Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of Kaposi's sarcoma (KS), and KSHV activation of mitogen-activated protein kinases (MAPKs) initiates a number of key pathogenic determinants of KS. Direct inhibition of signal transduction as a therapeutic approach presents several challenges, and a better understanding of KSHV-induced mechanisms regulating MAPK activation may facilitate the development of new treatment or prevention strategies for KS. MAPK phosphatases, including dual-specificity phosphatase-1 (DUSP1), negatively regulate signal transduction and cytokine activation through MAPK dephosphorylation or interference with effector molecule binding to MAPKs, including the extracellular signal-regulated kinase (ERK). We found that ERK-dependent latent viral gene expression, the induction of promigratory factors, and cell invasiveness following de novo infection of primary human endothelial cells are in part dependent on KSHV suppression of DUSP1 expression during de novo infection. KSHV-encoded miR-K12-11 upregulates the expression of xCT (an amino acid transporter and KSHV fusion/entry receptor), and existing data indicate a role for xCT in the regulation of 14-3-3β, a transcriptional repressor of DUSP1. We found that miR-K12-11 induces endothelial cell secretion of promigratory factors and cell invasiveness through upregulation of xCT-dependent, 14-3-3β-mediated suppression of DUSP1. Finally, proof-of-principle experiments revealed that pharmacologic upregulation of DUSP1 inhibits the induction of promigratory factors and cell invasiveness during de novo KSHV infection. These data reveal an indirect role for miR-K12-11 in the regulation of DUSP1 and downstream pathogenesis.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV) remains one of the most common etiologies for cancers arising in patients with immune suppression (1, 2), including Kaposi's sarcoma (KS) (3). KS remains one of the most common HIV/AIDS-associated malignancies in the modern era (2), and the current standard of care for systemic KS—highly active antiretroviral therapy (HAART) and nontargeted cytotoxic chemotherapies—yields little or no benefit for a substantial proportion, if not the majority, of HIV-infected patients with this disease (4). Chemotherapy also incurs toxicities compounding those already induced by antiretrovirals and other medications used for the treatment of HIV and its many complications (4, 5). A better understanding of how KSHV initiates cellular pathogenesis following de novo infection may give rise to novel therapeutic approaches.
Mitogen-activated protein kinases (MAPKs), including the extracellular signal-regulated kinase (ERK), the c-Jun N-terminal kinase (JNK), and p38, play critical roles in the regulation of cell growth, differentiation, and control of cellular responses to cytokines and stress (6). During de novo infection, KSHV proteins activate signal transduction mediated by MAPKs to facilitate the establishment of latent viral gene expression, the activation of promigratory cytokines and chemokines, and the induction of cell motility and angiogenesis (7–16). Although compounds directly targeting intracellular kinases are under evaluation in clinical trials for cancer, their lack of target specificity and toxicities limit their clinical utility (17). A better understanding of regulatory mechanisms for kinase activation by oncogenic viruses, including KSHV, may facilitate the development of more tractable therapies for viral tumors.
The MAPK phosphatases (MKPs) are dual-specificity phosphatases (DUSPs) grouped according to subcellular localization and substrate specificity (6, 18). The DUSP family consists of 11 members that dephosphorylate MAPKs through recognition of TXY motifs or interfere with MAPK binding to effector molecules (18, 19). The prototype of this family—DUSP1 (encoding the protein MKP-1)—regulates the activation of ERK, JNK, and p38, and alteration of DUSP1 expression has been associated with malignant progression for a variety of cancers (18, 20). Existing data suggest that DUSPs may be differentially regulated in a cell type-specific manner during KSHV infection (21–25) and that repression of DUSP3 (23), upregulation of DUSP5 (21, 22), and upregulation of DUSP7 (25) occur during KSHV infection. Another study reported upregulation of DUSP1 transcript expression within human foreskin fibroblasts and a B cell tumor line following KSHV infection, but whether DUSP1 expression was altered during KSHV infection of human adult dermal microvascular endothelial cells used in that study was not reported (24). With the exception of a single study reporting DUSP5 upregulation by the KSHV-encoded G protein-coupled receptor (vGPCR) (21), these studies employed gene array analyses to investigate the expression of DUSPs without confirmation of specific KSHV-associated mechanisms for DUSP regulation. Therefore, a better understanding of specific mechanisms for KSHV regulation of DUSPs and the functional consequences of this regulation are needed. Given the well-characterized suppression of ERK, JNK, and p38 activation by DUSP1, the inverse correlation between DUSP1 and progression for some tumors (18, 20), ample published data for KSHV activation of ERK, JNK, and p38, and the presence of a putative binding site for KSHV miR-K12-1 within the DUSP1 3′ untranslated region (UTR) cloned from KSHV-infected primary effusion lymphoma (PEL) cell lines (26), we sought to determine more definitively whether KSHV regulates DUSP1 expression and, if so, to define KSHV-encoded mechanisms and functional consequences for this effect.
MATERIALS AND METHODS
Cell culture, reagents, and infection assays.
KSHV-infected PEL cells (BCBL-1) were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES (pH 7.5), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 0.05 mM β-mercaptoethanol, and 0.02% (wt/vol) sodium bicarbonate. Primary human umbilical vein endothelial cells (HUVEC) were grown in Dulbecco's modified Eagle's medium (DMEM)–F-12 50/50 medium (Cellgro) supplemented with 5% FBS. Primary human foreskin fibroblasts (HFF) were maintained in DMEM (Gibco) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. To obtain KSHV for infection experiments, BCBL-1 cells were incubated with 0.6 mM valproic acid for 6 days, and purified virus was concentrated from culture supernatants as described previously (27). For negative controls using UV light-inactivated KSHV (UV-KSHV), viral aliquots were exposed to 1,200 J/cm2 UV light for 10 min using a CL-1000 UV cross-linker. Infectious titers were determined using both HUVEC and HFF and methods described previously (28). Dexamethasone was purchased from Sigma.
Immunofluorescence assays (IFA).
Briefly, 1 × 104 HUVEC or HFF per well were seeded in eight-well chamber slides (Nunc) and were incubated with serial dilutions of freshly prepared viral stocks in the presence of 8 μg/ml Polybrene (Sigma-Aldrich) for 2 h at 37°C. After remaining in culture overnight, cells were incubated first in methanol-acetone (1:1) at 20°C for fixation and permeabilization and then with a blocking reagent (10% normal goat serum, 3% bovine serum albumin, and 1% glycine) for an additional 30 min. Cells were then incubated for 1 h at 25°C with a 1:1,000 dilution of a rat monoclonal antibody against latency-associated nuclear antigen (LANA) (ABI), followed by a 1:100 dilution of a goat anti-rat secondary antibody conjugated to Texas Red (Invitrogen). For the identification of nuclei, cells were subsequently counterstained with 0.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma) in 180 mM Tris-HCl (pH 7.5). Slides were washed once in 180 mM Tris-HCl for 15 min and were prepared for visualization using a Leica TCPS SP5 AOBS confocal microscope. Relative LANA expression was calculated as the number of LANA dots per 100 cells in the experimental group divided by the number of LANA dots per 100 cells in the control group.
Transduction assays.
HUVEC or HFF were transduced for 48 h with a recombinant adenovirus (AdV) vector encoding DUSP1 or a control vector using a multiplicity of infection (MOI) of ∼20 as described previously (29). According to 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assays (see “Cell viability assays” below for methods), this protocol resulted in no discernible toxicity for transduced cells over the time course of our experiments (data not shown).
Cloning.
The 3′ UTR of DUSP1 was amplified from genomic DNA isolated from HUVEC and was cloned into the XbaI site of the pGL3 luciferase (3′ position relative to luciferase; Promega). The primer sequences are listed in Table 1. Insertion of the DUSP1 3′ UTR was confirmed through commercial gene sequencing (Operon).
Table 1.
Primer sequences for qRT-PCR/qPCR and cloning
| Gene | Primer orientation | Primer sequence (5′ → 3′) |
|---|---|---|
| DUSP1 3′ UTR | Sense | GCGCTCTAGAAAGGCCACGGGAGGTGAGG |
| Antisense | GCGCTCTAGACCATTGTATAAAAAAGTCAT | |
| ORF73 | Sense | TCCCTCTACACTAAACCCAATA |
| Antisense | TTGCTAATCTCGTTGTCCC | |
| ORF71 | Sense | GGGCACGGATGACAGGGAA |
| Antisense | TGTGATGGGCCGGAAAGG | |
| ORF72 | Sense | CCCTCGGGACTTCTGGAT |
| Antisense | CGTCGCTAAGACTGCCTC | |
| IL-6R | Sense | AGTTCCAGCTTCGATACCGAC |
| Antisense | GTATTGTCAGACCCAGAGCTG | |
| IL-8R1 | Sense | CTTTGCCCTGACCTTGCC |
| Antisense | AACACCATCCGCCATTTT | |
| IL-8R2 | Sense | TCTGCTACGGATTCACCC |
| Antisense | AGAGCCAACAAAGGAAGG | |
| VEGFR1 | Sense | GACTAGATAGCGTCACCAG |
| Antisense | AATACTCCGTAAGACCACA | |
| VEGFR2 | Sense | AAAGGGTGGAGGTGACTG |
| Antisense | GACATAAATGACCGAGGC | |
| DUSP1 | Sense | CGCTCCTTCTTCGCTTTCA |
| Antisense | GCTCGTCCAGCAACACCAC | |
| β-actin | Sense | GGAAATCGTGCGTGACATT |
| Antisense | GACTCGTCATACTCCTGCTTG |
Transfection and luciferase reporter assays.
HUVEC and HFF were transfected with pcDNA3.1-FLAG-ERK (pcERK) or a control vector as described previously (30). Constructs encoding miR-K12-1 (pc-mi1) or miR-K12-11 (pc-mi11), and luciferase reporter constructs encoding complementary sequences for miR-K12-11 (pGL3-mi11 sensor), have been validated previously in transfection assays for determining the expression and function of mature KSHV microRNAs (miRNAs) (31). For the inhibition of mature miRNAs, 2′ O-methyl (2′ OMe) RNA antagomirs were designed and purchased from Dharmacon (Chicago, IL) as described previously (31–33). Cells were transfected with 0.1 μg pc-mi11, 0.1 μg pGL3-mi11 sensor, 300 pmol 2′ OMe RNA antagomirs, or 0.1 μg control vector for negative controls in 12-well plates by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and/or the DharmaFECT transfection reagent (Dharmacon, Chicago, IL) for 24 to 48 h according to the manufacturer's instructions and prior to subsequent incubation with KSHV. For RNA interference assays, 14-3-3β small interfering RNA (siRNA) or negative-control siRNA (Santa Cruz) was delivered using commercial siRNA transfection reagents (Santa Cruz). xCT siRNA or negative-control siRNA (Dharmacon, Chicago, IL) was delivered using the DharmaFECT transfection reagent. For DUSP1 3′ UTR reporter assays, cells were transfected with 0.1 μg of the pGL3-DUSP1 3′ UTR construct with or without 0.1 μg pc-mi11 for 48 h using Lipofectamine 2000. For luciferase expression assays, cells were incubated with 100 μl lysis buffer according to the manufacturer's instructions (Promega, Madison, WI), and luciferase activity was determined using a Berthold FB12 luminometer (Titertek, Huntsville, AL). Light units were normalized to total protein levels for each sample using the bicinchoninic acid (BCA) protein assay kit according to the manufacturer's instructions (Pierce, Rockford, IL). Transfection efficiency was assessed through cotransfection of a lacZ reporter construct, and β-galactosidase activity was determined using a commercially available β-galactosidase enzyme assay system according to the manufacturer's instructions (Promega, Madison, WI). Two to three independent transfections were performed for each experiment, and all samples were analyzed in triplicate for each transfection. For some experiments, transfection efficiency was further assessed on a single-cell level through cotransfection with a green fluorescent protein (GFP) construct and subsequent IFA. This procedure was performed using primary cells and HEK cells as a positive control, since the transfection efficiency of GFP for HEK cells approaches 90%. Using this method, we confirmed a HUVEC transfection efficiency of approximately 50% of cells in our experiments.
Immunoblotting.
Cells were lysed in a buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM EDTA, 5 mM NaF, and 5 mM Na3VO4. Total-cell lysates (30 μg) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and immunoblotted with 100 to 200 μg/ml of antibodies against 14-3-3β, phospho-p44/42 ERK (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), phospho-JNK (Tyr185), t-p44/42 ERK, t-p38, t-JNK (Cell Signaling Technologies), DUSP1, and xCT (Santa Cruz). For loading controls, blots were reacted with antibodies detecting β-actin (Sigma). Immunoreactive bands were developed using an enhanced chemiluminescence reaction (Perkin-Elmer), visualized by autoradiography, and quantified using ImageJ software.
Transwell invasion assays.
Matrigel invasion chambers (Becton Dickinson) were hydrated for 4 h at 37°C with a culture medium. After hydration, the medium in the bottom of the well was replaced with fresh medium; then 2 × 104 HUVEC or 5 × 104 HFF were plated in the top of the chamber. After a 24-h incubation, the cells were fixed with 4% formaldehyde for 15 min at room temperature and the chambers were rinsed in phosphate-buffered saline (PBS) and stained with 0.2% crystal violet for 10 min. After the chambers were washed five times with dH2O, the cells at the top of the Matrigel membrane were removed with cotton applicators. The cells at the bottom of the membrane (representing cells that have penetrated/migrated through the membrane) were counted using a phase-contrast microscope. Relative invasion for cells in experimental groups was calculated as the number of invading cells in the experimental group divided by the number of invading cells in the control group (28).
ELISA.
Concentrations of vascular endothelial growth factor (VEGF), interleukin 8 (IL-8), and IL-6 in culture supernatants were determined by using human VEGF (Pierce Biotechnology), IL-8 (Becton Dickinson), and IL-6 (eBioscience) enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers' instructions.
PCR.
Total cellular DNA was prepared using the QIAamp DNA minikit according to the manufacturer's instructions (Qiagen). Briefly, cells (2 h or 24 h postinfection) were trypsinized for 5 min at 37°C and were collected with 1 ml of ice-cold DMEM. Cells were pelleted at 2,000 rpm for 5 min, washed, and resuspended in 200 μl of 1× PBS, and total DNA was prepared according to the manufacturer's instructions. To ensure that viral DNA amplification in these experiments was not the result of “carryover” viral DNA from culture supernatants rather than intracellular virus, cells were washed several times in fresh medium prior to trypsinization, and samples from culture supernatants following these washes were assessed for viral DNA content. Total RNA was isolated from infected or uninfected cells using the RNeasy minikit according to the manufacturer's instructions (Qiagen). cDNA was synthesized from 0.5 ng of total RNA by using the SuperScript III First-Strand Synthesis SuperMix kit (Invitrogen) according to the manufacturer's procedures. The primers designed for target genes are displayed in Table 1. Amplification experiments were carried out using an iCycler IQ real-time PCR detection system, and cycle threshold (CT) values were tabulated in triplicate (DNA) or in duplicate (cDNA) for each gene of interest for each experiment. For KSHV-miRNA measurement, cDNA was synthesized using the TaqMan microRNA reverse transcription (RT) kit (Applied Biosystems), and quantitative PCR (qPCR) was performed using the TaqMan microRNA assay kit (Applied Biosystems) on a 7500 real-time PCR system. “No-template” (water) controls were also used to ensure minimal background contamination. By use of mean CT values tabulated for different experiments, with CT values for β-actin as loading controls, fold changes for experimental groups relative to assigned controls were calculated using automated iQ5 software (version 2.0; Bio-Rad).
Cell viability assays.
Cell viability was assessed using a standard MTT assay (34). Briefly, a total of 5 × 103 cells were incubated in individual wells in a 96-well plate for 24 h. Twenty-four to 72 h after the addition of serial dilutions of dexamethasone or transduction with adenoviral vectors, cells were incubated first in a 1-mg/ml MTT solution (Sigma) at 37°C for 3 h and then in 50% dimethyl sulfoxide (DMSO) overnight prior to the determination of optical density at 570 nm by use of a spectrophotometer (Thermo Labsystems).
Statistical analysis.
Significance for differences between experimental and control groups was determined using the two-tailed Student t test (Excel, version 8.0). P values of <0.05 or <0.01 were considered significant or highly significant, respectively.
RESULTS
KSHV suppresses DUSP1 expression during de novo infection.
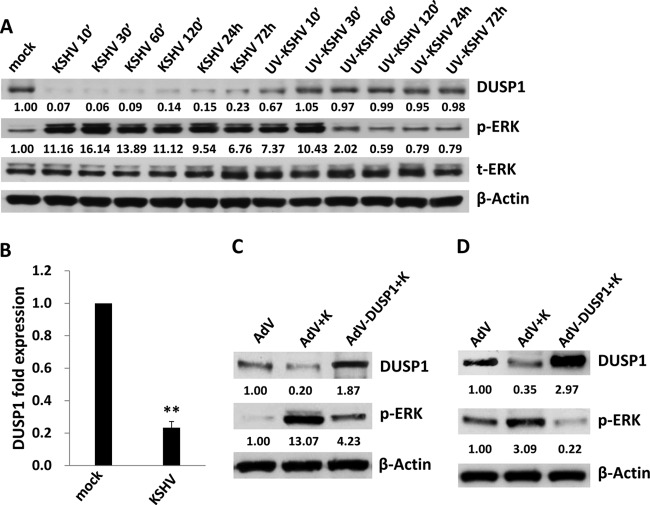
Immunoblotting was used to determine the kinetics of DUSP1 expression during de novo infection of HUVEC with KSHV. DUSP1 expression was reduced by >90% relative to control levels within 10 min of KSHV incubation (Fig. 1A) and remained reduced by >75% up to 72 h. These changes paralleled increases in phospho-ERK expression (Fig. 1A), as well as p38 and JNK expression (data not shown). DUSP1 expression was also reduced by UV-KSHV within 10 min, but by only approximately 30% from control levels, and returned to baseline within 30 min (Fig. 1A). Following an initial 10-fold increase, phospho-ERK expression induced by UV-KSHV returned to basal levels within 30 to 60 min (Fig. 1A). Using qRT-PCR, we also found that incubation of HUVEC with KSHV significantly reduced DUSP1 transcript expression within 48 h (Fig. 1B). To verify that ongoing ERK activation at later time points (>24 h) was in part mediated through KSHV suppression of DUSP1, we transduced KSHV-infected HUVEC with a recombinant adenoviral vector encoding DUSP1 and observed repression of KSHV activation of ERK (Fig. 1C), as well as p38 and JNK (data not shown). In contrast to the findings of another report (24), we also observed KSHV-induced suppression of DUSP1 expression 24 h after KSHV incubation with HFF, as well as repression of ERK activation with concurrent ectopic expression of DUSP1 in these cells (Fig. 1D).
Fig 1.
KSHV suppresses DUSP1 expression following de novo infection of endothelial cells and fibroblasts. (A) HUVEC were incubated with purified KSHV or UV-inactivated virus (UV-KSHV), and cell lysates were collected at the indicated time points for immunoblotting to identify DUSP1, as well as total and activated ERK (t-ERK and p-ERK, respectively). β-Actin was used as a loading control. Numbers indicate relative expression as determined using ImageJ software. (B) qRT-PCR was used to quantify DUSP1 transcripts 48 h after incubation of HUVEC with KSHV or medium alone (mock). Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01. (C and D) HUVEC (C) or HFF (D) were transduced using a recombinant human adenovirus encoding DUSP1 (AdV-DUSP1), or a control adenovirus (AdV), for 48 h prior to their incubation with purified KSHV (K) for 2 h. After an additional 24 h, protein expression was quantified by immunoblotting. Each immunoblot shown represents one of three independent experiments.
KSHV suppression of DUSP1 facilitates ERK-mediated latent viral gene expression during de novo infection.
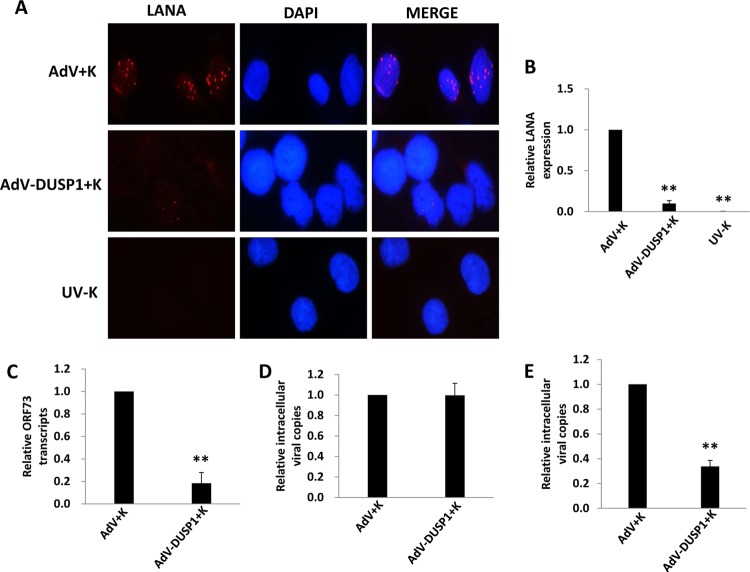
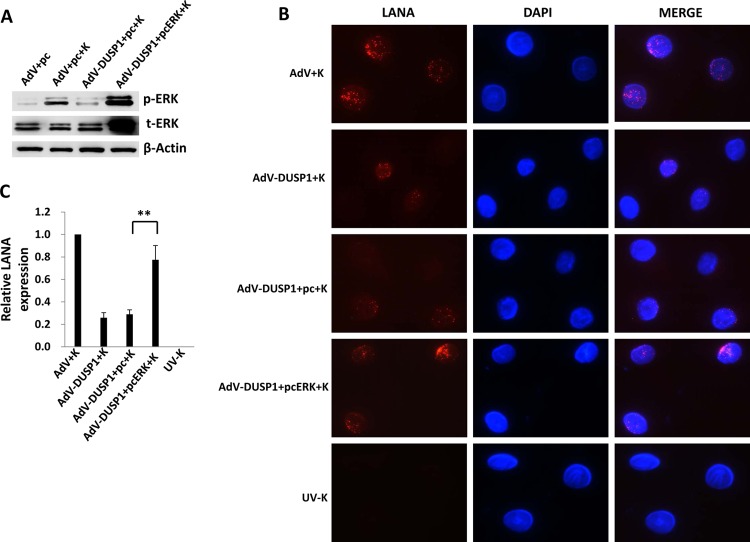
Published data indicate that ERK activation during KSHV infection of primary cells plays an essential role in the establishment of viral gene expression (10, 12, 15, 16, 35). Therefore, we sought to determine whether ectopic DUSP1 expression suppressed KSHV gene expression during de novo infection of primary human cells. IFA revealed that ectopic DUSP1 expression significantly reduced expression of the KSHV-encoded latency-associated nuclear antigen (LANA) during de novo infection of HUVEC (Fig. 2A and B) and HFF (data not shown). Moreover, qRT-PCR and qPCR confirmed that overexpression of DUSP1 reduced ORF73 (LANA) transcript expression but not the number of internalized viral episome copies, respectively, within 2 h of KSHV incubation with HUVEC (Fig. 2C and D) or HFF (data not shown). Within 24 h, intracellular viral episome copies were reduced in cells overexpressing DUSP1 (Fig. 2E), consistent with the transient nature of intracellular KSHV episomes in the absence of LANA expression (36). In addition, viral lytic gene expression remained at or below the relatively low levels exhibited by KSHV-infected control transductants (data not shown), consistent with published data implicating ERK as an important factor in the establishment of both latent and lytic viral gene expression (12, 30). In subsequent experiments, we transfected cells with a recombinant ERK construct validated previously (30) and found that restoration of ERK activation in the presence of ectopic DUSP1 expression partially restored the expression of LANA within KSHV-infected cells (Fig. 3).
Fig 2.
Ectopic expression of DUSP1 suppresses latent KSHV gene expression during de novo infection. HUVEC were transduced with AdV-DUSP1 (or a control AdV) for 48 h prior to their incubation with purified KSHV for 2 h. (A and B) IFA to identify LANA expression were performed 24 h after viral incubation using an anti-LANA monoclonal antibody and a secondary antibody conjugated to Texas Red, along with DAPI for nuclear colocalization (blue). LANA expression, signified by the typical punctate intranuclear staining pattern (red dots), was quantified relative to that in KSHV-infected/control-transduced cells as described in Materials and Methods. (C) qRT-PCR was used to quantify ORF73 (LANA) transcript expression 2 h after viral incubation. (D and E) qPCR was used to quantify intracellular KSHV DNA contents 2 h (D) and 24 h (E) after incubation of cells with the virus. Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01.
Fig 3.
ERK activation restores latent KSHV gene expression in the presence of DUSP1 overexpression. HUVEC were transduced with AdV-DUSP1 (or a control AdV) for 48 h and were then transfected with a recombinant ERK construct (pcERK) or a control vector (pc) for 24 h prior to their incubation with KSHV for 2 h. Cells transduced with the control AdV and cells incubated with UV-inactivated virus (UV-K) were used as controls. (A) Immunoblot analyses were performed 12 h after KSHV incubation to determine total and activated ERK expression. β-Actin was used as a loading control. (B and C) LANA expression was identified by IFA and was quantified relative to that in KSHV-infected/control-transduced cells as described above. Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01.
KSHV suppression of DUSP1 induces the secretion of promigratory factors and cell invasiveness during de novo infection.
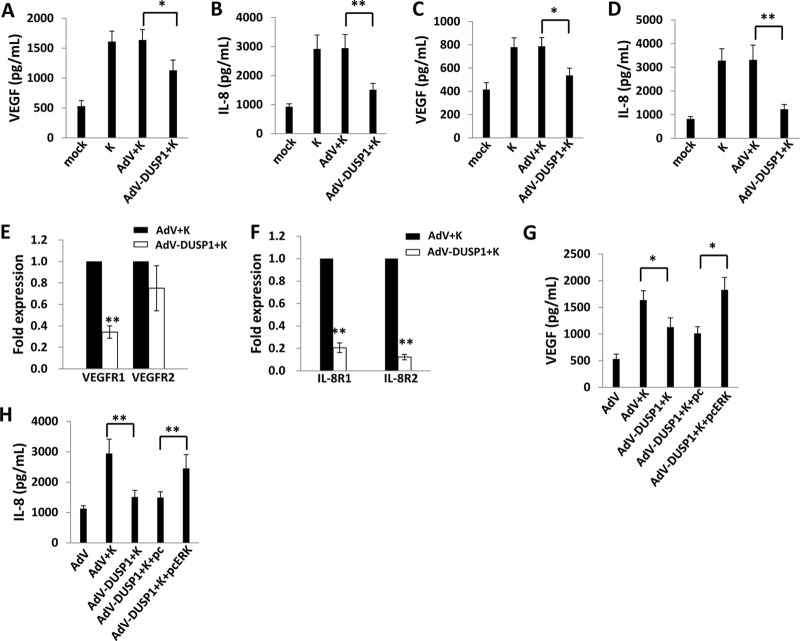
Negative regulation of MAPK activation by DUSP1 reduces inflammation associated with a variety of infectious and inflammatory diseases (37–41). Proinflammatory and promigratory factors induced by activated MAPKs, including VEGF, IL-8, and IL-6, are secreted by KSHV-infected cells, and their presence within KS lesions and the peripheral circulation of KS patients is thought to facilitate KSHV-associated cellular pathogenesis and angiogenesis (34, 42–50). To first determine whether KSHV-induced secretion of promigratory factors is, at least in part, mediated through KSHV repression of DUSP1, we quantified the secretion of VEGF and IL-8 by KSHV-infected primary cells in the presence or absence of ectopic DUSP1 expression. We found that ectopic DUSP1 expression suppressed KSHV-induced secretion of VEGF and IL-8 by HUVEC and HFF (Fig. 4A to D), as well as transcript expression for VEGF receptor-1 (VEGFR1) and IL-8 receptor isoforms (IL-8R1 and IL-8R2) (Fig. 4E and F). Furthermore, restoration of ERK activation derepressed VEGF and IL-8 secretion by KSHV-infected cells in the presence of ectopic DUSP1 expression (Fig. 4G and H). We also found that ectopic DUSP1 expression suppressed KSHV-induced secretion of IL-6 and the expression of transcripts for the IL-6 receptor (IL-6R), but restoration of ERK activation failed to restore IL-6 secretion in the presence of ectopic DUSP1 expression in KSHV-infected cells (data not shown). Using transwell invasion assays, we also found that ectopic DUSP1 expression suppressed KSHV-induced invasiveness following de novo infection and that invasiveness was restored under these conditions with concurrent reactivation of ERK (Fig. 5).
Fig 4.
Ectopic expression of DUSP1 suppresses ERK-dependent secretion of promigratory factors during de novo KSHV infection. (A to D) HUVEC (A and B) and HFF (C and D) were transduced with AdV-DUSP1 (or a control AdV) for 48 h prior to their incubation with purified KSHV for 2 h. Culture supernatants were collected 24 h later for the quantification of VEGF and IL-8 secretion by ELISA. (E and F) In parallel, qRT-PCR was used to quantify VEGF (E) and IL-8 (F) receptor transcript expression within HUVEC. (G and H) HUVEC were transduced for ectopic DUSP1 expression and, after 48 h, were transfected with a recombinant ERK construct (pcERK) or a control vector (pc) for 24 h; then they were incubated with purified KSHV for an additional 2 h. Culture supernatants were collected 24 h later for the quantification of VEGF and IL-8 secretion by ELISA. Error bars represent the standard errors of the means for three independent experiments. *, P < 0.05; **, P < 0.01.
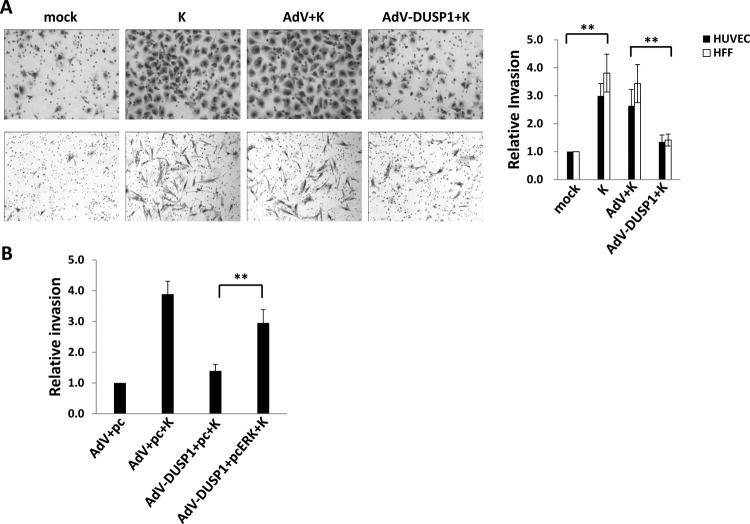
Fig 5.
Ectopic expression of DUSP1 suppresses cell invasiveness induced by de novo KSHV infection. (A) HUVEC (top row) or HFF (bottom row) were transduced as described above for DUSP1 overexpression prior to their incubation with KSHV for 2 h. Cells were then incubated for 24 h prior to the assessment of invasiveness. Relative invasiveness was calculated as described in Materials and Methods. (B) HUVEC were transduced with either a control AdV or AdV-DUSP1 for 48 h, transfected for an additional 24 h with constructs expressing ERK (pcERK) or a control vector (pc) as described above, and then incubated with KSHV for an additional 2 h. Transwell assays were used to quantify invasiveness 24 h following viral incubation. Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01.
miR-K12-11 induces endothelial cell invasion through indirect transcriptional repression of DUSP1.
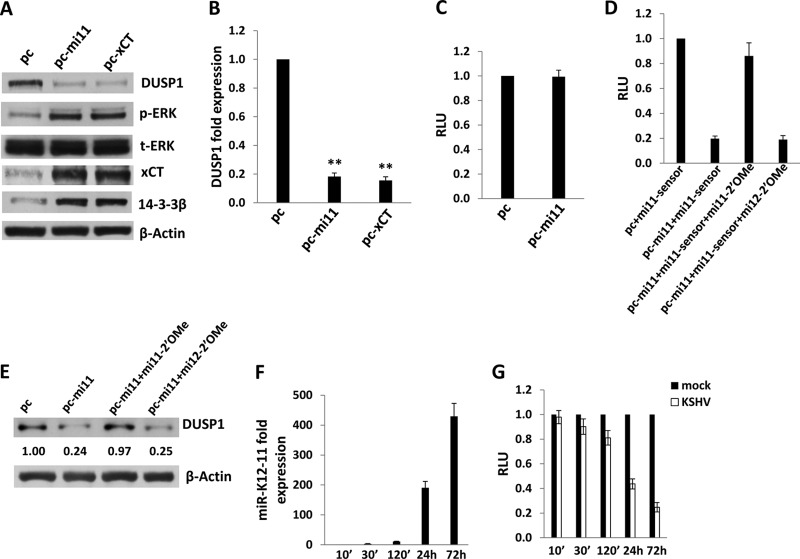
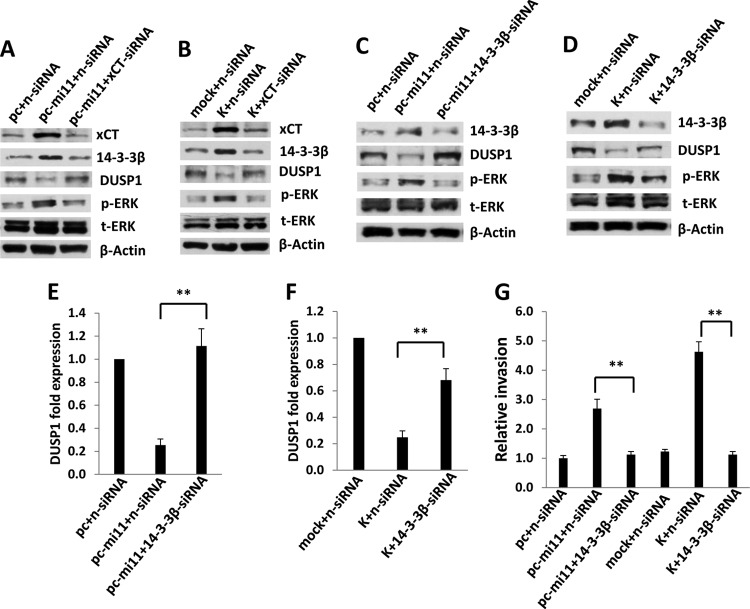
Next, we sought to define specific KSHV-encoded mechanisms responsible for the suppression of DUSP1 expression at later time points following the establishment of latent KSHV infection. Existing data suggest that the 14-3-3β/14-3-3β interactant 1 (FBI1) complex binds the promoter region of DUSP1 and acts as a transcriptional repressor, thereby increasing ERK activation and promoting anchorage-independent growth, tumorigenicity, and metastasis (51). One study also reported that overexpression of the amino acid transporter xCT (a fusion/entry receptor for KSHV [52]) induces upregulation of 14-3-3β expression in KSHV-infected cells through mechanisms not yet defined (53). We have reported previously that KSHV-encoded miR-K12-11 upregulates xCT expression through repression of BACH-1, a negative transcriptional regulator of xCT (32), and others have confirmed that miR-K12-11 targets the BACH-1 3′ UTR (33, 54). Based on these data, we hypothesized that miR-K12-11 indirectly suppresses DUSP1 expression through upregulation of xCT and 14-3-3β. To test this hypothesis, we first transfected HUVEC with a construct encoding miR-K12-11 and observed reduced DUSP1 protein and transcript expression, as well as a parallel increase in ERK activation (Fig. 6A and B). miR-K12-11 transfectants also exhibited increased expression of xCT, as previously demonstrated (32), as well as of 14-3-3β (Fig. 6A). Bioinformatics analyses revealed no putative binding sites for miR-K12-11 within the DUSP1 3′ UTR (data not shown), and luciferase reporter assays using a construct containing the full-length DUSP1 3′ UTR revealed that overexpression of miR-K12-11 failed to repress DUSP1 3′ UTR reporter activity (Fig. 6C). Using miR-K12-11 transfectants, we confirmed that miR-K12-11-specific 2′ OMe RNA antagomirs suppressed miR-K12-11 activity (Fig. 6D) and restored DUSP1 expression (Fig. 6E), whereas control antagomirs targeting miR-K12-12 had no effect. Using qRT-PCR to verify the kinetics of miR-K12-11 expression in HUVEC incubated with KSHV, we noted an increase in the expression of miR-K12-11 within 2 h (∼11-fold), with a more significant increase (∼430-fold) beginning at 24 h (Fig. 6F). For validation, luciferase reporter assays were performed, revealing discernible miR-K12-11 activity beginning 24 h after viral incubation (Fig. 6G).
Fig 6.
miR-K12-11 represses DUSP1 expression. (A and B) HUVEC were transfected with constructs encoding miR-K12-11 (pc-mi11), xCT (pc-xCT), or an empty control vector (pc). Twenty-four hours later, immunoblotting was performed to identify DUSP1, MAPK, xCT, and 14-3-3β protein expression (A), and qRT-PCR was performed to identify DUSP1 transcript expression (B). Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01. (C) Cells were cotransfected with the pGL3-DUSP1 3′ UTR and either a control vector or pc-mi11, and luciferase expression was quantified as described in Materials and Methods. (D) HUVEC were cotransfected with either a control vector or pc-mi11, along with the luciferase reporter construct for miR-K12-11 (mi11-sensor). Aliquots were also incubated with 300 pmol 2′ OMe RNA antagomirs targeting either miR-K12-11 or miR-K12-12 (the latter was used as a negative control). Forty-eight hours later, luciferase expression was quantified as described in Materials and Methods. (E) Immunoblotting was performed 48 h after the transfection of cells with either a control vector or pc-mi11 in the presence of 300 pmol of either a control antagomir or a miR-K12-11-specific antagomir. (F) HUVEC were incubated with purified KSHV; then miR-K12-11 expression was quantified at the time points indicated using qRT-PCR. Expression at each time point was quantified relative to that in cells incubated with KSHV for 10 min. (G) HUVEC were transfected with luciferase reporter constructs for miR-K12-11 for 24 h and were then incubated with purified KSHV or medium (mock). Luciferase expression was quantified as described in Materials and Methods for cells collected at the indicated time points. Error bars represent the standard errors of the means for three independent experiments.
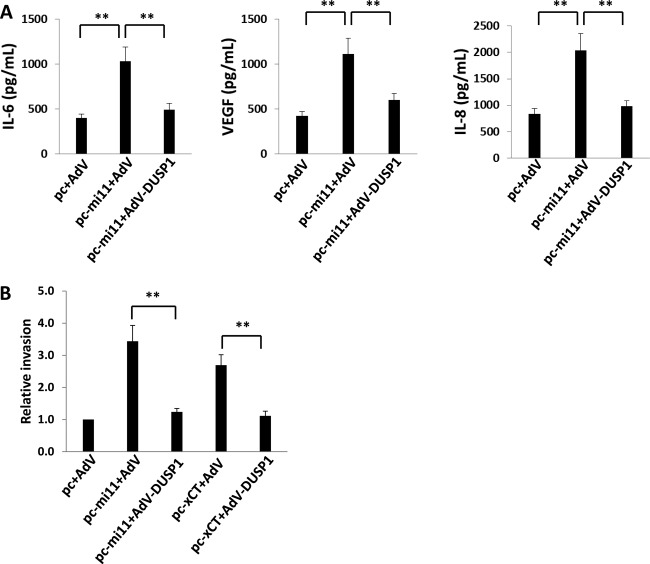
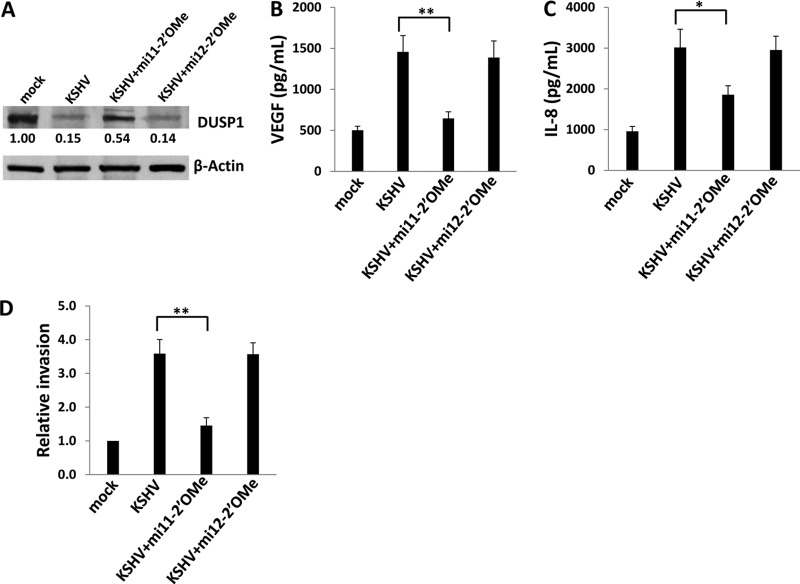
To begin to assess the functional relevance of miR-K12-11 regulation of DUSP1, we quantified IL-6, VEGF, and IL-8 secretion, as well as invasiveness, for miR-K12-11 transfectants in the presence or absence of ectopic DUSP1 expression. We found that miR-K12-11 significantly increased the secretion of IL-6, VEGF, and IL-8 by HUVEC, as well as HUVEC invasiveness, and that concurrent ectopic expression of DUSP1 (following the establishment of miR-K12-11 expression and function at 48 h) suppressed these effects (Fig. 7). To validate these data using KSHV-infected cells, we performed immunoblotting, confirming that repression of miR-K12-11 expression using miR-K12-11-specific antagomirs partially derepressed DUSP1 expression, whereas control miR-K12-12-specific antagomirs had no effect (Fig. 8A). Inhibition of miR-K12-11, but not that of miR-K12-12, also reduced VEGF and IL-8 secretion and invasiveness for KSHV-infected HUVEC (Fig. 8B to D).
Fig 7.
miR-K12-11 repression of DUSP1 induces the secretion of promigratory factors and endothelial cell invasiveness. (A) HUVEC were transfected with either a control vector or pc-mi11 and, after 24 h, were transduced with either a control AdV or AdV-DUSP1. After 48 h, ELISAs were used to quantify the levels of IL-6, VEGF, and IL-8 secreted in culture supernatants. (B) In parallel experiments, aliquots either were treated as described for panel A or were transfected with constructs encoding xCT (pc-xCT) for 24 h; then they were transduced with either a control AdV or AdV-DUSP1. After an additional 24 h, transwell assays were used to quantify invasiveness as described in Materials and Methods. Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01.
Fig 8.
Targeting miR-K12-11 restores DUSP1 expression and reduces the secretion of promigratory factors and invasiveness for KSHV-infected endothelial cells. (A) HUVEC were incubated with purified KSHV for 2 h and were then transfected with 300 pmol of either a miR-K12-11-specific or a miR-K12-12-specific antagomir. Forty-eight hours later, immunoblotting was performed for the identification of DUSP1 protein expression. β-Actin was used as a loading control. Numbers in immunoblots indicate relative expression as determined using ImageJ software. Data are the results of one of three independent experiments. (B and C) Cells were treated as described for panel A, and culture supernatants were collected for the quantification of VEGF and IL-8 secretion by ELISA. (D) Cells were treated as described for panel A, and transwell assays were used to quantify endothelial cell invasiveness. Error bars represent the standard errors of the means for three independent experiments. *, P < 0.05; **, P < 0.01.
To confirm that miR-K12-11 suppresses DUSP1 expression through upregulation of xCT, we first used a recombinant construct to overexpress xCT in HUVEC and noted reductions in basal DUSP1 protein and transcript expression, along with a parallel increase in ERK activation (Fig. 6A and B). We also observed increased HUVEC invasiveness with ectopic xCT expression, an effect repressed with concurrent ectopic expression of DUSP1 (Fig. 7B). Our RNA interference assays subsequently confirmed that targeting xCT resulted in the derepression of DUSP1 expression, as well as in the suppression of 14-3-3β expression and ERK activation, during either KSHV infection or miR-K12-11 transfection of HUVEC (Fig. 9A and B). To better confirm a role for 14-3-3β in miR-K12-11/xCT-induced suppression of DUSP1, we used RNA interference targeting 14-3-3β applied to both miR-K12-11-transfected cells and KSHV-infected cells. 14-3-3β suppression restored DUSP1 protein and transcript expression and reduced ERK phosphorylation under either condition (Fig. 9C to F). Furthermore, targeting 14-3-3β repressed cytokine and chemokine secretion induced by either miR-K12-11 transfection or KSHV infection (data not shown). Finally, targeting 14-3-3β reduced KSHV- or miR-K12-11-initiated invasiveness for HUVEC (Fig. 9G).
Fig 9.
Targeting xCT or 14-3-3β results in derepression of DUSP1 during KSHV infection. (A and B) HUVEC were transfected with control (n-siRNA) or xCT-specific siRNA, and after 24 h, the cells either were transfected with a control or a pc-mi11 vector (A) or were incubated with or without KSHV for 2 h (B). Twenty-four hours later, protein expression was determined by immunoblotting. (C to G) HUVEC were transfected with control (n-siRNA) or 14-3-3β-specific siRNA, and after 24 h, cells either were transfected with a control or a pc-mi11 vector or were incubated with or without KSHV for 2 h. Assays were performed 24 h after viral incubation. DUSP1 protein and transcript expression was determined by immunoblotting (C and D) and qRT-PCR (E and F), respectively. (G) Transwell assays were used to quantify invasiveness as described in Materials and Methods. Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01.
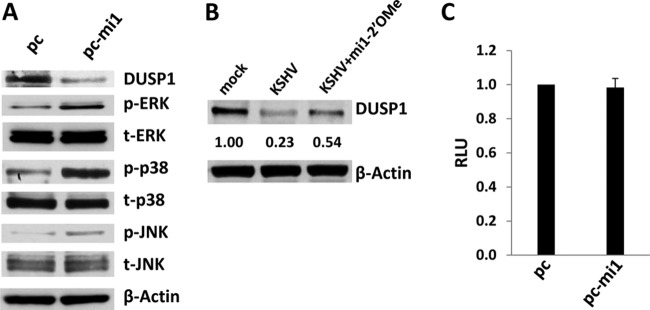
A recent study reported a putative binding site for miR-K12-1 within the DUSP1 3′ UTR cloned from KSHV-infected primary effusion lymphoma (PEL) cell lines (26). We found that ectopic expression of miR-K12-1 also suppresses basal DUSP1 expression and induces MAPK activation in HUVEC and that miR-K12-1-specific antagomirs partially restore DUSP1 expression in KSHV-infected cells (Fig. 10A and B). However, our luciferase reporter assays using a construct containing the full-length DUSP1 3′ UTR cloned from HUVEC indicated that overexpression of miR-K12-1 failed to repress DUSP 3′ UTR reporter activity (Fig. 10C), thus limiting our current understanding of how miR-K12-1 downregulates DUSP1 expression in HUVEC.
Fig 10.
miR-K12-1 represses DUSP1 expression. (A) HUVEC were transfected with constructs encoding miR-K12-1 (pc-mi1) or an empty control vector (pc). Twenty-four hours later, immunoblotting was performed to identify DUSP1 and MAPK protein expression. (B) Cells were incubated with or without (mock) purified KSHV for 2 h, and infected cells were subsequently transfected with or without 300 pmol 2′ OMe RNA antagomirs targeting miR-K12-1. Forty-eight hours later, immunoblotting was performed for the identification of DUSP1 protein expression. β-Actin was used as a loading control. Numbers in immunoblots indicate relative expression as determined using ImageJ software. Data in panels A and B represent one of three independent experiments. (C) Cells were cotransfected with the pGL3-DUSP1 3′ UTR and either a control vector or pc-mi1, and luciferase expression was quantified as described in Materials and Methods. Error bars represent the standard errors of the means for three independent experiments.
Pharmacologic induction of DUSP1 expression inhibits KSHV-associated cellular pathogenesis following de novo infection.
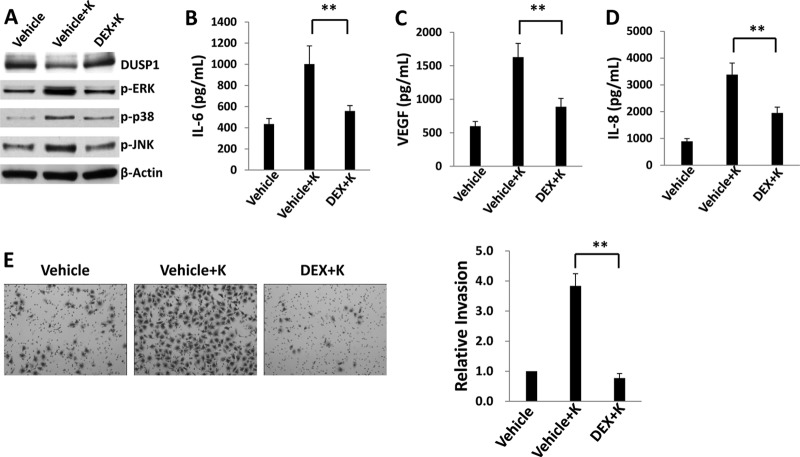
Glucocorticoids exert anti-inflammatory effects through the induction of glucocorticoid receptor (transcription factor)-mediated gene expression, and one glucocorticoid used frequently in clinical practice, dexamethasone, induces transcriptional activation of DUSP1 in a variety of cell types (55–58). Therefore, we conducted proof-of-principle experiments to determine whether pharmacologic upregulation of DUSP1 expression with dexamethasone is associated with KSHV-induced endothelial cell pathogenesis. First, we confirmed a lack of discernible toxicity for HUVEC incubated with dexamethasone over a wide range of concentrations (<10 μM) (data not shown). Subsequent experiments revealed that at noncytotoxic concentrations, dexamethasone restored DUSP1 expression in KSHV-infected HUVEC and suppressed KSHV activation of ERK, JNK, and p38 (Fig. 11A). Of note, dexamethasone exhibited no apparent effect on the expression of miR-K12-11 during KSHV infection of HUVEC (data not shown). In addition, dexamethasone suppressed KSHV-induced secretion of IL-6, VEGF, and IL-8 (Fig. 11B to D), as well as invasiveness for KSHV-infected cells (Fig. 11E). As observed during ectopic DUSP1 expression in the context of KSHV infection (Fig. 2), dexamethasone also reduced the expression of LANA and ORF73 transcripts during de novo infection of HUVEC with KSHV (data not shown).
Fig 11.
Pharmacologic derepression of DUSP1 suppresses KSHV-induced endothelial cell pathogenesis. (A) HUVEC were incubated first with either a vehicle or 1 μM dexamethasone (DEX) for 24 h and then with purified KSHV for 2 h. After an additional 24 h, immunoblotting was performed to identify the protein expression of DUSP1, activated MAPKs, and β-actin (loading control). (B to D) Cells were treated as described for panel A, and supernatants were collected for the quantification of IL-6, VEGF, and IL-8 secretion by ELISA. (E) Transwell assays were used to quantify invasiveness for cells treated as described for panel A. Relative invasiveness was determined as described in Materials and Methods. Error bars represent the standard errors of the means for three independent experiments. **, P < 0.01.
DISCUSSION
KSHV activation of MAPKs and associated cellular pathogenesis following de novo infection has been firmly established. KSHV-encoded IL-6 (vIL-6) and G protein-coupled receptor (vGPCR) upregulate angiopoietin-2 expression through the activation of ERK in lymphatic endothelial cells (14). vGPCR also induces VEGF expression and secretion through the activation of ERK and p38 (13). Furthermore, two KSHV genes, K15 and ORF49, initiate ERK and JNK/p38 activation, respectively (7, 8). Finally, ERK activation appears critically important for the establishment of KSHV gene expression during de novo infection (12, 30). Although these studies underscore the importance of MAPK activation for KS pathogenesis, clinical applications of small molecules targeting intracellular kinases are problematic given the nonselectivity of kinase inhibitors and associated toxicities (17). Identifying novel mechanisms for KSHV regulation of MAPK activation may, therefore, offer more clinically tractable approaches for the treatment and prevention of KS.
We found that KSHV represses DUSP1 expression during de novo infection. These data contradict results from one study mentioned previously (24) but are well supported by our observations of repression of DUSP1 protein and transcript expression under conditions of de novo infection of HUVEC and HFF by KSHV, repression of DUSP1 expression with ectopic expression of miR-K12-11, miR-K12-1, or xCT, and derepression of DUSP1 with miR-K12-11- or miR-K12-1-specific antagomirs applied to KSHV-infected HUVEC. Additional characterization of DUSP1 expression in a larger array of KSHV-infected primary cell types would help determine the cell type-specific nature of our findings. Although our work has initially focused on DUSP1 for reasons outlined above, “fine-tuning” of MAPK activation may occur in KSHV-infected cells as a result of simultaneous viral regulation of different DUSPs, and additional studies should confirm the functional relevance of these coordinated efforts.
Although our work to date has focused on KSHV miRNA-associated mechanisms for maintaining DUSP1 suppression at later time points during de novo infection, it is likely that multiple KSHV-associated mechanisms, including viral replication-independent mechanisms, participate in the suppression of DUSP1. This is supported by our observation that miR-K12-11 expression and function are optimal beginning 24 h after KSHV incubation with primary cells in our experiments, along with our kinetic assays that reveal DUSP1 suppression within 10 min of incubation with either UV-KSHV or KSHV. The half-lives of DUSP1 protein and transcripts are relatively short (less than 2 h and as short as 40 min [19]), so it is plausible that discernible differences in their expression may be observed over a short time frame. One possibility is that DUSP1 suppression in KSHV-infected cells is the result of ERK-dependent ubiquitin-proteasome degradation of DUSP1, as previously observed for other cancer cells (59). However, DUSP1 suppression with UV-KSHV is not observed at 30 min despite the induction of ERK activation to levels 10-fold greater than those for controls at this time point (the latter result is in accord with previously published data for the kinetics of ERK activation for cells following their incubation with UV-KSHV [12]). It is also possible that proteasome activity unrelated to ERK influences DUSP1 expression; proteasome activity influences signaling in human KSHV-infected lymphoma cell lines (60), so we cannot categorically dismiss this possibility.
A second possibility is that virus interactions with the cell surface may trigger DUSP1 downregulation. Our observation that UV-KSHV reduces DUSP1 expression within 10 min, but that heat-inactivated KSHV does not (data not shown), supports this concept. One report indicates that KSHV interactions with toll-like receptor-4 (TLR4) mediate innate immune responses to KSHV (61), and alterations in DUSP1 expression in response to TLR4 engagement (62) and dendritic cell interactions with influenza virus (63) have been reported. It is plausible, therefore, that in the absence of established KSHV gene expression (as with UV-KSHV), TLR-induced, MAPK-dependent activation of cytokines induces subsequent activation of DUSP1 in order to repress MAPK activation in a negative feedback loop.
A recent report indicates that murine herpesvirus-68 miRNAs are packaged within the virion (64). Therefore, a third possibility is that packaged KSHV miRNAs suppress DUSP1 expression at early time points, and the less-robust DUSP1 suppression observed with UV-KSHV within 10 min of viral incubation may result from an attenuated effect of these miRNAs due to UV-irradiation (and no effect at later time points).
In addition to the likelihood of DUSP1 regulation by replication-independent mechanisms in KSHV-infected cells, it is plausible that DUSP1 expression is regulated by other replication-dependent mechanisms. Previous work has indicated that multiple KSHV miRNAs upregulate xCT, possibly through the targeting of other transcriptional repressors, including c-Maf (32, 65, 66). In addition, despite our results for luciferase assays indicating a lack of direct interactions between miR-K12-11 or miR-K12-1 and the DUSP1 3′ UTR, we cannot categorically exclude direct interactions between these two miRNAs and DUSP1, since it has been recognized that both cellular and viral miRNAs bind sequences within the 5′ UTR and coding regions of target genes (67–70). Moreover, the binding of KSHV miRNA to DUSP1 UTRs may be cell type specific. Nevertheless, our data suggest that multiple KSHV miRNAs coordinately regulate DUSP expression.
Our data are consistent with published data indicating that the upregulation of xCT by miR-K12-11 results in xCT-mediated activation of 14-3-3β expression, which, in turn, leads to 14-3-3β-mediated transcriptional repression of DUSP1 (32, 33, 51, 53, 54). For additional confirmation, we show that targeting 14-3-3β restores DUSP1 expression and reduces the production of promigratory factors and endothelial cell invasiveness under conditions of either KSHV infection or ectopic miR-K12-11 expression. The expression of FBI1 and its effect on the transcriptional repression of DUSP1 were not directly addressed in our studies. Additionally, our data do not exclude the possibility that KSHV induction of 14-3-3β expression influences other genes which themselves regulate DUSP1 expression, cytokine expression, and/or endothelial cell invasiveness. For example, published data indicate that 14-3-3β facilitates Raf/Ras activation through the coupling of protein kinase C-ζ to Raf-1 (71), and we and others have demonstrated that KSHV induction of ERK-dependent VEGF expression and endothelial cell invasiveness are due, in part, to KSHV regulation of Ras/Raf activation (34, 72). Furthermore, the mechanisms through which xCT induces 14-3-3β expression require additional clarification.
DUSP1 expression is induced by growth factors, the initiation of cellular stress, or pharmaceuticals, including retinoids and glucocorticoids (55–58, 73–76). We found that the glucocorticoid dexamethasone induces DUSP1 expression while inhibiting KSHV activation of ERK during de novo infection. We cannot exclude the possibility that dexamethasone impacts the expression of KSHV genes that regulate ERK activation, or the expression of genes (viral or cellular) that impact cell invasiveness independent of DUSP1. Moreover, although glucocorticoids are used routinely for the treatment of KSHV-associated lymphoma (77), they also upregulate phosphatidylinositol 3-kinase (PI3K)/Akt activation and nitric oxide synthase activity (78), both of which are associated with KS progression (32, 79). Existing clinical data further suggest that glucocorticoids may increase the incidence of KS in organ transplant recipients or may exacerbate existing KS lesions (80–87), and it remains controversial whether glucocorticoids induce lytic viral gene expression in latently infected cells (82, 88). DNA-damaging agents such as doxorubicin may actually induce DUSP1 expression (19), and a recent study indicated that overexpression of DUSP1 protected breast cancer cells from chemotherapy-induced apoptosis through the repression of caspase activation and DNA fragmentation caused by doxorubicin or paclitaxel (89). The effects of DUSP1 induction may, therefore, prove undesirable in the context of KS treatment with either of these agents. Additional studies are clearly needed to determine whether pharmacologic upregulation of DUSP1 offers a tractable and safe approach to the treatment of KS.
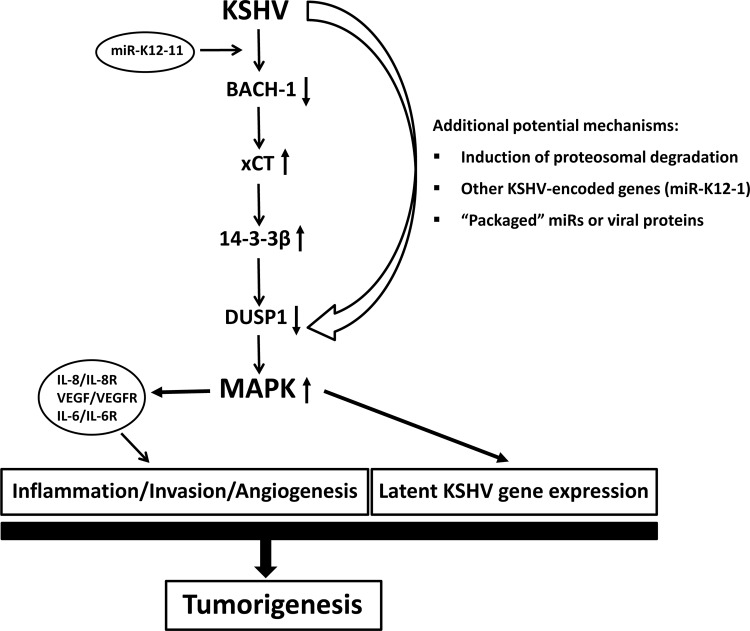
In summary, our data reveal suppression of DUSP1 expression by miR-K12-11 through indirect mechanisms involving xCT-mediated upregulation of a transcriptional repressor of DUSP1 (14-3-3β) in KSHV-infected cells. We hypothesize that, along with replication-independent mechanisms for KSHV suppression of DUSP1 expression, this contributes to the constitutive MAPK activation and pathogenesis related to KS (Fig. 12).
Fig 12.
Proposed schematic representation of DUSP1 regulation by KSHV.
ACKNOWLEDGMENTS
We thank Wei Li (Chinese Academy of Sciences, Beijing, China) for providing the pcDNA3.1-xCT construct, Yusuf Hannun (Medical University of South Carolina [MUSC], Charleston, SC) for providing LacZ constructs, Scott Eblen (MUSC, Charleston, SC) for providing pcDNA3.1-FLAG-ERK (pcERK) constructs, and Narayan Bhat (MUSC, Charleston, SC) for providing HFF. We especially thank Rolf Renne (University of Florida, Gainesville, FL) for providing constructs for the overexpression of miR-K12-11 and miR-K12-1, as well as pGL3-miRNA sensor vectors and bioinformatics analysis.
This work was supported by grants from the National Institutes of Health (R01-CA142362, to C.P., and R01-DE018290, to K.K.), a South Carolina COBRE for Oral Health (P20-RR017696; C.P., subproject investigator), the National Natural Science Foundation (81272191, to Z.Q.) and NNSF for Young Scientists of China (81101791, to Z.Q.).
Footnotes
Published ahead of print 24 October 2012
REFERENCES
- 1. Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, Costagliola D, Salmon D, Chene G, Morlat P. 2004. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer 101:317–324 [DOI] [PubMed] [Google Scholar]
- 2. Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ. 2008. Cancer risk in people infected with human immunodeficiency virus in the United States. Int. J. Cancer 123:187–194 [DOI] [PubMed] [Google Scholar]
- 3. Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 4. Nguyen HQ, Magaret AS, Kitahata MM, Van Rompaey SE, Wald A, Casper C. 2008. Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: characterizing the predictors of clinical response. AIDS 22:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanni T, Sprinz E, Machado MW, Santana RDC, Fonseca BA, Schwartsmann G. 2006. Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat. Rev. 32:445–455 [DOI] [PubMed] [Google Scholar]
- 6. Chang L, Karin M. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37–40 [DOI] [PubMed] [Google Scholar]
- 7. Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 77:9346–9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez CM, Wong EL, Bowser BS, Hong GK, Kenney S, Damania B. 2006. Identification and characterization of the Orf49 protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 80:3062–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuang E, Tang Q, Maul GG, Zhu F. 2008. Activation of p90 ribosomal S6 kinase by ORF45 of Kaposi's sarcoma-associated herpesvirus and its role in viral lytic replication. J. Virol. 82:1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan H, Xie J, Ye F, Gao SJ. 2006. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 80:5371–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. 2007. Kaposi's sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J. Virol. 81:3949–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 79:10308–10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873–4880 [PubMed] [Google Scholar]
- 14. Vart RJ, Nikitenko LL, Lagos D, Trotter MW, Cannon M, Bourboulia D, Gratrix F, Takeuchi Y, Boshoff C. 2007. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res. 67:4042–4051 [DOI] [PubMed] [Google Scholar]
- 15. Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. 2008. Reactivation of Kaposi's sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 371:139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie J, Pan H, Yoo S, Gao SJ. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 79:15027–15037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Yang PL, Gray NS. 2009. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 9:28–39 [DOI] [PubMed] [Google Scholar]
- 18. Wu GS. 2007. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 26:579–585 [DOI] [PubMed] [Google Scholar]
- 19. Boutros T, Chevet E, Metrakos P. 2008. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol. Rev. 60:261–310 [DOI] [PubMed] [Google Scholar]
- 20. Keyse SM. 2008. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 27:253–261 [DOI] [PubMed] [Google Scholar]
- 21. Glaunsinger B, Ganem D. 2004. Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J. Exp. Med. 200:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matta H, Surabhi RM, Zhao J, Punj V, Sun Q, Schamus S, Mazzacurati L, Chaudhary PM. 2007. Induction of spindle cell morphology in human vascular endothelial cells by human herpesvirus 8-encoded viral FLICE inhibitory protein K13. Oncogene 26:1656–1660 [DOI] [PubMed] [Google Scholar]
- 23. McAllister SC, Hansen SG, Ruhl RA, Raggo CM, DeFilippis VR, Greenspan D, Fruh K, Moses AV. 2004. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood 103:3465–3473 [DOI] [PubMed] [Google Scholar]
- 24. Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72–84 [DOI] [PubMed] [Google Scholar]
- 25. Poole LJ, Yu Y, Kim PS, Zheng QZ, Pevsner J, Hayward GS. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, Shamulailatpam P, Love CL, Dave SS, Tuschl T, Ohler U, Cullen BR. 2011. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe 10:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qin Z, Kearney P, Plaisance K, Parsons CH. 2010. Pivotal advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J. Leukoc. Biol. 87:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qin Z, Dai L, Slomiany MG, Toole BP, Parsons C. 2010. Direct activation of emmprin and associated pathogenesis by an oncogenic herpesvirus. Cancer Res. 70:3884–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu H, Li Q, Herbert B, Zinna R, Martin K, Junior CR, Kirkwood KL. 2011. Anti-inflammatory effect of MAPK phosphatase-1 local gene transfer in inflammatory bone loss. Gene Ther. 18:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qin Z, DeFee M, Isaacs JS, Parsons C. 2010. Extracellular Hsp90 serves as a co-factor for MAPK activation and latent viral gene expression during de novo infection by KSHV. Virology 403:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, Renne R. 2007. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 3:e65 doi:10.1371/journal.ppat.0030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, Parsons C. 2010. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog. 6:e1000742 doi:10.1371/journal.ppat.1000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. 2007. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J. Virol. 81:12836–12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin Z, Dai L, Toole B, Robertson E, Parsons C. 2011. Regulation of Nm23-H1 and cell invasiveness by Kaposi's sarcoma-associated herpesvirus. J. Virol. 85:3596–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roupelieva M, Griffiths SJ, Kremmer E, Meisterernst M, Viejo-Borbolla A, Schulz T, Haas J. 2010. Kaposi's sarcoma-associated herpesvirus Lana-1 is a major activator of the serum response element and mitogen-activated protein kinase pathways via interactions with the Mediator complex. J. Gen. Virol. 91:1138–1149 [DOI] [PubMed] [Google Scholar]
- 36. De Leon Vazquez E, Kaye KM. 2011. The internal Kaposi's sarcoma-associated herpesvirus LANA regions exert a critical role on episome persistence. J. Virol. 85:7622–7633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhattacharyya S, Brown DE, Brewer JA, Vogt SK, Muglia LJ. 2007. Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 109:4313–4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chi H, Flavell RA. 2008. Acetylation of MKP-1 and the control of inflammation. Sci. Signal. 1:pe44 doi:10.1126/scisignal.141pe44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nieminen R, Korhonen R, Moilanen T, Clark AR, Moilanen E. 2010. Aurothiomalate inhibits cyclooxygenase 2, matrix metalloproteinase 3, and interleukin-6 expression in chondrocytes by increasing MAPK phosphatase 1 expression and decreasing p38 phosphorylation: MAPK phosphatase 1 as a novel target for antirheumatic drugs. Arthritis Rheum. 62:1650–1659 [DOI] [PubMed] [Google Scholar]
- 40. Sartori R, Li F, Kirkwood KL. 2009. MAP kinase phosphatase-1 protects against inflammatory bone loss. J. Dent. Res. 88:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. 2006. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J. Exp. Med. 203:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ensoli B, Sturzl M. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63–83 [DOI] [PubMed] [Google Scholar]
- 43. Grossmann C, Podgrabinska S, Skobe M, Ganem D. 2006. Activation of NF-κB by the latent vFLIP gene of Kaposi's sarcoma-associated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J. Virol. 80:7179–7185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCubrey JA, Lahair MM, Franklin RA. 2006. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 8:1775–1789 [DOI] [PubMed] [Google Scholar]
- 45. Qian LW, Xie J, Ye F, Gao SJ. 2007. Kaposi's sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J. Virol. 81:7001–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Riva G, Barozzi P, Torelli G, Luppi M. 2010. Immunological and inflammatory features of Kaposi's sarcoma and other Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-associated neoplasias. AIDS Rev. 12:40–51 [PubMed] [Google Scholar]
- 47. Schwarz M, Murphy PM. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-κB and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505–513 [DOI] [PubMed] [Google Scholar]
- 48. Shin YC, Joo CH, Gack MU, Lee HR, Jung JU. 2008. Kaposi's sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1α to induce vascular endothelial growth factor expression. Cancer Res. 68:1751–1759 [DOI] [PubMed] [Google Scholar]
- 49. Sivakumar R, Sharma-Walia N, Raghu H, Veettil MV, Sadagopan S, Bottero V, Varga L, Levine R, Chandran B. 2008. Kaposi's sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors A and C early during in vitro infection of human microvascular dermal endothelial cells: biological implications. J. Virol. 82:1759–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Subramanian R, Sehgal I, D'Auvergne O, Kousoulas KG. 2010. Kaposi's sarcoma-associated herpesvirus glycoproteins B and K8.1 regulate virion egress and synthesis of vascular endothelial growth factor and viral interleukin-6 in BCBL-1 cells. J. Virol. 84:1704–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Komiya Y, Kurabe N, Katagiri K, Ogawa M, Sugiyama A, Kawasaki Y, Tashiro F. 2008. A novel binding factor of 14-3-3β functions as a transcriptional repressor and promotes anchorage-independent growth, tumorigenicity, and metastasis. J. Biol. Chem. 283:18753–18764 [DOI] [PubMed] [Google Scholar]
- 52. Kaleeba JA, Berger EA. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 311:1921–1924 [DOI] [PubMed] [Google Scholar]
- 53. Zeng Y, Li Y, Chen RS, He X, Yang L, Li W. 2010. Overexpression of xCT induces up-regulation of 14-3-3β in Kaposi's sarcoma. Biosci. Rep. 30:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. 2007. A viral microRNA functions as an orthologue of cellular miR-155. Nature 450:1096–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Engelbrecht Y, de Wet H, Horsch K, Langeveldt CR, Hough FS, Hulley PA. 2003. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology 144:412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. 2001. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 20:7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. 2002. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22:7802–7811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu W, Pew T, Zou M, Pang D, Conzen SD. 2005. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J. Biol. Chem. 280:4117–4124 [DOI] [PubMed] [Google Scholar]
- 59. Lin YW, Chuang SM, Yang JL. 2003. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J. Biol. Chem. 278:21534–21541 [DOI] [PubMed] [Google Scholar]
- 60. Saji C, Higashi C, Niinaka Y, Yamada K, Noguchi K, Fujimuro M. 2011. Proteasome inhibitors induce apoptosis and reduce viral replication in primary effusion lymphoma cells. Biochem. Biophys. Res. Commun. 415:573–578 [DOI] [PubMed] [Google Scholar]
- 61. Lagos D, Vart RJ, Gratrix F, Westrop SJ, Emuss V, Wong PP, Robey R, Imami N, Bower M, Gotch F, Boshoff C. 2008. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4:470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cho IJ, Woo NR, Kim SG. 2008. The identification of C/EBPβ as a transcription factor necessary for the induction of MAPK phosphatase-1 by toll-like receptor-4 ligand. Arch. Biochem. Biophys. 479:88–96 [DOI] [PubMed] [Google Scholar]
- 63. Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, Lander ES, Hacohen N. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870–875 [DOI] [PubMed] [Google Scholar]
- 64. Cliffe AR, Nash AA, Dutia BM. 2009. Selective uptake of small RNA molecules in the virion of murine gammaherpesvirus 68. J. Virol. 83:2321–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dhakshinamoorthy S, Jaiswal AK. 2002. c-Maf negatively regulates ARE-mediated detoxifying enzyme genes expression and anti-oxidant induction. Oncogene 21:5301–5312 [DOI] [PubMed] [Google Scholar]
- 66. Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36:683–685 [DOI] [PubMed] [Google Scholar]
- 67. Grey F, Tirabassi R, Meyers H, Wu G, McWeeney S, Hook L, Nelson JA. 2010. A viral microRNA down-regulates multiple cell cycle genes through mRNA 5′ UTRs. PLoS Pathog. 6:e1000967 doi:10.1371/journal.ppat.1000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee I, Ajay SS, Yook JI, Kim HS, Hong SH, Kim NH, Dhanasekaran SM, Chinnaiyan AM, Athey BD. 2009. New class of microRNA targets containing simultaneous 5′-UTR and 3′-UTR interaction sites. Genome Res. 19:1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Orom UA, Nielsen FC, Lund AH. 2008. MicroRNA-10a binds the 5′ UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 30:460–471 [DOI] [PubMed] [Google Scholar]
- 70. Tsai NP, Lin YL, Wei LN. 2009. MicroRNA mir-346 targets the 5′-untranslated region of receptor-interacting protein 140 (RIP140) mRNA and up-regulates its protein expression. Biochem. J. 424:411–418 [DOI] [PubMed] [Google Scholar]
- 71. Van Der Hoeven PC, Van Der Wal JC, Ruurs P, Van Dijk MC, Van Blitterswijk J. 2000. 14-3-3 isotypes facilitate coupling of protein kinase C-ζ to Raf-1: negative regulation by 14-3-3 phosphorylation. Biochem. J. 345(Part 2):297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Akula SM, Ford PW, Whitman AG, Hamden KE, Bryan BA, Cook PP, McCubrey JA. 2005. B-Raf-dependent expression of vascular endothelial growth factor-A in Kaposi sarcoma-associated herpesvirus-infected human B cells. Blood 105:4516–4522 [DOI] [PubMed] [Google Scholar]
- 73. Lornejad-Schafer MR, Schafer C, Graf D, Haussinger D, Schliess F. 2003. Osmotic regulation of insulin-induced mitogen-activated protein kinase phosphatase (MKP-1) expression in H4IIE rat hepatoma cells. Biochem. J. 371:609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Seta KA, Kim R, Kim HW, Millhorn DE, Beitner-Johnson D. 2001. Hypoxia-induced regulation of MAPK phosphatase-1 as identified by subtractive suppression hybridization and cDNA microarray analysis. J. Biol. Chem. 276:44405–44412 [DOI] [PubMed] [Google Scholar]
- 75. Sun H, Charles CH, Lau LF, Tonks NK. 1993. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487–493 [DOI] [PubMed] [Google Scholar]
- 76. Xu Q, Konta T, Furusu A, Nakayama K, Lucio-Cazana J, Fine LG, Kitamura M. 2002. Transcriptional induction of mitogen-activated protein kinase phosphatase 1 by retinoids. Selective roles of nuclear receptors and contribution to the antiapoptotic effect. J. Biol. Chem. 277:41693–41700 [DOI] [PubMed] [Google Scholar]
- 77. Chen YB, Rahemtullah A, Hochberg E. 2007. Primary effusion lymphoma. Oncologist 12:569–576 [DOI] [PubMed] [Google Scholar]
- 78. Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. 2002. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 8:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. 2006. Immortalization of primary endothelial cells by the K1 protein of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 66:3658–3666 [DOI] [PubMed] [Google Scholar]
- 80. Corda L, Benerecetti D, Ungari M, Facchetti F, Radaeli E. 1996. Kaposi's disease and sarcoidosis. Eur. Respir. J. 9:383–385 [DOI] [PubMed] [Google Scholar]
- 81. Farge D. 1993. Kaposi's sarcoma in organ transplant recipients. The Collaborative Transplantation Research Group of Ile de France. Eur. J. Med. 2:339–343 [PubMed] [Google Scholar]
- 82. Gill PS, Loureiro C, Bernstein-Singer M, Rarick MU, Sattler F, Levine AM. 1989. Clinical effect of glucocorticoids on Kaposi sarcoma related to the acquired immunodeficiency syndrome (AIDS). Ann. Intern. Med. 110:937–940 [DOI] [PubMed] [Google Scholar]
- 83. Gotti E, Remuzzi G. 1997. Post-transplant Kaposi's sarcoma. J. Am. Soc. Nephrol. 8:130–137 [DOI] [PubMed] [Google Scholar]
- 84. Penn I. 1991. The changing pattern of posttransplant malignancies. Transplant. Proc. 23:1101–1103 [PubMed] [Google Scholar]
- 85. Rady PL, Hodak E, Yen A, Memar O, Trattner A, Feinmesser M, David M, Hudnall SD, Tyring SK. 1998. Detection of human herpesvirus-8 DNA in Kaposi's sarcomas from iatrogenically immunosuppressed patients. J. Am. Acad. Dermatol. 38:429–437 [DOI] [PubMed] [Google Scholar]
- 86. Trattner A, Hodak E, David M, Sandbank M. 1993. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer 72:1779–1783 [DOI] [PubMed] [Google Scholar]
- 87. Vella JP, Mosher R, Sayegh MH. 1997. Kaposi's sarcoma after renal transplantation. N. Engl. J. Med. 336:1761. [DOI] [PubMed] [Google Scholar]
- 88. Zoeteweij JP, Rinderknecht AS, Davis DA, Yarchoan R, Blauvelt A. 2002. Minimal reactivation of Kaposi's sarcoma-associated herpesvirus by corticosteroids in latently infected B cell lines. J. Med. Virol. 66:378–383 [DOI] [PubMed] [Google Scholar]
- 89. Small GW, Shi YY, Higgins LS, Orlowski RZ. 2007. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 67:4459–4466 [DOI] [PubMed] [Google Scholar]