the monocyte-associated marker CD14 (Fig. 4A and B). Overall, CD14+ cells were more susceptible to transduction than CD14-negative cells, probably reflecting the high resistance to infection displayed by T and B cells that are mostly in a quiescent state. The incorporation of Src-Vpx within HIV-1 LVs ameliorated the transduction rates on CD14-positive cells by at least a factor of 4 to 7 depending on the viral dose; however, it exerted a negligible effect on infectivity in CD14-negative cells (Fig. 4B; note that the y axes use different scales).
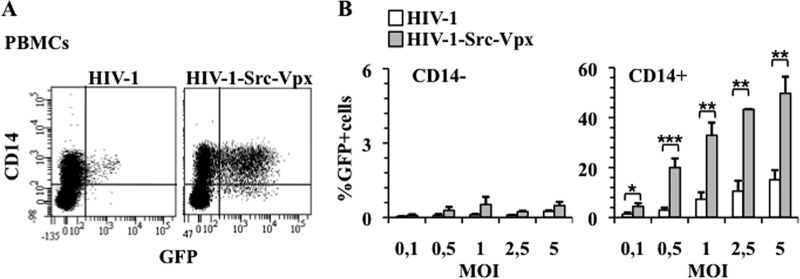
Fig 4.
HIV-1-Src-Vpx LVs specifically transduce monocytes within total PBMCs. (A and B) HIV-1 and HIV-1-Src-Vpx LVs were used to transduce nonstimulated and unseparated PBMCs at different MOIs prior to flow cytometry analysis 3 days later. PBMCs were labeled with an anti-CD14 antibody to distinguish monocytes from T and B lymphocytes (CD14− fraction). A representative flow cytometry image is shown in panel A, while the graphs present averages and SEM obtained from 6 independent experiments. Statistical analysis was performed according to an unpaired Student t test: *, P < 0,05; **, P < 0,01; ***, P < 0,001.
Thus, HIV-1-Src-Vpx LVs can be used to efficiently target a substantial fraction of monocytes within unsorted PBMCs.
The combined use of Src-Vpx and of a modified Envelope protein can further skew the cellular tropism of HIV-1 LVs toward MDDCs.
To our knowledge, Vpx is the first example of a nonstructural lentiviral protein that can be used for gene therapy purposes to alter the cellular tropism of LVs after viral entry into target cells. Other strategies toward the goal of cell type-specific targeting have involve the use of envelope proteins that recognize specific cellular receptors (38–41). In this respect, an engineered version of the Sindbis virus glycoprotein (SVGmu) has been described to be specific to DC-SIGN and thus to direct LVs specifically to MDDCs (41). To more stringently restrict the cellular tropism of HIV-1-Src-Vpx LVs, LVs were pseudotyped with SVGmu, an envelope protein that recognizes DC-SIGN, and compared to vectors pseudotyped with the pantropic envelope protein VSVg. LVs were normalized by exo-RT activity (as SVGmu-pseudotyped LVs are not infectious on HeLa cells) and used to transduced MDDCs or PHA-activated PBLs at an MOI of 10 prior to flow cytometry analysis 3 days posttransduction (Fig. 5A). As shown above, transduction with VSVg-pseudotyped LVs resulted in a high percentage of transduction of both MDDCs and activated PBLs, and if Vpx improved transduction rates in MDDCs by at least 10-fold, a substantial proportion of PHA-activated PBLs was nonetheless transduced. In contrast, we found that SVGmu-pseudotyped HIV-1 LVs were highly deficient in cell transduction in both MDDCs and activated PBLs compared to VSVg-pseudotyped ones, in line with what was previously reported for the same viral dose (24). However, when Src-Vpx was also incorporated into HIV-1 vectors, this envelope allowed for a specific transduction of MDDCs to the detriment of activated PBLs. In this case, the overall proportion of modified MDDCs was similar, albeit inferior, to the one observed with VSVg-pseudotyped HIV-1-Src-Vpx LVs but was strictly directed toward MDDCs. SVGmu has been shown to engage the DC-SIGN lectin on the surface of MDDCs (41), so this vector may affect the overall maturation phenotype of transduced MDDCs. To determine whether this was the case, MDDCs transduced with HIV-1-Src-Vpx SVGmu were compared to mock-transduced cells with respect to two clearly associated cell maturation markers, CD83 and CD86 (Fig. 5B and C). MDDC transduction with this vector induced a marked upregulation of the above-mentioned cell maturation markers, suggesting that SVGmu-pseudotyped vectors engage a cell maturation pathway, likely through their binding to DC-SIGN.
Fig 5.
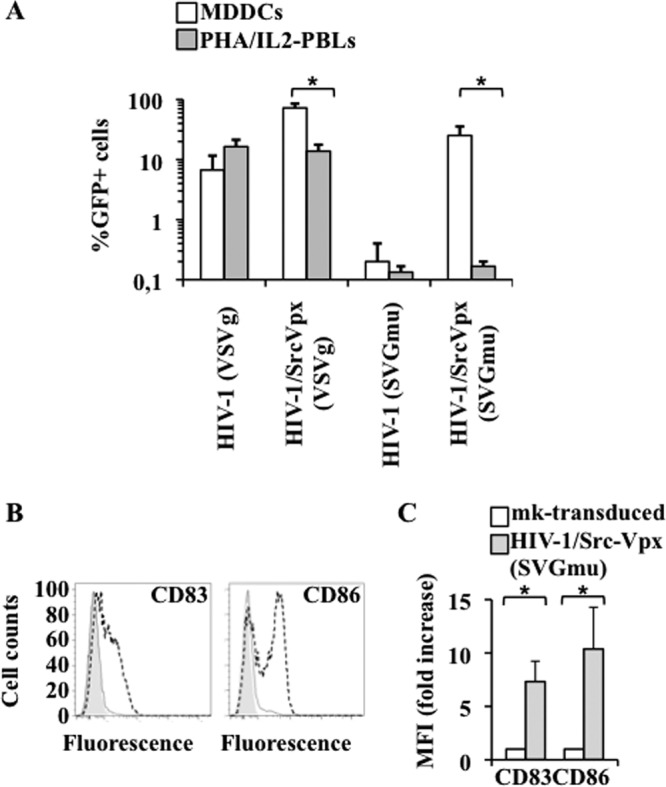
HIV-1-Src-Vpx LVs pseudotyped with the SVGmu Envelope protein strictly restrict the cellular tropism of HIV-1 LVs to MDDCs. (A) MDDCs and PHA-activated PBLs were transduced at a multiplicity of infection (MOI equivalent) of 10 with HIV-1 LVs containing or not containing Src-Vpx, and they were pseudotyped with either the pantropic Env VSVg or with the DC-specific Env derived from the Sindbis virus (SVGmu). LVs were produced by transfection of 293T cells, purified, and normalized by an exo-RT activity assay against standards of known infectivity. The percentage of transduced GFP-positive cells was determined 3 days later by flow cytometry. The graph displays results (averages and SEM) obtained with cells derived from 4 different donors. *, P < 0.05 according to an unpaired Student t test. (B and C) MDDCs transduced with HIV-1-Src-Vpx LVs pseudotyped with the SVGmu envelope were further analyzed by flow cytometry for the upregulation of known cell-associated markers of MDDC maturation. (B) Representative shifts observed between mock- and vector-transduced cells (filled gray and dotted lines, respectively). (C) Averages obtained from multiple experiments. *, P < 0.05 according to an unpaired Student t test.
Overall, these results indicate that the combined use of cell type-specific envelope proteins and of Src-Vpx is a particularly potent tool to redirect the cellular tropism of HIV-1 vectors toward cells of myeloid origins using both entry and postentry control steps. In the case of SVGmu Env pseudotypes, the induction of cell maturation underlines the need for further lentiviral vector optimization.
Transduction with HIV-1-Src-Vpx LVs does not induce MDDC maturation and does not modify the ability of transduced MDDCs to undergo maturation upon LPS stimulation.
To determine whether LV transduction more generally affected the maturation of MDDCs, a number of cell surface markers were analyzed by flow cytometry following transduction with viral doses required to obtain equivalent proportions of transduced cells (MOIs of 5 and 0.5 for HIV-1 and HIV-1-Src-Vpx, respectively) (Fig. 6A). This setup allows for the study of the effect that transductions carried out with equivalent MDDC transduction units exert on cell physiology. HIV-1 transduction significantly increased the cell surface expression of CD80, CD86, and major histocompatibility complex class I and of the total MDDC population, and most importantly it led to an increase in CD83 expression (Fig. 6B and C show representative histograms and a graph presenting the average mean fluorescent intensity [MFI] increase with respect to mock-transduced MDDCs observed in multiple donors). Thus, at these viral doses, HIV-1 upregulated a number of cell markers associated with MDDC maturation, although this induction was lower than the one observed with LPS on these and other markers, such as CD40 and CD80. On the contrary, no statistically significant changes were observed upon MDDC transduction with HIV-1-Src-Vpx LVs compared to mock-transduced cells (Fig. 6C), indicating that under these conditions MDDCs had no major effect on the phenotypic maturation of target MDDCs.
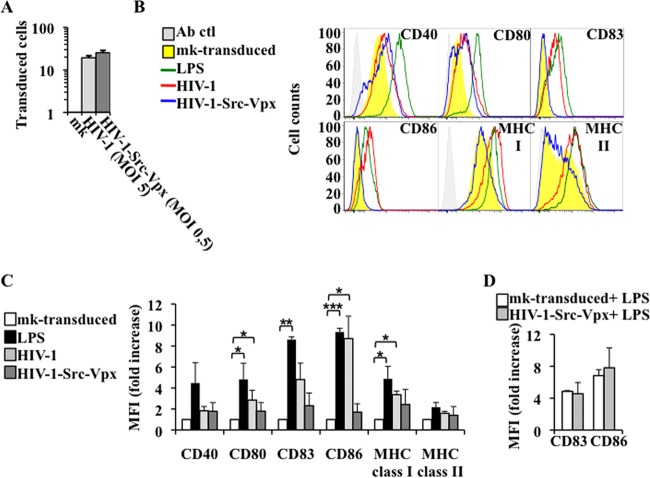
Fig 6.
Transduction of MDDCs with HIV-1-Src-Vpx LVs does not alter their immature phenotype or affect their ability to undergo subsequent LPS-mediated maturation. MDDCs were transduced with viral inputs yielding the same number of transduced cells between the two conditions (HIV-1 at an MOI of 5 and HIV-1-Src-Vpx at an MOI of 0.5) and examined 72 h later by flow cytometry for the analysis of the vector-based marker (A), as well as with antibodies directed against the indicated cell surface markers (B). Ab ctl, antibody control. As a control for maturation, mock (mk)-transduced MDDCs were matured with LPS for 24 h prior to analysis. The histogram plots display representative results. (C) The graph presents the normalized mean fluorescence intensities (MFI) and SEM calculated from 3 independent experiments between the indicated conditions and mock-transduced cells. Statistical analyses were performed by unpaired Student t test between each condition and mock transduction (P values of 0.05, 0.01, and 0.001 are indicated by one, two, or three asterisks, respectively). (D) Two days after transduction, cells were further incubated for 24 h with LPS to induce their maturation prior to staining with the indicated cell surface markers and flow cytometry analysis. The graph presents averages and SEM of the MFI increase observed upon LPS treatment of 3 different donors.
To determine whether transduced MDDCs possessed the ability to undergo maturation once a proper stimulus was provided, 2 days after transduction MDDCs were incubated for an additional 24 h with LPS prior to flow cytometry analysis of the two most prominent markers of MDDC maturation (CD83 and CD86) (Fig. 6D). The data obtained indicated that HIV-1-Src-Vpx LV-transduced cells were able to strongly upregulate these markers upon LPS stimulation, similar to mock-transduced cells.
Transduction with HIV-1-Src-Vpx lowers the extent of LV-induced MDDC cell death.
To determine whether HIV-1-Src-Vpx affected survival rates because of its higher level of infectivity, cells were challenged with doses that allowed the same percentage of transduced cells (between 20 and 25%, as described above), and cells were analyzed 3 days later by flow cytometry upon staining with annexin V and 7-amino-actinomycin (7-AAD), which allow the identification of viable cells (7-AAD negative, annexin V negative), early apoptotic cells (7-AAD negative, annexin V positive), and late apoptotic/necrotic cells (7-AAD positive, annexin V positive) (Fig. 7). To avoid interference with this staining, an LV-coded human-truncated CD8 reporter was used in place of GFP to identify transduced cells. Under these conditions, the total population of mock-transduced MDDCs contained an equal proportion of viable and early apoptotic cells (7-AAD negative/annexin V negative and 7-AAD negative/annexin V positive, respectively) (Fig. 7A). HIV-1 LV transduction decreased the proportion of viable cells to the benefit of the early apoptosis profile. However, the overall ratio between viable and early apoptotic cells was not significantly different from that of mock-transduced cells following transduction with HIV-1-Src-Vpx LVs. Similarly, the analysis of transduced cells (Fig. 7B) revealed that about 60% of transduced MDDCs displayed signs of early apoptosis upon transduction with HIV-1 LVs (67%; 7-AAD negative, annexin V positive), with a minor proportion of late apoptotic/necrotic cells (7%; 7-AAD positive, annexin V positive) and about 25% of viable cells (7-AAD negative, annexin V negative). Instead, transduction with HIV-1-Src-Vpx with a viral input that yielded the same percentage of transduced MDDCs resulted in fewer apoptotic cells and in an increased proportion of viable cells (37 and 52%, respectively).
Fig 7.
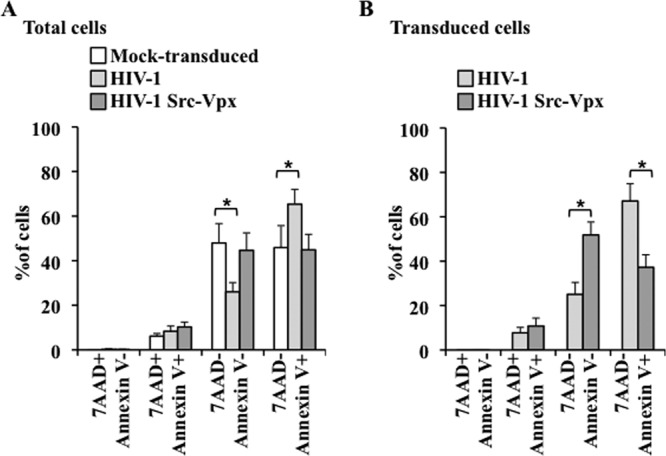
Transduction of MDDCs with HIV-1-Src-Vpx LVs protects from transduction-mediated apoptosis. MDDCs transduced with viral inputs resulting in equivalent percentages of GFP-positive cells (MOI of 0.5 for HIV-1-Src-Vpx and of 5 for HIV-1 LVs, yielding around 20% transduction) were examined 72 h later by flow cytometry with 7AAD and annexin V. To avoid staining interference, the LV-coded GFP was substituted for a truncated hCD8 molecule. The graph presents data obtained from 4 donors in total or from transduced CD8-positive MDDCs (A and B, respectively). Statistical analyses were performed by unpaired Student t test (*, P < 0.05).
Thus, transduction with Src-Vpx LVs reduces cell death associated with transduction, a phenomenon likely due to the important positive effect that Vpx has during the early phases of infection in myeloid cells which, in turn, makes it possible to considerably lower the viral input dose.
Cell transduction with Src-Vpx LVs results in only low levels of IFN-α secretion.
An extremely efficient transduction process has been linked to a potent secretion of IFN-α and the maturation phenotype in transduced MDDCs (24, 42). To determine whether this phenotype also could be observed here, MDDCs were transduced with the indicated vectors as described above, and the amount of IFN-α released in the supernatant was measured by ELISA (Fig. 8). As a control, primary plasmacytoid DCs that express high levels of IFN-α upon viral encounter were stimulated with inactivated influenza virus. As expected, pDCs secreted very high levels of IFN-α, and similarly, albeit to a lower extent, mock-transduced MDDCs stimulated with LPS were able to secrete high levels of IFN-α. On the contrary, transduced MDDCs either did not secrete interferon or did so to levels just above the background of our assay (as for HIV-1-Src-Vpx LVs). Thus, overall our results indicate that at least under the conditions used here, LV transduction results in only low to undetectable levels of IFN-α secretion.
Fig 8.
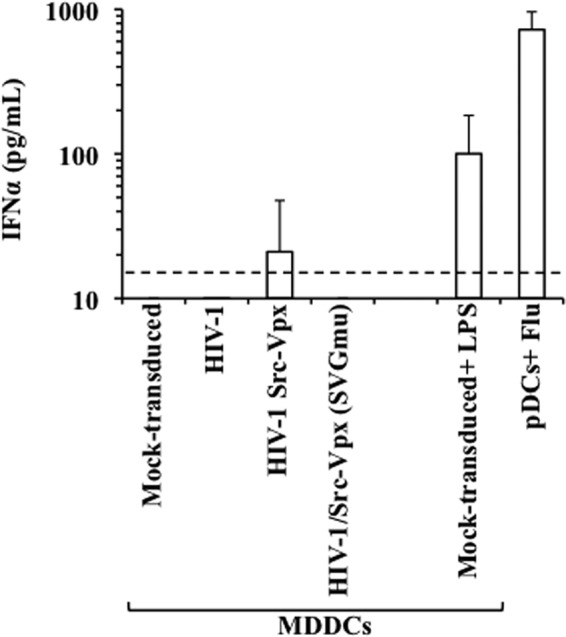
Cell transduction results in low levels of IFN-α secretion. The amount of IFN-α present in the supernatant of MDDCs obtained 2 days after transduction with the indicated vectors was quantified by ELISA. As a control, pDCs were stimulated for 30 min with inactivated influenza virus provided at an MOI of 8, while mock-transduced MDDCs were stimulated with LPS for 24 h. The graph presents averages and SEM obtained from 4 different donors. The dotted line indicates the limit of detection of the assay.
DISCUSSION
In the work presented here, we have successfully engineered HIV-1 LVs incorporating Vpx that display an increased infectivity toward purified monocytes and MDDCs and that rather selectively transduced monocytes within unsorted PBMCs. This preferential cellular tropism for myeloid cells can be further restricted through the use of cell type-specific envelopes, as the SVGmu envelope recognizes DC-SIGN (41). As a result of their higher level of infectivity, HIV-1-Src-Vpx LVs exert a lower impact on the physiology of transduced MDDCs, preserving their immature phenotype as well as their viability.
MDDCs are fairly resistant to lentiviral infection. While this restriction can be overcome with high viral doses of HIV-1, this induces secondary effects that modify, sometimes profoundly, the cell physiology (8–10). This effect is not surprising in light of the natural role of sentinels played by MDDCs against pathogens and therefore also against LVs that are sensed as such. The exact viral determinant(s) at the basis of these modifications (cell maturation, interferon activation, and cell death) is unknown, but a number of viral products are directly sensed by the cell, such as the capsid or the viral genome itself, are toxic, such as VSVg (43–45), or can lead to cell death, as reported when high levels of incomplete viral genomes are present (46).
A more efficient manner to remove this restriction is through the use of Vpx, a protein that counters it very efficiently by stimulating the efficiency of the process of reverse transcription (11, 17–23). Indeed, the HIV-1-Src-Vpx LVs described here efficiently degrade the restriction factor SAMHD1. SAMHD1 is a nuclear protein, so that, by virtue of its plasma membrane localization, Src-Vpx ought to be prevented from degrading it. However, the Src-Vpx construct we have devised here contains a processing sequence for the viral protease between the c-Src epitope and Flag-Vpx that is meant to release free Vpx once in the viral particle. The short size of the lost c-Src tag makes it impossible for us to determine whether and with what efficiency this processing occurs. However, Src-Vpx is proficient in mediating SAMHD1 degradation, indicating that either SAMHD1 can also be degraded in the cytoplasm, contrary to what has been reported (47, 48), or that Src-Vpx is processed in such a manner that it is able to access and degrade the nuclear pool of SAMHD1.
In this respect, the major advantage of HIV-1-Src-Vpx LVs over HIV-1 LVs is that by completing the early phases of infection more efficiently, the viral dose required to obtain the same proportion of transduced cells can be lowered by at least 10-fold. This difference is of the utmost importance, as it translates to a lower impact of viral transduction on cell physiology, as shown here through the analysis of the maturation phenotype and of the viability of MDDCs. Our results are in contrast to those indicating that incorporation of Vpx into HIV-1 LVs via modification of the p6 domain of HIV-1 Gag induce the strong maturation of MDDCs and a strong secretion of interferon (24), as we observed no maturation and little IFN-α secretion. At present, the reasons for these discrepancies are unknown, and differences in vector design or in the experimental system used may account for them. However, the HIV-1-Src-Vpx LVs developed here preserve the functionality of MDDCs, indicating that these vectors provide a notable advance for use in gene therapy applications to preserve the immature status of MDDCs.
Lastly, the results shown here offer for the first time the possibility of modifying the cellular tropism of HIV-1 LVs using a nonstructural viral protein that acts after viral entry and, more specifically, promotes reverse transcription in a cell type-specific manner. While HIV-1-Src-Vpx LVs remain as proficient as WT HIV-1 in the transduction of other activated cell types, selective targeting of primary myeloid cells with HIV-1-Src-Vpx LVs can be achieved either under conditions in which these cells are the main susceptible cell type (as in PBMCs) or by combining it with other mechanisms of control. Recently, Vpx has been shown to promote infection of purified quiescent PBLs (49). In our infection of PBMCs, however, the proportion of quiescent lymphocytes found to be positively transduced in the presence of Vpx was largely inferior to that of monocytes, indicating that if Vpx plays a role in the transduction of qPBLs, this role is certainly more modest than the one played in myeloid cells.
Importantly, we have been able to combine postentry events mediated by Vpx with an Env-mediated entry restriction through the use of SVGmu that recognizes DC-SIGN (41). Although SVGmu-pseudotyped HIV-1 LVs were poorly infectious under the conditions and the MOI used here, the addition of Vpx significantly improved their infectivity and did so to the detriment of their infectivity in activated PBLs. In this case, however, transduction was accompanied by a strong maturation of MDDCs. We believe this is because the SVGmu engages the DC-SIGN lectin, which in turn may lead to MDDC maturation. Therefore, while these results indicate that the combination of entry and postentry regulatory mechanisms are particularly potent to achieve cell type-specific transduction, they also indicate that certain ameliorations are needed to direct viral particle entry to MDDCs without engaging a specific maturation pathway.
In the future, inclusion in these vectors of other mechanisms of control of transgene expression, such as cell type-specific promoters, or posttranscriptional regulatory elements, such as microRNA target sequences, may prove particularly helpful to achieving the ultimate goal of selective targeting of a given cell type of interest directly in vivo.
ACKNOWLEDGMENTS
We thank Jeanine Bernaud and Dominique Rigal for help with blood sample collection and the AIDS Research and Reference Reagents Program of the NIH for providing some of the material used here, David Baltimore and Pin Wang (USC Viterbi School of Engineering, Los Angeles, CA) for providing the SVGmu envelope protein, Philippe Mangeot for providing farnesylated GFP (Department of Virology, INSERM U758, Lyon, France), and Bruno Lina and Manuel Rosa Calatrava for providing inactivated influenza virus (Faculty of Medicine, Laennec, Lyon, France).
A.C. is a CNRS researcher. J.T. is supported by ARC-1 Santé. S.D. is a former postdoctoral ANRS fellow and is presently supported by Sidaction. This work received the support of the ANRS, the ENS-L, the FRM, and Sidaction. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We declare no competing financial interests.
Footnotes
Published ahead of print 17 October 2012
REFERENCES
- 1. Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 [DOI] [PubMed] [Google Scholar]
- 2. Boudreau JE, Bonehill A, Thielemans K, Wan Y. 2011. Engineering dendritic cells to enhance cancer immunotherapy. Mol. Ther. 19:841–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dyall J, Latouche JB, Schnell S, Sadelain M. 2001. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood 97:114–121 [DOI] [PubMed] [Google Scholar]
- 4. Gruber A, Kan-Mitchell J, Kuhen KL, Mukai T, Wong-Staal F. 2000. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood 96:1327–1333 [PubMed] [Google Scholar]
- 5. Mangeot PE, Duperrier K, Negre D, Boson B, Rigal D, Cosset FL, Darlix JL. 2002. High levels of transduction of human dendritic cells with optimized SIV vectors. Mol. Ther. 5:283–290 [DOI] [PubMed] [Google Scholar]
- 6. Negre D, Duisit G, Mangeot PE, Moullier P, Darlix JL, Cosset FL. 2002. Lentiviral vectors derived from simian immunodeficiency virus. Curr. Top. Microbiol. Immunol. 261:53–74 [DOI] [PubMed] [Google Scholar]
- 7. Schroers R, Sinha I, Segall H, Schmidt-Wolf IG, Rooney CM, Brenner MK, Sutton RE, Chen SY. 2000. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system. Mol. Ther. 1:171–179 [DOI] [PubMed] [Google Scholar]
- 8. Tan PH, Beutelspacher SC, Xue SA, Wang YH, Mitchell P, McAlister JC, Larkin DF, McClure MO, Stauss HJ, Ritter MA, Lombardi G, George AJ. 2005. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood 105:3824–3832 [DOI] [PubMed] [Google Scholar]
- 9. Breckpot K, Emeagi P, Dullaers M, Michiels A, Heirman C, Thielemans K. 2007. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum. Gene Ther. 18:536–546 [DOI] [PubMed] [Google Scholar]
- 10. Chen X, He J, Chang LJ. 2004. Alteration of T cell immunity by lentiviral transduction of human monocyte-derived dendritic cells. Retrovirology 1:37 doi:10.1186/1742-4690-1-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2007. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4:2 doi:10.1186/1742-4690-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Brien WA, Namazi A, Kalhor H, Mao SH, Zack JA, Chen IS. 1994. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J. Virol. 68:1258–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. 1996. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J. Virol. 70:3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kloke BP, Schule S, Muhlebach MD, Wolfrum N, Cichutek K, Schweizer M. 2010. Functional HIV-2- and SIVsmmPBj-derived lentiviral vectors generated by a novel polymerase chain reaction-based approach. J. Gene Med. 12:446–452 [DOI] [PubMed] [Google Scholar]
- 15. Muhlebach MD, Wolfrum N, Schule S, Tschulena U, Sanzenbacher R, Flory E, Cichutek K, Schweizer M. 2005. Stable transduction of primary human monocytes by simian lentiviral vector PBj. Mol. Ther. 12:1206–1216 [DOI] [PubMed] [Google Scholar]
- 16. Wolfrum N, Muhlebach MD, Schule S, Kaiser JK, Kloke BP, Cichutek K, Schweizer M. 2007. Impact of viral accessory proteins of SIVsmmPBj on early steps of infection of quiescent cells. Virology 364:330–341 [DOI] [PubMed] [Google Scholar]
- 17. Bergamaschi A, Ayinde D, David A, Le Rouzic E, Morel M, Collin G, Descamps D, Damond F, Brun-Vezinet F, Nisole S, Margottin-Goguet F, Pancino G, Transy C. 2009. The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection. J. Virol. 83:4854–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. 2006. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther. 13:991–994 [DOI] [PubMed] [Google Scholar]
- 19. Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, Stevenson M. 1998. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: evidence of macrophage-dependent viral amplification. Nat. Med. 4:1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, Sharkey M, Stevenson M. 2008. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 4:e1000057 doi:10.1371/journal.ppat.1000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. 2008. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 4:e1000059 doi:10.1371/journal.ppat.1000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sunseri N, O'Brien M, Bhardwaj N, Landau NR. 2011. Human immunodeficiency virus type 1 modified to package simian immunodeficiency virus Vpx efficiently infects macrophages and dendritic cells. J. Virol. 85:6263–6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berger A, Munk C, Schweizer M, Cichutek K, Schule S, Flory E. 2010. Interaction of Vpx and apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A) correlates with efficient lentivirus infection of monocytes. J. Biol. Chem. 285:12248–12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berger A, Sommer AF, Zwarg J, Hamdorf M, Welzel K, Esly N, Panitz S, Reuter A, Ramos I, Jatiani A, Mulder LC, Fernandez-Sesma A, Rutsch F, Simon V, Konig R, Flory E. 2011. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutieres syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 7:e1002425 doi:10.1371/journal.ppat.1002425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. 2011. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7:e1002221 doi:10.1371/journal.ppat.1002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laguette N, Rahm N, Sobhian B, Chable-Bessia C, Munch J, Snoeck J, Sauter D, Switzer WM, Heneine W, Kirchhoff F, Delsuc F, Telenti A, Benkirane M. 2012. Evolutionary and functional analyses of the interaction between the myeloid restriction factor SAMHD1 and the lentiviral Vpx protein. Cell Host Microbe 11:205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. 2012. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim ES, Fregoso OI, McCoy CO, Matsen FA, Malik HS, Emerman M. 2012. The ability of primate lentiviruses to degrade the monocyte restriction factor SAMHD1 preceded the birth of the viral accessory protein Vpx. Cell Host Microbe 11:194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, Cimarelli A. 2011. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat. Protoc. 6:806–816 [DOI] [PubMed] [Google Scholar]
- 32. Naldini L, Blomer U, Gage FH, Trono D, Verma IM. 1996. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 93:11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gallina A, Mantoan G, Rindi G, Milanesi G. 1994. Influence of MA internal sequences, but not of the myristylated N-terminus sequence, on the budding site of HIV-1 Gag protein. Biochem. Biophys. Res. Commun. 204:1031–1038 [DOI] [PubMed] [Google Scholar]
- 34. Verderame MF, Nelle TD, Wills JW. 1996. The membrane-binding domain of the Rous sarcoma virus Gag protein. J. Virol. 70:2664–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou W, Parent LJ, Wills JW, Resh MD. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyake M, Mizutani S, Koide H, Kaziro Y. 1996. Unfarnesylated transforming Ras mutant inhibits the Ras-signaling pathway by forming a stable Ras.Raf complex in the cytosol. FEBS Lett. 378:15–18 [DOI] [PubMed] [Google Scholar]
- 37. Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. 2011. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480:379–382 [DOI] [PubMed] [Google Scholar]
- 38. Frecha C, Szecsi J, Cosset FL, Verhoeyen E. 2008. Strategies for targeting lentiviral vectors. Curr. Gene Ther. 8:449–460 [DOI] [PubMed] [Google Scholar]
- 39. Funke S, Maisner A, Muhlebach MD, Koehl U, Grez M, Cattaneo R, Cichutek K, Buchholz CJ. 2008. Targeted cell entry of lentiviral vectors. Mol. Ther. 16:1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Humbert JM, Frecha C, Bouafia FA, N′Guyen TH, Boni S, Cosset FL, Verhoeyen E, Halary F. 2012. Measles virus glycoprotein-pseudotyped lentiviral vectors are highly superior to vesicular stomatitis virus G pseudotypes for genetic modification of monocyte-derived dendritic cells. J. Virol. 86:5192–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang L, Yang H, Rideout K, Cho T, Joo KI, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, Wang P. 2008. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. 2007. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 362:304–313 [DOI] [PubMed] [Google Scholar]
- 44. Harman AN, Kraus M, Bye CR, Byth K, Turville SG, Tang O, Mercier SK, Nasr N, Stern JL, Slobedman B, Driessen C, Cunningham AL. 2009. HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood 114:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harman AN, Wilkinson J, Bye CR, Bosnjak L, Stern JL, Nicholle M, Lai J, Cunningham AL. 2006. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J. Immunol. 177:7103–7113 [DOI] [PubMed] [Google Scholar]
- 46. Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. 2010. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143:789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brandariz-Nunez A, Valle-Casuso JC, White TE, Laguette N, Benkirane M, Brojatsch J, Diaz-Griffero F. 2012. Role of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac. Retrovirology 9:49 doi:10.1186/1742-4690-9-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hofmann H, Logue EC, Bloch N, Daddacha W, Polsky SB, Schultz ML, Kim B, Landau NR. 2012. The Vpx lentiviral accessory protein targets SAMHD1 for degradation in the nucleus. J. Virol. [Epub ahead of print.] doi:10.1128/JVI.01657-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baldauf HM, Pan X, Erikson E, Schmidt S, Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg T, Panitz S, Flory E, Landau NR, Sertel S, Rutsch F, Lasitschka F, Kim B, Konig R, Fackler OT, Keppler OT. 2012. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. [Epub ahead of print.] Nat. Med. doi:10.1038/nm.2964 [DOI] [PMC free article] [PubMed] [Google Scholar]