Abstract
Objective
This study aimed to evaluate the cellular toxicity of two newly-released acrylic resins (Futura Gen and GC Reline Hard) in comparison with the conventional heat-cure resin (Meliodent).
Materials and Methods:
Sample discs from each acrylic resin were placed in 24-well culture plates along with L929 mouse fibroblast cell line. A mixture of the RPMI 1640 medium, antibiotics and 10% FBS was added to the plates and the specimens were incubated in a CO2 incubator. The amount of light absorption by each plate was determined after 1 hour, 24 hours and 1 week by the MTT assay and ELISA. The cytotoxic effect of the resins was compared among groups using the two-way ANOVA and further paired comparison was performed using the post-hoc Tukey’s test.
Results:
After 1 hour, Futura Gen displayed a significantly lower level of light absorption in comparison with Meliodent (P=0.03). After 24 hours, GC Reline Hard rendered a significantly lower level of light absorption compared to Meliodent (P=0.02). After one-week of incubation, the mean absorption rates for GC Reline Hard, Meliodent and Futura Gen were relatively similar (P>0.05). The lowest and highest level of cytotoxicity among all resins were observed after one week and 1 hour of immersion in water, respectively.
Conclusion:
All the tested resins induced some degree of cytotoxicity. Cytotoxicity of Futura Gen, GC Reline Hard and Meliodent resins failed to show any significant reduction from 24 hours to one week. Thus, it is recommended to immerse the dentures in water for 24 hours prior to delivery to the patient.
Keywords: Cytotoxicity, Fibroblasts, Acrylic Resins
INTRODUCTION
Acrylic resins are the most frequently used materials in the fabrication of removable dentures [1]. The majority of denture bases are made from heat-cure acrylic resins which are believed to result in the release of certain toxic chemicals such as formaldehyde, methyl methacrylate, methacrylate acid and benzoic acid, causing serious reactions in the surrounding tissues. The major element causing these reactions is the methyl methacrylate monomer present in the denture base which can be released into the saliva [2, 3]. The amount of released monomer depends on factors such as the type of resin, polymerization reaction, the length of the polymerization cycle and the thickness of the resin [4–6]. The degree of harmfulness of these acrylic resins is associated with their route of insertion and their availability in the environment [7]. On the other hand, frequent use of antigenic materials in dentistry can cause hypersensitivity reactions in the oral mucosa [8]. Denture base resins have exhibited variable degrees of cellular toxicity in vitro and tissue sensitivity reactions in vivo which may be attributed to the level of residual monomers after completion of polymerization reaction [9].
Variations in the components, structure and the purity level of the available resins in the market, the monomer conversion rate and manipulative variables may affect the physical and biochemical properties or the toxicity of the resins [10–12].
Weaver and Goebel reported that patients tend to suffer from less sensitivity reactions when their dentures are immersed in warm water [13]. In another study, Vallittu et al. evaluated the effect of polymerization time and temperature on the residual monomers in denture bases. Their results indicated that compared to the heat-cure type, self-cure acrylic resins release more free methyl methacrylate [4]. Huang et al. evaluated the toxicity of three denture base resins; a self-cure resin, a light-cure resin, and a heat-cure resin and evaluated the cytotoxic reaction of primary oral epithelial and fibroblast cells of the buccal mucosa. The order of the resins in terms of cytotoxicity in both the primary epithelial cells and buccal fibroblasts were as follows: light-cure>heat-cure>self-cure [11]. Futura Gen is a self-cure auto-polymerized acrylic resin used in the fabrication of denture bases via the injection method. Based on the manufacturers’ claim, contrary to the previous types of this product, Futura Gen is more acceptable in terms of color stability and because of the initiator system in this product i.e. copper ions and modified barbituric acid, less residual monomer remains in the denture base. Other positive characteristics of Futura Gen include dimensional stability, adaptability, ease of manipulation and cleanliness [14].
GC Reline Hard is an improved methyl methacrylate-free acrylic resin which is used for chairside reline. Reduced heat production, odor and chemical irritation, greater adaptability, less time consuming complicated laboratory procedure and minimal porosity are claimed to be some of the positive properties of this product [15].
To the best of our knowledge, there is lack of published articles and reliable evidence evaluating the cytotoxic effects of Futura Gen and GC Reline Hard acrylic resins. To ensure the safety of dental materials, tissue biocompatibility tests should be conducted. Thus, this study aimed to determine the cytotoxicity of these two products in comparison with conventional heat-cure resins in vitro.
MATERIALS AND METHODS
To evaluate the cytotoxicity of three denture base acrylic resins, this experimental study was designed according to Vojdani et al. research [16]. The cells were obtained from Pasteur Institute, Tehran, Iran and subjected to evaluation after 1-hour, 24-hours, and one-week incubation periods. The experimented resins were GC Reline Hard (GC America Inc.), Futura Gen (Schutz, Germany) and Meliodent (Heraeus Kulzer, Germany). The acrylic resin specimens were produced in molds prepared by the investment of circular dies (10 × 1 mm) within the flask. The heat cure specimens were polymerized using the long polymerization cycle (9 hours, 165°F/73.5°C). The acrylic resins were then deflasked and polished to the desired dimensions.
For the GC Reline Hard groups, the acrylic specimens were prepared by packing the GC Reline material into the molds subjected to pressure to undergo polymerization. The cured resins were eventually polished.
The Futura Gen resins were made according to the manufacturer’s instruction using the injection technique via Unipress system (Schutz-Dental GmbH, Rosbach, Germany). Prior to cytotoxicity testing, the dimensions of all specimens were measured by a digital vernier caliper.
To evaluate the cytotoxicity of acrylic base resins, we initially passaged the fibroblasts in the culture flasks.
After reaching an adequate volume of cultured cells, the cells were detached from the walls of the plate via a mixture of trypsin-EDTA. Cells were then plated in 24-well tissue culture plates (3×105 cell/cm2) [cell:RPMI media = 0.1:0.9ml] containing the acrylic discs.
In order to raise the number of cells to 3×105, after using trypsin and collecting the cells, the cells were counted using a neobar lam and the cell volume in the flask was raised to the desired level. A certain amount of these cells were subsequently extracted and exposed to Trypan blue (as a vital stain).
The cell vitality was evaluated under conventional microscope (×40 magnifications) where viable cells failed to absorb the stains. The number of viable cells required for the succeeding stages of the experiment should be over 90%. The fibroblast suspension was placed in the 24-well plates and the RPMI-1640 media along with antibiotics streptomycin and penicillin and 10% FBS (Fetal Bovine Serum) were added to the plates. The plates were then incubated (5% CO2, 37°C, over 90% humidity) and evaluated after 1 hour (for immediate inflammatory reactions), 24 hours (for acute inflammatory reactions) and 1 week (for chronic inflammatory reactions).
For MTT assay [3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide], 100 μl of the MTT solution was added to the plates and incubated for 4 hours in 37°C. Had the cells been able to reduce the solution, an insoluble, dark purple product called formazan was produced. The plates were then removed from the incubator and the excess volume of the solution in the wells was gently removed using a sampler and discarded. 200 μl of an acid-alcohol solution was added to each well. The acid and the alcohol were hydrochloric acid and isopropyl alcohol, respectively. The acid-alcohol solution was thoroughly mixed with the containments of the well using the sampler. Eventually, 100 μl of each resulting suspension was placed in ELISA plates and read via the ELISA reader.
Plates with no acrylic resin discs containing only the fibroblast and the RPMI-1640 media made up the negative control group. For the positive control group, however, we added distilled water to the fibroblasts. Distilled water lacks ions and therefore disturbs the osmotic balance of the cells resulting in cell lysis.
In order to minimize bias, the specimens were coded throughout the entire procedure of the experiment and the operator and the data assessor remained blind.
The effect of time and the type of resin were analyzed using the repeated measure ANOVA test. Furthermore, to evaluate the differences in light absorption on different intervals among the resin groups, one-way ANOVA and post-hoc Tukey’s test was implied and P values lower than 0.05 were considered significant. Data were analyzed using SPSS software, version 15.0.
RESULT
The statistical analysis revealed that time (P<0.001) and the type of resin (P<0.002) significantly affected cytotoxicity with each resin group demonstrating different levels of cytotoxicity on different intervals (except for the 1-week interval). However, the results failed to reveal any significant impact of time and the type of resin as combined covariates on the ultimate cytotoxicity (P>0.07). Thus, their effect is recognized as independent factors.
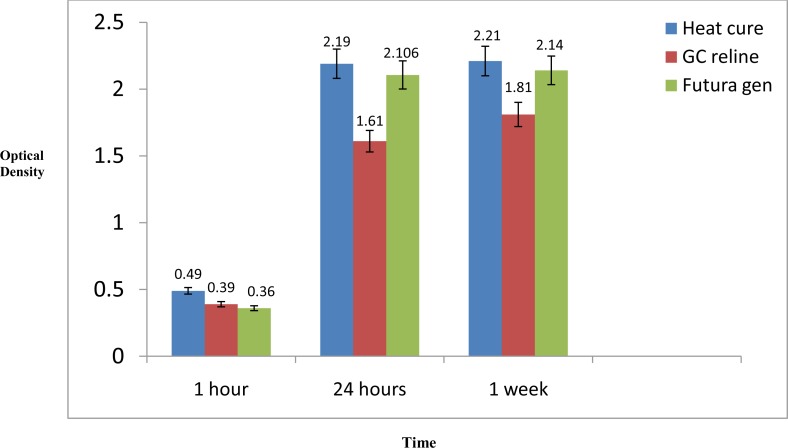
The mean (±SD) light absorption for GC Reline Hard, Meliodent and Futura Gen after 1 hour were 0.39(±0.058), 0.48(±0.11) and 0.36(±0.071), respectively. The two-way ANOVA revealed a significant difference among the groups in terms of cytotoxicity after 1 hour representing immediate inflammatory response to the resins (P<0.03). Furthermore, Tukey’s test results providing paired comparisons between the groups demonstrated a significantly less light absorption in the Futura Gen group compared to the Meliodent group (P=0.03). The test failed to reveal any significant differences between the Futura Gen and GC Reline Hard groups or Meliodent and GC Reline Hard groups, respectively.
The mean (±SD) light absorption for the Meliodent, Futura Gen, and GC Reline Hard groups after 24 hours were 2.19 (±0.49), 2.106 (±0.34) and 1.61 (±0.36), respectively. The two-way ANOVA revealed a marked difference in terms of cytotoxicity between the groups after 24 hours (P=0.02), which represented the acute phase of inflammation. The post-hoc Tukey’s test further demonstrated that light absorption in the GC Reline Hard group was significantly less than the Meliodent group (P=0.02). The test failed to reveal any significant differences between the GC Reline Hard and Futura Gen groups or Meliodent and Futura Gen groups, respectively.
The mean (±SD) light absorption for the Meliodent, Futura Gen, and GC Reline Hard groups after 24 hours were 2.21 (±0.13), 2.14 (±0.31) and 1.81 (±0.307), respectively. One-way ANOVA results revealed no significant difference among the groups in terms of cytotoxicity (P>0.09). Because of lack of significant difference among the groups, we failed to perform additional paired comparison between them (Figure 1).
Fig 1.
Average optical density of acrylic resins at different time intervals
DISCUSSION
The results of the present study indicated that the three groups of resins displayed various levels of cytotoxicity after 1 and 24 hours. The cytotoxicity level, however failed to show significant differences among groups after one week. The immediate inflammatory reaction to Futura Gen resin was significantly higher than Meliodent resin after a 1-hour incubation period; however, no significant difference was observed between Futura Gen and the GC Reline Hard resins or GC Reline Hard and the Meliodent group. After 24 hours (acute phase of inflammation), the cytotoxic effect of GC Reline Hard resin was shown to be significantly greater than the Meliodent resin and the Futura Gen resin, respectively. Futura Gen and Meliodent resins failed to show significant differences in cytotoxicity during the acute phase of inflammation. All groups showed relatively the same level of toxicity after one week (chronic phase of inflammation).
In general, Futura Gen and GC Reline Hard resins rendered a lower degree of light absorption indicating a higher level of toxicity, which varied among groups and the time intervals in terms of statistical significance.
Given the importance of rinsing in the elimination of toxic chemicals, it is recommended to immerse dentures in water before delivery. This has been said to reduce tissue hypersensitivity to different denture base resins to varying degrees [13]. Sheridan et al. reported that the cytotoxic effect of acrylic resins was greater in the first 24 hours after polymerization and decreased with time for all the resins evaluated in their study [17]. Lack of significant difference in cytotoxicity among the tested resins after one week in comparison with the cytotoxicity pattern after 24 hours may be because of the differences in the released substances and their cytotoxic effect on tissues. Moreover, different patterns of cytotoxicity may also be associated with the methods of polymerization and the chemical composition of the resin systems [18].
The method of polymerization is a decisive feature in the cytotoxicity of denture base acrylic resins. According to Hensten-Pettersen and Wictorin [19], the cytotoxic effect is greater in autopolymerized resins than in heat-polymerized resins. The cytotoxic effect of heat-activated, chemically-activated and microwave-activated acrylic resins on gingival fibroblasts were also reported by Sheridan et al. [17], who observed that among the tested materials, the greatest cytotoxic effect was produced by the chemically activated acrylic resins. The presence and the amount of free monomers in the resin is one of the most important factors in inducing the cytotoxic reaction and greater residual monomer has been shown to cause greater cytotoxicity [20]. According to the manufacturers of the Futura Gen and GC Reline Hard, these resins have been claimed to have less residual monomer remains in the denture base, yet, the results of the present study have proven otherwise. Huang et al. revealed that cold-cure acrylic resins are significantly more cytotoxic to oral epithelial cells (CCL-17) and human buccal mucosa fibroblasts compared to heat-cure and light-cure acrylic resins. The present study resembled Huang’s report and demonstrated that the peak level of reaction is observed after 24 hours subsiding gradually after 5 days [18]. Similarly, Ata and coworkers revealed that auto polymerized acrylic resins display greater levels of cytotoxicity [20].
Furthermore, the concentration of residual monomers varies with the methods and conditions of polymerization [20, 21]. Vallitu et al. performed a study with two autopolymerized resins in which the reaction was initiated by barbituric acid and two heat-polymerized resins activated by benzoyl peroxide. The results showed that autopolymerized resins exhibited higher contents of residual methyl methacrylate than the heat-polymerized resins. This may be due to the rise of temperature in heat-polymerized resins, which resulted in mobility of the molecular chains, thereby facilitating the conversion of monomer into polymer [4]. Moreover, Lamb et al. observed that levels of residual monomers in autopolymerized resins were higher for specimens polymerized at 22°C, as compared with those polymerized at 55°C. Therefore, it seems reasonable to suggest that the autopolymerized acrylic resins should be heat-treated to decrease cytotoxic effects [22]. The toxic elements in resins include formaldehyde, methyl methacrylate, methacrylic acid, benzoic acid, dibutyl phthalate, phenyl benzoate, phenyl salicylate, dicyclohexyl phthalate and additives such as hydroquinone, benzoyl peroxide and N-N dimethyl toluidine [21,23,24]. Formaldehyde in comparison with methyl methacrylate is a more potent material requiring more attention [25]. The origin of formaldehyde is not completely clear and may be a result of methacrylate oxidation or decomposition of oxygen-methacrylate copolymer. The amount of formaldehyde is also related to the residual methyl methacrylate and the means of polymerization [26]. When methyl methacrylate and methacrylate acid bind with an oxidizing agent as the initiator of heat polymerization reaction, a significant amount of formaldehyde is released. The oxidation reactions per se have also been deemed effective in the production and release of formaldehyde [21]. The release of formaldehyde in air saturated water was found to be larger than in argon-saturated water. The differences in quantity represent formaldehyde formed by oxidation of unreacted methacrylate in the polymerized material. The results also demonstrate that the quantity of released formaldehyde is dependent on the processing conditions of the denture base polymer. The quantity of released formaldehyde from different heat-cured materials is of the same magnitude. The release of relatively small quantities of formaldehyde from heat-cured materials is probably due to the fact that the oxygen-methyl methacrylate copolymer is thermally unstable. At the processing conditions of the heat-cured materials with 30 min in boiling water, the polymeric peroxide will decompose to a great extent and the volatile formaldehyde will diffuse out of the denture base materials. Prolonged time at high temperatures should further decrease the quantity of releasable formaldehyde [27].
The results indicated that the greatest level of cytotoxicity is observed after one-hour incubation period. This may be because of the culture conditions during the first hour. In other words, fibroblasts should be able to adapt themselves with the cell growth conditions during the first hours. The least level of cytotoxicity was observed after 24 hours and after one week, cytotoxicity was at a median level. It appears that after the first 24 hours, the residual monomers start to leak in water and thus less monomer is present to cause tissue damage [27, 28]. During the polymerization reaction, the free radicals react with oxygen and thus, the inhibition rate should be a function of oxygen gradient [29]. Numerous studies have evaluated the cellular toxicity of denture base resins [13,17, 30–32]. The findings of the present study confirming some degree of cytotoxicity among all the tested resins, was in line with the other reports [12,33]. Lai and colleagues demonstrated that the cytotoxic effect of the resin monomer (containing MMA) on human PDL fibroblast is dose dependent [34]. Our findings regarding the cytotoxic effect of cold-cure and heat-cure resins on L929 mouse fibroblast (except the GC Reline Hard resin which is claimed to lack MMA) confirmed the latter. Fibroblasts are the dominant cells in the gingival connective tissue and since the acrylic resin is in close contact with the attached epithelium, even in complete absence of inflammation (which is theoretically impossible), molecules lower than 100 Kda (kilodalton) can penetrate into the underlying connective tissue, providing a passage for the resin monomer to reach the connective tissue cells. Another reason for choosing mouse fibroblasts was their availability [35]. Various tests have been implemented to evaluate the cytotoxicity of different biomaterials in vitro. H-thymidine assay is a precise test in which the number of DNA synthesizing cells is counted. However, because of the high cost, advanced technology and the associated radioactive wastes, this test is seldom used in experiments [36]. But due to wide range of applicability and ease of access of MTT assay, we implemented this method in our evaluation. According to Wagner et al., the MTT assay is recommended as a screening tool and should not be used as a precise means of quantitative measurement of lymphocyte proliferation in canines [36]. Furthermore, in experimental evaluations of cytotoxicity, in order to avoid depletion of toxic chemicals in the environment and to obtain more precise results, it is recommended to evaluate the immediate inflammatory reaction to these substances, which in our study, was performed after the first hour. The results of the present study may not be completely applicable in clinical conditions; however, as a simple means of evaluation and with the elimination of confounding variables, in vitro studies are frequently regarded as sources of evidence in evaluating the cytotoxicity of denture base resins [27]. Hence, in generalizing the results of in vitro studies to clinical practice, limitations in the simulation of in vivo conditions should be taken into consideration. On the other hand, acrylic resins are increasingly used in reline and denture base repair procedures and since they have long been thought to impose very little risk on oral tissues, no biological analysis has been performed on them prior to their application in clinical practice [36]. Considering that all the acrylic resins in the present study demonstrated some degree of cytotoxicity, further experimental and clinical evaluation, using different methods is required. Moreover, further long term studies (over one week) evaluating the effect of different substances associated with denture base resins can provide the researchers with further details of the nature of the tissue irritation [37].
CONCLUSION
We found that all the tested resins displayed cytotoxic effects on fibroblasts. The Futura Gen and GC Reline Hard displayed greater levels of cytotoxicity at all times compared to the heat-cure Meliodent resin and the highest and lowest levels of cytotoxicity among all groups were observed after 1 hour and one week-incubation period, respectively.
Acknowledgments
The author thank the vice-chancellery of Shiraz University of Medical Science, for supporting the research (Grant# 5550). This manuscript relevant thesis of Dr M Ebrahimi Saravi.
REFERENCES
- 1.Sadamori S, Kotani H, Hamada T. The usage period of dentures and their residual monomer contents. J Prosthet Dent. 1992 Aug;68(2):374–6. doi: 10.1016/0022-3913(92)90349-f. [DOI] [PubMed] [Google Scholar]
- 2.Schuster GS, Lefebvre CA, Dirksen TR, Knoernschild KL, Caughman GB. Relationships between denture base resin cytotoxicity and cell lipid metabolism. Int J Prosthodont. 1995 Nov-Dec;8(6):580–6. [PubMed] [Google Scholar]
- 3.Tsuchiya H, Hoshino Y, Tajima K, Takagi N. Leaching and cytotoxicity of formaldehyde and methyl methacrylate from acrylic resin denture base materials. J Prosthet Dent. 1994 Jun;71(6):618–24. doi: 10.1016/0022-3913(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 4.Vallittu PK, Ruyter IE, Buykuilmaz S. Effect of polymerization temperature and time on the residual monomer content of denture base polymers. Eur J Oral Sci. 1998 Feb;106(1):588–93. doi: 10.1046/j.0909-8836.1998.eos106109.x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison A, Huggett R. Effect of the curing cycle on residual monomer levels of acrylic resin denture base polymers. J Dent. 1992 Dec;20(6):370–4. doi: 10.1016/0300-5712(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 6.Yunus N, Harrison A, Huggett R. Effect of microwave irradiation on the flexural strength and residual monomer levels of an acrylic resin repair material. J Oral Rehabil. 1994 Nov;21(6):641–8. doi: 10.1111/j.1365-2842.1994.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 7.Craig RG, Powers JM. Restorative dental materials. 12th ed. St Louis: The CV Mosby Co; 2006. pp. 125–62. [Google Scholar]
- 8.American National Standards Institute/American Dental Association Document no. 41 for Recommended Standard Practices for Biological Evaluation of Dental Materials Council on Dental Materials and Devices. J Am Dent Assoc. 1979 Oct;99(4):697–8. [PubMed] [Google Scholar]
- 9.Jorge JH, Giampaolo ET, Machado AL, Vergani CE. Cytotoxicity of denture base acrylic resin: a literature review. J Prosthet Dent. 2003 Aug;90(2):190–3. doi: 10.1016/s0022-3913(03)00349-4. [DOI] [PubMed] [Google Scholar]
- 10.Bahnen Kamp DM. Traumatic stomatitis following an intraoral denture reline: a clinical report. J Prosthet Dent. 1996 Aug;76(2):113–4. doi: 10.1016/s0022-3913(96)90292-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang FM, Hu CC, Chang YC, Chou MY. The level of monomer release from acrylic denture base materials into water. Chin Dent J. 2000;19:17–22. [Google Scholar]
- 12.Vallittu PK. The effect of surface treatment of denture acrylic resin on the residual monomer content and its release into water. Acta Odontol Scand. 1996 Jun;54(3):188–92. doi: 10.3109/00016359609003522. [DOI] [PubMed] [Google Scholar]
- 13.Weaver RE, Goebel WM. Reactions to acrylic resin dental prostheses. J Prosthet Dent. 1980 Feb;43(2):138–42. doi: 10.1016/0022-3913(80)90176-6. [DOI] [PubMed] [Google Scholar]
- 14.FuturaGen. Available at: http://www.developementschuetzdental.de/Futura_Gen.306.0.html?&L=2
- 15.GC RELINE. Hard Denture, Chair side Reline Material. Available at: http://www.gcamerica.com/products/operatory/GC_Reline/index.php
- 16.Vojdani M, Satari M, Khajeh Hosseini SH, Farzin M. Cytotoxicity of resin-based cleansers: an in vitro study. IRCMJ Iranian Red Crescent Med J. 2010 Mar;12(2):158–62. [Google Scholar]
- 17.Sheridan PJ, Koha S, Ewoldsen NO, Lefebvre CA, Lavin MT. Cytotoxicity of denture base resins. Int J Prosthodont. 1997 Jan-Feb;10(1):73–7. [PubMed] [Google Scholar]
- 18.Huang FM, Tai KW, Hu CC, Chang YC. Cytotoxic effects of denture base materials on a permanent human oral epithelial cell line and on primary human oral fibroblast in vitro. Int J Prosthodont. 2001 Sep-Oct;14(5):439–43. [PubMed] [Google Scholar]
- 19.Hensten-Pettersen A, Wictorin L. The cytotoxic effect of denture base polymers. Acta Odontol Scand. 1981;39(2):101–6. doi: 10.3109/00016358109162267. [DOI] [PubMed] [Google Scholar]
- 20.Ata SO, Yavuzyilmaz H. In vitro comparison of the cytotoxicity of acetal resin, heat-polymerized resin, and auto-polymerized resin as denture base materials. J Biomed Mater Res B Appl Biomater. 2009 Nov;91(2):905–9. doi: 10.1002/jbm.b.31473. [DOI] [PubMed] [Google Scholar]
- 21.Kedjarune U, Charoenworaluk N, Koontongkaew S. Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J. 1999 Mar;44(1):25–30. doi: 10.1111/j.1834-7819.1999.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 22.Lamb DJ, Ellis B, Priestley D. The effects of process variables on levels of residual monomer in autopolymerizing dental acrylic resin. J Dent. 1983 Mar;11(1):80–8. doi: 10.1016/0300-5712(83)90051-9. [DOI] [PubMed] [Google Scholar]
- 23.Koda T, Tsuchiya H, Yamauchi M, Ohtani S, Takagi N, Kawano J. Leachability of denture base acrylic resins in artificial saliva. Dent Mater. 1990 Jan;6(1):13–6. doi: 10.1016/0109-5641(90)90037-f. [DOI] [PubMed] [Google Scholar]
- 24.Lygre H, Solheim E, Gjerdet NR. Leaching from denture base materials in vitro. Acta Odontol Scand. 1995 Apr;53(2):75–80. doi: 10.3109/00016359509005950. [DOI] [PubMed] [Google Scholar]
- 25.Koda T, Tsuchiya H, Yamauchi M, Hoshino Y, Takagi N, Kawano J. High-performance liquid chromatographic estimation of eluates from denture base polymers. J Dent. 1989 Apr;17(2):84–9. doi: 10.1016/0300-5712(89)90137-1. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya H, Hoshino Y, Kato H, Takagi N. Flow injection analysis of formaldehyde leached from denture-base acrylic resins. J Dent. 1993 Aug;21(4):240–3. doi: 10.1016/0300-5712(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 27.Ruyter IE. Release of formaldehyde from denture base polymers. Acta Odontol Scand. 1980;38(1):17–27. doi: 10.3109/00016358008997715. [DOI] [PubMed] [Google Scholar]
- 28.Lamb DJ, Ellis B, Priestley D. Loss into water of residual monomer from autopolymerizing dental acrylic resin. Biomaterials. 1982 Jul;3(3):155–9. doi: 10.1016/0142-9612(82)90005-9. [DOI] [PubMed] [Google Scholar]
- 29.Baker S, Brooks SC, Walker DM. The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: an assay for monomer in saliva. J Dent Res. 1988 Oct;67(10):1295–9. doi: 10.1177/00220345880670101001. [DOI] [PubMed] [Google Scholar]
- 30.Lee SY, Lai YL, Hsu TS. Influence of polymerization conditions on monomer elution and microhardness of autopolymerized polymethyl methaclylate resin. Eur J Oral Sci. 2002 Apr;110(2):179–83. doi: 10.1034/j.1600-0722.2002.11232.x. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre CA, Schuster GS. Biocompatibility of visible light-cured resin systems in prosthodontics. J Prosthet Dent. 1994 Feb;7(2):178–85. doi: 10.1016/0022-3913(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 32.Jorge JH, Giampaolo ET, Vergani CE, Machado AL, Pavarina AC, Carlos IZ. Cytotoxicity of denture base resins: effect of water bath and microwave post-polymerization heat treatments. Int J Prosthodont. 2004 May-Jun;17(3):340–4. [PubMed] [Google Scholar]
- 33.Moharamzadeh K, Van Noort R, Brook IM, Scutt AM. Cytotoxicity of resin monomers on human gingival fibroblasts and HaGaT keratinocytes. Dent Mater. 2007 Jan;23(1):40–4. doi: 10.1016/j.dental.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Lai YL, Chen YT, Lee SY, Shieh TM, Hung SL. Cytotoxic effects of dental resin liquids on primary gingival fibroblasts and periodontal ligament cells in vitro. J Oral Rehabil. 2004 Dec;31(12):1165–72. doi: 10.1111/j.1365-2842.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 35.Mahshid M, Satari M, Mehdizade Dastjerdy M. Comparison of cellular toxicity of Iranian and non-Iranian self cure acryle and their effects on production of mouse IL-6 by L929 gingival fibroblast. Shiraz Univ Dent J. 2007;7:63–74. [Google Scholar]
- 36.Wagner U, Burkhardt E, Failing K. Evaluation of canine lymphocyte proliferation: comparison of three different colorimetric methods with the 3H-thymidine incorporation assay. Vet Immunol Immunopathol. 1999 Sep;70(3–4):151–9. doi: 10.1016/s0165-2427(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith DC. Recent development and prospects in dental polymers. J Prosthet Dent. 1962;12:1066–10. [Google Scholar]