Abstract
OBJECTIVE
To describe the spectrum of visits to US emergency departments (EDs) for acute dizziness and determine whether ED patients with dizziness are diagnosed as having a range of benign and dangerous medical disorders, rather than predominantly vestibular ones.
PATIENTS AND METHODS
A cross-sectional study of ED visits from the National Hospital Ambulatory Medical Care Survey (NHAMCS) used a weighted sample of US ED visits (1993–2005) to measure patient and hospital demographics, ED diagnoses, and resource use in cases vs controls without dizziness. Dizziness in patients 16 years or older was defined as an NHAMCS reason-for-visit code of dizziness/vertigo (1225.0) or a final International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis of dizziness/vertigo (780.4) or of a vestibular disorder (386.x).
RESULTS
A total of 9472 dizziness cases (3.3% of visits) were sampled over 13 years (weighted 33.6 million). Top diagnostic groups were otologic/vestibular (32.9%), cardiovascular (21.1%), respiratory (11.5%), neurologic (11.2%, including 4% cerebrovascular), metabolic (11.0%), injury/poisoning (10.6%), psychiatric (7.2%), digestive (7.0%), genitourinary (5.1%), and infectious (2.9%). Nearly half of the cases (49.2%) were given a medical diagnosis, and 22.1% were given only a symptom diagnosis. Predefined dangerous disorders were diagnosed in 15%, especially among those older than 50 years (20.9% vs 9.3%; P<.001). Dizziness cases were evaluated longer (mean 4.0 vs 3.4 hours), imaged disproportionately (18.0% vs 6.9% undergoing computed tomography or magnetic resonance imaging), and admitted more often (18.8% vs 14.8%) (all P<.001).
CONCLUSION
Dizziness is not attributed to a vestibular disorder in most ED cases and often is associated with cardiovascular or other medical causes, including dangerous ones. Resource use is substantial, yet many patients remain undiagnosed.
Dizziness is estimated to account for 5% of walk-in clinic1 and 4% of emergency department (ED) visits.2 Although most ED patients with dizziness are said to have benign vestibular or cardiovascular disorders,3 possible etiologies include numerous diseases from various organ systems; in one study, 46 different diagnoses were given to 106 patients presenting with dizziness.4 In contrast to the outpatient setting, where only a few cases are attributed to dangerous causes, such as cerebrovascular accident (6%) or cardiac arrhythmia (1%),5 in the ED, small studies have estimated that up to 30% of patients with dizziness have a serious disorder causing their symptoms, including 15% with stroke, transient ischemic attack, cardiac arrhythmia, acute infection, or anemia.3
Emergency department physicians must differentiate dizziness requiring only symptom management from that requiring further diagnostic work-up for serious, yet treatable, causes. Some consider dizziness the most difficult symptom to diagnose,6 and there is growing evidence that misdiagnosis of ED patients with dizziness is not rare.7–9 A lack of access to robust estimates for disease prevalence could hinder ED physicians’ ability to make accurate diagnoses. Using data from the National Hospital Ambulatory Medical Care Survey (NHAMCS), we sought to estimate the prevalence of dizziness presentations across demographic groups in the ED, the spectrum of diagnoses identified, the frequency of imaging and other diagnostic tests, and the disposition of ED patients with dizziness. We expected to corroborate findings from other settings indicating dizziness is common, particularly among older patients and women.6 We speculated that the spectrum of identified causes would be broad and more “medical” than “vestibular,” with frequent diagnoses of serious underlying disorders, particularly among older patients. Finally, we anticipated that diagnostic tests (especially advanced imaging) would be used frequently and that overall ED resource use for patients with dizziness would be substantial, as seen in small samples.10
PATIENTS AND METHODS
This cross-sectional study of US ED patients with dizziness analyzed public-use data from NHAMCS sampled from all US ED visits occurring between January 1, 1993, and December 31, 2005. Study years (1993–2005) were determined on the basis of data availability. NHAMCS is a 4-stage probability sample of visits to randomly selected US hospitals, including noninstitutional general and short-stay hospitals but excluding federal, military, and Veterans Affairs hospitals.11 NHAMCS data are gathered annually, and the sampling protocol, which covers geographic primary sampling units, hospitals within primary sampling units, EDs within hospitals, and patients within EDs, has been described previously.11
Because children have a different spectrum of causes than older adolescents and adults and are much less likely to experience dizziness, we restricted our study population to ED patients aged 16 years or older. Dizziness cases were defined as any patient with an NHAMCS-assigned patient reason-for-visit classification (RFV code)12 of vertigo/dizziness (1225.0) in any of the 3 RFV code fields, or a final International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) symptom diagnosis of vertigo/ dizziness (780.4), or a final ICD-9-CM disease diagnosis of a vestibular disorder (386.x) in any of the 3 final diagnosis fields.11 Controls without dizziness were defined as not NHAMCS RFV code 1225.0, or ICD-9-CM 386.x, or ICD-9-CM 780.4.
Study Procedures
As part of the NHAMCS protocol, trained hospital staff members gather data from ED visit records during a randomly assigned 4-week data period for each sampled hospital. 11 A structured data entry form is used.11 Completed forms are sent to Constella Group (Durham, NC), where data abstraction and medical coding are performed.13 Data entry and coding have previously been verified using a 2- way independent 10% subsample,11 and keying and coding error rates are known to be very low (0%–2%).13 National population estimates are obtained from the raw sampled data through use of assigned patient visit weights, which account for probability of visit selection, nonresponse, and ratio of sampled hospitals to hospital universe.11
Outcome measures were taken directly or derived from the NHAMCS data set, including patient demographic and hospital characteristics, ICD-9-CM diagnoses, ED visit details (eg, mode of arrival, length of stay), reason for visit (ie, primary symptom or problem), diagnostic tests (eg, mean number of tests performed; proportion undergoing computed tomography [CT] or magnetic resonance imaging [MRI]), and final disposition (admit, discharged, other). Missing data are reported as missing or unknown, as coded in the NHAMCS data set.
Simple analysis of the most frequent ICD-9-CM diagnosis codes in NHAMCS is subject to potential biases related to differences in clinical coding specificity. To eliminate such bias, we grouped ICD-9-CM diagnoses using the Healthcare Cost and Utilization Project’s Clinical Classifications Software (HCUP-CCS) for multilevel diagnoses.14 This standardized coding schema groups all ICD-9-CM diagnosis codes into 16 mutually exclusive, top-level etiologic classes familiar to most physicians (eg, “infectious and parasitic diseases,” “neoplasms,” “mental disorders”), as well as pertinent subclasses (eg, “eye” and “ear” within the major class “diseases of the nervous system and sense organs”) (Appendix 1). We prospectively identified 2 HCUP-CCS subclasses of particular interest for dizziness diagnosis (“cerebrovascular” [HCUP-CCS 7.3] and “ear” [HCUPCCS 6.8]), along with several individual ICD-9-CM diagnoses selected a priori, chosen to reflect the spectrum of benign and dangerous disorders known to cause dizziness in the ED (Appendix 2).
Finally, we identified the proportion of patients receiving any symptom diagnosis (eg, “dizziness” or “chest pain”), only a symptom diagnosis, and specifically a dizziness symptom diagnosis (ICD-9-CM 780.4 “dizziness and giddiness; light-headedness; vertigo NOS” [not otherwise specified]). Patients were considered to have a symptom diagnosis if they had 1 or more symptom diagnosis codes (ICD-9-CM 780–789) but no diagnosis codes listed outside that range (ie, no etiologic diagnosis) in the other 2 diagnosis fields.
The study was exempted from institutional board review by the Partners Healthcare Institutional Review Board.
Statistical Analyses
Data across years (1993–2005) were combined for analysis, except as noted. For data available only from particular years in the NHAMCS data set, analyses reflect combined data from that subset of years (eg, mean visit length, 2001–2004). For demographic and visit outcomes, we compare dizziness cases to controls without dizziness and report number of visits sampled, national weighted proportion or mean national estimate, and 95% confidence interval (CI). Crude or group-specific rates per 1000 US population were calculated using data from the US Census Bureau,15 and rates per 1000 ED visits were calculated using projected NHAMCS estimates.
Visits were classified by urgency (urgent, nonurgent, unknown). Coding for this variable has shifted several times in the NHAMCS data set during the years studied (1993–2005). Visits from 1993–1996 and 2001–2004 were coded simply as urgent/emergent or nonurgent. However, data in years 1997–2000 and 2005 were coded using expected wait times at triage. We coded visits with expected wait time less than 1 hour as “urgent,” those with expected wait times over 1 hour as “nonurgent,” and those with this field blank or coded unknown as “unknown.”
We compare resource use parameters, including mode of arrival, length of ED visit, and diagnostic tests across dizziness cases and controls without dizziness. Data regarding type of diagnostic imaging are limited in the NHAMCS data set. NHAMCS advanced imaging data are not subclassified by body part scanned, and the type of imaging (ie, CT vs MRI) was only recorded in certain years (1995–2000, 2005). As a result, we provide 2 separate analyses, the first across all years with CT and MRI results combined; the second for available years with CT and MRI results separate. We also assessed imaging trends for dizziness cases and controls over time by comparing scan rates (overall vs CT vs MRI) in individual years 1995 and 2005.
NHAMCS ICD-9-CM diagnosis codes were linked to HCUP-CCS multilevel codes in Microsoft Access 2003 (Microsoft Corporation, Redmond, WA). We report the frequency of etiologic class (HCUP-CCS) diagnoses, individual benign and dangerous diagnoses of interest, and proportion of symptom-only diagnoses, comparing dizziness cases to controls without dizziness and providing an odds ratio (OR) estimate for relative disease frequency in those with dizziness vs controls. For HCUP-CCS etiologic classes, we assessed frequency for all top-level diagnostic categories and for the 2 subclasses “cerebrovascular” (HCUP-CCS 7.3) and “ear” (HCUP-CCS 6.8). We also defined that subset with any “general medical” diagnosis (HCUP-CCS 1 [infectious], 3 [metabolic], 4 [hematologic], 7 [circulatory except for 7.3 cerebrovascular], 8 [respiratory], 9 [digestive], 10 [genitourinary]) (Appendix 1) and those with only a “general medical” diagnosis (ie, no other diagnostic class listed in either of the 2 remaining diagnostic fields).
We calculated 95% CIs using the relative standard error of the estimate, using a method approved of by the National Center for Health Statistics (NCHS).16 As NHAMCS recommends for standard analysis, we did not calculate 95% CIs for samples with fewer than 30 cells.17 When appropriate, we offer subgroup comparisons by demographic category. Comparison of proportions was assessed by χ2 test, and comparison of means was assessed by t test. All P values are 2-sided with P<.05 considered significant. Statistical analyses were performed using SAS 9.1 SURVEYFREQ, SURVEYMEANS, and SURVEYREG procedures for survey data (SAS Institute, Cary, NC). Area-proportional Venn diagrams were drawn using Microsoft Visio 2003 (Microsoft Corporation).
RESULTS
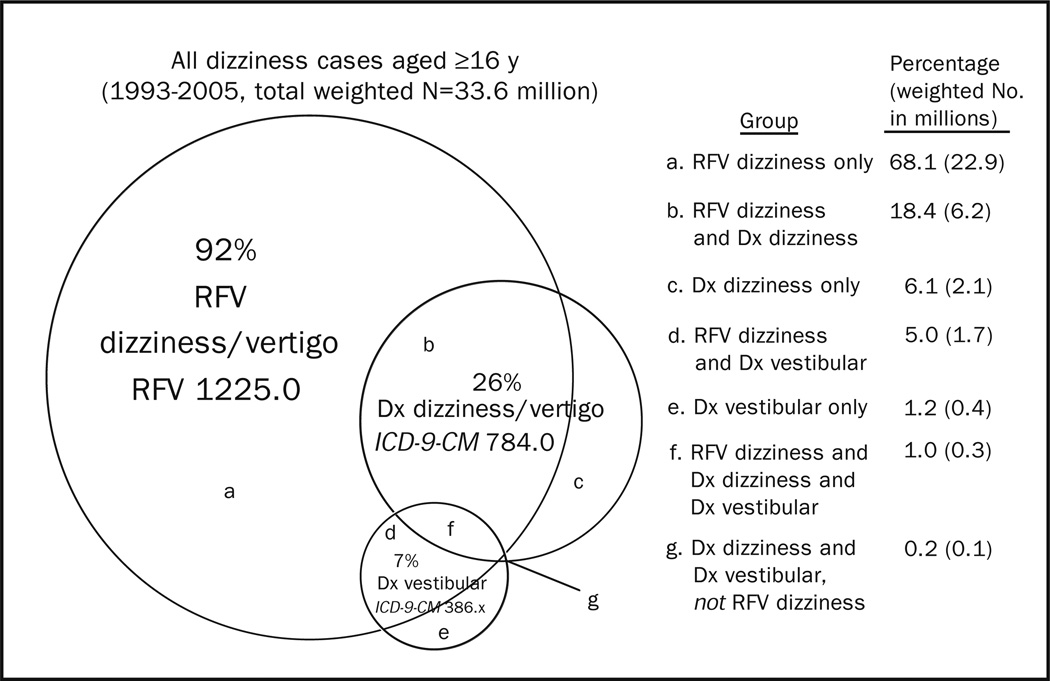
The total 13-year sample of dizziness cases was 9472, yielding a weighted estimate of 33.6 million ED visits nationally over that same period. This estimate corresponds to 2.6 million visits annually in the United States and 3.3% of all ED visits during that period. Among these, 92% were coded with dizziness as a presenting symptom (Figure 1). There was a bimodal age distribution for ED dizziness visits with a small peak in the third decade and an escalating frequency among those 50 years and older, peaking in the oldest (≥ 80 years) group (Table 1). Patients with dizziness were somewhat older (mean age, 51.0 vs 43.7 years; P<.001) than their counterparts without dizziness, and a greater fraction were female (61.4% vs 55.1%; P<.001) (Table 2). Dizziness cases were slightly more likely to use private (42.7% vs 41.3%) or public (34.7% vs 28.8%) insurance than controls, rather than self-pay (13.2% vs 16.9%) or other insurance (4.3% vs 7.3%) (P<.001 for the aggregate comparison). There were minor differences between cases and controls by race, urban status, and geographic region (Table 2).
Figure 1.
Proportional and absolute makeup of weighted study population. Numbers do not sum because of rounding. Dx = Diagnosis; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; RFV = National Hospital Ambulatory Medical Care Survey reason for visit.
TABLE 1.
Population and Emergency Department (ED) Visit Rates for Dizziness by Demographic Groupa
| Demographic group | No. of sampled visits |
US ED visits (millions) |
Per 1000 US populationb |
Per 1000 ED visits |
|---|---|---|---|---|
| No. (95% CI) | Rate (95% CI) | Rate (95% CI) | ||
| Overall | 9472 | 33.6 (31.3–35.9) | 12.0 (11.2–12.9) | 32.9 (32.0–33.8) |
| Age (y) | ||||
| 16–19 | 448 | 1.7 (1.4–1.9) | 8.2 (7.0–9.5) | 20.6 (18.1–23.0) |
| 20–29 | 1456 | 5.3 (4.8–5.8) | 10.6 (9.6–11.6) | 23.9 (22.2–25.6) |
| 30–39 | 1399 | 5.1 (4.6–5.5) | 9.0 (8.2–9.8) | 25.1 (23.6–26.7) |
| 40–49 | 1335 | 4.5 (4.1–5.0) | 8.4 (7.6–9.2) | 27.1 (25.2–29.0) |
| 50–59 | 1269 | 4.5 (4.1–5.0) | 11.5 (10.5–12.8) | 41.3 (38.3–44.4) |
| 60–69 | 1168 | 3.9 (3.5–4.3) | 14.4 (12.9–15.8) | 47.9 (44.4–51.5) |
| 70–79 | 1302 | 4.7 (4.3–5.1) | 22.7 (20.7–24.7) | 57.2 (53.1–61.2) |
| ≥80 | 1095 | 3.9 (3.4–4.3) | 32.5 (28.9–36.1) | 51.8 (47.8–55.9) |
| Sex | ||||
| Female | 5799 | 20.6 (19.1–22.2) | 11.6 (10.7–12.5) | 36.5 (35.2–37.9) |
| Male | 3673 | 13.0 (12.0–13.9) | 7.8 (7.2–8.4) | 28.4(27.2–29.6) |
| Race | ||||
| White | 7057 | 25.9 (24.0–27.9) | 10.7 (9.9–11.6) | 33.0 (31.9–34.0) |
| Black | 1989 | 6.6 (5.8–7.3) | 19.4 (17.1–21.7) | 31.6 (29.7–33.5) |
| Other | 426 | 1.1 (0.9–1.4) | 5.9 (4.5–7.1) | 41.5 (34.5–48.5) |
| Ethnicityc | ||||
| Hispanic | 1076 | 3.2 (2.9–3.6) | 9.2 (8.0–10.5) | 33.1 (30.4–35.8) |
| Non-Hispanic | 7626 | 27.2 (25.2–29.2) | 9.4 (8.6–10.2) | 33.0 (32.0–34.0) |
| Urban statusb | ||||
| Metropolitan statistical area | 8177 | 27.3 (24.8–29.8) | … | 33.7 (32.6–34.7) |
| Nonmetropolitan | 1245 | 6.3 (4.6–8.0) | … | 30.0 (27.9–32.1) |
| US regionb | ||||
| Northeast | 2506 | 7.2 (6.3–8.1) | … | 34.4 (32.5–36.3) |
| Midwest | 2159 | 8.5 (7.2–9.8) | … | 33.1 (31.4–34.9) |
| South | 2896 | 11.6 (10.1–13.1) | … | 31.3 (29.7–32.8) |
| West | 1911 | 6.3 (5.5–7.2) | … | 34.2 (31.5–36.9) |
CI = confidence interval.
Missing data reflect the fact that the US census bureau does not provide annual population demographic data broken down by urban status or US region for all age ranges necessary to complete these calculations.
Ethnicity categories do not total 100% because of 9% missing values in National Hospital Ambulatory Medical Care Survey data set.
TABLE 2.
Demographic Characteristics of Dizziness Cases and Controls Without Dizzinessa
| Dizziness cases |
Controls without dizziness |
||||
|---|---|---|---|---|---|
| Demographic group | No. | % or mean (95% CI) | No. | % or mean (95% CI) | P value |
| Age (y) | |||||
| Mean age | 9472 | 51.0 (50.4–51.6) | 281,158 | 43.7 (43.4–44.0) | <.001 |
| Sex | |||||
| Female | 5799 | 61.4 (60.1–62.7) | 153,718 | 55.1 (54.8–55.4) | <.001 |
| Race | |||||
| White | 7057 | 77.1 (75.3–79.0) | 209,361 | 77.0 (75.4–78.6) | |
| Black | 1989 | 19.5 (17.8–21.3) | 62,237 | 20.4 (18.9–21.9) | |
| Other | 426 | 3.3 (2.6–4.0) | 9560 | 2.6 (2.3–2.9) | .03 |
| Ethnicityb | |||||
| Hispanic | 1076 | 9.6 (8.4–10.8) | 31,214 | 9.5 (8.7–10.4) | |
| Non-Hispanic | 7626 | 81.0 (79.2–82.7) | 226,015 | 80.8 (79.2–82.4) | .86 |
| Urban status | |||||
| Metropolitan statistical area | 8177 | 81.3 (76.3–86.3) | 240,378 | 79.4 (74.2–84.7) | .02 |
| US region | |||||
| Northeast | 2506 | 21.4 (19.0–23.9) | 71,934 | 20.5 (17.9–23.0) | |
| Midwest | 2159 | 25.2 (22.0–28.5) | 62,098 | 25.1 (22.1–28.1) | |
| South | 2896 | 34.5 (31.0–38.0) | 92,340 | 36.4 (32.7–40.0) | |
| West | 1911 | 18.8 (16.4–21.3) | 54,786 | 18.1 (15.5–20.8) | .05 |
| Insurance type | |||||
| Private | 3165 | 42.7 (40.9–44.6) | 115,206 | 41.3 (40.2–42.4) | |
| Publicc | 3629 | 34.7 (33.1–36.4) | 115,886 | 28.8 (27.8–29.8) | |
| Self-pay | 1147 | 13.2 (12.2–14.2) | 51,037 | 16.9 (16.3–17.4) | |
| Other | 562 | 4.3 (3.7–4.9) | 31,068 | 7.3 (7.1–8.0) | |
| Missing | 484 | 5.0 (4.3–5.8) | 19,102 | 5.3 (5.0–6.0) | <.001 |
CI = confidence interval.
Ethnicity sums do not total 100% because of 9% missing values in National Hospital Ambulatory Medical Care Survey data set.
Public insurance includes State Children’s Health Insurance Program for 2001.
Dizziness cases were given an average of 1.7 diagnoses, with 22.1% receiving only a symptom diagnosis (eg, “dizziness…vertigo NOS” and “headache”), and nearly half of those (9.6%) specifically only a symptom diagnosis of dizziness (ie, “dizziness…vertigo NOS”). Symptom diagnoses without accompanying etiologic diagnoses were more common among dizziness cases than controls (22.1% vs 8.4%; OR, 3.1; P<.001). Among both cases and controls, 91% of diagnoses were classifiable using the HCUPCCS schema (codes 1–16). Top ICD-9-CM diagnostic categories (grouped using HCUP-CCS) for dizziness cases and controls are listed in Table 3. Considering oto-vestibular diagnoses separate from other neurologic disorders, and placing cerebrovascular disorders with neurologic (rather than cardiovascular) disorders, the 10 most frequent classes of diagnoses made were oto-vestibular (32.9%), cardiovascular (21.1%), respiratory (11.5%), neurologic (11.2%, including 4% cerebrovascular), metabolic (11.0%), injury/poisoning (10.6%), psychiatric (7.2%), digestive (7.0%), genitourinary (5.1%), and infectious (2.9%). In total, 49.2% (95% CI, 47.9%-50.5%) of ED dizziness cases were given at least 1 general medical diagnosis, and 40.3% (95% CI, 39.1%-41.5%) were given only a general medical diagnosis (with no associated oto-vestibular, neurologic, psychiatric, or other diagnosis). Several diagnostic groups were at least twice as likely among dizziness cases: oto-vestibular (OR, 34.4), cerebrovascular (OR, 4.0), metabolic (OR, 2.7), and cardiovascular (OR, 2.1). Others were at least twice as likely among controls without dizziness: dermatologic (OR, 0.2), musculoskeletal (OR, 0.3), and injury/poisoning (OR, 0.3).
TABLE 3.
Diagnoses of Dizziness Cases vs Controls Grouped by HCUP-CCS Level 1 Diagnostic Category, Listed by Diagnostic Frequency for Dizziness Cases a
| Dizziness cases |
Controls without dizziness |
||||
|---|---|---|---|---|---|
| Diagnostic categoryb | No. | % (95% CI) | No. | % (95% CI) | OR (95% CI) |
| Diseases of nervous system and sense organs | 3764 | 39.4 (38.1–40.7) | 21,543 | 7.8 (7.6–8.0) | 7.7 (7.3–8.1) |
| Oto-vestibular | 3125 | 32.9 (31.6–34.1) | 3768 | 1.4 (1.3–1.5) | 34.4 (31.9–37.1) |
| All others | 639 | 6.6 (5.9–7.2) | 17,775 | 6.4 (6.2–6.6) | 1.0 (0.9–1.1) |
| Diseases of the circulatory system | 2357 | 25.1 (23.8–26.3) | 33,625 | 12.2 (11.9–12.4) | 2.4 (2.3–2.6) |
| Cerebrovascular | 358 | 4.0 (3.5–4.5) | 2730 | 1.0 (1.0–1.1) | 4.0 (3.5–4.6) |
| All othersc | 1999 | 21.1 (19.9–22.2) | 30,895 | 11.1 (10.9–11.4) | 2.1 (2.0–2.3) |
| Diseases of the respiratory system | 1023 | 11.5 (10.7–12.4) | 40,480 | 14.9 (14.6–15.1) | 0.7 (0.7–0.8) |
| Endocrine, nutritional, and metabolic diseases and immunity disorders | 1047 | 11.0 (10.2–11.8) | 12,765 | 4.4 (4.3–4.6) | 2.7 (2.5–2.9) |
| Injury and poisoning | 992 | 10.6 (9.8–11.4) | 84,152 | 30.7 (30.3–31.1) | 0.3 (0.2–0.3) |
| Mental disorders | 714 | 7.2 (6.5–8.0) | 20,839 | 6.0 (5.8–6.2) | 1.2 (1.1–1.4) |
| Diseases of the digestive system | 669 | 7.0 (6.3–7.6) | 25,790 | 9.3 (9.1–9.4) | 0.7 (0.7–0.8) |
| Diseases of the genitourinary system | 482 | 5.1 (4.5–5.7) | 23,198 | 8.3 (8.2–8.5) | 0.6 (0.5–0.7) |
| Infectious and parasitic disease | 291 | 2.9 (2.5–3.4) | 8812 | 3.0 (2.9–3.1) | 1.0 (0.8–1.1) |
| Diseases of the musculoskeletal system and connective tissue | 268 | 2.8 (2.3–3.2) | 22,764 | 8.1 (7.9–8.3) | 0.3 (0.3–0.4) |
| Diseases of blood and blood-forming organs | 203 | 2.1 (1.7–2.4) | 3091 | 1.1 (1.0–1.1) | 1.9 (1.6–2.3) |
| Complications of pregnancy, childbirth, and the puerperium | 155 | 1.7 (1.3–2.0) | 7146 | 2.3 (2.2–2.4) | 0.7 (0.6–0.9) |
| Neoplasms | 101 | 0.9 (0.7–1.2) | 3230 | 1.0 (0.9–1.0) | 1.0 (0.7–1.3) |
| Diseases of the skin and subcutaneous tissue | 69 | 0.7 (0.5–0.9) | 8916 | 3.1 (2.9–3.2) | 0.2 (0.2–0.3) |
| Congenital anomalies/certain conditions originating in perinatal period | 12 | 0.2 (NC) | 263 | 0.1 (0.1–0.1) | 1.6 (0.8–3.2) |
| Medical diagnosis onlyd | 3772 | 40.3 (39.1–41.5) | 123,674 | 44.8 (44.4–45.2) | 1.0 (0.9–1.1) |
| Symptom diagnosis onlye | 2130 | 22.1 (20.8–23.3) | 22,855 | 8.4 (8.2–8.7) | 3.1 (2.9–3.3) |
CI = confidence interval; HCUP-CCS = Healthcare Cost and Utilization Project’s Clinical Classifications Software; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; NC = not calculated because sample had fewer than 30 observations; OR = odds ratio.
Although HCUP-CCS has 18 level 1 categories (Appendix 1), only the first 16 describe an etiologic diagnosis. Therefore, we analyzed only these 16 categories. Because of their diagnostic similarity and infrequency in the patient population under study, we combined HCUP–CCS level 1 categories 14 (congenital anomalies) and 15 (certain conditions originating in the perinatal period) for analysis.
Other diagnoses within the “circulatory system” include cardiovascular and peripheral vascular disease (Appendix 1).
“Medical” diagnoses were defined to include the following HCUP-CCS categories: Diseases of the circulatory system (excluding cerebrovascular); Diseases of the respiratory system; Endocrine, nutritional, and metabolic diseases and immunity disorders; Diseases of the digestive system; Diseases of the genitourinary system; Infectious and parasitic diseases; and Diseases of the blood and blood-forming organs. To be considered a “medical diagnosis only,” the emergency visit had to be coded with one or more diagnoses in these specific categories but no diagnoses in any other HCUP-CCS diagnostic category.
Symptom diagnoses were not derived from HCUP-CCS categories, but directly from ICD-9-CM codes. Patients diagnosed only with 1 or more symptom codes (ICD-9-CM 780–789) but no etiologic diagnostic codes (ie, ICD-9-CM codes outside this range) were considered to have been given a symptom-only diagnosis.
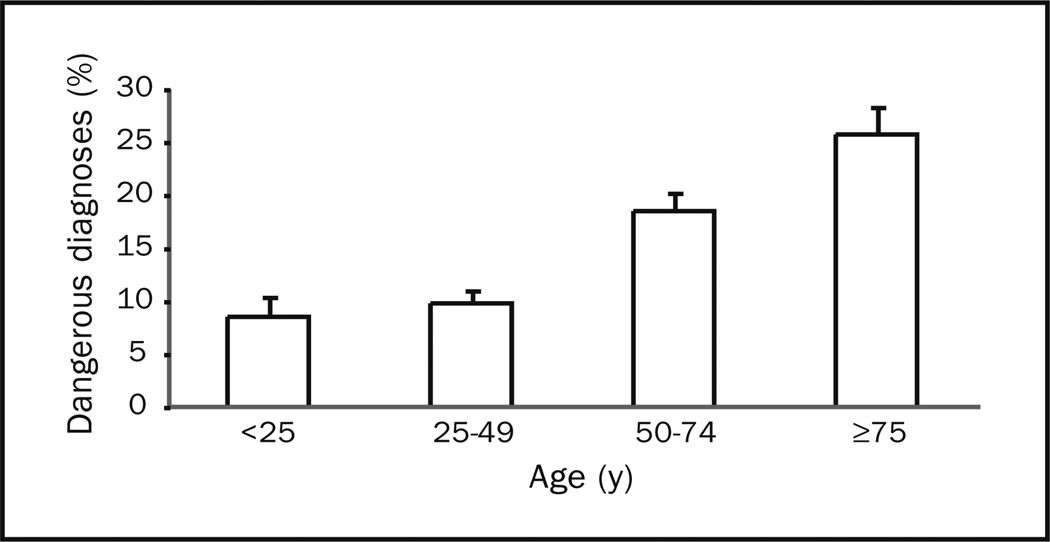
The frequency of prospectively defined benign and dangerous diagnoses is listed in Table 4. Vestibular neuritis/ labyrinthitis, benign paroxysmal positioning vertigo, and Meniere disease diagnoses were found only among dizziness cases, because we included vestibular disorders in our case definition. Among other benign diagnoses assessed, several were at least twice as likely among dizziness cases: orthostatic hypotension (OR, 22.4), vasovagal syncope (OR, 8.8), and panic disorder (OR, 3.9). Among dangerous diagnoses assessed, several were at least twice as likely among dizziness cases: carbon monoxide poisoning (OR, 7.4); transient ischemic attack (OR, 5.7); stroke/intracerebral hemorrhage (OR, 5.4); subarachnoid hemorrhage/ intracranial aneurysm/cervicocranial vascular dissection (OR, 4.4); arrhythmia (OR, 3.5); hypoglycemia (OR, 3.2); fluid and electrolyte disorders (OR, 3.1); aortic dissection/ ruptured aneurysm (OR, 2.0); and anemia (OR, 2.0). Among the prospectively defined benign diagnoses, the “top 10” represented 16% of the diagnoses made among dizziness cases. Similarly, among the prospectively defined dangerous diagnoses, the “top 10” represented 15% of the diagnoses made among dizziness cases. The frequency of dangerous diagnoses was high across age groups but rose with increasing age (Figure 2) and was substantially greater in those older than 50 years than in those younger than 50 years (20.9%; 95% CI, 19.2%–22.6% vs 9.3%; 95% CI, 8.3%–10.3%; P<.001).
TABLE 4.
Benign and Dangerous Diagnoses of Dizziness Cases vs Controls, Listed by Diagnostic Frequency for Dizziness Casesa
| Dizziness cases |
Controls without dizziness |
||||
|---|---|---|---|---|---|
| Diagnosis group | No. | % (95% CI) | No. | % (95% CI) | OR (95% CI) |
| Benign | |||||
| Vasovagal syncope | 628 | 6.6 (5.9–7.3) | 2122 | 0.8 (0.8–0.8) | 8.8 (7.8–9.9) |
| Vestibular neuritis/labyrinthitisb | 498 | 5.6 (5.0–6.2) | … | … | … |
| Migraine | 98 | 1.1 (0.8–1.4) | 2894 | 1.1 (1.0–1.2) | 1.0 (0.7–1.3) |
| Benign paroxysmal positional vertigob | 84 | 0.7 (0.6–0.9) | … | … | … |
| Orthostatic hypotension | 61 | 0.6 (0.4–0.7) | 66 | 0.0 (0.0–0.0) | 22.4 (14.3–35.1) |
| Depression | 82 | 0.6 (0.4–0.8) | 3725 | 1.0 (1.0–1.1) | 0.6 (0.5–0.8) |
| Panic disorder | 46 | 0.5 (0.3–0.7) | 408 | 0.1 (0.1–0.2) | 3.9 (2.6–5.7) |
| Alcohol intoxication | 51 | 0.5 (0.3–0.7) | 3220 | 1.0 (0.9–1.1) | 0.5 (0.3–0.7) |
| Meniere diseaseb | 32 | 0.3 (0.2–0.5) | … | … | … |
| Multiple sclerosis | 10 | 0.1 (NC) | 157 | 0.1 (0.0–0.1) | 1.6 (0.8–3.5)c |
| Dangerousd | |||||
| Fluid and electrolyte disorders | 539 | 5.6 (5.0–6.2) | 5245 | 1.9 (1.8–1.9) | 3.1 (2.8–3.5) |
| Arrhythmia | 296 | 3.2 (2.7–3.8) | 2550 | 0.9 (0.9–1.0) | 3.5 (3.0–4.2) |
| Transient ischemic attack | 154 | 1.7 (1.4–2.0) | 787 | 0.3 (0.3–0.3) | 5.7 (4.6–7.0) |
| Anemia | 167 | 1.6 (1.3–1.9) | 2255 | 0.8 (0.7–0.9) | 2.0 (1.7–2.5) |
| Hypoglycemia | 110 | 1.4 (1.0–1.7) | 1127 | 0.4 (0.4–0.5) | 3.2 (2.5–4.1) |
| Angina | 87 | 0.9 (0.6–1.1) | 2107 | 0.8 (0.8–0.9) | 1.0 (0.8–1.4) |
| Myocardial infarction | 73 | 0.8 (0.5–1.0) | 2149 | 0.8 (0.7–0.9) | 1.0 (0.7–1.3) |
| Stroke/intracerebral hemorrhage | 39 | 0.5 (0.3–0.7) | 262 | 0.1 (0.1–0.1) | 5.4 (3.6–7.9) |
| Carbon monoxide poisoning | 16 | 0.2 (NC) | 59 | 0.0 (0.0–0.0) | 7.4 (4.0–13.6)c |
| Subarachnoid hemorrhage/intracranial aneurysm/cervicocranial vascular dissection |
3 | 0.1 (NC) | 41 | 0.0 (0.0–0.0) | 4.4 (0.9–22.0)c |
| Alcohol withdrawal | 7 | 0.0 (NC) | 292 | 0.1 (0.1–0.1) | 0.6 (0.3–1.4)c |
| Aortic dissection/ruptured aneurysm | 2 | 0.0 (NC) | 34 | 0.0 (0.0–0.0) | 2.0 (0.4–9.1)c |
CI = confidence interval; NC = not calculated because sample had fewer than 30 observations; OR = odds ratio.
These vestibular diagnoses were included a priori within dizziness cases; thus, no comparison can be made with the control group without dizziness.
These ORs are potentially unstable estimates given the relatively few sampled diagnoses among dizziness cases.
There were no sampled cases among dizziness cases of pulmonary embolism, bacterial meningitis, or adrenal insufficiency. Likewise, there were too few sampled cases among controls without dizziness to calculate stable point estimates. Thus, we did not report these prospectively defined groups.
Figure 2.
Frequency of dangerous diagnoses in 25-year age groups. Note that these point estimates are likely conservative given that only prospectively defined dangerous diagnoses are considered. Error bars represent 95% confidence interval upper bounds on point estimates.
Dizziness cases were associated with greater health care resource use than were controls without dizziness (Table 5). They were more likely to arrive by ambulance (23.5% vs 17.1%), be seen as an urgent visit (57.2% vs 50.8%), have a longer ED stay (4.0 hours vs 3.4 hours), be tested extensively (mean number of diagnostic tests 4.6 vs 3.2), undergo cardiac monitoring (18.5% vs 9.2%), undergo imaging by CT or MRI (18.0% vs 6.9%), and be admitted to the hospital (18.8% vs 14.8%) (all listed comparisons, P<.001). Rates of imaging were higher in recent years for both cases (24.0% in 2005 vs 10.0% in 1995; P<.001) and controls (12.6% in 2005 vs 3.4% in 1995; P<.001), but there was no evidence that MRI had displaced CT as the primary imaging modality in the ED for either group as of 2005 (Table 5).
TABLE 5.
Emergency Department Resource Use of Dizziness Cases vs Controlsa
| Dizziness cases | Controls without dizziness |
||||
|---|---|---|---|---|---|
| Diagnosis group | No. | % or mean (95% CI) | No. | % or mean (95% CI) | P value |
| Arrival | |||||
| Ambulanceb | 1274 | 23.5 (21.9–25.0) | 26,724 | 17.1 (16.6–17.7) | <.001 |
| Initial visitc | 3725 | 90.8 (89.5–92.2) | 97,971 | 87.5 (86.7–88.3) | <.001 |
| Return visit (within 72 h)d | 131 | 2.8 (2.2–3.5) | 4566 | 3.4 (3.1–3.6) | .11 |
| Injury-relatede | 1342 | 15.9 (14.9–16.9) | 89,654 | 37.3 (36.9–37.8) | <.001 |
| Urgent visit | 5486 | 57.2 (55.1–59.4) | 143,679 | 50.8 (49.1–52.5) | <.001 |
| Wait time (min),b mean | 5274 | 266.4 (244.0–288.7) | 149,377 | 290.5 (272.4–308.7) | .001 |
| Testing | |||||
| Electrocardiography | 4295 | 45.2 (43.7–46.7) | 49,623 | 17.7 (17.3–18.1) | <.001 |
| Cardiac monitore | 1494 | 18.5 (17.0–19.9) | 21,000 | 9.2 (8.8–9.7) | <.001 |
| CT/MRI scane | 1556 | 18.0 (16.8–19.2) | 16,998 | 6.9 (6.5–7.3) | <.001 |
| 1995 | 57 | 10.0 (6.6–13.4) | 516 | 3.4 (2.9–3.8) | <.001 |
| 2005 | 242 | 24.0 (20.2–27.8) | 3096 | 12.6 (11.7–13.6) | <.001 |
| CT scanf | 656 | 14.5 (13.2–15.9) | 7302 | 5.7 (5.3–6.0) | <.001 |
| 1995 | 54 | 9.4 (6.0–12.8) | 496 | 3.2 (2.8–3.7) | <.001 |
| 2005 | 231 | 22.8 (19.1–26.4) | 2993 | 12.3 (11.3–13.2) | <.001 |
| MRIf | 39 | 0.8 (0.5–1.1) | 373 | 0.3 (0.2–0.3) | .001 |
| 1995 | 5 | 1.2 (NC) | 26 | 0.2 (NC) | <.001 |
| 2005 | 19 | 1.8 (NC) | 151 | 0.5 (0.4–0.7) | <.001 |
| Electroencephalographyc | 39 | 0.8 (0.5–1.2) | 649 | 0.5 (0.4–0.6) | .01 |
| Diagnostic tests, mean No.g | 7275 | 4.6 (4.4–4.8) | 205,660 | 3.2 (3.1–3.3) | <.001 |
| Procedures, mean No.g | 7330 | 4.6 (4.0–5.3) | 208,383 | 4.4 (4.0–4.8) | .37 |
| Medications, mean No. | 9472 | 1.7 (1.6–1.8) | 281,158 | 1.8 (1.7–1.8) | .13 |
| Visit length (min), mean No.c | 4502 | 242.4 (232.4–252.4) | 123,316 | 201.8 (196.1–207.5) | <.001 |
| Disposition | |||||
| Admit | 1777 | 18.8 (17.7–19.9) | 43,042 | 14.8 (14.4–15.3) | <.001 |
| ICUe | 157 | 2.2 (1.7–2.7) | 4090 | 1.8 (1.7–1.9) | .15 |
| Left without careg | 143 | 1.8 (1.4–2.3) | 4072 | 1.8 (1.7–2.0) | .90 |
| Transferred | 125 | 1.4 (1.0–1.7) | 6154 | 2.1 (1.9–2.2) | <.001 |
| Died | 5 | 0.0 (NC) | 918 | 0.4 (0.3–0.4) | <.001 |
Aggregate data 1993–2005, except as noted. CI = confidence interval; CT = computed tomography; ICU = intensive care unit; MRI = magnetic resonance imaging; NC = not calculated because sample had fewer than 30 observations.
Data available 1997–2000; 2003–2005.
Data available 2001–2004.
Data available 2001–2005.
Data available 1995–2005.
Data available 1995–2000, 2005.
Data available 1997–2005.
DISCUSSION
Our study demonstrates that dizziness is an extremely common ED symptom that preferentially affects older adults. We confirm prior literature that suggests the most frequent diagnostic category is oto-vestibular; however, our results also indicate general medical diagnoses are prevalent in this acute care population, and the proportion harboring a dangerous underlying disorder is high. Resource use for dizziness is disproportionate, particularly for diagnostic imaging, yet many patients leave the ED without an etiologic diagnosis.
From these nationally representative data, at least 3.3% of all ED visits are associated with dizziness or vertigo as a presenting symptom. This fraction is similar to those obtained from chart reviews at single institutions (1.7% ED chief complaint; 6.7% any charted complaint10) but lower than those obtained with prospective case capture (4.0% chief complaint18) or direct patient interview (4.4% main reason for the ED visit; 28.8% at least part of the reason for visit; 50.4% any recent dizziness2). These differences probably reflect differences in sensitivity across techniques for determining the presence of dizziness or its relevance to the visit.19
Our findings corroborate a higher prevalence of dizziness among older ED patients, in accordance with community-based estimates.6 Among those older than 50 years, dizziness represented roughly 5% of all ED visits, about twice that of younger adults. As hypothesized, there was a strong association between the frequency of dangerous diagnoses and increasing age. Notably, even among younger patients, nearly 1 in 10 harbored a dangerous underlying diagnosis. Our findings also confirm a slightly higher frequency of dizziness symptoms among women, described previously in population-based studies.20,21 None of the minor differences we identified between cases and controls in geographic distribution, race, or insurance status appear to be important from a clinical or public health standpoint.
The results confirm our hypothesis that dizziness is often medical. When considered in aggregate, general medical diagnoses (49.2%) were more common than otovestibular ones (32.9%). Nearly half of the medical disorders diagnosed were cardiovascular, in keeping with prior data from both EDs and general medical settings.6 Psychiatric diagnoses were less common (7.2%) than reported in settings other than acute care (eg, chronic dizziness in primary care 40%22 or subspecialty clinic 21%23).
Because we did not exhaustively classify all diagnoses as benign or dangerous, we cannot provide a robust estimate of absolute prevalence of benign or dangerous conditions. However, we can offer an estimate of relative prevalence: prospectively defined dangerous diagnoses (15%) were about as frequent as prospectively defined benign diagnoses (16%). Cerebrovascular diagnoses were not rare (4.0%) and, in aggregate, were the second most common dangerous diagnosis after fluid and electrolyte disturbances (5.6%), outpacing cardiac arrhythmias (3.2%), angina and myocardial infarction (1.7%), anemia (1.6%), and hypoglycemia (1.4%). Three dangerous disorders, although rare (<0.5%), were associated in our sample with a presenting symptom of dizziness (carbon monoxide poisoning [OR, 7.4; 95% CI, 4.0–13.6], subarachnoid hemorrhage/intracranial aneurysm/cervicocranial vascular dissection [OR, 4.4; 95% CI, 0.9–22.0], and aortic dissection/ruptured aneurysm [OR, 2.0; 95% CI, 0.4–9.1]). These disorders were chosen prospectively because of demonstrated dizziness symptoms in disease-based case reports or series,24–29 but their small numbers even in this very large data set underscore the difficulty in describing predictors of rare but critical diagnoses.
Our findings extend prior work suggesting that patients with dizziness consume substantial resources in EDs.13 Given the broad diagnostic spectrum and high risk of dangerous disorders, this consumption is probably appropriate. However, the frequent and disproportionate use of diagnostic imaging technology among dizziness cases vs controls (18.0% vs 6.9% across all years, and 24.0% vs 12.6% in 2005) deserves consideration. Although we cannot be sure that all imaging obtained for dizziness cases was focused on the head or brain, prior research indicates a high rate of neuroimaging among ED patients with dizziness.10 Virtually all imaging was by CT, rather than MRI, even in more recent years (22.8% vs 1.8% in 2005). However, for ED patients with dizziness, the diagnostic yield of head CT is known to be low,30 and CT has recently been shown to identify only about 16% of all acute ischemic strokes (even those imaged 12 hours or more after symptom onset).31 Computed tomography probably identifies even fewer strokes in the posterior cranial fossa because of radiographic artifacts emanating from the skull base.32 Some ED physicians might be falsely reassured by normal results from head CT,33 so heavy use of CT in these patients could be both costly and dangerous. Emergency department physicians worldwide would apparently welcome guidance in making imaging decisions among patients with dizziness or vertigo,33,34 so this represents an important area for future study.
Despite greater ED length of stay and extensive testing, dizziness cases are admitted with greater frequency than controls. Because admissions to the intensive care unit were comparable (2.2% vs 1.8%; P=.15), dizziness cases were probably not appreciably “sicker” (in a medical sense) than controls without dizziness. Considering the high frequency of symptom-only diagnoses (22.1% vs 8.4%), perhaps many of the non-ICU admissions among dizziness cases (16.6% vs 13.0%; P<.001) reflect residual diagnostic uncertainty, rather than admission for specific treatment. This suggests a need for new approaches to diagnosis, among them perhaps clinical decision rules, diagnostic protocols, or computer-based diagnostic decision support.35
Data from NHAMCS on ED visits are from a large, nationally representative sample that offers many advantages in ascertaining the overall spectrum of dizziness presentations to the ED. However, the lack of independent diagnostic confirmation and follow-up makes it impossible to precisely gauge the accuracy of diagnoses and appropriateness of diagnostic testing. We identified several potential limitations to our study findings.
The NHAMCS data set does not provide sufficient data to analyze details of clinical presentation. For instance, we cannot be sure, in those cases in which dizziness was one of 2 or 3 presenting symptoms, whether dizziness was the primary symptom. Also, we cannot know how the type of dizziness (vertigo, presyncope, disequilibrium, other) or presence of certain signs (eg, nystagmus) influenced the final diagnosis; nor can we use such data to generate clinical prediction rules.
Because ED diagnoses were unconfirmed, we cannot have absolute confidence that the diagnoses given were accurate, which might bias results. Misdiagnoses could be common with certain vestibular disorders, including cerebrovascular causes of dizziness.7,8 Perhaps 35% of those presenting to the ED with dizziness of confirmed cerebrovascular cause are misdiagnosed.7 However, at least for cerebrovascular disorders, ED overdiagnosis and underdiagnosis appear to be of similar magnitude,36 likely making our prevalence estimate roughly accurate despite diagnostic misclassification. In support of this notion, the one study of a population-based community sample of cerebrovascular diagnoses among ED patients with dizziness estimated a rate of confirmed cerebrovascular cases (3.2%)7 similar to that seen in our study (4.0%).
Overdiagnoses might be frequent for some benign disorders (eg, vasovagal syncope) but are unlikely among acute, life-threatening diseases with well-established diagnostic tests (eg, myocardial infarction, aortic dissection). Underdiagnoses might also be frequent, given the high rate of symptom-only diagnoses. This might be particularly so for certain vestibular disorders, such as multisensory disequilibrium (common among older patients37 but not available as a specific ICD-9-CM diagnosis code) or vestibular migraine (common at any age but probably unrecognized by many physicians38). Finally, perhaps some diagnoses were incidental to the symptom of dizziness. This is plausible for some diagnoses (eg, diabetes, hypertension) but less so for others (eg, labyrinthitis, aortic dissection).
CONCLUSION
There are roughly 2.6 million visits for dizziness in the United States annually. Emergency department patients with dizziness tend to be older and to use more medical resources than their counterparts without dizziness. They do not conform to traditional textbook notions of “the dizzy patient.” Dizziness is not attributed to a vestibular disorder in most cases and is often associated with cardiovascular or other medical causes. Dangerous disorders are frequently identified, even among younger adults. Despite extensive testing in the ED (including advanced imaging in approximately 1 of every 4 patients in recent years), dizziness often is undiagnosed at the end of ED assessment. Detailed studies in cohorts of unselected patients with dizziness are required to better understand this common ED symptom. Future research should focus on ways to streamline the diagnosis of ED patients with dizziness, taking into consideration the full range of associated dangerous medical and neurologic disorders.
Acknowledgments
This study was supported principally by the National Institutes of Health, National Center for Research Resources (NCRR) grant K23 RR 17324-01.
Glossary
- CI
confidence interval
- CT
computed tomography
- ED
emergency department
- HCUP-CCS
Healthcare Cost and Utilization Project’s Clinical Classifications Software
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MRI
magnetic resonance imaging
- NHAMCS
National Hospital Ambulatory Medical Care Survey
- NOS
not otherwise specified
- OR
odds ratio
- RFV code
NHAMCS reason-for-visit classification
APPENDIX 1
Multilevel Classification Using Healthcare Cost and Utilization Project’s Clinical Classifications Software (HCUPCCS) (Level 1 Codes and Some Level 2 Codes of Interest)
| 1 | Infectious and parasitic diseasesa | |
| 2 | Neoplasms | |
| 3 | Endocrine, nutritional, and metabolic diseases and immunity disorders | |
| 4 | Diseases of the blood and blood-forming organs | |
| 5 | Mental disorders | |
| 6 | Diseases of the nervous system and sense organs | |
| 6.1 | Central nervous system infection | |
| 6.2 | Hereditary and degenerative nervous system conditions | |
| 6.3 | Paralysis | |
| 6.4 | Epilepsy, convulsions | |
| 6.5 | Headache, including migraine | |
| 6.6 | Coma, stupor, and brain damage | |
| 6.7 | Eye disorders | |
| 6.8 | Ear conditionsb | |
| 6.9 | Other nervous system disorders | |
| 7 | Diseases of the circulatory system | |
| 7.1 | Hypertension | |
| 7.2 | Diseases of the heart | |
| 7.3 | Cerebrovascular diseasec | |
| 7.4 | Diseases of arteries, arterioles, and capillaries | |
| 7.5 | Diseases of veins and lymphatic system | |
| 8 | Diseases of the respiratory system | |
| 9 | Diseases of the digestive system | |
| 10 | Diseases of the genitourinary system | |
| 11 | Complications of pregnancy, childbirth, and the puerperium | |
| 12 | Diseases of the skin and subcutaneous tissue | |
| 13 | Diseases of the musculoskeletal system and connective tissue | |
| 14 | Congenital anomalies | |
| 15 | Certain conditions originating in the perinatal period | |
| 16 | Injury and poisoning | |
| 17 | Symptoms, signs, and ill-defined conditions and factors influencing health status | |
| 18 | Residual codes, unclassified, all E codes [259 and 260] | |
Infectious and parasitic diseases category includes systemic infections. However, in the HCUP-CCS system, localized infections (eg, meningitis, ocular infections) are generally listed within the affected organ system.
Ear conditions are listed with “Diseases of the nervous system and sense organs” in the HCUP-CCS system. In our study, we analyzed these otovestibular diagnoses (HCUP-CCS 6.8) as a separate category.
Cerebrovascular disorders are listed with “Diseases of the circulatory system” in the HCUP-CCS system. In our study, we analyzed these cerebrovascular diagnoses (HCUP-CCS 7.3) as a special subcategory of “Diseases of the nervous system and sense organs.”
APPENDIX 2
Benign and Dangerous Diagnoses of Interest Among Emergency Department Patients With Dizziness (ICD-9-CM or HCUP-CCS Codes)a
| Benign disorders |
| Orthostatic hypotension (458.0) |
| Vasovagal syncope (780.2) |
| Migraine (346.x) |
| Multiple sclerosis (340) |
| Benign paroxysmal positional vertigo (386.11) |
| Vestibular neuritis/labyrinthitis (386.12 or 386.3x) |
| Meniere disease (386.0x) |
| Panic disorder (300.01 or 300.21) |
| Depression (296.2, 296.3, 296.5, 296.82, 300.4, 311, 309.0, or 309.1) |
| Alcohol intoxication (305.0, 291.4, or 303.0) |
| Dangerous disorders |
| Angina (413.x or 411.1) |
| Myocardial infarction (410.x) |
| Arrhythmia (427.x not 427.5 [cardiac arrest]) |
| Aortic dissection/ruptured aneurysm (441.0, 441.1, 441.3, 441.5, or 441.6) |
| Pulmonary embolism (451.1x) |
| Transient ischemic attack (435.x) |
| Stroke/intracerebral hemorrhage (433.x, 434.x, or 431) |
| Subarachnoid hemorrhage/intracranial aneurysm/cervicocranial vascular dissection (430, 443.21, or 443.24) |
| Bacterial meningitis (320.x or 036.0) |
| Fluid and electrolyte disorders (HCUP-CCS 3.8.xb) |
| Hypoglycemia (251.0, 251.1, 251.2, or 250.8) |
| Anemia (HCUP-CCS 4.1.xc) |
| Adrenal insufficiency (255.4 or 255.5) |
| Alcohol withdrawal (291.81 or 291.0) |
| Carbon monoxide poisoning (986) |
Diagnoses were designated “benign” or “dangerous” on the basis of imminent risk of preventable morbidity or mortality. HCUP-CCS = Healthcare Cost and Utilization Project’s Clinical Classifications Software;ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
HCUP-CCS 3.8 “Fluid and electrolyte disorders” includes the following: 3.8.1 Hyposmolality, 3.8.2 Hypovolemia, 3.8.3 Hyperpotassemia, 3.8.4 Hypopotassemia, and 3.8.5 Other fluid and electrolyte disorders. These incorporate ICD-9-CM codes 276.x as follows: 276.0, 276.1, 276.2, 276.3, 276.4, 276.5, 276.50, 276.51, 276.52, 276.6, 276.7, 276.8, and 276.9.
HCUP-CCS 4.1 “Anemia” includes the following: 4.1.1 Acute posthemorrhagic anemia; 4.1.2 Sickle cell anemia; 4.1.3 Deficiency and other anemia; 4.1.3.1 Iron deficiency anemia; 4.1.3.2 Other deficiency anemia; 4.1.3.3 Aplastic anemia; 4.1.3.4 Chronic blood loss anemia; 4.1.3.5 Acquired hemolytic anemia; 4.1.3.6 Other specified anemia; 4.1.3.7 Anemia; unspecified. These incorporate ICD-9-CM codes in the 280–285 range, as follows: 280.0, 280.1, 280.8, 280.9, 281.0, 281.1, 281.2, 281.3, 281.4, 281.8, 281.9, 282.0, 282.1, 282.2, 282.3, 282.4, 282.41, 282.42, 282.49, 282.5, 282.60, 282.61, 282.62, 282.63, 282.64, 282.68, 282.69, 282.7, 282.8, 282.9, 283.0, 283.1, 283.10, 283.11, 283.19, 283.2, 283.9, 284.0, 284.8, 284.9, 285.0, 285.1, 285.21, 285.22, 285.29, 285.8, and 285.9.
References
- 1.Kroenke K, Jackson JL. Outcome in general medical patients presenting with common symptoms: a prospective study with a 2-week and a 3-month follow-up. Fam Pract. 1998;15(5):398–403. doi: 10.1093/fampra/15.5.398. [DOI] [PubMed] [Google Scholar]
- 2.Newman-Toker DE, Cannon LM, Stofferahn ME, Rothman RE, Hsieh YH, Zee DS. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. 2007;82(11):1329–1340. doi: 10.4065/82.11.1329. [DOI] [PubMed] [Google Scholar]
- 3.Herr RD, Zun L, Mathews JJ. A directed approach to the dizzy patient. Ann Emerg Med. 1989;18(6):664–672. doi: 10.1016/s0196-0644(89)80524-4. [DOI] [PubMed] [Google Scholar]
- 4.Skiendzielewski JJ, Martyak G. The weak and dizzy patient. Ann Emerg Med. 1980;9(7):353–356. doi: 10.1016/s0196-0644(80)80111-9. [DOI] [PubMed] [Google Scholar]
- 5.Kroenke K, Hoffman RM, Einstadter D. How common are various causes of dizziness? a critical review. South Med J. 2000;93(2):160–167. [PubMed] [Google Scholar]
- 6.Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med. 2001;134(9, pt 2):823–832. doi: 10.7326/0003-4819-134-9_part_2-200105011-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37(10):2484–2487. doi: 10.1161/01.STR.0000240329.48263.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulin T, Sablot D, Vidry E, et al. Impact of emergency room neurologists on patient management and outcome. Eur Neurol. 2003;50(4):207–214. doi: 10.1159/000073861. [DOI] [PubMed] [Google Scholar]
- 9.Newman-Toker DE. Charted records of emergency department dizzy patients suggest overemphasis on symptom quality may be associated with diagnostic errors [abstract] Ann Emerg Med. 2003;42(suppl 4):S80. [Google Scholar]
- 10.Dallara J, Lee C, McIntosh L, Sloane PD, Morris D. ED length-of-stay and illness severity in dizzy and chest-pain patients. Am J Emerg Med. 1994;12(4):421–424. doi: 10.1016/0735-6757(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 11.McCaig LF, McLemore T. Plan and operation of the National Hospital Ambulatory Medical Survey, series 1: programs and collection procedures. Vital Health Stat 1. 1994 Jul;34:1–78. [PubMed] [Google Scholar]
- 12.Schneider D, Appleton L, McLemore T. A reason for visit classification for ambulatory care. Vital Health Stat 2. 1979 Feb;78:1–63. [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. [Accessed June 4, 2008];NHAMCS Data Collection and Processing, 2007. US Department of Health and Human Services, Centers for Disease Control and Prevention. Web site. http://www.cdc.gov/nchs/about /major/ahcd/imputham.htm.
- 14.Elixhauser A, Steiner C, Palmer L. [Accessed June 4, 2008];Clinical Classifications Software (CCS), 2008. US Agency for Healthcare Research and Quality. Web site. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 15.Population Estimates Program. [Accessed June 4, 2008.];US Census Bureau Web site. http://www.census.gov/popest/estimates.php.
- 16.Nawar E. [Accessed June 4, 2008];Understanding and Using NAMCS and NHAMCS Data: A Hands-On Workshop, part 2: Using Public Use Data Files. 2006 Data Users Conference Presentations Web site. http://www.cdc.gov/nchs/ppt/duc2006 /Nawar_41_exercises.pdf.
- 17.McCaig LF, Woodwell D. [Accessed June 4, 2008];Analyzing data from the NAMCS and the NHAMCS. 2006 Data Users Conference Presentations Web site 2007. http://www.cdc.gov/nchs/ppt/duc2006/Mccaig_28.ppt.
- 18.Lam JMY, Siu WS, Lam TS, Cheung NK, Graham CA, Rainer TH. The epidemiology of patients with dizziness in an emergency department. Hong Kong J Emerg Med. 2006;13:133–139. [Google Scholar]
- 19.Guardabascio LM, Rothman RE, Zee DS, Newman-Toker DE. “Chief complaint screening”—a new method for symptom-oriented research in the emergency department [abstract] Acad Emerg Med. 2006;13(5 suppl 1):S146. [Google Scholar]
- 20.Colledge NR, Wilson JA, Macintyre CC, MacLennan WJ. The prevalence and characteristics of dizziness in an elderly community. Age Ageing. 1994;23(2):117–120. doi: 10.1093/ageing/23.2.117. [DOI] [PubMed] [Google Scholar]
- 21.Yardley L, Owen N, Nazareth I, Luxon L. Prevalence and presentation of dizziness in a general practice community sample of working age people. Br J Gen Pract. 1998;48(429):1131–1135. [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Lucas CA, Rosenberg ML, et al. Causes of persistent dizziness: a prospective study of 100 patients in ambulatory care. Ann Intern Med. 1992;117(11):898–904. doi: 10.7326/0003-4819-117-11-898. [DOI] [PubMed] [Google Scholar]
- 23.Nedzelski JM, Barber HO, McIlmoyl L. Diagnoses in a dizziness unit. J Otolaryngol. 1986;15(2):101–104. [PubMed] [Google Scholar]
- 24.Heckerling PS, Leikin JB, Maturen A, Perkins JT. Predictors of occult carbon monoxide poisoning in patients with headache and dizziness. Ann Intern Med. 1987;107(2):174–176. doi: 10.7326/0003-4819-107-2-174. [DOI] [PubMed] [Google Scholar]
- 25.Hampson NB, Hampson LA. Characteristics of headache associated with acute carbon monoxide poisoning. Headache. 2002;42(3):220–223. doi: 10.1046/j.1526-4610.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 26.Hauerberg J, Andersen BB, Eskesen V, Rosenorn J, Schmidt K. Importance of the recognition of a warning leak as a sign of a ruptured intracranial aneurysm. Acta Neurol Scand. 1991;83(1):61–64. doi: 10.1111/j.1600-0404.1991.tb03960.x. [DOI] [PubMed] [Google Scholar]
- 27.Seet CM. Clinical presentation of patients with subarachnoid haemorrhage at a local emergency department. Singapore Med J. 1999;40(6):383–385. [PubMed] [Google Scholar]
- 28.Demiryoguran NS, Karcioglu O, Topacoglu H, Aksakalli S. Painless aortic dissection with bilateral carotid involvement presenting with vertigo as the chief complaint. Emerg Med J. 2006;23(2):e15. doi: 10.1136/emj.2005.027862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kung SW, Ng WS, Ng MH. Aortic dissection in an accident and emergency department in Hong Kong. Hong Kong Med J. 2007;13(2):122–130. [PubMed] [Google Scholar]
- 30.Wasay M, Dubey N, Bakshi R. dizziness and yield of emergency head CT scan: is it cost effective [letter]? Emerg Med J. 2005;22(4):312. doi: 10.1136/emj.2003.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons Z, Biller J, Adams HP, Jr, Dunn V, Jacoby CG. Cerebellar infarction: comparison of computed tomography and magnetic resonance imaging. Ann Neurol. 1986;19(3):291–293. doi: 10.1002/ana.410190312. [DOI] [PubMed] [Google Scholar]
- 33.Stanton VA, Hsieh YH, Camargo CA, Jr, et al. Overreliance on symptom quality in diagnosing dizziness: results of a multicenter survey of emergency physicians. Mayo Clin Proc. 2007;82(11):1319–1328. doi: 10.4065/82.11.1319. [DOI] [PubMed] [Google Scholar]
- 34.Eagles D, Stiell IG, Clement CM, et al. International survey of emergency physicians’ priorities for clinical decision rules. Acad Emerg Med. 2008;15(2):177–182. doi: 10.1111/j.1553-2712.2008.00035.x. [DOI] [PubMed] [Google Scholar]
- 35.Newman-Toker DE. Diagnosing Dizziness in the Emergency Department— Why “What do you mean by ‘dizzy’?” Should Not Be the First Question You Ask [dissertation] Clinical Investigation, Bloomberg School of Public Health] Baltimore, MD: The Johns Hopkins University; 2007. [Accessed April 22, 2008]. Publication No. AAT 3267879. ProQuest Digital Dissertations Web site. http://www.proquest.com/products_umi/dissertations/pqdt.shtml. [Google Scholar]
- 36.Newman-Toker DE, Robinson KA, Edlow JA. Frontline misdiagnosis of cerebrovascular events in the era of modern neuroimaging: a systematic review [abstract]. Abstracts of the 133rd Annual Meeting of the American Neurological Association; Salt Lake City, UT; September 21–24, 2008. Ann Neurol. In press. [Google Scholar]
- 37.Ekvall Hansson E, Mansson NO, Hakansson A. Benign paroxysmal positional vertigo ampong elderly patients in primary health care. Gerontology. 2005;51(6):386–389. doi: 10.1159/000088702. [DOI] [PubMed] [Google Scholar]
- 38.Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028–1033. doi: 10.1212/01.wnl.0000237539.09942.06. [DOI] [PubMed] [Google Scholar]