Abstract
Psychopathy is believed to be associated with brain abnormalities in both paralimbic (i.e., orbitofrontal cortex, insula, temporal pole, parahippocampal gyrus, posterior cingulate) and limbic (i.e., amygdala, hippocampus, anterior cingulate) regions. Recent structural imaging studies in both community and prison samples are beginning to support this view. Sixty six participants, recruited from community corrections centers, were administered the Hare Psychopathy Checklist Revised (PCL R), and underwent magnetic resonance imaging (MRI). Voxel based morphometry was used to test the hypothesis that psychopathic traits would be associated with gray matter reductions in limbic and paralimbic regions. Effects of lifetime drug and alcohol use on gray matter volume were covaried. Psychopathic traits were negatively associated with gray matter volumes in right insula and right hippocampus. Additionally, psychopathic traits were positively associated with gray matter volumes in bilateral orbital frontal cortex and right anterior cingulate. Exploratory regression analyses indicated that gray matter volumes within right hippocampus and left orbital frontal cortex combined to explain 21.8% of the variance in psychopathy scores. These results support the notion that psychopathic traits are associated with abnormal limbic and paralimbic gray matter volume. Furthermore, gray matter increases in areas shown to be functionally impaired suggests that the structure function relationship may be more nuanced than previously thought.
Keywords: structural MRI, voxel based morphometry (VBM), paralimbic cortex, limbic structures, substance use
1. Introduction
Psychopathy is a personality disorder characterized by abnormal affective, interpersonal, and behavioral functioning. Psychopathic traits include emotional deficits such as shallow affect and an inability to feel remorse and guilt; behavioral problems such as irresponsibility and impulsivity, stimulation seeking, and hot headedness are also noted features of the disorder (Cleckley, 1976; Hare, 2003). Psychopathy is typically assessed with the Psychopathy Checklist Revised (PCL R; Hare, 1991, 2003), a rating scale that consists of 20 affective, interpersonal, and behavioral items with higher scores indicating more severe psychopathic traits.
Identifying the neural basis of psychopathy using neuropsychological, psychophysical, and functional and structural neuroimaging techniques has been an active area of research. Given the pervasiveness of both emotional and behavioral symptoms in psychopathy, it is not surprising that dysfunction in a number of brain regions has been associated with the disorder (Kiehl et al., 2001; Veit et al., 2002; Muller et al., 2003; Birbaumer et al., 2005). For instance, Blair (2007) put forth a neurobiological theory of psychopathy that emphasizes ventromedial/orbital frontal cortex (OFC) and amygdala dysfunction, and the literature includes support for these abnormalities (Birbaumer et al., 2005; Kiehl et al., 2001; Veit et al., 2002; Harenski et al., 2010; Glenn et al., 2009). An alternative view asserts that the dysfunction is more widespread, encompassing additional limbic and paralimbic areas (Kiehl, 2006). In support of this model, work has identified abnormalities within the hippocampus and posterior cingulate during an affective memory task (Kiehl et al., 2001), parahippocampal gyrus during negative picture viewing (Muller et al., 2003), and anterior cingulate during aversive conditioning (Veit et al., 2002).
Though evaluating functional impairments in psychopathy has been fruitful, recently, interest in investigating structural abnormalities associated with adult psychopathy has increased. Many studies employ voxel based morphometry (VBM; Ashburner & Friston, 2000) to assess gray and/or white matter volume and/or concentration in the whole brain. Other morphometric studies have used anatomically based tracing techniques that focus on a single region. Either way, the growing literature on gray matter abnormalities in adult psychopathy is beginning to create an understanding of the abnormal neural architecture that may be related to observed emotional and behavioral deficits. For instance, within limbic and nearby paralimbic regions, abnormalities have been found in the hippocampus (Laakso et al., 2001; Raine et al., 2004), amygdala (Yang et al., 2009), insula (de Oliveira Souza et al., 2008), and striatum (Glenn et al., 2010).
Findings in the prefrontal cortex have been somewhat less consistent, but tend to also report reduced volume associated with psychopathy. For instance, Yang and colleagues (2005) found reduced prefrontal gray matter volumes related to high PCL R scores. Gray matter decreases have also been reported in lateral and left medial OFC (VBM measured concentration; de Oliveira Souza et al., 2008) and right cingulate (VBM measured volume; Muller et al., 2008a). In a large prison sample (N = 296), Ermer et al., 2012 found decreased gray matter volume and concentration in widespread areas of the OFC associated with psychopathy. Others have, however, reported increases in prefrontal gray matter volume and concentration in boys with CU traits (De Brito et al., 2009) or failed to find any differences in prefrontal gray matter related to PCL R score (Laakso et al., 2002).
In summary, most studies investigating brain morphometry in psychopathy have found decreases in the limbic, temporal, and prefrontal regions. It should be noted, however, that at least two studies have found gray matter increases in samples with high psychopathic traits (De Brito et al., 2009; Glenn et al., 2010). Noting that both functional and structural investigations have frequently found abnormalities in paralimbic and limbic regions, Kiehl (2006) has hypothesized that psychopathy may involve dysfunction of a distributed network of related regions that consists of anterior and posterior cingulate, anterior superior temporal gyrus (temporal pole), OFC, insula, parahippocampal gyrus, amygdala, and hippocampus. Inconsistencies regarding gray matter increases and decreases from study to study may be due to methodological variations in sample characteristics or analysis techniques; this may also suggest that a more nuanced explanation for the structure function relationship in psychopathy is appropriate.
It should also be noted that many of the studies reviewed above have focused on a single region (e.g., hippocampus: Laakso et al., 2001; Raine et al., 2004; amygdala: Yang et al., 2009; striatum: Glenn et al., 2010), rather than undertaking a whole brain approach. The present study considered multiple brain regions that have been implicated in psychopathy. Given the variety of previous findings, this approach may be preferred over more restrictive single region approaches. In addition, the current study utilized PCL R scores on a continuum, in line with recent studies (e.g., Muller et al., 2008a; de Oliveira Souza et al., 2008), and as consistently advocated recently (Edens et al., 2006; Guay et al., 2007). This also reduces potential cross study variation due to the use of different PCL R cutoff scores (e.g., cut off of 23: Raine et al., 2004) and is preferable when the number of participants scoring 30 or above on the PCL R is low, as is the case in non incarcerated samples such as this. Some previous studies have also not accounted for the potential confounding factors of drugs and alcohol (e.g., Laakso et al., 2001; de Oliveira Souza et al., 2008), which are well established as being comorbid with psychopathy (Smith and Newman, 1990), and to have a significant relationship with gray matter morphometry (Makris et al., 2008; Tanabe et al., 2009; Yuan et al., 2009). In line with prior studies that did consider substance use, and given the substance abusing nature of the present sample, an important aim of this paper was to separate the influences of psychopathic traits from those of drug and alcohol use. Given the inconsistencies in findings and the limitations of some previous work, the current study was undertaken in an effort to clarify the literature on heightened levels of psychopathic traits in individuals who are not currently incarcerated (see Kirkman, 2002). This research also helps fill a gap between incarcerated samples with high psychopathy scores and community samples (e.g., college students) with very low psychopathy scores.
To this end, the present study utilized VBM to search for abnormalities in gray matter volume related to heightened psychopathic traits in a sample of 66 participants recruited from community corrections (i.e., parole, probation, and drug treatment) centers. Regression analyses were used to evaluate the relationship between PCL R scores and gray matter volumes, and secondary statistical analyses quantitatively evaluated the contribution of gray matter abnormalities in specific a priori regions to the prediction of psychopathic traits. Given that most structural MRI studies have found volume and concentration reductions, and most functional studies have found paralimbic hypoactivity, we expected to see reduced gray matter volume in paralimbic and limbic regions. However, we also note that some structural studies have found gray matter increases and the structure function relationship remains poorly defined, enabling the possibility that some regions would instead show increased gray matter.
2. Methods
2.1 Participants
Seventy seven individuals were recruited from probation, parole, and drug treatment centers in the Hartford, CT area. Participants were excluded if they were under 18 or over 55 years of age, were currently pregnant, had a history of seizures or epilepsy, had a history of psychosis or a first degree family member with a psychotic disorder, had a history of loss of consciousness exceeding 30 minutes, or had any other MRI incompatibility (e.g., pacemaker or metal implants). All participants were fluent in English at or above a grade four reading level. Four participants were excluded for excessive head motion in the scanner, and seven did not have complete drug and alcohol use data, leaving 66 participants in the final sample (36 male, 30 female; mean age: 36.9 years, SD = 7.9). The majority of participants met Diagnostic and Statistical Manual of Mental Disorders (APA, 1994) criteria for alcohol abuse or dependence (74.2% [n = 49]), drug abuse and/or dependence (100.0% [n = 66]), or antisocial personality disorder1 (57.6% [n = 38]). Participants were 47% Caucasian (n = 31), 32% African American (n = 21), 17% Hispanic (n = 11), and 1.5% Asian (n = 1). Seventeen percent of the sample participants did not wish to respond to questions of race or ethnicity, and race or ethnicity information was not available for an additional 3% of the sample. Left handed participants were excluded in the present analyses. All aspects of the study were performed in accordance with the institution’s guidelines and regulations. Participants also provided informed consent and were compensated for their time.
2.2 Assessment materials
2.2.1. Psychopathy Checklist – Revised (PCL R)
The Psychopathy Checklist Revised (PCL R; Hare, 1991, 2003) is a widely used 20 item rating scale used to assess the construct of psychopathy. The interviewer evaluates how well each item applies to the interviewee and is scored 0 (doesn’t apply), 1 (applies somewhat), or 2 (definitely applies). Factor analysis of the PCL R has revealed two correlated factors (Hare, 1991): an interpersonal and affective factor (Factor 1; F1) and a social deviance factor (Factor 2; F2). F1 includes items such as glibness and superficial charm, pathological lying, and callousness. F2 consists of items related to impulsive and antisocial behavior, and includes poor behavioral controls, irresponsibility, and lack of realistic, long-term goals. The present study evaluated relationships with Total PCL R scores, as well as with both factor scores, in order to be consistent with the extant literature on structural deficits in psychopathy (e.g., de Oliveira Souza et al., 2008; Muller et al., 2008a). However, a four facet model of the PCL R has also been proposed (Hare, 2003; reviewed in Hare and Neumann, 2008), where Facet 1 is comprised of the interpersonal items from Factor 1 (glibness/superficial charm, grandiosity, pathological lying, and manipulation) and Facet 2 is defined by the affective items from Factor 1 (lack of remorse, shallow affect, callousness, and failure to accept responsibility). Facet 3 is comprised of the lifestyle items from Factor 2 (need for stimulation, parasitic lifestyle, lack of realistic goals, impulsivity, and irresponsibility), and Facet 4 is defined by the antisocial items from Factor 2 (poor behavioral controls, early behavioral problems, juvenile delinquency, revocation of conditional release, and criminal versatility). Thus, facet scores were also evaluated in supplementary analyses for completeness.
For participants recruited from local probation/parole offices, official criminal files were obtained; these files contained detailed criminal histories, as well as background information regarding school, family, and work histories. For participants recruited through drug and alcohol treatment facilities, professional credit and background checks were completed by SSC Inc. (Hartford, CT), which provided information regarding criminal, driving, employment, and credit history.
2.2.2. Cognitive, clinical, and substance use assessments
A measure of IQ was estimated using the Hopkins Adult Reading Test (HART; Schretlen et al., 2009). To evaluate for comorbid DSM IV Axis I and Axis II disorders (Table 1), participants were assessed using the Structured Clinical Interview for DSM Disorders (SCID; First et al., 2002). In terms of SCID diagnoses, only participants diagnosed with a lifetime psychotic disorder were excluded; 38 individuals met criteria for lifetime mood and/or anxiety disorders. No participants had a current severe mood or anxiety disorder according to DSM criteria2. A modified Addiction Severity Index (ASI; McLellan et al., 1992) was used to obtain lifetime drug and alcohol use severity measures. Drug (methamphetamine, cocaine, and heroin) history was defined as the cumulative years of “regular use” (defined as three or more times per week). Alcohol history was likewise calculated as the number of cumulative years of regular use. All participants self reported no current drug or alcohol use.
Table 1.
Diagnostic and Statistical Manual of Mental Disorders diagnoses.
| Axis I Diagnosis | n |
|---|---|
| Alcohol Use Disorder | 49 |
| Cannabis Use Disorder | 47 |
| Sedative, Hypnotic, or Anxiolytic Use Disorder | 3 |
| Cocaine Use Disorder | 60 |
| Opioid Use Disorder | 19 |
| Amphetamine Use Disorder | 2 |
| Hallucinogen Use Disorder | 11 |
| Other/Unknown Substance Use Disorder | 2 |
| Anxiety Disorder | 17 |
| Mood Disorder | 21 |
| Axis II Diagnosis | n |
| Antisocial Personality Disorder | 38 |
| Borderline Personality Disorder | 2 |
| Obsessive-Compulsive Personality Disorder | 1 |
| Narcissistic Personality Disorder | 1 |
| Personality Disorder Not Otherwise Specified | 1 |
Alcohol and drug use disorders include both abuse and dependence. Anxiety Disorder includes Anxiety Disorder Not Otherwise Specified (3), Social Phobia (3), Post-Traumatic Stress Disorder (5), Obsessive-Compulsive Disorder (1), Cocaine-Induced Anxiety Disorder (1), Other/Unknown Substance-Induced Anxiety (1), Panic Disorder (2), and Anxiety Disorder Due to General Medical Condition (1). Mood Disorder includes Major Depressive Disorder (13) and Substance- Induced Mood Disorder (6), Dysthymic Disorder (1), Mood Disorder Not Otherwise Specified (1).
2.3 MRI acquisition and analysis
High resolution T1 weighted structural MRI scans were acquired on a Siemens 3T Allegra scanner at the Olin Neuropsychiatry Research Center in Hartford, CT, using an MPRAGE pulse sequence (repetition time = 2500 ms, echo time = 2.74 ms, inversion time = 900 ms, flip angle = 8°, slice thickness = 1 mm, matrix size = 176 × 256) yielding 256 sagittal slices with an in plane resolution of 1 mm × 1 mm. Data were pre processed and analyzed using Statistical Parametric Mapping software (SPM5; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). T1 images were manually inspected by an operator blind to subject identity and realigned if improper spatial normalization was likely due to gross misalignment. In SPM5, tissue classification, bias correction, and image registration are performed in one integrated step3 (Ashburner and Friston, 2005). Images were spatially normalized to the SPM5 T1 template, segmented into gray matter, white matter, and cerebrospinal fluid, modulated to preserve total volume, and resampled to 2 × 2 × 2 mm. Voxels with a matter value of < 0.15 were excluded in order to remove possible edge effects between gray matter and white matter. Finally, segmented images were smoothed with a 10 mm full width at half maximum (FWHM) Gaussian kernel.
2.4 Analytic strategy
2.4.1. Primary whole brain and SVC analyses
Multiple regression analyses were performed on a voxel by voxel basis using the general linear model. We used total gray matter volume to account for individual variation in brain size (Bassitt et al., 2007; De Brito et al., 2009; Tanabe et al., 2009). Drug and alcohol history were also entered as control covariates. All whole brain analyses were thresholded at P < 0.005, uncorrected for multiple comparisons, with an extent threshold of 5 voxels (after de Oliveira Souza et al., 2008)4. The effects of PCL R Total score, F1, and F2 were each examined separately in primary analyses.
A priori regions of interest (ROIs) were defined by creating 10 mm spheres around coordinates obtained from the literature on structural and functional differences in psychopathy (see Table 2 for a complete list of all ROI coordinates). Although the present study employed a correlational approach, previous findings in psychopathy (whether they come from correlational or categorical analyses) represent the best tool for defining ROIs for this analysis. For each ROI, a small volume correction (SVC) was applied using a model identical to the primary whole brain analyses (i.e., with PCL R score as the predictor of interest, controlling for total gray matter volume and drug and alcohol history), and regions that survived SVC with a family wise error (FWE) corrected threshold of P < 0.05 are reported.
Table 2.
Regions of interest.
| Region | MNI Coordinates | Studies | |
|---|---|---|---|
| Anterior Cingulate Cortex | |||
| 1 | Rostral ACC | −1 35 7 | Birbaumer et al., 2005 |
| Muller et al., 2003 | |||
| Kiehl et al., 2001 | |||
| 2 | Dorsal ACC | −2 24 21 | Muller et al., 2003 |
| Kiehl et al., 2001 | |||
| Amygdala | |||
| 3 | L Amygdala | −19 2 –25 | Birbaumer et al., 2005 |
| Kiehl et al., 2001 | |||
| Veit et al., 2002 | |||
| 4 | R Amygdala | 18 −9 –15 | Muller et al., 2003 |
| Anterior Temporal Cortex | |||
| 5 | L Temporal Pole | −48 6 −34 | Muller et al., 2008a |
| de Oliveira-Souza et al., 2008 | |||
| Kiehl et al., 2001 | |||
| 6 | R Temporal Pole | 45 17 –31 | Muller et al., 2008 |
| Muller et al., 2008a | |||
| Kiehl et al., 2001 | |||
| Kiehl et al., 2004 | |||
| Hippocampus | |||
| 7 | L Hippocampus | −34 −11 −24 | bilateral counterpart of R hippocampus |
| 8 | R Hippocampus | 34 −11 −24 | Kiehl et al., 2001 |
| Insula | |||
| 9 | L Insula | −36 2 10 | de Oliveira-Souza et al., 2008 |
| 10 | R Insula | 33 −8 14 | de Oliveira-Souza et al., 2008 |
| Birbaumer et al., 2005 | |||
| Muller et al., 2003 | |||
| Orbital Frontal Cortex | |||
| 11 | L OFC | −26 53 −14 | de Oliveira-Souza et al., 2008 |
| 12 | L OFC | −28 20 –18 | de Oliveira-Souza et al., 2008 |
| 13 | R OFC | 10 34 –17 | Birbaumer et al., 2005 |
| de Oliveira-Souza et al., 2008 | |||
| 14 | R OFC | 22 14 –19 | de Oliveira-Souza et al., 2008 |
| Parahippocampal Cortex | |||
| 15 | L PHC | −32 −45 −16 | Tiihonen et al., 2008 |
| Muller et al., 2003 | |||
| Kiehl et al., 2001 | |||
| 16 | R PHC | 32 −45 −16 | bilateral counterpart of L PHC |
| Posterior Cingulate Cortex | |||
| 17 | L PCC | −9 −32 31 | de Oliveira-Souza et al., 2008 |
| Kiehl et al., 2001 | |||
| 18 | R PCC | 4 −20 46 | Muller et al., 2003 |
| Striatum | |||
| 19 | L Striatum | −19 14 2 | bilateral counterpart of R striatum |
| 20 | R Striatum | 19 14 2 | de Oliveira-Souza et al., 2008 |
These paralimbic regions were derived from the imaging literature on psychopathic traits. They were used for small volume correction (SVC), with a 10 mm sphere around coordinates. MNI, Montreal Neurological Institute; ACC, anterior cingulate cortex; L, left; R, right; OFC, orbital frontal cortex; PHC, parahippocampal cortex; PCC, posterior cingulate cortex.
2.4.2. Supplemental whole brain analyses
We performed three sets of supplemental analyses. In this sample, IQ was negatively correlated with both Total and F2 scores. Thus, this variable was entered as a covariate in a supplemental analysis in order to examine the effects of psychopathic traits on regional gray matter volume with the effects of IQ removed (PCL R Total score predicting regional gray matter volume, controlling for total gray matter volume, drug and alcohol history, and IQ; PCL R F2 score predicting regional gray matter volume, controlling for total gray matter volume, drug and alcohol history, and IQ). Second, the effects of Facets 1 4 were examined in supplemental analyses for completeness (PCL R facets predicting regional gray matter volume, controlling for total gray matter volume, and drug and alcohol history [one model for each facet]). Finally, given that nearly half of this sample was female, we also controlled for gender in a supplemental analysis (PCL R Total score predicting regional gray matter volume, controlling for total gray matter volume, drug and alcohol history, and gender).
2.4.3. Secondary regression analyses
An analysis was performed to ascertain the proportion of variance in psychopathy scores accounted for by gray matter volumes in our a priori coordinates. Volumetric values, indicating the average gray matter for the given voxel from each ROI were extracted from SPM5 and entered into three separate multiple regression equations (one for each of Total, F1, and F2) with regional brain volumes predicting PCL R scores using a forward selection method in SPSS (SPSS Inc., Chicago, IL). In forward selection, the predictor with the highest simple correlation with the outcome variable is first added and is retained if it significantly increases the ability of the model to predict the outcome, above and beyond the constant alone. Next, the predictor with the highest semi partial correlation with the outcome is added and is retained if it makes a significant contribution to the predictive power of the model. This process continues until no predictors significantly improve the model (Field, 2009).
3. Results
3.1 Psychopathy and individual differences variables
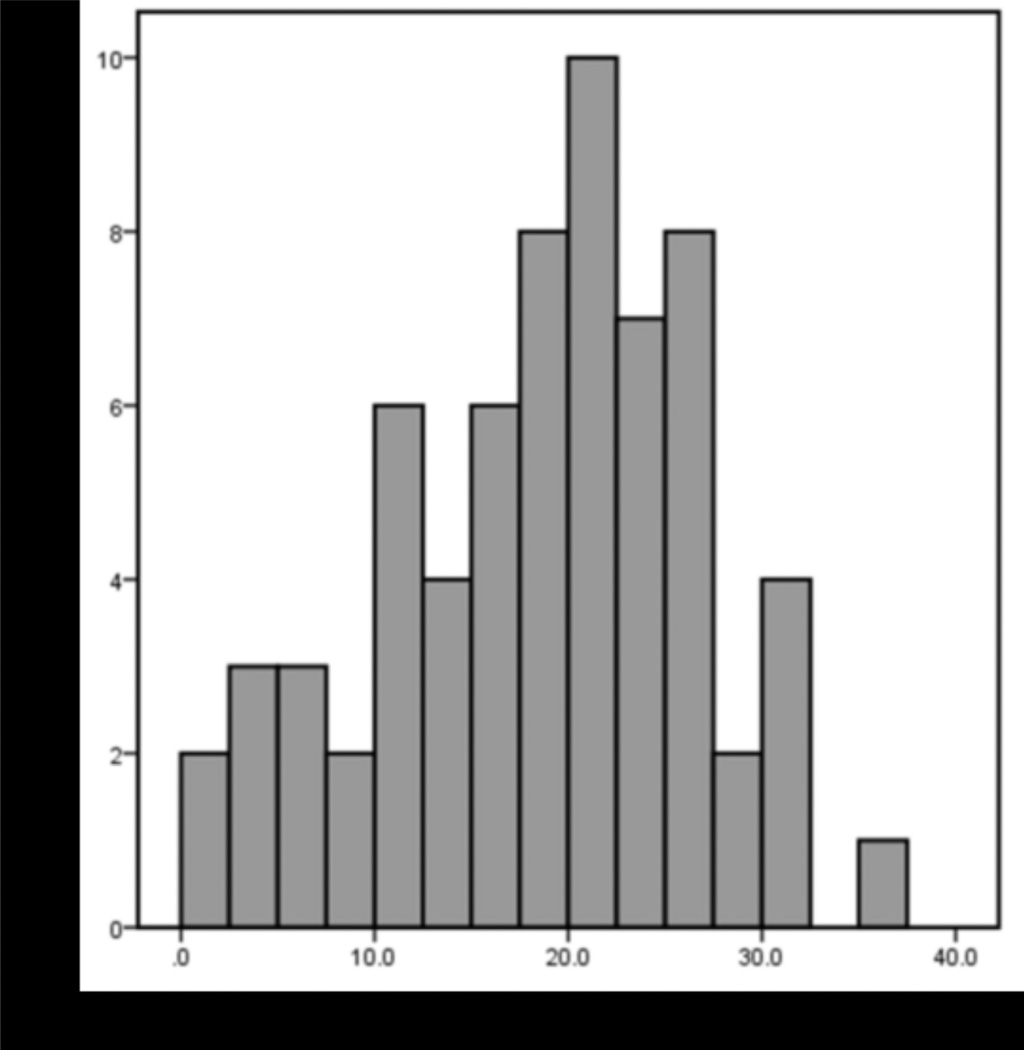
Total PCL R scores ranged from 2.1 to 36.0 (M = 18.4; SD = 8.0; Figure 1). F1 (interpersonal and affective traits) ranged from 0.0 to 14.0 (M = 4.8; SD = 3.4), and F2 (social deviance traits) ranged from 2.2 to 20.0 (M = 11.6; SD = 4.7). Facet 1 (interpersonal) ranged from 0.0 to 7.0 (M = 2.1; SD = 1.6); Facet 2 (affective) ranged from 0.0 to 7.0 (M = 2.7; SD = 2.1); Facet 3 (lifestyle) ranged from 1.0 to 10.0 (M = 6.2; SD = 2.4); Facet 4 (antisocial) ranged from 0.0 to 10.0 (M = 5.4; SD = 2.9). The difference between PCL R scores for males and females was not statistically significant, t(64) = 0.53; P = 0.60. The mean HART score, estimating IQ (Schretlen et al., 2009), was 97.3 (SD = 9.8). Estimated IQ and psychopathy scores were negatively correlated (PCL R Total: r = 0.28, P < 0.05; F2: r = 0.33, P < 0.01). The correlation between IQ and F1 was not significant. The relationships between drug/alcohol use and PCL R scores were investigated, given their potential for a relationship with brain structure. There was a significant positive correlation between self reported drug use and PCL R Total score (r = 0.37, P < 0.01), and between drug use and F2 (r = 0.39, P < 0.01). There was no significant relationship between drug use and F1 or between alcohol use and PCL R scores (all Ps > 0.05). Correlations between PCL R scores and all covariates can be found in Table 3.
Figure 1.
Histogram of Psychopathy Checklist-Revised (PCL-R) Total scores in 66 adults (36 male, 30 female; mean age: 36.9 years, SD = 7.9). Total PCL-R scores ranged from 2.1 to 36.0 (M = 18.4; SD = 8.0).
Table 3.
Correlations among individual differences variables.
| PCL-R Total | PCL-R F1 | PCL-R F2 | Facet 1 | Facet 2 | Facet 3 | Facet 4 | Age | Estimated IQ |
Total GMV | Total Intracranial Volume |
Drug History | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCL−R F1 | 0.83** | |||||||||||
| PCL−R F2 | 0.92** | 0.58** | ||||||||||
| Facet 1 | 0.73** | 0.87** | 0.46** | |||||||||
| Facet 2 | 0.77** | 0.93** | 0.57** | 0.62** | ||||||||
| Facet 3 | 0.80** | 0.46** | 0.89** | 0.36** | 0.46** | |||||||
| Facet 4 | 0.86** | 0.58** | 0.92** | 0.47** | 0.57** | 0.64** | ||||||
| Age | −0.16 | −0.12 | −0.17 | −0.2 | −0.18 | −0.11 | −0.19 | |||||
| Estimated IQ | −0.28* | −0.12 | −0.33** | −0.4 | −0.16 | −0.22 | −0.38** | −0.03 | ||||
| Total GMV | −0.02 | −0.01 | 0.01 | −0.09 | 0.06 | −0.02 | 0.04 | −0.40** | 0.19 | |||
| Total Intracranial Volume | −0.17 | −0.13 | −0.17 | −0.14 | −0.09 | −0.16 | −0.15 | −0.09 | 0.07 | 0.44** | ||
| Drug History | 0.37** | 0.23 | 0.39** | 0.21 | 0.20 | 0.41** | 0.32 | 0.46** | −0.26* | −0.15 | −0.14 | |
| Alcohol History | 0.18 | 0.19 | 0.15 | 0.15 | 0.19 | 0.12 | 0.15 | 0.56** | −0.11 | −0.19 | −0.32** | 0.50** |
correlation is significant at the 0.01 level (two-tailed)
correlation is significant at the 0.05 level (two-tailed)
PCL-R, Psychopathy Checklist-Revised; F1, Factor 1; F2, Factor 2; GMV, gray matter volume.
3.2 Voxel based morphometry
3.2.1. Primary whole brain and SVC analyses
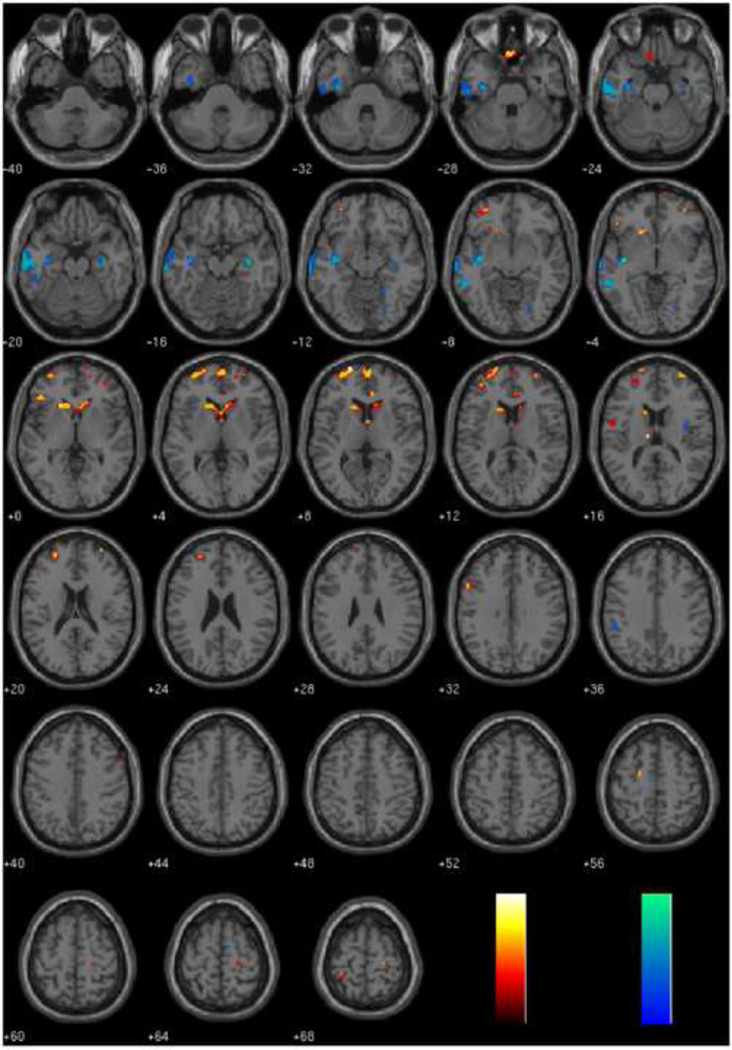
In the whole brain analyses, PCL R scores were negatively correlated with regional gray matter in temporal and limbic areas (Table 4), specifically left inferior temporal gyrus (Total and F2), left middle temporal gyrus (Total and F2), left uncus (Total), right inferior temporal gyrus (F1), right hippocampus (Total), and right insula (F2). PCL R scores were also positively correlated with regional gray matter in frontal and subcortical areas (Table 5), specifically medial OFC (Total, F1, and F2), bilateral superior frontal gyrus (Total, F1, and F2), and bilateral thalamus (Total). Full results can be found in Tables 4 and 5 and Figure 2.
Table 4.
Brain regions showing significant negative associations with PCL-R scores, controlling for total gray matter volume and drug and alcohol history.
| Total | F1 | F2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | x | y | z | t-value | x | y | z | t-value | x | y | z | t-value | |
| Negative Associations | |||||||||||||
| Frontal Lobes | |||||||||||||
| Medial Frontal Gyrus | 6 | 12 | −4 | 64 | 2.97 | ||||||||
| Temporal Lobes | |||||||||||||
| Inferior Temporal Gyrus | 20 | −62 | −16 | −22 | 3.58 | −60 | −14 | −22 | 3.81 | ||||
| Inferior Temporal Gyrus | 20 | 60 | −48 | −16 | 2.78 | ||||||||
| Inferior Temporal Gyrus | 20 | −50 | −34 | −20 | 3.16 | ||||||||
| Parahippocampal Gyrus | 20 | −36 | −12 | −22 | 3.41* | ||||||||
| Fusiform Gyrus | 20 | −52 | −20 | −28 | 3.66 | ||||||||
| Inferior Temporal Gyrus | 21 | −64 | −6 | −22 | 2.87 | ||||||||
| Middle Temporal Gyrus | 21 | −68 | −20 | −8 | 2.98 | −68 | −20 | −8 | 3.76 | ||||
| Middle Temporal Gyrus | 21 | −58 | −42 | −4 | 3.45 | −58 | −40 | −6 | 3.34 | ||||
| Middle Temporal Gyrus | 21 | −36 | −6 | −32 | 3.19* | ||||||||
| Middle Temporal Gyrus | 21 | −62 | −8 | −16 | 3.41 | ||||||||
| Insula | 13 | −38 | −12 | −8 | 3.32 | −40 | −16 | −12 | 3.83 | ||||
| Insula | 13 | 38 | −4 | 18 | 3.09 | ||||||||
| Uncus | 20 | −34 | −14 | −32 | 2.97 | ||||||||
| Hippocampus | 36 | −14 | −18 | 3.16* | |||||||||
| Occipital Lobes | |||||||||||||
| Lingual Gyrus | 18 | 24 | −76 | −10 | 3.00 | ||||||||
| Lingual Gyrus | 19 | 24 | −68 | −2 | 2.91 | ||||||||
| Parietal Lobes | |||||||||||||
| Inferior Parietal Lobule | 40 | −50 | −38 | 36 | 3.27 | ||||||||
| Subcortical | |||||||||||||
| Claustrum | −38 | −12 | −6 | 2.99 | |||||||||
| Caudate Tail | 38 | −16 | −16 | 3.17 | |||||||||
| Cerebellum | 20 | −52 | −12 | 2.81 | |||||||||
Results are significant at P < 0.005, uncorrected for multiple comparisons, unless bolded (P < 0.001, uncorrected).
Coordinates with an * survived small volume correction with a family-wise error (FWE) corrected threshold of P < 0.05. Coordinates are in Montreal Neurological Institute (MNI) space. BA, Brodmann area, F1, Factor 1; F2, Factor 2.
Table 5.
Brain regions showing significant positive associations with PCL-R scores, controlling for total gray matter volume and drug and alcohol history.
| Total | F1 | F2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | x | y | z | t-value | x | y | z | t-value | x | y | z | t-value | |
| Positive Associations | |||||||||||||
| Frontal Lobes | |||||||||||||
| Superior Frontal Gyrus | 10 | −20 | 66 | 8 | 3.55 | −20 | 64 | 8 | 3.39 | ||||
| Superior Frontal Gyrus | 10 | 14 | 70 | −2 | 2.99 | ||||||||
| Superior Frontal Gyrus | 10 | −18 | 68 | 12 | 3.47 | ||||||||
| Superior Frontal Gyrus | 10 | 30 | 62 | 4 | 2.86 | ||||||||
| Superior Frontal Gyrus | 10 | 18 | 56 | 2 | 2.87 | ||||||||
| Middle/Superior Frontal Gyrus | 10 | −26 | 52 | 20 | 2.97 | −24 | 50 | 20 | 3.57 | ||||
| Middle Frontal Gyrus | 10 | 32 | 60 | 16 | 3.20 | 34 | 60 | 16 | 3.46 | ||||
| Middle Frontal Gyrus | 10 | 36 | 50 | −2 | 3.18 | ||||||||
| Middle Frontal Gyrus | 10 | −38 | 46 | 14 | 2.86 | −36 | 46 | 12 | 3.06 | ||||
| Middle Frontal Gyrus | 10 | −32 | 58 | 6 | 3.08 | ||||||||
| Medial Frontal Gyrus | 10 | 2 | 64 | 8 | 3.12 | 4 | 66 | 8 | 3.27 | ||||
| Gyrus Rectus | 11 | 2 | 28 | −28 | 3.20 | ||||||||
| Middle Frontal Gyrus | 11 | −32 | 48 | −8 | 2.93 | −32 | 48 | −8 | 3.26 | ||||
| Middle Frontal Gyrus | 11 | −30 | 48 | −8 | 3.16* | ||||||||
| Gyrus Rectus | 11 | −2 | 26 | −28 | 3.04 | −4 | 28 | −28 | 2.78 | ||||
| Inferior Frontal Gyrus | 47 | −46 | 32 | −2 | 3.03 | ||||||||
| Middle Frontal Gyrus | 47 | −44 | 32 | −2 | 3.18 | ||||||||
| Inferior/Middle Frontal Gyrus | 9 | −52 | 16 | 32 | 3.02 | −54 | 14 | 32 | 3.10 | ||||
| Middle Frontal Gyrus | 9 | 54 | 14 | 38 | 2.90 | ||||||||
| Superior Frontal Gyrus | 6 | −20 | −4 | 56 | 2.93 | −20 | −4 | 56 | 3.14 | ||||
| Precentral Gyrus | 6 | −52 | 0 | 16 | 3.29 | ||||||||
| Precentral Gyrus | 6 | 22 | −24 | 66 | 2.88 | ||||||||
| Precentral Gyrus | 4 | 22 | −26 | 70 | 2.78 | ||||||||
| Middle Frontal Gyrus | 46 | −46 | 26 | 22 | 2.78 | ||||||||
| Anterior Cingulate | 24 | 8 | 36 | 8 | 3.20* | 8 | 36 | 8 | 2.90 | ||||
| Parietal Lobes | |||||||||||||
| Postcentral Gyrus | 2 | −28 | −40 | 68 | 2.93 | ||||||||
| Subcortical | |||||||||||||
| Caudate Head | −10 | 24 | 0 | 3.62 | |||||||||
| Caudate Head | 2 | 18 | 2 | 3.17 | |||||||||
| Caudate Head | −16 | 22 | 0 | 3.27* | −14 | 22 | 2 | 3.63* | |||||
| Caudate Head | −14 | 24 | 2 | 3.77 | |||||||||
| Caudate Head | 14 | 26 | 2 | 3.67 | |||||||||
| Caudate Head | 12 | 22 | 2 | 3.32* | |||||||||
| Caudate Head | 14 | 22 | 6 | 3.26* | |||||||||
| Caudate Head | 2 | 4 | 6 | 2.90 | |||||||||
| Caudate Head | −14 | 22 | −10 | 2.80 | |||||||||
| Caudate Body | −16 | 18 | 14 | 3.26 | |||||||||
| Thalamus | −10 | −14 | 16 | 3.00 | |||||||||
| Thalamus | 4 | 0 | 10 | 2.81 | |||||||||
| Lentiform Nucleus, Putamen | −20 | 22 | −4 | 3.15 | |||||||||
Results are significant at P < 0.005, uncorrected for multiple comparisons, unless bolded (P < 0.001, uncorrected).
Coordinates with an * survived small volume correction with a family-wise error (FWE) corrected threshold of P < 0.05. Coordinates are in Montreal Neurological Institute (MNI) space. BA, Brodmann area, F1, Factor 1; F2, Factor 2.
Figure 2.
Regions where gray matter volume is related to PCL-R Total, Factor 1, or Factor 2, controlling for total gray matter volume, drug history, and alcohol history.
Negative associations are depicted in blue colors and positive associations are depicted in red colors. Results are overlaid on a canonical high-resolution structural image and thresholded at P < 0.005, uncorrected for multiple comparisons. See tables for t-values.
A priori ROI analyses supported the whole brain analyses, and identified several paralimbic regions as being significantly associated with psychopathy scores. These regions that survived SVC with an FWE corrected threshold of P < 0.05 were: rostral anterior cingulate cortex (positive; Total), left orbital frontal cortex (positive; F1), left and right striatum (positive; Total and F2), right hippocampus (negative; Total), and near the left hippocampus (negative; F2). These areas are denoted with an asterisk in Tables 4 and 5.
3.2.2. Supplemental whole brain analyses
In this sample, IQ was correlated with psychopathy Total and F2 scores. Thus, we conducted an additional analysis with this variable as a covariate (Table S1). This analysis, with PCL R predicting regional gray matter volume, controlling for drug and alcohol use, IQ, and total gray matter volume (GMV), yielded similar results to those of the primary analysis (with PCL R predicting regional gray matter volume, controlling for drug and alcohol use and total GMV).
We also evaluated the effects of the four facets on regional gray matter volume. For the negative associations, Facets 3 and 4 were not substantially different from each other, and showed the strongest associations with regional gray matter volumes. BA 21 (middle temporal gyrus) and BA 13 (insula) showed notable associations with the lifestyle and antisocial facets. Facet 2 (affective) was negatively related to BA 22 (superior temporal gyrus) and the claustrum, while Facet 1 (interpersonal) was negatively related to BA 37 (middle temporal gyrus), BA 19 (middle occipital gyrus), and the lingual gyrus. For the positive associations, BA 24 (anterior cingulate) was positively related to Facets 1 (interpersonal) and 4 (antisocial), while the putamen and temporoparietal junction were only related to Facet 2 (affective). The precuneus was positively related only to Facet 4 (antisocial). Full results can be found in Tables S2 and S3.
Finally, we conducted an additional analysis gender with gender as a covariate (with PCL R predicting regional gray matter volume, controlling for drug and alcohol use, total GMV, and gender; Figure S1). This analysis yielded similar results to those of the primary analysis.
3.2.3. Secondary regression analyses
A final regression model was conducted to evaluate the proportion of variance in PCL R scores accounted for by specific a priori regional gray matter volumes. To this end, gray matter values from a priori coordinates (Table 2) were entered as predictors of PCL R Total, F1 or F2 scores (one model for prediction of each PCL R score; forward selection method in SPSS). These models indicated that gray matter volumes explained 21.8% of the variance in PCL R Total score (right hippocampus and left OFC), and 26.8% of the variance in F2 score (left hippocampus, left OFC, and left temporal pole). There were no significant predictors of F1 scores.
4. Discussion
In the present study, we found negative associations between regional gray matter volumes and psychopathic traits in a community corrections sample, which is consistent with previous functional and structural work. For instance, hippocampal volume was found to be negatively related to PCL R Total score in the present study. The hippocampus, a region important for aversive conditioning (Buchel et al., 1999), has been shown to be negatively related to psychopathy scores in violent offenders with comorbid alcoholism (Laakso et al., 2001).
We also found positive associations in several regions. Here we report a positive relationship between anterior cingulate gray matter volume and psychopathic traits. The rostral anterior cingulate is connected to the amygdala, anterior insula, hippocampus, and OFC, and is known to be involved in a wide variety of affective processes, including emotion regulation and assessing the salience of affective information (Bush et al., 2000). This region was also found to be dysfunctional during fear conditioning in psychopaths compared to healthy controls (Birbaumer et al, 2005). Additionally, our findings replicate the positive association between regional gray matter volumes and severity of psychopathy in the striatum (caudate nucleus and putamen), which was shown in a recent study by Glenn and colleagues (2010).
We also found a positive relationship between OFC volumes (BAs 11 and 47) and psychopathic traits. Orbital frontal dysfunction has been found in studies of emotional processing and aversive conditioning in psychopathy (Veit et al., 2002; Muller et al., 2003), and is characteristic of brain lesioned patients who display psychopathic like behaviors (Bechara et al., 1994; Anderson et al., 1999). Additionally, abnormalities in the above regions were shown in a preliminary regression analysis to account for a substantial portion of variance in psychopathy scores. Right hippocampus and left OFC explained 21.8% of the variance in PCL R Total score, and left hippocampus, left OFC, and left temporal pole explained 26.8% of the variance in F2 score. Together, these findings converge to suggest the possibility that abnormalities within these regions may underlie important characteristics of psychopathic traits. These results, which suggest that psychopathy may be a disorder of a distributed network of regions, are more consistent with the paralimbic hypothesis put forth by Kiehl (2006) than with the more restricted model put forth by Blair (2007). These results support those obtained from neuropsychological, psychophysical, and functional and structural neuroimaging studies, suggesting that psychopathy is associated with abnormalities in a network of limbic and closely related paralimbic regions, including the OFC, amygdala, hippocampus, temporal pole, and anterior cingulate.
It is worth noting that we found generally decreased volume within temporal and limbic regions, yet generally increased volume within frontal and subcortical structures. One potential explanation for the observed results is that psychopathy is associated with abnormal interactions between frontal/subcortical and temporal/limbic structures. In support of this idea, a recent study of white matter in psychopathy found abnormalities in the amygdala OFC network (Craig et al., 2009). Giorgio and colleagues (2010) have shown in a sample of healthy adolescents that gray matter volume decreases with age in several distinct clusters, including medial and lateral prefrontal cortex, whereas in the present study, gray matter increases were observed in these regions. Similarly, it has been shown that in healthy individuals caudate volumes decrease during adolescence (Giedd, 2004) while amygdala and hippocampal volumes increase during this time (Giedd et al., 1996). It is thus possible that abnormal neurodevelopment lies at the heart of the manifestation of psychopathic traits. This explanation is supported by the findings of De Brito et al. (2009), where boys with callous unemotional traits had increased gray matter concentration in the medial orbitofrontal cortex and anterior cingulate compared to typically developing boys. Disruption in the normal developmental trajectory during adolescence could result in increased frontal and subcortical but decreased temporal and limbic gray matter in adulthood, leading to dysfunction such as the kind seen in psychopathy. One possibility is that the dysfunction occurs at the cellular level, where a premature arrest in synaptic and neuronal pruning in some areas, coupled with deficient growth in others, results in ineffective and/or dysfunctional processing. Our data do not address this level of analysis directly, but they do suggest an interesting avenue to investigate.
Some previous studies have failed to account for the potential confounding factors of drugs and alcohol, which are well established as being comorbid with psychopathy (Smith and Newman, 1990), and to have a significant relationship with gray matter morphometry (Makris et al., 2008; Tanabe et al., 2009; Yuan et al., 2009). Here we statistically controlled for drug and alcohol use, and still found structural differences in frontal, subcortical, temporal, and limbic areas associated with psychopathic traits.
The results from the present study can be contrasted with those from a recent large scale study on prisoners (N = 296, with 42 individuals scoring at or above 30; Ermer et al., 2012). In both studies, negative associations between PCL R Total scores and regional gray matter volumes were found in the left inferior temporal gyrus (BA 20), left parahippocampal gyrus (uncus/hippocampus; BA 20), and right hippocampus specifically. More generally, negative associations were found in temporal and limbic/paralimbic regions in both samples. One difference between the two results is the positive association found in the present community corrections study between PCL R Total scores and regional gray matter volumes in frontal and subcortical areas and the negative correlation found in these regions in Ermer et al. Due to the small sample size in the present study and inconsistent nature of findings in the OFC in previous studies (including this one), these frontal gray matter increases should be replicated before definitive conclusions are drawn regarding the structure function relationship in psychopathy. Differences between the results of the present study and those of the Ermer et al. study could be due to any number of the following methodological factors: population (community vs. prison), participant gender (54.5% vs. 100% male), number of individuals scoring at or above 30 on the PCL R (5 vs. 42), and covariates in the whole brain analysis (GM and regular substance use vs. BV, regular substance use, and age). Note that sample size, race/ethnicity, mean PCL R scores (P = 0.005), and reported thresholds are also different. Although the small sample size (n = 30 females) does not allow direct comparisons to be made between males and females in this study, we controlled for gender in a supplemental analysis, and future studies should indeed examine whether structural abnormalities in females with psychopathic traits differ from those in males.
Studying psychopathy in different samples drawn from different populations allows us to characterize the disorder more comprehensively. Given strong evidence that psychopathy is a dimensional construct (Edens et al., 2006; Guay et al., 2007), studying both incarcerated and nonincarcerated individuals, as well as samples scoring at all levels of the PCL R, could be fruitful endeavors (Kirkman, 2002). It should be noted that these individuals were recruited from community corrections centers, and as such, should be considered part way between the general population and prison populations. One potential limitation of this study is that we were not able to statistically control for depression and anxiety scores in the imaging analysis. Additionally, we did not perform drug tests to definitively rule out the possibility that participants were using drugs or alcohol at the time of the study (though note that they self reported no current use). On the other hand, comorbidities including drug and alcohol use history and anxiety and mood disorders make this sample both heterogeneous and representative.
4.1. Conclusions
These results support the hypothesis that psychopathic traits are associated with structural abnormalities in a number of related brain regions, including parahippocampal gyrus, orbital frontal cortex, insula, anterior cingulate, and striatum. Undoubtedly, the relation between functional abnormalities and volumetric increases or decreases needs to be more fully elucidated, as does the functional and structural connectivity of regions important in psychopathy. In line with Craig and colleagues (2009), imaging analyses that evaluate white matter volume and integrity, such as diffusion tensor imaging, might be useful in this regard. It is important to keep in mind the potential differences between studies that have a large number of individuals scoring high on the PCL R (i.e., that address psychopathy per se) versus those that address heightened psychopathic traits. Indeed, future studies should continue to investigate the role of paralimbic and limbic regions in the manifestation of psychopathic traits, as well as the precise relationship between morphometric and functional abnormalities.
Supplementary Material
Acknowledgment
This research was supported in part by grants R01 MH070539 01 (PI: Kiehl) and R21 MH086880 01 (PI: Shane) from the National Institute of Mental Health, and R01 EB000840 (PI: Calhoun) from the National Institute of Biomedical Imaging and Bioengineering. The authors wish to thank Drs. B. Fink and J. Selig for their technical assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Antisocial personality disorder (ASPD) taps mainly the social deviance component of psychopathy (Factor 2), while missing the interpersonal/affective component (Factor 1; Hare, 2003). So, with respect to assessing psychopathy and its neurobiological correlates, ASPD – as conceptualized in the DSM IV – is not a meaningful construct; here we included it for the purposes of reporting complete comorbid Axis I and Axis II diagnoses.
Though we did not have enough responses to covary depression (Beck Depression Inventory II; Beck, Steer, & Brown, 1996; n = 44) and anxiety (Spielberger State Trait Anxiety Inventory; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983; n = 29) in the imaging analysis, psychopathy (PCL R Total score) was uncorrelated with both depression, r = .22, P = .15, and state and trait anxiety: state, r = .12, P = .54; trait, r = .26, P = .19.
We chose not to use a customized template because the participants were adults without gross brain pathology. However, we also analyzed the data using a customized template and found no substantial differences between the original analysis and those done with the customized template.
There were no significant corrected for multiple comparisons results with 66 subjects in the whole brain. See SVC analyses with ROIs for corrected for multiple comparisons results.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel based morphometry—The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bassitt DP, Neto MRL, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:58–62. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Science. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala hippocampal involvement in human aversive trace conditioning revealed through event related functional magnetic resonance imaging. Journal of Neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cleckley HM. The Mask of Sanity: An Attempt to Reinterpret the So Called Psychopathic Personality. 5th Edition. St. Louis: Mosby; 1976. [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, Picchioni M, McGuire PK, Fahy T, Murphy DGM. Altered connections on the road to psychopathy. Molecular Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S, Viding E. Size matters: Increased grey matter in boys with conduct problems and callous unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Lilienfeld SO, Poythress NG. Psychopathic, not psychopath: Taxometric evidence for the dimensional structure of psychopathy. Journal of Abnormal Psychology. 2006;115:131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using SPSS. 3rd Edition. Los Angeles: SAGE Publications; 2009. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM IV TR Axis I Disorders – Patient Edition (SCID I/P) Washington, D. C.: American Psychiatric Press; 2002. [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18-years. Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, De Stefano N, Matthews PM, Smith SM, Johansen Berg H, James AC. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in the psychopathic individuals. Biological Psychiatry. 2010;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Guay JP, Ruscio J, Knight RA, Hare RD. A taxometric analysis of the latent structure of psychopathy: Evidence for dimensionality. Journal of Abnormal Psychology. 2007;116:701–716. doi: 10.1037/0021-843X.116.4.701. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist Revised. Toronto: Multi Health Systems; 1991. [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist Revised. 2nd ed. Toronto: Multi Health Systems; 2003. [Google Scholar]
- Hare RD, Neumann CS. Psychopathy as a clinical and empirical construct. Annual Review of Clinical Psychology. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Mendrek A, Forster BB, Hare RD, Liddle PF. Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Research: Neuroimaging. 2004;130:27–42. doi: 10.1016/S0925-4927(03)00106-9. [DOI] [PubMed] [Google Scholar]
- Kirkman CA. Non incarcerated psychopaths: why we need to know more about the psychopaths who live among us. Journal of Psychiatric and Mental Health Nursing. 2002;9:155–160. doi: 10.1046/j.1365-2850.2002.00462.x. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Gunning Dixon F, Vaurio O, Repo Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2-alcoholism. Psychiatry Research: Neuroimaging. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Koivisto E, Savolainen L, Eronen M, Aronen HJ, Hakola P, Repo E, Soinien H, Tiihonen J. Psychopathy and the posterior hippocampus. Behavioural Brain Research. 2001;118:187–193. doi: 10.1016/s0166-4328(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Grissom G, Pettinati H, Argeriou M. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Muller JL, Ganssbauer S, Sommer M, Dohnel K, Weber T, Schmidt Wilcke T, Hajak G. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel based morphometry. Psychiatry Research: Neuroimaging. 2008a;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Dohnel K, Weber T, Schmidt Wilcke T, Hajak G. Disturbed prefrontal and temporal brain function during emotion and cognition interaction in criminal psychopathy. Behavioral Sciences and the Law. 2008;26:131–150. doi: 10.1002/bsl.796. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roeder CH. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: Evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- de Oliveira Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar Moll F, Moll J. Psychopathy as a disorder of the moral brain: Fronto temporo limbic grey matter reductions demonstrated by voxel based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, LaCasse L, Colletti P. Hippocampal structural asymmetry in unsuccessful psychopaths. Biological Psychiatry. 2004;55:185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Winicki JM, Meyer SM, Testa SM, Pearlson GD, Gordon B. Development, psychometric properties, and validity of the Hopkins Adult Reading Test (HART) The Clinical Neuropsychologist. 2009;23:926–943. doi: 10.1080/13854040802603684. [DOI] [PubMed] [Google Scholar]
- Smith SS, Newman JP. Alcohol and drug abuse dependence disorders in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology. 1990;99:430–439. doi: 10.1037//0021-843x.99.4.430. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance dependent individuals. Biological Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, Repo-Tiihonen E, Vaurio O, Soininen H, Aronen HJ, Kononen M, Thompson PM, Frisoni GB. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Research: Neuroimaging. 2008;163:201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry. 2005;57:1103–1108. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr K, Colletti P, Toga A. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhu ZD, Shi JF, Zou ZL, Yuan F, Liu YJ, Lee TMC, Weng XC. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin dependent individuals. Brain and Cognition. 2009;71:223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.