Abstract
The pedunculopontine tegmental nucleus (PPN) is being explored as a site for deep brain stimulation (DBS) for the treatment of patients with medically refractory gait and postural abnormalities (MRGPA) associated with Parkinson's disease (PD). The PPN is involved in initiation and modulation of gait and other stereotyped motor behaviors and is inter-connected with the pallido-thalamo-cortical circuit. GPi DBS is effective at treating the motor signs associated with PD, however its impact on MRGPA is limited and its effect on PPN neuronal activity is unknown. The current work characterizes the effect of therapeutically-effective GPi DBS on PPN neuronal activity in a single rhesus monkey made parkinsonian using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). A scaled-down, quadripolar DBS lead was implanted into sensorimotor GPi under electrophysiological and stereotactic guidance. Single neuron activity was recorded from PPN before, during and after DBS. GPi DBS reduced the mean discharge rate of PPN neurons from 16.8 Hz to 12.8Hz, with 34 (66.7%) neurons showing a decreased mean rate, 3 (6.7%) increased and 12 (26.7%) unchanged. Consistent with known GABAergic projections from GPi to PPN, and with previous observations that stimulation increases output from the stimulated structure, GPi DBS suppressed activity in PPN. The present observations, together with previous reports of improvement in MRGPA during low frequency stimulation in PPN, suggest that activation of PPN output may be required to improve MRGPA and may account for the lack of improvement in MRGPA typically observed with GPi or STN DBS.
Keywords: deep brain stimulation; pedunculopontine tegmental nucleus; globus pallidus; MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine); monkey; Parkinsonism
Introduction
Deep brain stimulation (DBS) has revolutionized the treatment of Parkinson's disease (PD) and renewed exploration of surgical therapy for a wide range of neurological disorders (Constantoyannis, et al., 2004, Greenberg, et al., 2008, Hariz, et al., 2002, Houeto, et al., 2005, Magarinos-Ascone, et al., 2008, Witjas, et al., 2005, Wyckhuys, et al., 2009). Chronic, high-frequency stimulation of the internal segment of the globus pallidus (GPi) is an effective therapy for tremor, rigidity, bradykinesia as well as dopamine responsive gait, balance and freezing disorders. For a subset of patients however, particularly those in the advanced stages of the disease, difficulties with gait, balance and freezing may be unresponsive to GPi or STN DBS as well as to levodopa replacement therapy. This has led some to hypothesize that the pathophysiological basis underlying these axial motor symptoms involves anatomical pathways outside of the traditional pallido-thalamo-cortical neuronal circuitry.(Allert, et al., 2001, Bonnet, et al., 1987, Faist, et al., 2001, Ferrarin, et al., 2005, Liu, et al., 2005, Lubik, et al., 2006, Tagliati, et al., 2008), including brainstem areas like the peduncupontine tegmental nucleus (PPN). The PPN receives input from both the basal ganglia and the spinal cord and its cholinergic and glutamatergic neurons project, in turn, to widespread basal ganglia, thalamic, brainstem and spinal cord targets. It is known to play a role in the initiation, maintenance and modulation of gait and postural stability (Lee, et al., 2000), which has led a number of researchers to explore the PPN as a possible therapeutic target for patients with medically refractory gait and postural abnormalities (Jenkinson, et al., 2004, Mazzone, et al., 2005, Nandi, et al., 2002, Pereira, et al., 2008, Plaha and Gill, 2005, Stefani, et al., 2007). To date, the results of those preliminary studies have been mixed, as some have reported significant improvement in response to low frequency PPN DBS (Jenkinson, et al., 2004, Mazzone, et al., 2005, Nandi, et al., 2002, Pereira, et al., 2008, Plaha and Gill, 2005) while others have failed to observe benefit (Stefani, et al., 2007).
Given current interest in the PPN as a therapeutic target and its proposed mechanism of action, i.e. activation of PPN output, an understanding of the effect of GPi and STN DBS on PPN activity is critically important. In this study, we investigated the effect of therapeutic GPi DBS on PPN neuronal activity in a rhesus monkey rendered parkinsonian using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). By examining the effect of GPi DBS on PPN neuronal activity, we may better understand why GPi DBS has limited effect on medically refractory gait and postural abnormalities and devise alternative approaches and targets for its treatment.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee and complied with United States Public Health Service policy on the humane care and use of laboratory animals. Surgical procedures were performed in aseptic conditions using isoflurane anesthesia. A single, female monkey (Macaca mulatta, 5 kg, 9 yrs) was used for this study.
Behavioral assessments
The severity of the parkinsonian state was assessed weekly using a version of the Unified Parkinson's Disease Rating Scale (UPDRS) modified for the non-human primate. A four-point scale is used to evaluate each of ten key parkinsonian features, including rigidity, bradykinesia and tremor. Scoring ranges from 0 to 3, with a score of zero representing the “normal” motor state for a given feature. The scale was used to confirm the attainment and stability of the moderate (overall score between 18 and 28) parkinsonian condition, with specific sub-scale items, including rigidity, used individually to evaluate the effect of GPi DBS.
Quantitative evaluation of bradykinesia was achieved using a reach and retrieval task. After comfortably restraining one of the animal's arms, small food items were placed in each of three wells of a modified Klüverboard and the time required to retrieve the items with the unrestrained arm recorded. The animal was timed from initial contact with the first piece of food until contact with the mouth with the last piece. Once performance stabilized, task performance video was captured directly to PC computer for off-line analysis. The duration of each of three aspects of the motor behavior was determined: reach, manipulation and retrieval time. Reach time is defined as the time from the hand leaving the mouth to the time when the hand enters the food well. Manipulation time represents the amount of time that the hand remains within the food well. Finally, retrieval time is the time from when the animal's hand leaves the food well to when it arrives at the mouth. The sum of the reach and retrieval time components was used to represent the total movement time for the reach and retrieval task. Only those trials where the hand started and ended at the same position in space were included in the analysis.
Quantitative evaluation of rigidity was performed using the automated rigidity testing (ART) system. The ART system(Nitta, et al., 2009) was used to record the force normal to the transducer (Fz, 1/40N resolution), which best reflected the rigidity about the elbow joint by directly opposing the direction of arm rotation. Rigidity was quantified with multiple analytical parameters on the Fz versus angle plot. Five measures of rigidity were calculated: total area (TA), extension area (EA), flexion area (FA), extension slope (ES), and flexion slope (FS). We evaluated the rigidity measures under both ON and OFF conditions. The loading and unloading hysteresis plot was used to represent the extension and flexion curves. Details of the ART system and the rigidity measures can be found elsewhere (Nitta, et al., 2009).
In addition to the reach and retrieval task an index of spontaneous motor activity was created through manual scoring of upper and lower extremity movement time during videotaped cage behavior period. This was achieved by transferring the animal from its home cage into a specially-designed observation cage where its behavior was videotaped over a period of thirty minutes. The first ten minutes of video was excluded as the animal acclimated to the cage environment, while activity during the remaining twenty minutes was reviewed manually off-line to generate the total movement time in seconds.
Chamber placement
Prior to placing the recording chamber high-resolution MRI and CT imaging was performed. The imaging data were imported into the Cicerone software package and used to target and position the cephalic recording chambers on the skull (Miocinovic, et al., 2007). This software also allows the user to interactively visualize the X, Y, and Z coordinates of recorded neurons superimposed on to the pre-operative MRI. Once the stereotaxic coordinates of the target and entry point were determined for each cephalic chamber, and with the head positioned in a primate stereotaxic instrument, a pair of craniotomies was performed. Over each, a cephalic recording chamber was placed: one oriented in the parasagittal plane targeted at PPN and a second oriented in the coronal plane targeted at GPi. Titanium screws were secured to the skull, and the implant system consisting of screws, recording chambers and a head stabilization receptacle were bonded together with dental acrylic.
Administration of MPTP
The parkinsonian state was induced using the neurotoxin MPTP (0.1% solution in saline). A symmetric, systemic parkinsonian state was achieved by repeated intramuscular injections until the animal reached a persistent, moderate state of disability marked by an average score of 24.2 (±0.8 SD) on the modified UPDRS across the experimental period.
Neuronal recording and analysis
The GPi was mapped electrophysiologically in the awake animal using single-neuron microelectrode techniques. With the animal placed in a primate chair and its head restrained, a tungsten microelectrode (impedance 0.5 – 1.0 MΩ at 1 KHz) was advanced using a hydraulic microdrive (Narishige Scientific Instruments) attached to the recording chamber. The sensorimotor portion of GPi was identified, including its medial, lateral and posterior borders, using techniques similar to those applied during human functional neurosurgery.(Hutchison, et al., 1998, Vitek, et al., 1998). Once the sensorimotor region of GPi was identified, a scaled-down version of a DBS lead used in humans (Model 3387, Medtronic Inc.) was implanted (Elder, et al., 2005). The DBS lead had four metal contacts, each with a diameter of 0.76 mm, a height of 0.50 mm and separated from each other by a distance of 0.50 mm. The lead was connected to a programmable pulse generator (Itrel II, Medtronic Inc.) surgically implanted subcutaneously between the scapulae. Stimulation parameters were assessed and those that induced the greatest improvement in motor signs at the lowest voltage were used for the study. Stimulation parameters were pre-set to include a 90 μs pulse width and a pulse frequency of 135 Hz.
Following placement of the DBS lead within the GPi, the PPN was electrophysiologically mapped with the analog signal from the microelectrode amplified (15,000×), filtered (100 Hz -10,000Hz) and digitized to a PC computer using the Spike 2 data acquisition system (50,000 Hz) for offline analysis. The PPN recording chamber was oriented such that the microelectrode penetrations were made in the sagittal plane at an angle of 25 degrees from vertical. The microelectrode was advanced toward the target and the acoustically transduced neuronal activity was monitored continuously while the animal performed a voluntary movement or was examined passively by the investigator to determine the receptive field characteristics of each neuron. Typical PPN neurons include Type I bursting cell and Type II tonic cell (Pahapill and Lozano, 2000). Type I neurons are characterized by high-frequency clustered bursting; Type II neurons have a much more regular, tonic pattern. It is our experience that both types generally are not responsive to passive limb movement. Microstimulation up to 40 μA was delivered intermittently to help to further identify the location of the microelectrode tip.
Once a neuron was isolated, spontaneous neuronal activity was collected under the following conditions: pre-stimulation “control” for 1 minute, during GPi stimulation (using therapeutic voltage) for 1 minute and following cessation of stimulation for 1 minute. The after-stimulation state was examined to confirm persistence of neuronal activity across the recording period. Stimulation artifact was removed from the digitized neuronal recordings as previously described (Hashimoto, et al., 2002) with subsequent identification of spikes made using custom-designed software written in Matlab. The timestamps of both the neuronal action potentials and the DBS stimulus pulses were stored for off-line analysis. A post-stimulation time histogram (PSTH) of neuronal activity before, during, and after stimulation was constructed by aligning the occurrence of action potentials with the onset of each stimulus pulse during stimulation, or to computer generated trigger pulses with the same frequency as the stimulation pulses during collection of spontaneous activity before and after stimulation. The discharge rate within each bin of the PSTH during stimulation was assessed for a significant increase or decrease by evaluating the probability that the change in discharge rate within each bin could have occurred by chance compared to the discharge rate predicted by a Poisson distribution with a mean rate of the corresponding bin in the pre-stimulation time histogram (P ≤ 0.05). Changes in the overall pattern of each PSTH were evaluated by comparing the distribution of bins in the PSTH during stimulation with the distribution of bins in the PSTH before stimulation using a Kolmogorov-Smirnov goodness of fit test.
Statistical comparisons of the mean of behavioral data and firing rates were performed using t-tests (α = 0.05). Oscillatory activity was assessed by evaluating the power change (ON state - OFF state) in different frequency ranges as the ratio of the power in each range to the total power in the entire spectrum. Power was examined in 5 different frequency ranges, 3-8Hz, 8-13Hz, 13-30Hz, 30-60Hz, and 60-90Hz. All values were expressed as mean ± standard error. Burst characteristics of each neuron was analyzed using a modified Poisson surprise algorithm originally described by Legendy and Salcman (Legendy and Salcman, 1985) to detect the occurrences of bursts. This detection algorithm iteratively evaluates the probability that the number of action potentials within successive time intervals could have occurred by chance by comparing the actual number of spikes to the number of spikes predicted by a Poisson distribution. After bursts are identified, the frequency of bursting (bursts per minute) was calculated. The bursting index was defined by the number of interspike intervals less than 10 ms divided by the number of interspike intervals greater than 10 ms in the examined period of time.
Results
Behavioral changes
Optimal therapeutic parameters for GPi DBS at a pre-set pulse rate of 135 Hz were identified as using contacts 2 and 1 as the cathode and anode, respectively, with a pulse amplitude of 6.0 V. Stimulation at these settings was associated with improvements across all activity metrics applied. A significant improvement in spontaneous cage behavior activity was marked by an increase in movement of the affected upper (93 seconds to 214 seconds per 20 min.) and lower extremity (73 seconds to 161 seconds per 20 min.) as shown in Figure 1A. Reach and retrieval time also improved (p < 0.01) from an average of 0.285 (+/- 0.045 SD) seconds without stimulation to an average of 0.25 (+/- 0.038 SD) during GPi DBS (Figure 1B). Marked improvements in rigidity were observed, denoted by a reduction in the UPDRS subscale score of rigidity from 2 to 0 during GPi DBS. Quantitative measurement of rigidity using the ART system revealed a significant decrease across all five rigidity measures (p < 0.05), with reductions ranging from 25% for FA to 70% for FS (Nitta, et al., 2009). Figure 1C (reprinted from (Nitta, et al., 2009), with permission) compares the DBS OFF to DBS ON conditions in this monkey, and shows a reduction in the loading and unloading hysteresis plot represented by a decreased shaded area and flatter extension and flexion curves.
Figure 1.
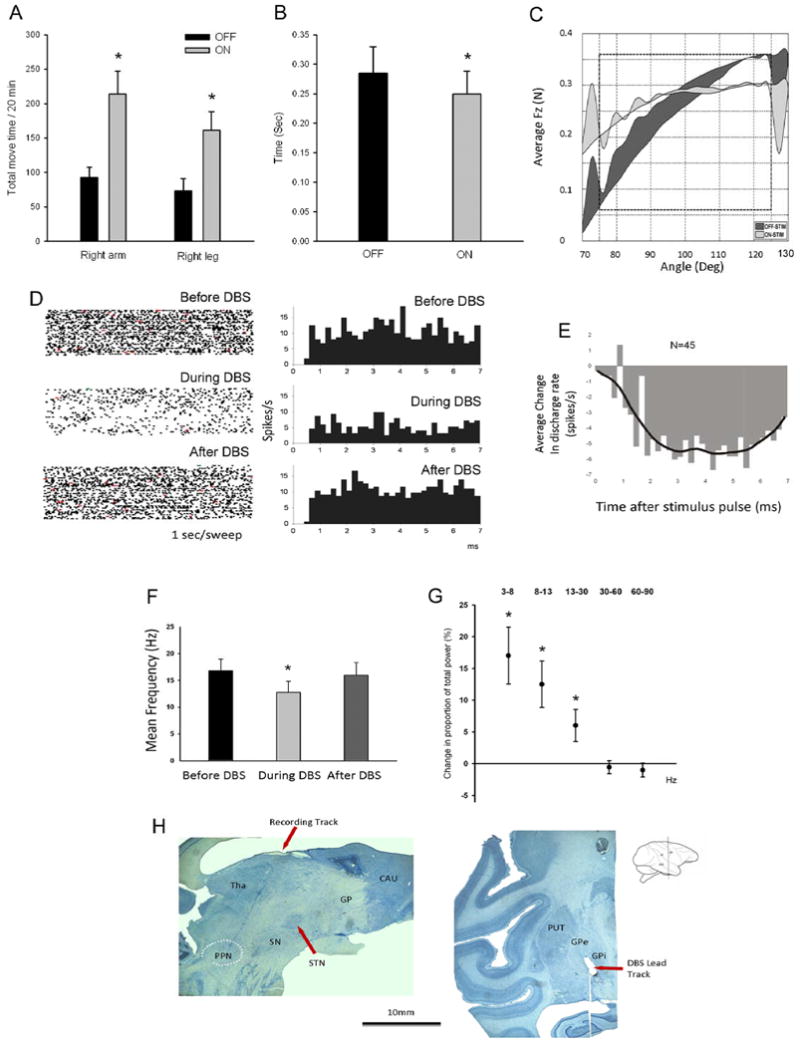
Panel A: total movement time of the right upper and lower extremity during a 20-minute cage behavior analysis. The y-axis shows the total movement time in seconds. Panel B: sum of total movement time in the reach and retrieval task before and during GPi DBS, showing behavioral improvement in bradykinesia. Y axis is in seconds. For this and the following panels, significance is marked by *, and standard errors are shown by bar plots around the mean. Panel C: changes in all rigidity measures induced by GPi-DBS in this monkey (reprint from (Nitta, et al., 2009) with permission). In the DBS-on state, the averaged Fz versus angle plot showed a substantial decrease in area and slope. Panel D: raster plots (left) at 1 second/sweep and perievent histograms (right) illustrating discharge activity for one, typical PPN neuron, before, during and after GPi DBS. During the stimulation, the events are counted on a 7 ms cycle (corresponding to the 135 pulse per second DBS). Events of the before and after states were counted also in a 7 ms cycle, based on a computer-generated pulse-timing event. The first bin is omitted because of signal saturation and residual stimulation artifacts. X axis: time in milliseconds after the stimulation pulse or computer-generated pulse-timing event. Y axis: spikes per second. Panel E: changes (OFF-ON) in the averaged post stimulation time histogram (PSTH) of all the PPN neurons during GPi DBS, showing inhibition of neuron firing. The responses for each animal are illustrated by the bars and the continuous line (Kolmogorov-Smirnov goodness of fit test). The bars were determined by subtracting the response during stimulation from that which occurs during the control period before stimulation. X axis: time in milliseconds post the stimulation pulse. Y axis: spikes per second. Panel F: Mean discharge frequency of PPN neurons before, during and after GPi DBS, showing decreased neuronal activities during the stimulation. Panel G: The mean change in proportion of total power in the PPN population neuronal activity during the DBS ON relative to the DBS OFF state. Five frequency bands are examined: 3-8 Hz, 8-13 Hz, 13-30 Hz, 30-60 Hz and 60-90 Hz. Panel H: Histology of monkey brain. Left: Sagittal view of the brain showing the recording track going through the thalamus to the PPN (white dashed oval). Right: Coronal view of the brain showing the tip of DBS lead located in the dorsal posterior area of GPi.
Electrophysiological Changes
Mean discharge rate
Pre-, peri- and post-stimulation data were collected for a total of 45 PPN neurons, with the predominant response to GPi DBS being inhibition of PPN neuronal activity. The raster plots (left) and PSTH (right) in Figure 1D, provide an example of the effect of GPi DBS on the activity of a single neuron in PPN during GPi DBS. Following the reduction in activity observed during the stimulation period, the firing rate returned to baseline level after discontinuation of GPi DBS. Figure 1E shows the effect of stimulation on the PSTH for the population of PPN neurons, and illustrates that GPi DBS is associated with a persistent reduction in neuronal activity throughout approximately 7 ms interval between stimulus pulses. Overall, the mean discharge rate of PPN neurons decreased from a pre-stimulation level of 16.8 (+/- 2.12 SE) Hz to 12.8 (+/- 2.06 SE) Hz during stimulation (p<0.001), as demonstrated in Figure 1F. Overall for the population of PPN neurons recorded during pre, during and post DBS, thirty-four (66.7%) neurons decreased firing rate, 3 (6.7%) increased and 12 (26.7%) remained unchanged.
Burst and Oscillatory Activity
GPi DBS was associated with a tendency for reduced bursting activity, with the burst index decreasing from 0.17 to 0.12 (p=0.072) and the burst frequency dropping from 32.52 to 27.61 (P=0.08), however these changes did not reach statistical significance. The average change of power for PPN neuronal activity during GPi DBS is shown in Figure 1G. A significant increase was identified in the 3-8Hz (p=0.017), 8-13Hz (p=0.027) and 13-30Hz (p=0.027) frequency ranges.
Histology
Recording sites in the PPN were confirmed histologically. Figure 1H(left) shows the recording track in a parasagittal section of the brain passing through the thalamus into the region of the PPN, the approximate position of which is designated by the white dashed oval. The DBS lead was confirmed to be positioned in the dorsal posterior region of GPi as illustrated in the coronal section in Figure 1H (right).
Discussion
In this study, we examined the effect of high-frequency stimulation of the GPi on PPN neuronal activity in a single non-human primate made moderately parkinsonian with the neurotoxin MPTP. We observed a reduction in the mean discharge rate and burst activity of the majority of PPN neurons during GPi DBS. The inhibition of PPN observed in our study is consistent with the activation of GABAergic output of the GPi during DBS as previously reported by our group (Hashimoto, et al., 2003, Vitek, et al., 2004) as well as with observations from others suggesting that stimulation increases output from the stimulated structure (Hershey, et al., 2003, Jech, et al., 2001, Perlmutter, et al., 2002, Windels, et al., 2003, Xu, et al., 2008). Changes were also observed in the oscillatory activity of neurons in the PPN, and support recent hypotheses concerning the role of oscillatory patterns in the pathophysiology of parkinsonism (Brown, 2006). Contrary to the reduction in oscillatory activity observed in GPi during STN DBS or VLo during GPi DBS (Xu, et al., 2008), oscillatory activity was increased in the theta and beta bands. While the relationship between changes in rate and oscillatory patterns and the behavioral improvement associated with GPi stimulation requires further investigation, these data are consistent with the hypothesis that GPi DBS produces a change in the pattern and periodicity of neuronal activity in the basal ganglia network and that this change extends beyond the traditional basal ganglio-thalamo-cortical network to include the PPN.
Embedded in an area packed with nuclei and vasculatures, the PPN has extensive connections. The output of the PPN is comprised of ascending, cholinergic fibers that project to thalamic nuclei and ascending non-cholinergic fibers that project to the globus pallidus, substantia nigra, subthalamic nucleus, and ventral tegmental area. In addition to the ascending projections, the PPN also has descending projections to the medullary and pontine reticular formation as well as bilaterally to the spinal cord(Semba and Fibiger, 1992, Shute and Lewis, 1967, Spann and Grofova, 1989). Based on the hypothesis that stimulation activates output from the stimulated structure and the known anatomical inputs to PPN, we would expect that DBS of either the STN or the GPi would ultimately have a net inhibitory effect on PPN activity. In the case of STN, we anticipate this inhibitory effect would derive from activation of the excitatory projection from STN to GPi increasing the inhibitory impact of that structure on PPN. Thus, while DBS of either structure produces improvements in rigidity and tremor, the inhibitory effect of GPi or STN stimulation on PPN could compromise the beneficial effect of stimulation in these sites on motor symptoms mediated by PPN. This may account for, or contribute to, the observations by others of a worsening of gait over time out of proportion to that observed for other parkinsonian motor signs with chronic DBS ON state(Tagliati, et al., 2008). Indeed it is surprising that DBS in these sites improves gait at all given our observations of suppression of PPN neurons during stimulation. One explanation is that compensatory mechanisms may play a role during the early phases of stimulation that wane over time. Further evidence for the suppression of PPN by STN and GPi DBS as a cause for gait dysfunction in PD comes from a recent study by Jabre et al (Jabre, et al., 2008) who reported on a cohort of patients who, after an average of three years of STN DBS treatment, showed a gradual worsening of axial symptoms, including freezing of gait, that was reversed significantly through a reduction of stimulation frequency (average 150 Hz to 81.7 Hz). It is possible that this lower frequency, while still sufficient to desynchronize the pathological activity present in the basal ganglia-thalamocortical circuitry, was low enough to minimize the profound inhibitory effect of the GPi on PPN.
If the inhibitory drive from GPi and SNr to PPN is overly active in PD and thus contributing to problems initiating programmed movements, then interventions that reduce the excessive inhibition of PPN from GPi and SNr, or interventions that directly increase output from the PPN, should facilitate a return towards normal function. If PPN stimulation acts by way of a pathway independent from that influenced by L-dopa (or STN and GPi DBS), then it may be that both can be incorporated into optimal treatment of advanced PD that more equally impacts tremor, rigidity and gait dysfunction. This could explain the results of the 2007 study of Stefani et al. where both STN and PPN stimulation were required to improve medically refractory gait and balance problems in PD patients.
The major limitation of previous PPN studies is an incomplete understanding of the electrophysiological features of the PPN allowing identification of this structure during electrophysiological mapping prior to lead placement. In human studies the final lead location is hard to assess since histological confirmation lead location is not possible and rarely accomplished even after death of the patient. Furthermore susceptibility artifact associated with MR scanning following placement precludes accurate lead localization on MRI; thus the actual site of implantation remains unclear. Located in such a dense mass of nuclei and fiber pathways, PPN stimulation outcomes are likely to be highly location specific. The controversy in current PPN studies underscores the complexity and limited characterization of this brainstem region and its potential role in the treatment of gait and other motor symptoms associated with PD. As such, studies in nonhuman primates that can provide an understanding of the changes in neuronal activity occurring with DBS, and how these correlate to the motor symptoms of PD with histological confirmation of the lead, have become even more important to the scientific community if we are to determine the utility of this target for the treatment of PD. In addition to understanding the electrophysiological hallmarks of PPN neuronal activity these data will facilitate the safe and accurate targeting of the PPN in human PD patients.
Conclusion
GPi DBS suppressed neuronal activity in the PPN in the MPTP primate. This is consistent with activation of GABAergic projections from the GPi to the PPN and previous observations that stimulation increases output from the stimulated structure. This occurred coincident with improvement in rigidity and bradykinesia. Given the reported lack of improvement in MRGPA with GPi DBS, the present study together with previous reports of improvement in MRGPA during low frequency stimulation in PPN suggest activation of PPN output may be required for optimal treatment of MRGPA in advanced PD, and provides the rationale for why PD patients with chronic STN or GPi DBS may improve gait and balance with reduction in stimulation frequency as reported by others.
Acknowledgments
This work was supported by National Institutes of Health Grant NS037019.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allert N, Volkmann J, Dotse S, Hefter H, Sturm V, Freund HJ. Effects of bilateral pallidal or subthalamic stimulation on gait in advanced Parkinson's disease. Mov Disord. 2001;16:1076–1085. doi: 10.1002/mds.1222. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet AM, Loria Y, Saint-Hilaire MH, Lhermitte F, Agid Y. Does long-term aggravation of Parkinson's disease result from nondopaminergic lesions? Neurology. 1987;37:1539–1542. doi: 10.1212/wnl.37.9.1539. [DOI] [PubMed] [Google Scholar]
- 3.Brown P. Bad oscillations in Parkinson's disease. Journal of neural transmission. Supplementum. 2006:27–30. doi: 10.1007/978-3-211-45295-0_6. [DOI] [PubMed] [Google Scholar]
- 4.Constantoyannis C, Kumar A, Stoessl AJ, Honey CR. Tremor induced by thalamic deep brain stimulation in patients with complex regional facial pain. Mov Disord. 2004;19:933–936. doi: 10.1002/mds.20047. [DOI] [PubMed] [Google Scholar]
- 5.Elder CM, Hashimoto T, Zhang J, Vitek JL. Chronic implantation of deep brain stimulation leads in animal models of neurological disorders. Journal of neuroscience methods. 2005;142:11–16. doi: 10.1016/j.jneumeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Faist M, Xie J, Kurz D, Berger W, Maurer C, Pollak P, Lucking CH. Effect of bilateral subthalamic nucleus stimulation on gait in Parkinson's disease. Brain. 2001;124:1590–1600. doi: 10.1093/brain/124.8.1590. [DOI] [PubMed] [Google Scholar]
- 7.Ferrarin M, Rizzone M, Bergamasco B, Lanotte M, Recalcati M, Pedotti A, Lopiano L. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson's disease. Exp Brain Res. 2005;160:517–527. doi: 10.1007/s00221-004-2036-5. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariz GM, Lindberg M, Bergenheim AT. Impact of thalamic deep brain stimulation on disability and health-related quality of life in patients with essential tremor. J Neurol Neurosurg Psychiatry. 2002;72:47–52. doi: 10.1136/jnnp.72.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto T, Elder CM, Vitek JL. A template subtraction method for stimulus artifact removal in high-frequency deep brain stimulation. Journal of neuroscience methods. 2002;113:181–186. doi: 10.1016/s0165-0270(01)00491-5. [DOI] [PubMed] [Google Scholar]
- 12.Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- 13.Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P, Pidoux B, Dormont D, Cornu P, Agid Y. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998;44:622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 15.Jabre MG, Nohra G, Habib KG, Bejjani BP, Lebanon B. Medium Frequency Subthalamic Stimulation for Axial Symptoms in Advanced Parkinsons Disease. American Academy of Neurology, Chicago 2008 [Google Scholar]
- 16.Jech R, Urgosik D, Tintera J, Nebuzelsky A, Krasensky J, Liscak R, Roth J, Ruzicka E. Functional magnetic resonance imaging during deep brain stimulation: a pilot study in four patients with Parkinson's disease. Mov Disord. 2001;16:1126–1132. doi: 10.1002/mds.1217. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson N, Nandi D, Miall RC, Stein JF, Aziz TZ. Pedunculopontine nucleus stimulation improves akinesia in a Parkinsonian monkey. Neuroreport. 2004;15:2621–2624. doi: 10.1097/00001756-200412030-00012. [DOI] [PubMed] [Google Scholar]
- 18.Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J. 2000;41:167–184. doi: 10.3349/ymj.2000.41.2.167. [DOI] [PubMed] [Google Scholar]
- 19.Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, McIntire K, Kim SH, Zhang J, Dascalos S, Lyons KE, Pahwa R. Quantitative assessments of the effect of bilateral subthalamic stimulation on multiple aspects of sensorimotor function for patients with Parkinson's disease. Parkinsonism Relat Disord. 2005;11:503–508. doi: 10.1016/j.parkreldis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Lubik S, Fogel W, Tronnier V, Krause M, Konig J, Jost WH. Gait analysis in patients with advanced Parkinson disease: different or additive effects on gait induced by levodopa and chronic STN stimulation. J Neural Transm. 2006;113:163–173. doi: 10.1007/s00702-005-0310-8. [DOI] [PubMed] [Google Scholar]
- 22.Magarinos-Ascone CM, Regidor I, Gomez-Galan M, Cabanes-Martinez L, Figueiras-Mendez R. Deep brain stimulation in the globus pallidus to treat dystonia: Electrophysiological characteristics and 2 years' follow-up in 10 patients. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport. 2005;16:1877–1881. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- 24.Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97:561–567. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- 25.Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the pedunculopontine region in the normal non-human primate. J Clin Neurosci. 2002;9:170–174. doi: 10.1054/jocn.2001.0943. [DOI] [PubMed] [Google Scholar]
- 26.Nitta T, Itoh T, Matsuoka N, Mera T, Kojima D, Nakano M, Yamashita Y, Yasunami Y. Prevention of early loss of transplanted islets in the liver of mice by adenosine. Transplantation. 2009;88:49–56. doi: 10.1097/TP.0b013e3181aa6c9b. [DOI] [PubMed] [Google Scholar]
- 27.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain : a journal of neurology. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 28.Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson's disease. Preliminary experience at Oxford. Br J Neurosurg. 2008;22:S41–44. doi: 10.1080/02688690802448335. [DOI] [PubMed] [Google Scholar]
- 29.Perlmutter JS, Mink JW, Bastian AJ, Zackowski K, Hershey T, Miyawaki E, Koller W, Videen TO. Blood flow responses to deep brain stimulation of thalamus. Neurology. 2002;58:1388–1394. doi: 10.1212/wnl.58.9.1388. [DOI] [PubMed] [Google Scholar]
- 30.Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson's disease. Neuroreport. 2005;16:1883–1887. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- 31.Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat:a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- 32.Shute CC, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- 33.Spann BM, Grofova I. Origin of ascending and spinal pathways from the nucleus tegmenti pedunculopontinus in the rat. J Comp Neurol. 1989;283:13–27. doi: 10.1002/cne.902830103. [DOI] [PubMed] [Google Scholar]
- 34.Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007;130:1596–1607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 35.Tagliati M, Barnaure I, Martin C, Alterman RL. Long-Term Gait Deterioration after Bilateral STN DBS is not due to the Natural Progression of Parkinson's Disease. American Academy of Neurology, Chicago, IL 2008 [Google Scholar]
- 36.Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson's disease. J Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 37.Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay RA. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Mov Disord. 2004;19:907–915. doi: 10.1002/mds.20137. [DOI] [PubMed] [Google Scholar]
- 38.Windels F, Bruet N, Poupard A, Feuerstein C, Bertrand A, Savasta M. Influence of the frequency parameter on extracellular glutamate and gamma-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J Neurosci Res. 2003;72:259–267. doi: 10.1002/jnr.10577. [DOI] [PubMed] [Google Scholar]
- 39.Witjas T, Baunez C, Henry JM, Delfini M, Regis J, Cherif AA, Peragut JC, Azulay JP. Addiction in Parkinson's disease: impact of subthalamic nucleus deep brain stimulation. Mov Disord. 2005;20:1052–1055. doi: 10.1002/mds.20501. [DOI] [PubMed] [Google Scholar]
- 40.Wyckhuys T, Geerts PJ, Raedt R, Vonck K, Wadman W, Boon P. Deep brain stimulation for epilepsy: knowledge gained from experimental animal models. Acta Neurol Belg. 2009;109:63–80. [PubMed] [Google Scholar]
- 41.Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11916–11924. doi: 10.1523/JNEUROSCI.2027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


