Summary
There is an urgent need to develop new drugs for treatment of tuberculosis, particularly against latent/persistent forms of the causative agent, Mycobacterium tuberculosis. In this issue of Chemistry & Biology, Krieger and colleagues use a structure-guided approach to develop novel inhibitors of malate synthase, a target in the glyoxylate shunt that is critical for pathogen survival in chronic infection.
Tuberculosis (TB) is one the “big-three” infectious diseases worldwide, killing ~1-2 million people every year, according to the 2012 WHO Global Tuberculosis Report. Almost 9 million people suffer from active disease, while another 2 billion (a third of the world’s population) harbor persistent infection with Mycobacterium tuberculosis (Mtb), the bacterium that causes TB. Current drug treatments are prolonged and complicated, and, to make matters even worse, multiple or extensively drug-resistant strains of Mtb (MDR-TB and XDR-TB, respectively) are rapidly spreading. Thus, there is an urgent need to develop new drugs against novel targets, particularly against latent/persistent forms of Mtb.
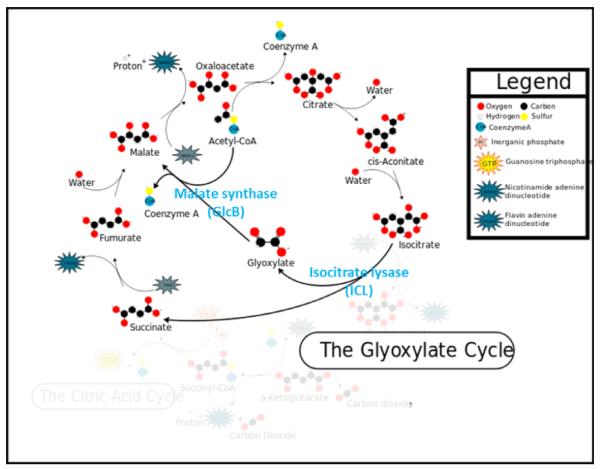
The glyoxylate shunt has received attention as an important pathway in Mtb, since it is up-regulated to maintain the TCA cycle in the absence of glycolysis during the persistent phase of infection (McKinney et al., 2000). A functional glyoxylate shunt has recently been shown to be critical for establishing and maintaining Mtb infection of macrophages and animal models (Marrero et al., 2010). The glyoxylate shunt is able to bypass the CO2-generating steps of the tricarboxcylic acid (TCA) cycle, allowing synthesis of oxaloacetate (and subsequent gluconeogenesis) under carbon-limiting conditions (see Figure 1). This is accomplished by utilizing two non-TCA enzymes: isocitrate lyase (ICL), which hydrolyzes the TCA isocitrate into glyoxylate and succinate; and malate synthase (GlcB), which combines glyoxylate with acetyl-CoA to produce malate. While the glyoxylate shunt is active in most prokaryotes, lower eukaryotes, and plants; mammals appear to lack both enzymes in the pathway (Kondrashov et al., 2006), making it even more attractive as an anti-bacterial drug target. Examination of the Mtb ICL and GlcB crystal structures (Sharma et al., 2000 and Smith et al., 2003) suggested that the latter would provide a more druggable target, due to its deeper and more hydrophobic active site.
Figure 1.
The glyoxylate cycle uses isocitrate lyase (ICL) and malate synthase (GlcB) to shunt isocitrate (a TCA cycle intermediate) to malate, via glyoxylate. This figure was modified from one on Wikipedia (http://en.wikipedia.org/wiki/File:Glyoxylatepath.svg).
In this issue of Chemistry & Biology, Krieger et al., 2012 present a “text-book” example of structure-based drug design to develop potent phenyl-diketo acid (PKDA) inhibitors of malate synthase that are active against a mouse model of TB. To achieve this important milestone, they harnessed an impressive array of technologies, including structural biology, medicinal chemistry, pharmacokinetics/pharmacodynamics, and activity testing using both whole-cell and mouse model assays. They started by screening a focused library of 35 glyoxylate-like compounds for inhibition of GlcB activity, and found that 19 (all phenyl-diketo acids) exhibit activity. Substitution at the ortho-position of the phenyl ring increased stability by 10-fold and improved the IC50, but further attempts to stabilize the framework resulted in loss of activity against GlcB. In order to obtain co-crystals of GlcB with the phenyl-diketo acid (PKDA) inhibitors, it proved necessary to employ a Cys619Ala mutant of the Mtb enzyme, which has a constricted entrance to the active site channel. The co-crystals revealed close contact between the carboxylic acid of Asp633 and the face of the PKDA aromatic ring via unusual anion-Π interactions. Based on the information obtained from the inhibitor-bound structures, a series of substitutions to the PKDA framework were synthesized and tested for activity, resulting in a considerable body of structure-activity relationship (SAR) and modest improvement in activity. Whole-cell testing of growth inhibition by the PKDAs using Mtb grown on acetate-supplemented M9 medium revealed activity in the low micromolar range for most compounds. Esterification of these compounds to mask the acid lowered the minimum inhibitory concentration (MIC) by ~8-fold, presumably by improving cellular uptake. When the most potent inhibitor (Z-methyl 4-(2-chloro-6-fluoro-3-methylphenyl)-2-hydroxy-4-oxobut-2-enoate) was tested on a GlcB-overexpressing strain of Mtb, the MIC increased by 8-fold after induction of GlcB expression, supporting on-target activity. The compound above was selected for pharmacokinetic (PK), pharmacodynamics (PD) and toxicity studies in mice, and found to be suitable for testing in the murine model of acute TB infection. Dosing strategies of 300-600 mg/kg once- or twice-daily resulted in significant (>100-fold) reduction in the Mtb load and inability to establish an acute infection.
Thus, this study has chemically validated the Mtb glyoxylate pathway (and malate synthase, in particular) as a viable new drug target, and identified a lead series of compounds (methyl esters of PKDA) for further pre-clinical development. It is particularly interesting to note that the efficacy in the murine model of acute infection was comparable to that of moxifloxacin, suggesting that GlcB is essential for growth on carbon sources other than fatty acids (although whole-cell activity of the PKDA compounds was 4-fold higher on acetate than dextrose). Obviously, much more work remains to be done before we can expect to see PKDA derivatives in clinical use, but this report offers renewed optimism for development of novel chemotherapeutic agents to combat the rising tide of MDR- and XDR-TB. Given the presence of the glyoxylate pathway in other pathogens and its absence from humans, there must also be hope for development of broad-spectrum antibiotics based on this target. It should also be noted that much of this work was made possible by the establishment and success of the TB Structural Genomics Consortium (Chim et al., 2011). Of the 1523 structures in the Protein Data Bank (PDB) from Mycobacterium, the TBsgc has submitted 245 (16%), while another 156 (10%) have come from the Seattle Structural Genomics Center for Infectious Disease (SSGCID). It is heartening to see these large-scale efforts beginning to bear fruit.
Acknowledgments
This work was funded with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institute of Health, Department of Health and Human Services, under Contract Number HHSN27220120025C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- McKinney JD, zu Bentrup KH, Muñoz-Elías EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR, Russsell DG. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- Marrero J, Rhee KY, Schnappinger D, Pether K, Ehrt S. Proc. Natl. Acad. Sci. USA. 2010;107:9819–9824. doi: 10.1073/pnas.1000715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashov FA, Koonin EV, Morgunov IG, Finogenova TV, Kondrashova MV. Biology Direct. 2006;1:31. doi: 10.1186/1745-6150-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Sharma S, zu Bentrup KH, McKinney JD, Russell DG, Jacobs WR, Sacchettini JC. Nat. Struct. Biol. 2000;7:663–668. doi: 10.1038/77964. [DOI] [PubMed] [Google Scholar]
- Smith CV, Huang C, Miczak A, Russell DG, Sacchettini JC, zu Bentrup KH. J. Biol. Chem. 2003;278:1735–1743. doi: 10.1074/jbc.M209248200. [DOI] [PubMed] [Google Scholar]
- Krieger IV, Freundlich JS, Gawandi VB, Roberts JP, Gawandi VB, Sun Q, Owen JL, Fraile MT, Huss SI, Duncan K, Lavandera J-L, Ioerger TR, Sacchettini JC. Chem. Biol. 2012;19(this issue) doi: 10.1016/j.chembiol.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chim N, Habel JE, Johnston JM, Krieger I, Miallau L, Sankaranarayanan R, Morse RP, Bruning J, Swanson S, Kim H, Kim C-Y, Li H, Bulloch EM, Payne RJ, Manos-Turvey A, Hung L-W, Baker EN, Lott JS, James MNG, Terwilliger TC, Eisenberg DS, Sacchettini JC, Goulding CW. Tuberculosis (Edinb) 2011;91:155–172. doi: 10.1016/j.tube.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]