Abstract
Malignant pleural mesothelioma (MPM) can be a challenging diagnosis for clinicians to make as it is often difficult to distinguish from benign asbestos pleural effusions and metastatic carcinomas. In this review, we present a case of MPM and discuss clinical manifestations, traditional diagnostic techniques, and the role of cytopathologic immunostains and serum biomarkers in the diagnosis of MPM.
INTRODUCTION
Malignant mesothelioma is a rare, highly aggressive malignancy involving the serosa of the pleura and peritoneum [1]. Malignant pleural mesothelioma (MPM) arising from the parietal pleura is much more frequent because inhalation is the typical route of asbestos pathogenicity [2]. The relationship between asbestos and mesothelioma was established in the 1960s; nearly a century after industrial production of asbestos began in the 1850s [3]. While occupational asbestos exposure accounts for over 80% of the cases of MPM, only a minority of heavily exposed workers (5–17%) will develop MPM highlighting the importance of host factors and genetic predisposition [4]. Given the protean clinical manifestations and poor prognosis of MPM, a prompt, accurate diagnosis challenges clinicians in distinguishing MPM from benign asbestos pleural effusions and metastatic carcinomas. In this review we present a patient with MPM and then review the emerging epidemiologic trends and clinical manifestations. Our primary focus is to review the accumulating evidence supporting an important role for cytopathologic immunostains and serum biomarkers in the diagnosis and management of MPM.
CASE REPORT
A 77-year-old Caucasian man with a 20 pack-year history of tobacco smoking presented with several months of slowly progressive dyspnea on exertion and intermittent right-sided pleuritic chest pain. His occupational history revealed over a 50-year period of employment as a pipefitter with known asbestos exposure with limited use of personal protective devices, such as a respiratory mask. Physical examination revealed decreased breath sounds on the right with dullness to percussion. A chest radiograph showed a large right pleural effusion with associated atelectasis as well as pleural plaques (Figure 1). The patient underwent thoracentesis with removal of a large volume of bloody pleural fluid. Cytologic examination of pleural fluid showed abundant clusters of polygonal epithelial cells with slightly dense cytoplasm and enlarged nuclei containing prominent nucleoli, some of which were arranged in papillary architecture (Figure 2A). Immunohistochemical (IHC) study showed that the atypical epithelial cells were positive for calretinin, cytokeratin 5/6 and WT-1 but were negative for pCEA and CD15 (Figure 2C and D). Based on cytomorphology and IHC results, a diagnosis of malignant mesothelioma was rendered. He ultimately underwent video-assisted thoracoscopic pleural biopsy that showed infiltrating malignant epithelioid cells and confirmed the diagnosis of malignant mesothelioma, epithelioid subtype (Figure 3).
Figure 1.
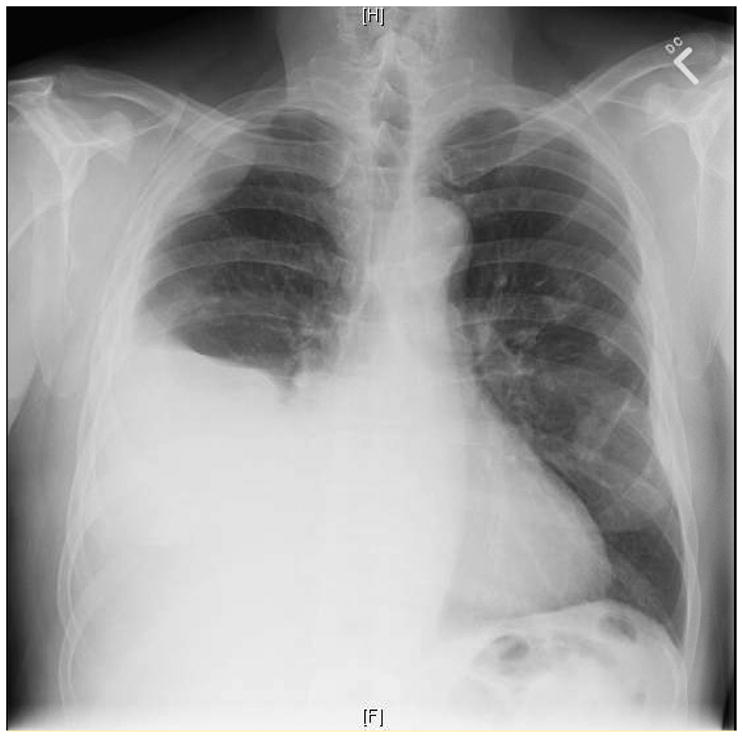
Chest radiograph demonstrating large right pleural effusion and calcified pleural plaques involving the left hemithorax.
Figure 2.
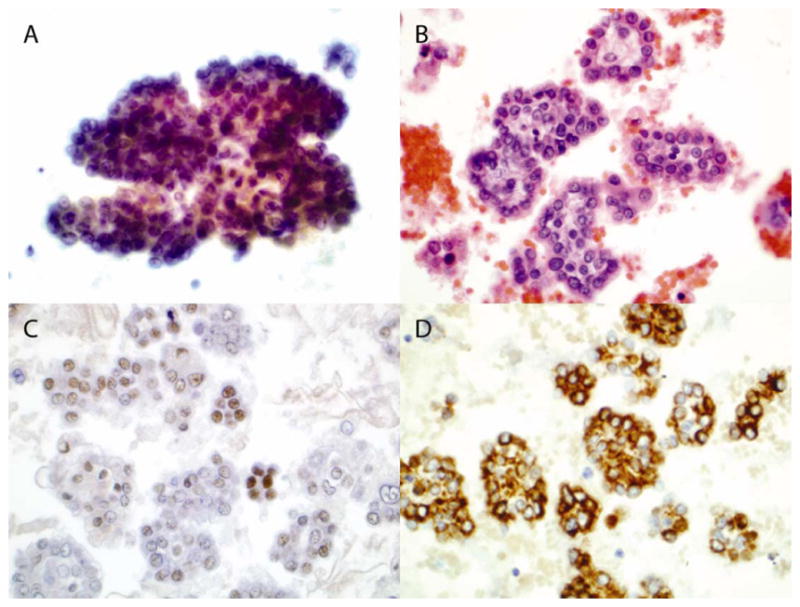
Cytology of pleural fluid (amplification, 600x). Cytospinned slide was stained with Papanicolaou stain (A). Section of cellblock was stained with H & E stain (B). Sections of cellblock were stained with antibody against WT-1 (C) and cytokeratin 5/6 (D).
Figure 3.
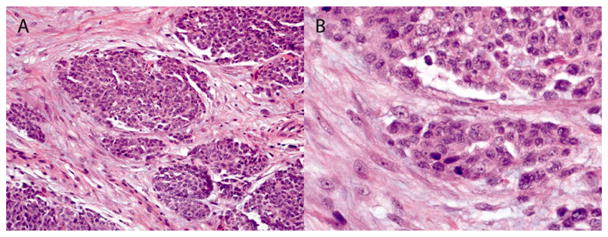
Histology of pleural biopsy. Section of paraffin-embedded pleural biopsy tissue was stained with H & E stain (A: 200x, and B: 600x).
EPIDEMIOLOGY
Despite a dramatic reduction in asbestos consumption in industrial countries since the 1970s, asbestos-related lung diseases, including MPM, remain a challenge for multiple reasons including: (1) an estimated 27 million workers in the United States were occupationally exposed to asbestos between 1940 and 1979, (2) the long latency period between asbestos exposure and the development of disease (25–71 years), and (3) asbestos exposure can occur from fibers released from former open mines (e.g. Libby, Montana or Wittenoom, Western Australia) or from consumer products, especially during structural remodeling (see for reviews: [2,5]). The incidence of MPM in the United States steadily increased from the 1950s until around 2004 when it stabilized at 2,500–3000 per year [5]. While the incidence in the United States may have peaked, there is concern that the increased use of asbestos in other countries may increase the worldwide incidence of MPM for years to come unless occupational health regulations are enacted [6]. Nearly 100% of individuals diagnosed with MPM will die of it, with a median survival of less than 14 months from the time of diagnosis [5]. Given the dismal prognosis of MPM, early detection and diagnosis is critical in order to increase the proportion of patients who are candidates for surgical resection and multimodality therapy, which offer the best chance for improving long-term outcomes [1].
CLINICAL FEATURES AND IMAGING
Malignant pleural mesothelioma typically presents between 50 and 70 years of age and more commonly occurs in males (male/female ratio 5:1) [7,8,9]. As in our patient, the onset of symptoms is insidious and patients often present with dyspnea (50–70%), chest-wall pain (60–70%), and cough (20–30%) [9]. Occasionally, patients may present with nonspecific complaints such as fatigue, weight loss, and fever. Findings consistent with pleural effusion are found on physical exam and chest radiograph in 80 to 95% of patients, however, pleural-based masses and diffuse pleural thickening may also be seen and lung entrapment may occur [7]. Computed tomography (CT) scanning is the primary modality for diagnosis and staging of MPM as it is more sensitive than chest radiograph for identifying pleural fluid and pleural masses that may be masked by the fluid and can also better assess the extent of lymph node involvement [2]. Magnetic resonance imaging (MRI) may provide useful information regarding the extent of tumor invasion of MPM and may be superior to CT in identifying chest wall and diaphragm invasion [10]. Fluorodeoxyglucose positron-emission tomography (FDG-PET) has also been demonstrated to have a growing role in the evaluation of patients with malignant pleural mesothelioma and can be used to distinguish benign from malignant pleural disease (up to 97% sensitivity) as well as to detect extrathoracic disease, which is useful in tumor staging [11]. Although beyond the scope of this review, staging of MPM is controversial with at least 5 systems described, as summarized elsewhere [9,12]. The International Mesothelioma Interest Group TNM staging system was approved in 2010 by the National Comprehensive Cancer Network and American Joint Committee on Cancer but was not widely accepted in Europe [9,12]. In general, patients with stage IV MPM are considered unresectable while stages I–III describe progressively more invasive disease that may be amendable to multimodality therapy and surgical resection [9].
TRADITIONAL DIAGNOSTIC TECHNIQUES
Because MPM can be difficult to differentiate from adenocarcinoma, the gold standard for a definitive diagnosis is pathologic tissue examination. Further, MPM is a very heterogeneous malignancy resulting in misdiagnosis with other malignant and non-malignant conditions. The low yield of pleural fluid cytology (25–33% sensitivity) and closed-needle pleural biopsy (21–77% sensitivity) requires that most patients undergo direct thoracoscopic pleural biopsy to establish a diagnosis (> 90% sensitivity) [7,12,13]. Mesothelioma is classified into 3 major histologic subtypes: epithelioid, sarcomatoid, and biphasic. The epithelioid subtype is most common and has the best prognosis. The European Respiratory Society (ERS)/European Society of Thoracic Surgeons (ESTS) Task Force gave Grade 1A recommendation (unequivocal benefit/risk ratio; high quality evidence) that clinical and radiographic evaluation alone is insufficient to diagnosis MPM and that thoracoscopy is the best method to obtain the diagnosis [12].
The main differential diagnoses of malignant mesothelioma are adenocarcinoma and mesothelial hyperplasia. Table 1 lists the important immunohistochemical markers that are commonly used to distinguish MPM from adenocarcinoma. Since no single immunohistochemical marker is pathognomonic for MPM or adenocarcinoma, it is important that a panel of stains be performed [12,13]. Epithelioid malignant mesothelioma typically stains positive for cytokeratin 5/6, calretinin, Wilms tumor-1 (WT-1), D2-40 and podoplanin. Pleural metastatic adenocarcinoma, with which MPM is often confused, stains positively with polyclonal carcinoembryonic antigen (pCEA), epithelial membrane antigen (EMA; also known as CD15/LeuM1), MOC-31, B72.3, and, in the case of lung tumors, thyroid transcription factor 1 (TTF-1) and Napsin A. It is sometimes difficult to distinguish malignant mesothelioma from mesothelial hyperplasia in fluid cytology. Although a few immune-histochemical (IHC) markers were reported to be useful, such as EMA IHC pattern and desmin, these are not reliable according to our experience. Recently, it was reported that IHC stain and especially fluorescence in situ hybridization (FISH) for p16 are excellent to separate malignant mesothelioma from mesothelial hyperplasia [14]. The deletion of the p16 gene was associated with poor prognosis. However, according to our experience, diagnosis of malignant mesothelioma was rendered in most epithelioid or mixed type malignant mesothelioma cases based on fluid cytology alone. Only rarely is a surgical biopsy needed to confirm the diagnosis of malignant mesothelioma. Table 2 highlights the relevant immunohistochemical markers that are commonly used to distinguish sarcomatoid MPM from squamous and transitional cell carcinomas. Detection of calretinin, D2-40, CK5/6, and WT-1 expression combined with lack of staining with p63 and epithelial markers (Ber-EP4 and/or MOC31) are required for distinguishing sarcomatoid MPM from metastatic sarcomatoid carcinoma. Notably, cytokeratin 5/6 immunostaining is not helpful in this regard since it is positive in both sarcomatoid MPM and metastatic sarcomatoid carcinoma. Collectively, based upon the above findings, most experts, including the ERS/ESTS, recommend that a panel of at least 4 sensitive markers, 2 of mesothelial lineage (usually calretinin, WT-1 and/or cytokeratin 5/6) and 2 of epithelial lineage (usually pCEA, CD15, MOC31, BER-EP4, and/or TTF-1), be used in the initial immunohistochemical evaluation of suspected MPM (Grade 1A) [12,13]. In distinguishing epithelioid MPM from metastatic pleural adenocarcinoma, the reported sensitivities range from 96–98% while specificity ranges from 88–90%.
Table 1.
Sensitivity and Specificity Ranges for Immunohistochemistry Markers in Patients with Epithelioid Mesothelioma and Adenocarcinoma 13, 41–43
| Sensitivity (%) | Specificity (%) | Adenocarcinomas | |
|---|---|---|---|
| Mesothelioma markers | |||
| Keratin CK5/6 | 70–99% | 58–89% | 2–41% focal positive |
| Calretinin | 82–98% | 67–89% | 5–33% positive (cytosol) |
| WT-1 | 77–99% | 62–96% | 0% |
| Podoplanin | 84–90% | 99–100% | 7% focal positive |
| Vimentin | 62% | 75% | 47% |
| D2-40 | 85% | 100% | Negative |
| Adenocarcinoma markers | MPM | ||
| pCEA | 63–100% | 17–98% | 7–13% |
| CD15 (LeuM1; EMA) | 51–92% | 36–97% | 0% |
| Ber-EP4 | 74–94% | 13–95% | 0–33% focal positive |
| MOC-31 | 69–100% | 38–93% | 6% |
| TTF-1 | 72–100% | 36–100% | 0% |
Table 2.
Sensitivity and Specificity Ranges for Immunohistochemistry Markers in Patients with Sarcomatoid Mesothelioma from Squamous and Transitional Cell (TC) Carcinoma *
| Sensitivity (%) | Specificity (%) | Squam; TC Carcinomas | |
|---|---|---|---|
| Mesothelioma markers | |||
| Keratin CK5/6 | 60–100% | 0% | 100% positive (not useful) |
| Calretinin | 80–100% | 60–95% | 5–40% positive (useful) |
| WT-1 | 43–93% | 100% | 0 % (very useful) |
| Squamous Cell Carcinoma markers | MPM | ||
| P63 | ~100% | ~100% | ~0% (very useful) |
| Ber-EP4 | 80–100% | 80% | ~20% (useful) |
| MOC-31 | 97–100% | 90–98% | 2–10% (useful) |
Adapted from Scherpereel A., et al ERJ 2010;35:479–95.
Some newer antibodies under investigation look very promising. The levels of tenascin-X, an extracellular matrix glycoprotein, are significantly higher in effusions due to MPM (n=56) as compared to those caused by metastatic adenocarcinoma (n=122) with a reported 73% sensitivity and 100% specificity for MPM diagnosis [15]. A small study by Saad et al. [16] showed that D2-40, a monoclonal antibody that has been used as a lymphatic endothelial marker, differentiated MPM (n=20) from metastatic lung adenocarcinomas (n=10) and squamous cell carcinomas (n=10). D2-40 staining was 85% sensitive and 100% specific for the diagnosis of epithelioid MPM. Others have reported similar promising results with D2-40 for staining MPM (n=61 epitheliod; n=19 biphasic) but it remains unclear whether this marker adds much to better-validated MPM immunostains, such as calretinin, cytokeratin 5/6 and WT-1 [17]. Another exciting new marker, caveolin-1, a membrane associated protein, was found to have 100% sensitivity and 93% specificity for diagnosing epithelioid MPM from a group of 80 MPM and 80 lung adenocarcinomas [18]. However, the reproducibility of these findings by others and its use in the differentiation of epithelioid mesothelioma from metastatic adenocarcinoma from other sites are unclear [18].
While immunohistochemical evaluation has largely replaced electron microscopy (EM) in the diagnosis of malignant mesothelioma, EM examination is still often used to confirm ultrastructural features that can be helpful when the immunohistochemical results are equivocal. The cells of epithelioid malignant mesothelioma have a multitude of elongated, curved and branched surface microvilli that do not have a glycocalyx, whereas adenocarcinomas usually have shorter, less numerous, unbranched microvilli with a glycocalyx [19]. Perinuclear tonofilament bundles, the presence of a basal lamina, and long desmosomes are also common features in epithelioid mesotheliomas [20]. EM is typically less helpful to distinguish sarcomatoid malignant mesothelioma from sarcomas and other spindle cell pleural tumors as the sarcomatoid type infrequently has characteristic microvilli [19]. However, the ERS/ESTS recommended that electron microscopy and molecular biology should not be routinely performed for diagnosing MPM (Grade 1A) and that freezing pleural tissue should not be routinely performed (Grade 1A) [12].
POTENTIAL MALIGNANT MESOTHELIOMA BIOMARKERS
Recent interest in the use of serum and pleural fluid biomarkers to allow early detection of malignancies has spurred research to determine if these biomarkers might be useful for discriminating between asbestos-exposed individuals with MPM as compared to benign (i.e. asbestosis, pleural plaques, etc) and other malignant conditions (i.e. lung cancer) [2]. An ideal biomarker for MPM would detect all subtypes of mesothelioma, differentiate mesothelioma from benign pleural diseases and metastatic cancer, predict the development of mesothelioma in asbestos-exposed subjects, and be useful in assessing disease severity, prognosis, and response to treatment [21]. Herein we focus our attention on the three most promising and widely studied biomarkers, soluble mesothelin-related protein (SMRP; also termed soluble mesothelin), megakaryocyte potentiation factor (MPF), and osteopontin. It should be emphasized that a major limitation of this approach is that because MPM is a rare tumor, most positive test results are false positives. Accordingly, the ERS/ESTS stated that “there is no place for screening of MPM” using radiologic approaches or any of the biomarkers (Grade 1A) [12]. As reviewed below, a potential role for these biomarkers is emerging for monitoring treatment responses and predicting prognosis.
SMRP
Mesothelin, a 40-kD cell surface glycoprotein that plays a role in cell adhesion and signaling, is expressed by normal mesothelial cells; however, it is highly expressed in cancers such as epithelioid MPM as well as pancreatic, ovarian, and some other cancers [22]. Mesothelin is synthesized as a precursor 69-kD protein and yields two proteins; the membrane bound mesothelin and a SMRP [23]. SMRP, a spliced variant of mesothelin, is detectable in the bloodstream as a result of cleavage of the membrane bound mesothelin [24]. In an early retrospective study, Robinson and colleagues [25] demonstrated that serum SMRP levels were elevated in 84% of patients with mesothelioma whereas only 2% of patients with other cancers or lung/pleural diseases were found to have elevated SMRP levels. SMRP levels in patients with mesothelioma were found to be significantly higher than asbestos-exposed controls. SMRP levels also directly correlated with tumor size and were observed to increase in instances of tumor progression.
Scherpereel and colleagues [22] prospectively evaluated the diagnostic role of serum and pleural SMRP levels in patients with MPM (n=74), pleural metastasis of carcinomas (n=35), and asbestos-related benign pleural lesions (n=28). They reported that mean serum SMRP levels were higher in patients with MPM using a cutoff value of 0.93 nM/L yielding an 80% sensitivity and 83% specificity for differentiating MPM from benign pleural lesions, but only a 58% sensitivity and 73% specificity for differentiating MPM from metastasis. In order to achieve similar specificity to Robinson and colleagues, the sensitivity dropped to just 63.3% indicating that SMRP is not sufficiently sensitive and specific to be used as a screening marker for MPM. Park and colleagues [26] found a high false positive rate of serum SMRP in healthy asbestos-exposed individuals (n=223) and those with benign asbestos-related diseases (n=292), despite using a higher cutoff value for SMRP than previous studies. In the 15 out of 538 patients found to have an abnormally elevated SMRP, none were found to have malignant mesothelioma, corroborating that SMRP is not likely to be a useful screening test. Additionally, SMRP is limited as it is only sensitive for the detection of the epithelioid subtype of malignant mesothelioma
It is important to note that different investigators have used varying absolute cut-off levels for an “abnormal” SMRP, producing a range of different sensitivities and specificities, and no definite cut-off has yet been determined. Additionally, most of the studies evaluating the utility of SMRP as a potential marker for malignant mesothelioma suffer from the caveat that as the cutoff value for SMRP is adjusted to maximize sensitivity, specificity drops to unacceptable levels. In a meta-analysis of 11 studies examining the diagnostic value of SMRP for MPM, the pooled sensitivity was 64% (95% confidence interval [CI] 61–68%) and specificity was 89% (95% CI 88–90%) [23].
Megakaryocyte Potentiating Factor
MPF, which originates from the 31-kD N-terminal fraction of the mesothelin gene, whereas the 40-kD C terminal fraction yields the SMRP glycoprotein, is a protein initially identified as a cytokine with megakaryocyte-stimulating activity [1]. It was initially postulated that MPF, which is secreted directly into the blood, may be detected earlier and have a higher sensitivity than SMRP in MPM [27]. Onda and colleagues demonstrated elevated MPF levels in 91% (51 of 56) of patients with mesothelioma, whereas no elevation in MPF was demonstrated in 70 healthy control subjects [28]. Additionally, they demonstrated that MPF might be useful to monitor treatment response as levels were noted to fall following tumor debulking in 2 patients. In another study, serum MPF levels were noted to be higher in mesothelioma patients when compared with healthy controls, patients with nonmalignant exudative effusions and patients with benign asbestos-related disease; however, there was not a detectable difference in MPF levels among patients with mesothelioma, lung cancer, or malignant effusion [29]. At a specificity of 95%, MPF was noted to have a sensitivity of only 34%, much less than the sensitivity of SMRP in the same study.
In contrast, Hollevoet and colleagues [24] reported that serum MPF and SMRP have a comparable diagnostic equivalence in a prospective, multicenter study involving 507 patients comprised of healthy controls (n=101), healthy asbestos-exposed individuals (n=89), and patients with lung cancer (n=63), benign asbestos-related respiratory diseases (n=123), and MPM (n=85). They found that SMRP and MPF comparably distinguished MPM from all the other cohorts; at 95% specificity, SMRP and MPF had a sensitivity of 64% and 68%, respectively. However, at a specificity of 99%, sensitivity was reduced to ~50% for both. Also, combining both markers failed to improve the diagnostic yield. The authors suggested that SMRP and MPF are most useful in guiding diagnostic and therapeutic decisions rather than in risk assessment in asbestos-exposed individuals. In this regard, increasing serum levels of SMRP were associated with MPM disease progression and shorted survival in 40 patients followed over 6 months [30]. However, a recent study showed that SMRP and MPF levels are independently associated with age, glomerular filtration rate (GFR), and body mass index (BMI) in control subjects and with tumor stage and GFR in patients with MPM [31]. Since MPF and SMRP are both low-molecular weight proteins, a reduction in GFR can cause an accumulation of these biomarkers in the blood.
Osteopontin
Osteopontin is a glycoprotein that plays a role in cell adhesion and bone-matrix interactions and is elevated in breast, ovarian, lung and gastric cancer, as well as MPM. In malignant transformation, osteopontin may augment tumor growth, invasiveness, and metastasis [29]. Osteopontin, in contrast to SMRP and MPF, is elevated in both epithelioid and sarcomatoid MPM. Pass and colleagues [32] retrospectively studied 190 patients with MPM, benign asbestos related disease, and healthy subjects without asbestos exposure, and found that serum osteopontin levels were significantly higher in patients with MPM than in patients with asbestosis and healthy non-asbestos exposed subjects but did not appear useful in patients with radiographic evidence of pleural disease as the levels were also elevated in these patients. Increased serum osteopontin levels had a sensitivity of 77.6% and specificity of 85.5% for diagnosing patients with MPM as compared to the group with benign asbestos-related pleural plaques. Other studies suggest that osteopontin is unlikely to be a useful screening test for MPM in asbestos-exposed patients. Park and colleagues [33] evaluated serum osteopontin levels in 525 patients, including both healthy controls with asbestos exposure as well as patients with other nonmalignant asbestos-related disorders. Although the mean serum osteopontin levels were higher in patients with MPM as compared to the healthy asbestos-exposed group and all patients with benign asbestos related disease, ~14% (72 subjects) had an elevated osteopontin level (≥ 64.2 ng/mL) as defined by Pass and colleagues [32] without evidence of MPM (false positive).
While serum levels of osteopontin may discriminate between MPM patients and healthy controls, the diagnostic utility of the test is confounded by the fact that osteopontin levels are elevated in other malignancies as well as some nonmalignant conditions such as coronary artery disease and other benign respiratory disease [34,35,36]. Creaney and colleagues [29] found that at a level of specificity of 95% relative to healthy controls and patients with benign asbestos-related disease, the sensitivity of serum osteopontin for MPM was only 47%. Combining data from three serum biomarkers (SMRP, MPF, and osteopontin) did not improve the diagnostic yield of MPM above that of SMRP alone [29].
Others
As reviewed in detail elsewhere [37] a number of potentially useful serum biomarkers in MPM are under investigation including various microRNAs (miRNAs), loss of p16, overexpression of vascular endothelial growth factor (VEGF), and others. Herein we briefly focus on the emerging role of microRNAs in MPM. MicroRNAs (miRNAs), which are small noncoding single stranded RNAs that are prominently implicated in playing a key role in cancer incidence and progression, function as oncogenes or tumor suppressor genes [38]. Busacca and colleagues [39] reported that reduced expression of specific miRNAs (miRNA-17-5p and miRNA-30c) correlated with a better survival in patients with sarcomatoid MPM subtype. Others have noted the down-regulation of 7 miRNAs in MPM (n=100) as compared to lung adenocarcinomas (n=32) and suggested that these may facilitate early diagnosis and identify novel treatment targets [38]. There is some evidence that increased expression of miRNA-29c, which regulates epigenetics via effects on DNA methylation, predicts a more favorable prognosis of MPM and that overexpression of miRNA-29c into mesothelioma cell lines reduces cell proliferation, migration and colony forming units [37]. Future studies exploring the role of specific miRNAs in the diagnosis and management of MPM will be of considerable interest.
CONCLUSIONS
Malignant mesothelioma is an uncommon, rapidly fatal malignancy that is closely associated with exposure to asbestos. While the incidence of MPM in the United States may have reached its peak, underdeveloped countries lacking appropriate asbestos regulatory constraints will see a rise in the incidence of MPM for years to come, resulting in the deaths of hundreds of thousands of people worldwide [5]. Early detection and diagnosis of MPM is crucial if we hope to significantly improve the management and survival of this devastating disease. Despite the advances in our understanding of the pathobiology of MPM, definitive diagnosis of MPM still requires histopathologic tissue examination, usually via direct thoracoscopic biopsy, combined with a panel of immunohistochemical markers (Tables 1 and 2).
Tumor-related serum and pleural biomarkers, such as SMRP, MPF, and osteopontin, have been intensively investigated in the management of MPM over the past decade. However, the evidence to date suggests that none of the biomarkers are sufficiently sensitive and specific to be used as a diagnostic screening tool that would preclude the need for a tissue diagnosis [9, 12]. Elevated MPM biomarker levels may lend credence to a suspected diagnosis of mesothelioma, however they should not be used alone as a diagnostic measure. Accumulating evidence supports that biomarkers, especially serum SMRP, may play a role in prognostication as well as to help guide therapeutic decision-making and monitor disease course. To date, there is insufficient evidence that combining serum MPM biomarkers improves the diagnostic or prognostic utility as compared to SMRP alone [29]. Clinicians using these biomarkers should be cognizant that clinical covariates, such as age, GFR and BMI may affect the diagnostic and prognostic value of SMRP and MPF for MPM [31]. Given the many clinical and legal challenges underlying MPM, continued research into its fundamental biology as well as the translational significance of emerging findings are of considerable interest. In particular, the evolving role of molecular biomarkers in providing novel insights into diagnosis and prognosis appears very promising for the management of these patients
Acknowledgments
Support/Acknowledgements: VA Merit Award (DWK); NIEHS-RO1ES020357 (DWK)
Footnotes
Disclosures: None
References
- 1.Ray M, Kindler HL. Malignant pleural mesothelioma: an update on biomarkers and treatment. Chest. 2009;136:888–896. doi: 10.1378/chest.08-2665. [DOI] [PubMed] [Google Scholar]
- 2.Kamp DW. Asbestos-induced lung diseases: An update. Transl Res. 2009;153:143–152. doi: 10.1016/j.trsl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neri M, Ugolini D, Dianzani I, et al. Genetic susceptibility to malignant pleural mesothelioma and other asbestos-associated diseases. Mutat Res. 2008;659:126–136. doi: 10.1016/j.mrrev.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol. 2012;227:44–58. doi: 10.1002/jcp.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le GV, Takahashi K, Park EK, et al. Asbestos use and asbestos-related diseases in Asia: past, present and future. Respirology. 2011;16:767–775. doi: 10.1111/j.1440-1843.2011.01975.x. [DOI] [PubMed] [Google Scholar]
- 7.Cugell DW, Kamp DW. Asbestos and the pleura: a review. Chest. 2004;125:1103–1117. doi: 10.1378/chest.125.3.1103. [DOI] [PubMed] [Google Scholar]
- 8.Boutin C, Schlesser M, Frenay C, et al. Malignant pleural mesothelioma. Eur Respir J. 1998;12:972–981. doi: 10.1183/09031936.98.12040972. [DOI] [PubMed] [Google Scholar]
- 9.Fuhrer G, Lazarus AA. Mesothelioma. Dis Mon. 2011;57:40–54. doi: 10.1016/j.disamonth.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Heelan RT, Rusch VW, Begg CB, et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol. 1999;172:1039–1047. doi: 10.2214/ajr.172.4.10587144. [DOI] [PubMed] [Google Scholar]
- 11.Duysinx B, Nguyen D, Louis R, et al. Evaluation of pleural disease with 18-fluorodeoxyglucose positron emission tomography imaging. Chest. 2004;125:489–493. doi: 10.1378/chest.125.2.489. [DOI] [PubMed] [Google Scholar]
- 12.Scherpereel A, Astoul P, Bass P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479–495. doi: 10.1183/09031936.00063109. [DOI] [PubMed] [Google Scholar]
- 13.Betta PG, Magnani C, Bensi T, et al. Immunohistochemistry and molecular diagnostics of pleural malignant mesothelioma. Arch Pathol Lab Med. 2012;136:253–261. doi: 10.5858/arpa.2010-0604-RA. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimura T, Torii I, Sato A, et al. Pathological and molecular biological approaches to early mesothelioma. Int J Clin Oncol. 2012;17:40–47. doi: 10.1007/s10147-011-0369-1. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Nymoen DA, Stavnes HT, et al. Tenascin-X is a novel diagnostic marker of malignant mesothelioma. Am J Surg Pathol. 2009;33:1673–1682. doi: 10.1097/PAS.0b013e3181b6bde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad RS, Lindner JL, Lin X, et al. The diagnostic utility of D2-40 for malignant mesothelioma versus pulmonary carcinoma with pleural involvement. Diagn Cytopathol. 2006;34:801–806. doi: 10.1002/dc.20556. [DOI] [PubMed] [Google Scholar]
- 17.Kao SC, Griggs K, Lee K, et al. Validation of a minimal panel of antibodies for the diagnosis of malignant pleural mesothelioma. Pathology. 2011;43:313–317. doi: 10.1097/PAT.0b013e32834642da. [DOI] [PubMed] [Google Scholar]
- 18.Amatya VJ, Takeshima Y, Kohno H, et al. Caveolin-1 is a novel immunohistochemical marker to differentiate epithelioid mesothelioma from lung adenocarcinoma. Histopathology. 2009;55:10–19. doi: 10.1111/j.1365-2559.2009.03322.x. [DOI] [PubMed] [Google Scholar]
- 19.Chirieac LR, Corson JM. Pathologic evaluation of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:121–124. doi: 10.1053/j.semtcvs.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 21.Scherpereel A, Lee YC. Biomarkers for mesothelioma. Curr Opin Pulm Med. 2007;13:339–443. doi: 10.1097/MCP.0b013e32812144bb. [DOI] [PubMed] [Google Scholar]
- 22.Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med. 2006;173:1155–1160. doi: 10.1164/rccm.200511-1789OC. [DOI] [PubMed] [Google Scholar]
- 23.Luo L, Shi HZ, Liang QL, et al. Diagnostic value of soluble mesothelin-related peptides for malignant mesothelioma: a meta-analysis. Respir Med. 2010;104:149–156. doi: 10.1016/j.rmed.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Hollevoet K, Nackaerts K, Thimpont J, et al. Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am J Respir Crit Care Med. 2010;181:620–625. doi: 10.1164/rccm.200907-1020OC. [DOI] [PubMed] [Google Scholar]
- 25.Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 26.Park EK, Sandrini A, Yates DH, et al. Soluble mesothelin-related protein in an asbestos-exposed population: the dust diseases board cohort study. Am J Respir Crit Care Med. 2008;178:832–837. doi: 10.1164/rccm.200802-258OC. [DOI] [PubMed] [Google Scholar]
- 27.Maeda M, Hino O. Molecular tumor markers for asbestos-related mesothelioma: serum diagnostic markers. Pathol Int. 2006;56:649–654. doi: 10.1111/j.1440-1827.2006.02024.x. [DOI] [PubMed] [Google Scholar]
- 28.Onda M, Nagata S, Ho M, et al. Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res. 2006;12:4225–4231. doi: 10.1158/1078-0432.CCR-06-0472. [DOI] [PubMed] [Google Scholar]
- 29.Creaney J, Yeoman D, Demelker Y, et al. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J Thorac Oncol. 2008;3:851–857. doi: 10.1097/JTO.0b013e318180477b. [DOI] [PubMed] [Google Scholar]
- 30.Grigoriu BD, Chahine B, Vachani A, et al. Kinetics of soluble mesothelin in patients with malignant pleural mesothelioma during treatment. Am J Respir Crit Care Med. 2009;179:950–954. doi: 10.1164/rccm.200807-1125OC. [DOI] [PubMed] [Google Scholar]
- 31.Hollevoet K, Nackaerts K, Thas O, et al. The effect of clinical covariates on the diagnostic and prognostic value of soluble mesothelin and megakaryocyte potentiating factor. Chest. 2012;141:477–484. doi: 10.1378/chest.11-0129. [DOI] [PubMed] [Google Scholar]
- 32.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. NEJM. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 33.Park EK, Thomas PS, Johnson AR, et al. Osteopontin levels in an asbestos-exposed population. Clin Cancer Res. 2009;15:1362–1366. doi: 10.1158/1078-0432.CCR-08-0360. [DOI] [PubMed] [Google Scholar]
- 34.Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res. 2007;13:2928–2935. doi: 10.1158/1078-0432.CCR-06-2144. [DOI] [PubMed] [Google Scholar]
- 35.Kadota J, Mizunoe S, Mito K, et al. High plasma concentrations of osteopontin in patients with interstitial pneumonia. Respir Med. 2005;99:111–117. doi: 10.1016/j.rmed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Ohmori R, Momiyama Y, Taniguchi H, et al. Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis. 2003;170:333–337. doi: 10.1016/s0021-9150(03)00298-3. [DOI] [PubMed] [Google Scholar]
- 37.Zucali PA, Ceresoli GL, De Vincenzo F, et al. Advances in the biology of malignant pleural mesothelioma. Cancer Treat Rev. 2011;37:543–558. doi: 10.1016/j.ctrv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Gee GV, Koestler DC, Christensen BC, et al. Downregulated microRNAs in the differential diagnosis of malignant pleural mesothelioma. Int J Cancer. 2010;127:2859–2869. doi: 10.1002/ijc.25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busacca S, Germano S, De Cecco L, et al. MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol. 2010;42:312–319. doi: 10.1165/rcmb.2009-0060OC. [DOI] [PubMed] [Google Scholar]
- 40.Ordonez NG. D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372–380. doi: 10.1016/j.humpath.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Padgett DM, Cathro HP, Wick MR, et al. Podoplanin is a better immunohistochemical marker for sarcomatoid mesothelioma than calretinin. Am J Surg Pathol. 2008;32:123–127. doi: 10.1097/PAS.0b013e31814faacf. [DOI] [PubMed] [Google Scholar]
- 42.King JE, Thatcher N, Pickering CA, et al. Sensitivity and specificity of immunohistochemical markers used in the diagnosis of epithelioid mesothelioma: a detailed systematic analysis using published data. Histopathology. 2006;48:223–232. doi: 10.1111/j.1365-2559.2005.02331.x. [DOI] [PubMed] [Google Scholar]


