Abstract
Probably the foremost hypothesis of depression is the 5-hydroxytryptamine (5-HT, serotonin) deficiency hypothesis. Accordingly, anomalies in putative 5-HT biomarkers have repeatedly been reported in depression patients. However, whether such anomalies in fact reflect deficient central 5-HT neurotransmission remains unresolved. We employed a naturalistic model of 5-HT deficiency, the tryptophan hydroxylase 2 (Tph2) R439H knockin mouse, to address this question. We report that Tph2 knockin mice have reduced basal and stimulated levels of extracellular 5-HT (5-HTExt). Interestingly, cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) and fenfluramine-induced plasma prolactin levels are markedly diminished in the Tph2 knockin mice. These data seemingly confirm that low CSF 5-HIAA and fenfluramine-induced plasma prolactin reflects chronic, endogenous central nervous system (CNS) 5-HT deficiency. Moreover, 5-HT1A receptor agonist-induced hypothermia is blunted and frontal cortex 5-HT2A receptors are increased in the Tph2 knockin mice. These data likewise parallel core findings in depression, but are usually attributed to anomalies in the respective receptors rather than resulting from CNS 5-HT deficiency. Further, 5-HT2A receptor function is enhanced in the Tph2 knockin mice. In contrast, 5-HT1A receptor levels and G-protein coupling is normal in Tph2 knockin mice, indicating that the blunted hypothermic response relates directly to the low 5-HTExt. Thus, we show that not only low CSF 5-HIAA and a blunted fenfluramine-induced prolactin response, but also blunted 5-HT1A agonist-induced hypothermia and increased 5-HT2A receptor levels are bona fide biomarkers of chronic, endogenous 5-HT deficiency. Potentially, some of these biomarkers could identify patients likely to have 5-HT deficiency. This could have clinical research utility or even guide pharmacotherapy.
Keywords: 5-HT, 5-HT1A, 5-HT2A, biomarkers, depression, Tph2
Introduction
Chronic, endogenous 5-hydroxytryptamine (5-HT, serotonin) deficiency has been prominently implicated in the etiology of depression for over 40 years. The hypothesis originally derived from the clinical observation that drugs enhancing 5-HT neurotransmission, by inhibiting the serotonin-reuptake transporter (SERT) or monoamine oxidase (MAO), displayed antidepressant activity.1 However, that enhancing 5-HT function alleviates depression does not necessarily mean that depression is caused by a primary 5-HT deficit. It also remains an outstanding question whether depression is accompanied, or can be caused, by central nervous system (CNS) 5-HT deficiency, although it should be noted that decreased midbrain 5-HT levels have been reported in depressed suicide victims.2 Unfortunately, this question is difficult to address since brain extracellular 5-HT (5-HTExt, the functionally active pool acting at 5-HT receptors) cannot currently be assessed in humans.3 In lieu of direct measures, aberrations of putative biomarkers of central 5-HT neurotransmission in depression and other psychiatric disorders have been interpreted as reflecting CNS 5-HT deficiency.4 Prominently, low cerebrospinal fluid (CSF) levels of 5-hydroxyindoleacetic acid (5-HIAA, the main 5-HT metabolite)5 and a blunted plasma prolactin response to the 5-HT releaser fenfluramine6,7 have repeatedly been reported in depression, particularly when suicidality is present, and suggested to reflect CNS 5-HT deficiency. Indeed, acute tryptophan depletion, a procedure that likely reduces CNS 5-HTExt,8 produces reductions in both CSF 5-HIAA and fenfluramine-induced plasma prolactin in healthy volunteers.9,10 However, starving the organism of the essential amino acid tryptophan may well have numerous effects apart from lowering 5-HT. For instance, tryptophan occurs in the amino acid chain of prolactin and is the precursor of the endogenous glutamatergic/cholinergic receptor ligand kynurenic acid.11,12 Moreover, it is not necessarily obvious that low CSF 5-HIAA and fenfluramine-induced plasma prolactin would reflect a CNS 5-HT deficit. Often 5-HTExt and extracellular 5-HIAA dynamics do not correlate,13,14 and prolactin secretion is regulated by a myriad of neurotransmitter systems in addition to 5-HT, notably dopamine.15,16 Thus, whether low CSF 5-HIAA and/or blunted fenfluramine-induced plasma prolactin reflects chronic endogenous CNS 5-HT deficiency remains to be directly demonstrated.
Similarly, blunted hypothermic responses to 5-HT1A receptor (5-HT1AR) agonists17–19 and increased levels of frontal cortex 5-HT2A receptors (5-HT2ARs)20–22 have repeatedly been reported in depression and suicidality. The pathobiologies of such observations are not known but are usually ascribed to anomalies in 5-HT1AR and 5-HT2AR function rather than CNS 5-HT-deficiency per se. The mechanism of 5-HT1AR agonist-induced hypothermia is poorly defined and the observation of a blunted response in depression is somewhat counterintuitive. Chronic enhancement of 5-HTExt actually results in blunted 5-HT1AR agonist-induced hypothermia via 5-HT1AR autoreceptor desensitization.13,23–25 Reduced extracellular levels of, for instance, dopamine can lead to increases in dopamine receptors.26 A similar causal relationship could be envisioned for 5-HTExt and 5-HT2AR. However, it is not given a priori that increased 5-HT2ARs would result from CNS 5-HT deficiency in depression. For instance, pharmacological 5-HT depletion in rats has inconsistent effects on 5-HT2AR levels.27,28 Moreover, reduced 5-HT1ARs have been reported in depression.29,30
We investigated whether chronic, endogenous 5-HT deficiency results in aberrations in putative CNS 5-HT biomarkers as reported in depression. To this end, we employed a naturalistic mouse model of 5-HT deficiency, the tryptophan hydroxylase 2 (Tph2) R439H knockin mouse.31 This murine model harbors a single point mutation producing a R439H substitution in Tph2, the rate-limiting enzyme in brain 5-HT synthesis.31 The analogous human mutation, R441H, was originally identified in a late life depression cohort.32 Tph2 R439H mice (Tph2 knockin mice henceforth) have markedly reduced 5-HT synthesis and tissue levels and exhibit increased depression-, anxiety- and aggression-like behaviors.31 Here, we report that Tph2 knockin mice have robustly reduced 5-HTExt levels and dynamics that are accompanied by a depression-like putative 5-HT biomarker profile: low CSF 5-HIAA, reduced fenfluramine-induced plasma prolactin, a blunted 5-HT1AR agonist hypothermic response and increased frontal cortex 5-HT2ARs.
Subjects and methods
Animals
The generation of the Tph2 knockin mice has been described previously.31 Littermate mice were housed 3–5 per cage with food and water available ad libitum on a 12-h light–dark cycle at an ambient temperature of 21±2 °C. Unless noted, experiments were conducted during the light phase. All experiments used age- and gender-matched wild type (WT) and Tph2 knockin littermate mice (3–6 months old) maintained on a mixed 129S6/SvEv × C57BL/6J background (see Supplementary Table 1 for gender distribution within groups). Experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals and an approved animal protocol from the Duke University Animal Care and Use Committee.
Materials
Escitalopram was a generous gift from Lundbeck (Valby, Denmark). Dexfenfluramine, 8-OH-DPAT, DOI, 5-HT, 5-hydroxytryptophan (5-HTP), 5-HIAA, 3,4-dihydroxyphenylacetic acid, homovanillic acid and clonidine were purchased from Sigma (St Louis, MO, USA). NAN-190 and DPCPX were purchased from Tocris (Ellisville, MO, USA). [125I]p-MPPI was obtained from Perkin Elmer (Waltham, MA, USA). [3H]WAY100635, [3H]ketanserin, [3H]citalopram and [35S]GTPγS were purchased from Perkin Elmer. All other reagents used were of analytic grade. All drugs were administered in a volume of 10 ml kg−1 in saline, intraperitoneal (i.p.) or subcutaneous (s.c.), as indicated.
Microdialysis
Surgery
Mice were anesthetized by ketamine/ xylazine (100 and 10 mg kg−1, respectively, in saline, i.p) and a guide cannula (CMA7, CMA Microdialysis, Chelmsford, MA, USA) was stereotactically implanted into the frontal cortex (AP: 2.3 mm, ML: 0.3 mm, DV: 0.7 mm), hippocampus (AP: −3.3 mm, ML: 3.0 mm, DV: 1.5 mm) or lateral ventricle (AP: 0.3 mm; ML: 0.9 mm; DV: 2.0 mm) according to the atlas by Franklin and Paxinos33 and fixed in place with two anchor screws (CMA) and carboxylate dental cement (CMA). Operated mice were singly housed, treated with antibiotics (1.2 mg sulfamethoxazole ml−1 and 0.24 mg trimethoprim ml−1) in the drinking water and allowed to recover 48–96 h post-surgery.
Dialysate collection
The microdialysis probe was inserted into the guide cannula approximately 16 h before the start of sample collection to allow for the stabilization of 5-HTExt levels. It has been previously demonstrated that dialysate analyte levels are usually only influenced by the tissue trauma caused by probe insertion for a few hours and that the blood–brain barrier is usually not compromised in brain microdialysate experiments.34 However, we employed a relatively long (overnight) stabilization period to ensure that we reached stable baseline 5-HTExt levels unaffected by peripheral (that is, Tph1 derived) 5-HT. In confirmation of the neuronal origin of the dialysate 5-HT, the 5-HT1AR agonist 8-OH-DPAT (1 mg kg−1, i.p.) decreased 5-HTExt by approximately 50% (Supplementary Figure S1), a decrement that is within the range typically reported after 8-OH-DPAT administration.23,35 The mouse was gently restrained and the microdialysis probe (CMA7 7/2 for frontal cortex and hippocampus, CMA7 7/1 for lateral ventricle) was inserted into the guide cannula. The mouse was then placed in a circular chamber with bedding, chow and water available. A two-channel swivel (cat. no. 375/D/22QM; Instech, Plymouth Meeting, PA, USA) allowed for unimpeded movement of the mouse. Artificial CSF (147 mM NaCl, 2.7 mM KCl, 0.85 mM MgCl2, 1.2 mM CaCl2, CMA) was delivered at a flow rate of 0.45 μl min−1 from probe insertion until the end of experiment. Following the stabilization period, samples (30- or 120-min sample duration, as noted) were collected on ice in the dark, immediately frozen on dry ice and stored at −80 °C. Dialysates were analyzed off-line by high-performance liquid chromatography-electrochemical detection (HPLC-EC, see Supplementary Methods) for 5-HT, 5-HIAA, 3,4-dihydroxyphenylacetic acid and homovanillic acid, or by the SymDAQ HPLC-tandem-mass spectrometry technology (see Supplementary Methods) for simultaneous determination of monoamines and amino acids.
Microdialysis experiment 1
In all mice, 120-min dialysates were collected at zeitgeber noon or midnight from frontal cortex or hippocampus. A 22.5 μl aliquot of each dialysate was transferred to 7.5 μl 20 mM formic acid. This sample was used for monoamine and amino acid analysis by SymDAQ HPLC-tandem-mass spectrometry. The remaining dialysate was used for 5-HT analysis using HPLC-EC. Thereafter, the following noon, two 30-min baseline dialysates were collected and then KCl raised to 100 mM in the artificial CSF to produce neuronal depolarization proximal to the probe membrane via reverse dialysis and four more dialysates collected.
Microdialysis experiment 2
Dialysates were collected from either frontal cortex or hippocampus. One 30-min baseline dialysate was collected followed by escitalopram (10 mg kg−1, i.p., 10 ml mg−1 in saline) injection and four more dialysates collected. 5-HT was analyzed by HPLC-EC (see Supplementary Methods).
Microdialysis experiment 3
The probe membrane was placed in the lateral ventricle to collect CSF. One 30-min sample was collected at noon. 5-HIAA was analyzed by HPLC-EC (see Supplementary Methods).
Tissue 5-HT and 5-HIAA analysis
Frontal cortex and hippocampus were rapidly dissected and frozen on dry ice. Tissues were homogenized by sonication in 20 volumes of ice-cold 100 mM HClO4. The supernatants were recovered and passed through 0.2 μm filters and 5-HT and 5-HIAA quantified in the filtrates by HPLC-EC (see Supplementary Methods).
Marble burying
The mouse was introduced into a novel arena (25 × 36 cm; height: 18 cm) with 24 marbles latticed on top of four inches of lightly compressed aspen wood shavings. After 30 min, the mouse was removed, a photograph taken from above and the number of marbles still visible on the photograph counted by an observer blind to the genotype. Marbles buried = 24 minus number of visible marbles.
Plasma prolactin and corticosterone
For assessment of naïve/diurnal plasma prolactin and corticosterone, unhandled mice were transferred from the holding room to an adjacent room, rapidly decapitated and trunk blood collected in EDTA-coated tubes. Such plasma samples were collected at zeitgeber time 0 and 12 h. For the response to dexfenfluramine (10 mg kg−1, i.p.), mice were killed and plasma samples collected as described above 60 min after saline or dexfenfluramine administration. Plasma prolactin levels were determined by an enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (GenWay Biotech, San Diego, CA, USA) and corticosterone levels were determined using a competitive binding enzyme immunoassay kit according to the manufacturer’s instructions (Assay Designs, Ann Arbor, MI, USA).
5-HT1AR agonist-induced hypothermia
The 5-HT1AR or α2R agonist-induced hypothermic response was assessed as described.36 In brief, following an initial conditioning rectal probe insertion, three baseline body temperature readings were recorded with 15-min intervals using a digital rectal probe thermometer. Thereafter, 8-OH-DPAT (1 mg kg−1, i.p.) or clonidine (0.2 mg kg−1, i.p.) was injected and six additional readings recorded.
5-HT2AR mediated head-twitches/head-shakes
Three different pharmacological manipulations were employed to induce head-twitches/head-shakes. These behaviors are part of the complex syndrome induced by supranormal 5-HT neurotransmission and are mainly mediated by 5-HT2ARs.37 The number of head-twitches or head-shakes was scored from 1-min videos by an observer blind to time, treatment and genotype.
Head-twitches induced by combined MAO A and SERT inhibition
Under these conditions, where 5-HT breakdown and reuptake is simultaneously blocked, the head-twitch response will closely relate to Tph2 enzymatic activity. Clorgyline (10 mg kg−1, i.p.) was injected immediately after recording the initial video. Sixty min later, the second video was recorded and then escitalopram (10 mg kg−1, s.c.) was injected. Hereafter videos were recorded of each animal every 30 min for 150 min.
Head-twitches induced by combined 5-HTP administration and SERT inhibition
5-HTP is the enzymatic product of Tph2. Hence, the head-twitch response is expected to be largely de-coupled from Tph2 enzymatic activity under these conditions. 5-HTP (100 mg kg−1, i.p.) and escitalopram (10 mg kg−1, s.c.) were injected immediately after recording the initial video. Hereafter videos were recorded every 30 min for 150 min.
Head-shakes induced by DOI
Here the head-shakes response is induced by direct 5-HT2AR stimulation by the 5-HT2A/CR agonist DOI. DOI (3 mg kg−1, i.p.) was injected immediately after recording the initial video and hereafter videos were recorded every 30 min for 90 min.
Ligand saturation binding of 5-HT1AR, 5-HT2AR and SERT in brain tissue
[3H]WAY100635, [3H]ketanserin and [3H]citalopram were used for 5-HT1AR, 5-HT2AR and SERT binding, respectively. Saturation binding was performed on rapidly dissected tissues stored at −80 °C until used, employing standard protocols (see Supplementary Methods).
5-HT1AR autoradiography
Mice were quickly euthanized by cervical dislocation and the brains collected and rapidly frozen on dry ice. In all, 20 μm cryo-sections were cut at the level of the hippocampus/hypothalamus and dorsal raphe, −2.06 and −4.5 mm relative to Bregma,33 respectively, onto silanized microscope slides. The sections were desiccated at 4 °C overnight and stored at −80 °C. [125I]p-MPPI binding for quantitation of 5-HT1AR levels and [35S]GTPγS binding for assessment of 5-HT1AR G-protein coupling were performed using standard protocols38,39 (see Supplementary Methods).
Statistical analysis
Data were analyzed with unpaired Student’s t-test, two-way analysis of variance (ANOVA) or two-way repeated measures (RM)-ANOVA using Prism statistical software version 5.0c (GraphPad Software, La Jolla, CA, USA). When significant factor effects or factor interactions were detected, analysis of variances were followed by Bonferroni post hoc analysis. Data are presented as mean±s.e.m.
Results
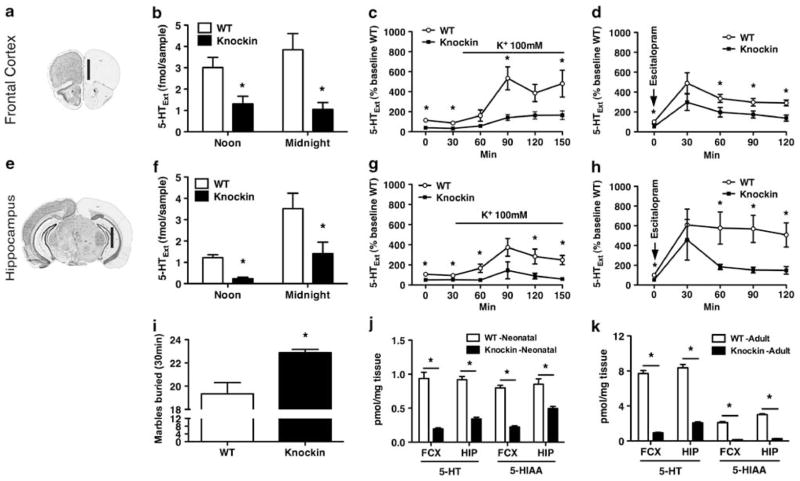
To assess 5-HTExt levels in the Tph2 knockin mice, we employed microdialysis and documented that basal 5-HTExt levels are decreased by more than half in both the frontal cortex and hippocampus (Figures 1b and f), two regions believed to be functionally compromised in depression.40 Evoked neuron terminal 5-HT release induced by either K+ depolarization (Figures 1c and g) or a selective serotonin reuptake inhibitor (SSRI; Figures 1d and h) was preserved but markedly attenuated in the Tph2 knockin mice. This attests to a loss, but not absence, of central 5-HT function. SSRIs decrease marble burying in mice with remarkable reliability across reports.41 In fact, mouse marble burying behavior has been reported to show a close, inverse relationship to frontal 5-HTExt levels.42,43 This indicates that marble burying could represent a functional reflection of frontal cortex 5-HTExt levels, with increased 5-HTExt levels yielding decreased marble burying and decreased 5-HTExt levels yielding increased marble burying. Hence, we hypothesized that 5-HT deficiency in the Tph2 knockin mice would cause these mice to bury more marbles. Indeed, that is what we observed (Figure 1i), indicating that the observed frontal 5-HTExt decrement in the knockin mice has direct functional sequelae. Conversely, acutely increasing 5-HTExt levels by treatment with the SSRI escitalopram (10 mg kg−1, i.p.) or the 5-HT precursor 5-HTP (50 mg kg−1, i.p.) robustly decreased marble burying in both WT and Tph2 knockin mice (Supplementary Figure S2). Similar to data obtained from adult mice (Figure 1j)31 tissue levels of 5-HT and 5-HIAA were markedly reduced in frontal cortex and hippocampus from neonatal mice (P2; Figure 1k). Thus, endogenous 5-HT neurotransmission is reduced, but not abolished, in the Tph2 knockin mice and this state appears to be present from birth.
Figure 1.
Functional 5-hydroxytryptamine (5-HT) deficiency in tryptophan hydroxylase 2 (Tph2) knockin mice. (a–d) Microdialysis, frontal cortex. (e–h) Microdialysis, hippocampus. (a, e) Probe location in the frontal cortex and hippocampus. Vertical black bar indicates probe membrane location. (Image modified from http://www.brain-map.org). (b, f) Basal extracellular 5-HT (5-HTExt) at noon and midnight (N = 9–10). (c, g) Net 5-HTExt release stimulated by reverse dialysis of 100 mM K+ via the probe (N = 8–10). (d, h) Net 5-HTExt release stimulated by the selective serotonin reuptake inhibitor (SSRI) escitalopram (10 mg/kg, i.p.) (N = 11–18). (i) Marbles buried in 30 min (N = 8–9). (k) Adult (12 weeks) tissue levels of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) (N = 7–8). (j) Neonatal (P2) tissue levels of 5-HT and 5-HIAA (N = 6–9). Data represent means±s.e.m. *P < 0.05 WT vs Tph2 knockin. (b–d, f–h) Repeated-measures (RM)-analysis of variance (ANOVA) followed by Bonferroni post hoc test. (i–k) Student’s t-test.
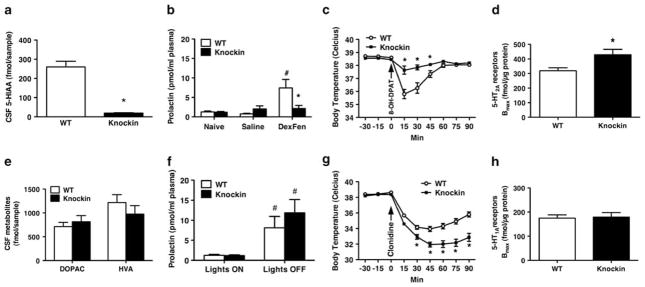
To determine whether chronic endogenous 5-HTExt deficiency is reflected in reduced CSF 5-HIAA, we collected CSF by microdialysis and found that Tph2 knockin mice have CSF 5-HIAA levels < 10% of WT mice (Figure 2a). The size of this decrement is within the range reported in suicidal individuals.44 In contrast, CSF levels of the dopamine metabolites 3,4-dihydroxyphenylacetic acid and homovanillic acid were unchanged in the knockin mice (Figure 2e). Next, we injected WT and knockin mice with saline or dexfenfluramine (the active fenfluramine enantiomer) and collected plasma 60 min later. In WT mice dexfenfluramine robustly increased plasma prolactin while no changes were seen in Tph2 knockin mice (Figure 2b). A similarly blunted prolactin response to fenfluramine has been reported in for instance suicidal depression inpatients.45 There were no genotype differences in plasma prolactin in naïve or saline treated mice (Figure 2b). Similarly, diurnal trough and peak levels of prolactin were unchanged in Tph2 knockin mice (Figure 2f), attesting to normal diurnal prolactin secretion. Plasma corticosterone at diurnal trough and peak or following dexfenfluramine administration did not differ between genotypes (Supplementary Figure S3). Next, we investigated the hypothermic response to the 5-HT1AR agonist 8-OH-DPAT. In the Tph2 knockin mice, the core body temperature drop was markedly attenuated compared with WT (Figure 2c). These data mirror clinical studies reporting markedly attenuated 5-HT1AR-induced hypothermic responses in unmedicated depression inpatients.17,18 In contrast, the hypothermic response to the α2 receptor agonist clonidine was preserved and even enhanced in the Tph2 knockin mice (Figure 2g). The hyperthermic response to rectal probe insertion (stress-induced hyperthermia46) was likewise preserved (Supplementary Figure S4), further demonstrating that thermo-regulation is not generally impaired in the Tph2 knockin mice. Next, we investigated 5-HT2AR levels in the frontal cortex using radioligand saturation binding and found a 23% increase in the Tph2 knockin mice (Figure 2d). A frontal cortex 5-HT2AR increase of similar magnitude has been reported postmortem in suicide victims.20 A general upregulation of 5-HT receptors in the Tph2 knockin mice did not seem to occur because 5-HT1AR levels were unchanged in the hippocampus (Figures 2h and 3e) as well as in the dorsal raphe and hypothalamus (Figure 3e).
Figure 2.
Alterations in putative 5-hydroxytryptamine (5-HT) biomarkers reported in depression and suicidality are recapitulated in tryptophan hydroxylase 2 (Tph2) knockin mice. (a) Tph2 knockin mice have decreased cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA) (N = 5–6), (b) a blunted prolactin response to dexfenfluramine (DexFen, 10 mg kg−1, i.p., N = 6–9), (c) an attenuated hypothermic response to the 5-HT1A receptor (5-HT1AR) agonist 8-OH-DPAT (1 mg kg−1, i.p., N = 10) and (d) increased frontal cortex 5-HT2A receptors (5-HT2ARs) (N = 7). On the other hand, (e) Tph2 knockin mice have WT levels of CSF 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) and of (f) plasma prolactin. (g) Tph2 knockin mice displayed a more pronounced hypothermic response to the α2R agonist clonidine compared with WT. (h) Tph2 mice have WT levels of 5-HT1ARs in the hippocampus. Data represent means±s.e.m. (a, d, e, h) Student’s t-test. (b, f) Two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. (c, g) Repeated-measures (RM)-ANOVA followed by Bonferroni post hoc test. *P < 0.05 WT vs Tph2 knockin. #P < 0.05 WT saline vs WT dexfenfluramine.
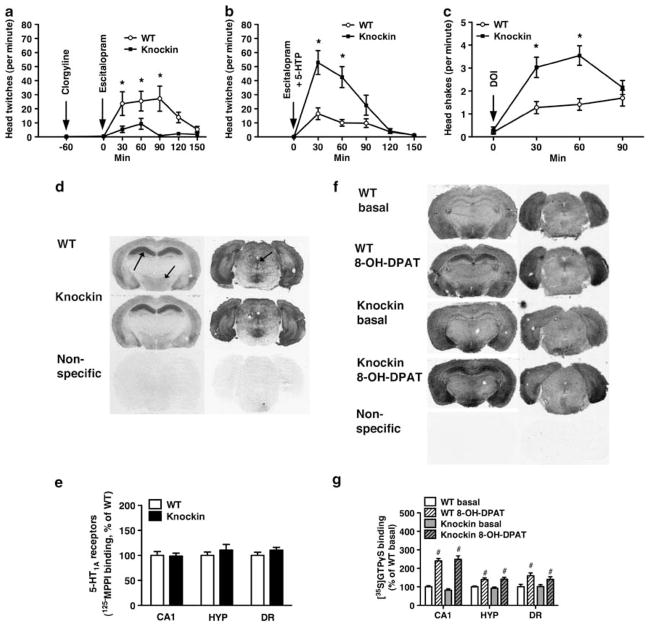
Figure 3.
5-Hydroxytryptamine2A receptors (5-HT2AR), but not 5-HT1A receptor (5-HT1AR), function is altered in tryptophan hydroxylase 2 (Tph2) knockin mice. (a) Decreased head-twitches induced by administration of clorgyline (10 mg kg−1, s.c.) 60 min before escitalopram (10 mg kg−1, i.p.), a Tph2 dependent condition, in Tph2 knockin mice (N = 10). (b) Increased head-twitches induced by co-administration of 5-hydroxytryptophan (5-HTP) (100 mg kg−1, s.c.) and escitalopram (10 mg kg, i.p.), a Tph2 independent condition, in Tph2 knockin mice (N = 10). (c) Increased head shakes induced by the 5-HT2A/CR agonist DOI (3 mg kg−1, i.p.), a Tph2 independent condition, in Tph2 knockin mice (N = 30–33). (d) Representative autoradiograms and (e) quantitation of [125I]p-MPPI binding to 5-HT1ARs in the CA1 of the hippocampus (left arrow), hypothalamus (HYP, middle arrow) and dorsal raphe (DR, right arrow) (N = 8–10). (f) Representative autoradiograms and (g) quantitation of basal and 8-OH-DPAT-stimulated [35S]GTPγS binding in CA1, hypothalamus and dorsal raphe (N = 7–10). (a–c) Repeated-measures (RM)-analysis of variance (ANOVA) followed by Bonferroni post hoc test. (d–g) Student’s t-test. *P < 0.05, WT vs Tph2 knockin. #P < 0.05, basal vs 8-OH-DPAT treated, within genotype.
Supranormal 5-HTExt can elicit quantifiable 5-HT syndrome-like behaviors in rodents, including 5-HT2AR-dependent head-twitches and head-shakes.37 Co-administration of the MAO A inhibitor clorgyline and the SSRI escitalopram simultaneously abolishes 5-HT degradation and active extracellular clearance (passive clearance via diffusion remains). This condition presumably creates a state where 5-HTExt levels and 5-HT receptor occupancy more directly reflect Tph2 activity. Hence, as would be predicted given the reduced Tph2 activity in the knockin mice,31 the head-twitch response to clorgyline + escitalopram in Tph2 knockin mice was markedly attenuated (Figure 3a). Interestingly, however, when Tph2 was bypassed by administering its enzymatic product, the 5-HT precursor 5-HTP, together with escitalopram the head-twitch response was markedly enhanced in the Tph2 knockin mice (Figure 3b). The latter finding signifies increased frontal cortex 5-HT2AR function in the Tph2 knockin mice, in accord with the increased frontal cortex 5-HT2AR levels (Figure 2d). This notion was further corroborated by the observation that the Tph2 knock-in mice displayed exaggerated head-shake responses after injection of DOI, a direct 5-HT2A/2CR agonist (Figure 3c).
Somatic 5-HT1ARs in the dorsal raphe have a key role in negative feedback control of 5-HT release47 and hippocampal postsynaptic 5-HT1ARs may be important for SSRI antidepressant responses.48 Further, evidence implicates the hypothalamus as the locus mediating 5-HT1AR agonist hypothermia.13 Conceivably, 5-HT1AR signaling in these regions could be compensatorily altered in the Tph2 knockin mice. In fact, the reduced 5-HT1AR agonist hypothermic response in depression has been suggested to be caused by impaired 5-HT1AR function.49 We therefore assessed regional 5-HT1AR levels and G-protein coupling by parallel [125I]p-MPPI radioligand and 5-HT1AR agonist-stimulated [35S]GTPγS binding auto-radiography, respectively, in the dorsal raphe, hippocampal CA1 region and the hypothalamus. We found that Tph2 knockin mice displayed WT levels of 5-HT1ARs and G-protein coupling in all three regions (Figures 3d and e). This indicates no compensations in 5-HT1AR function in the Tph2 knockin mice. Similarly, neither SERT levels (Supplementary Figure S5) nor MAO mRNA expression or enzymatic activity in the frontal cortex or hippocampus (Supplementary Figure S6) were changed in the Tph2 knockin mice.
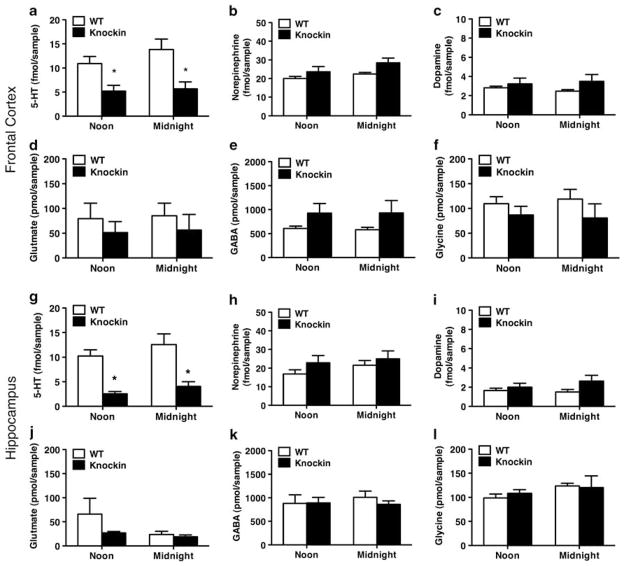
Finally, extensive crosstalk between 5-HT and other monoamine and amino acid neurotransmitters have been previously reported.50–52 It could therefore be hypothesized that 5-HT deficiency would affect extracellular levels of monoamine and amino acid neurotransmitters. We used the sensitive SymDAQ HPLC-tandem-mass spectrometry technology53 that allows the simultaneous determination of 5-HTExt, dopamineExt, noradrenalineExt, GABAExt, glycineExt and glutamateExt in microdialysates from frontal cortex and hippocampus. We replicated the marked decrease in 5-HTExt at noon and midnight in the Tph2 knockin mice (Figures 4a and g) determined using HPLC-EC (Figures 1b and f). Remarkably, however, no other neurotransmitter assessed was significantly altered, at either time point or brain region (Figures 4b–f and h–l). This indicates that at least under basal conditions 5-HT deficiency has no major effects on extracellular homeostasis of the monoamines and amino acid neurotransmitters assessed. Still, the enhanced hypothermic response to clonidine in the Tph2 knockin mice seems to suggest that 5-HT deficiency leads to changes in some noradrenergic responses.
Figure 4.
Basal extracellular levels of dopamine, noradrenaline and amino acid neurotransmitters are unchanged in tryptophan hydroxylase 2 (Tph2) knockin mice. (a–f) Frontal cortex (N = 7–8). (g–l) Hippocampus (N = 9–10). (a, g) Extracellular 5-hydroxytryptamine (5-HTExt) was decreased in Tph2 knockin mice. No significant genotype differences were found for any of the other assessed neurotransmitters (b–f, h–l). Repeated-measures (RM)- analysis of variance (ANOVA) followed by Bonferroni post hoc test. *P < 0.05 WT vs Tph2 knockin.
Discussion
The present report contains two major findings. First, we demonstrate for the first time that a polymorphism in the tph2 gene affects 5-HT neurotransmission, as assessed by 5-HTExt. This finding is important and could extrapolate to other tph2 polymorphisms. Although the human R441H analogue of the mouse R439H polymorphism is probably rare54 a number of distinct polymorphisms in tph2 have been associated with psychopathology, including depression.55–61 We found no evidence of compensatory changes in SERT, MAO and somatic 5-HT1ARs, major regulatory elements of 5-HT neurotransmission. Our data therefore suggest that loss-of-function mutations in Tph2 may not be significantly offset by homeostatic mechanisms and that brain 5-HT neurotransmission is susceptible to impaired Tph2 function. Studying hypomorphic murine models, particularly when induced by introduction of naturally occurring polymorphisms, may have an advantage over knock-out approaches because a level of functionality is preserved. Arguably, this provides a more naturalistic deficit model with greater translational potential. Although the 5-HT deficiency in this study was a consequence of Tph2 hypofunction, the current findings could potentially extrapolate to 5-HT deficiency stemming from a variety of primary causes, for instance reduced brain uptake of tryptophan, storage of 5-HT or decreased 5-HT neuronal firing.
A second, and perhaps even more salient finding, was the observation that chronic, endogenous brain 5-HT deficiency results in reductions in CSF 5-HIAA, fenfluramine-induced plasma prolactin and 5-HT1AR agonist-induced hypothermia in conjunction with increased frontal cortex 5-HT2ARs. These findings in mice closely parallel findings in depressed humans. Although low CSF 5-HIAA and a blunted prolactin response to fenfluramine have long been suggested to reflect reduced central 5-HT levels4,6 until now these notions had never actually been directly substantiated. On the other hand, the blunted 5-HT1AR agonist-induced hypothermia and increased frontal cortex 5-HT2ARs have rather been ascribed to ‘abnormalities’ in 5-HT receptors in depression.20,49 We demonstrate here that in fact also the two latter aberrations can arise as a result of chronic functional 5-HT deficiency. Thus, our findings provide hitherto unavailable cause-effect evidence for all four parameters as bona fide biomarkers of central 5-HT function. Importantly, the findings corroborate the long-standing hypothesis of a central 5-HT deficit in depressive disorders, particularly when suicidality is present.4–7,62
The most parsimonious explanations for the reduced CSF 5-HIAA and blunted fenfluramine-induced prolactin response in the Tph2 knockin mice relates directly to the low 5-HT brain levels. Decreased 5-HT synthesis would yield low 5-HIAA formation, which subsequently manifests as low CSF 5-HIAA. Decreased 5-HT storage would result in blunted fenfluramine-induced 5-HT release and subsequently reduced 5-HT-mediated prolactin secretion by pituitary lactotrophs, likely mediated by hypothalamic oxytonergic and vasoactive intestinal peptidergic projection neurons from the hypothalamus.16 At first glance, the blunted hypothermic response to the 5-HT1AR agonist 8-OH-DPAT in the 5-HT deficient Tph2 knockin mice appears paradoxical. Specifically, a blunted hypothermic response to a 5-HT1AR agonist resulting from enhancement of 5-HTExt levels via genetic or pharmacological means is well documented.23,24 Downregulation of presynaptic 5-HT1AR function seems unlikely because we found no changes in 5-HT1AR levels or G-protein-coupling. This seeming lack of adaptation is hardly surprising since micro-dialysis studies using 5-HT1AR antagonists indicate that there is little inhibitory signaling via presynaptic 5-HT1ARs at baseline 5-HTExt levels.63,64 Still, we cannot completely rule out 5-HT1AR signaling adaptations downstream of receptor expression and G-protein coupling. One report presented evidence that acute modulations in hypothalamic 5-HTExt levels regulate body temperature with a direct, bidirectional relationship.13 However, another report did not find this exact relationship.65 In turn, somatic 5-HT1AR autoreceptors in the raphe area appears crucial in mediating the hypothermic response to 5-HT1AR agonists66,67 as well as the apparently pan-CNS decrease in 5-HTExt induced by such ligands.25,68 It can therefore reasonably be hypothesized that decreased baseline 5-HTExt levels could blunt the hypothermic response to a 5-HT1AR agonist in the absence of any change in 5-HT1AR function, namely by limiting the potential downward amplitude in Δ5-HTExt. Thus, low baseline 5-HTExt levels per se could blunt the hypothermic response to a 5-HT1AR agonist, though via a distinct mechanism than high baseline 5-HTExt levels. Still, further examinations will be needed to delineate why low 5-HTExt levels cause blunted 5-HT1AR-agonist hypothermia. Both 5-HT2ARs69,70 and 5-HT1ARs71 internalize upon ligand binding.69,70 We found frontal cortex 5-HT2ARs, but not 5-HT1ARs in several regions, upregulated in the Tph2 knockin mice. A tentative explanation for these differential consequences of 5-HT deficiency on different receptor populations could pertain to differential sensitivity in terms of receptor turnover to baseline levels of 5-HTExt. This remains speculative, though. Moreover, for instance 5-HT2ARs beyond those in the frontal cortex may respond differently to changes in ambient 5-HTExt levels. Although chronic treatment with SSRIs can dramatically reduce brain SERT levels, MAO inhibitors do not have this effect.72 This indicates that while SERT downregulates in response to SSRI binding ambient 5-HTExt levels have little influence on SERT levels. Consistent with this notion, we found no change in SERT levels in the frontal cortex and hippocampus of Tph2 knockin mice.
Several clinically efficacious antidepressants are antagonists of 5-HT2ARs.73 Further, atypical antipsychotics with 5-HT2AR antagonistic activity are effective as add-on to SSRIs in many treatment-resistant patients.74 It is therefore intriguing that 5-HT2AR levels and function are increased in the Tph2 knockin mice as well as in at least some depression populations.20,21 It could be cautiously speculated that 5-HT2AR hyperfunction in the frontal cortex has a role in depression symptomatology.
The depression-related changes in 5-HT biomarkers reported here were found in Tph2 knockin mice that still maintain 25–40% of normal 5-HTExt levels. It would be relevant to extend our findings by investigating whether a similar or even more dramatic 5-HT biomarker phenotype might be present in Tph2 or Pet1 knockout mice that are almost completely devoid of CNS 5-HT.75,76 Future studies clarifying the mechanisms for 5-HT biomarker and behavioral31 changes in the Tph2 knockin mouse, or similar models of 5-HT deficiency, may contribute to elucidating the neurobiology of affective disorders. In turn, such investigations could ultimately point toward novel therapeutic targets. Further, 5-HT biomarkers may have utility in a clinical research setting, if not in general practice. Assessing 5-HT biomarkers, several of which are reasonably non-invasive, could assist in determining whether sub-populations of psychiatric patients could selectively benefit from 5-HT augmentation strategies.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institutes of Health MH79201 and MH60451 (MGC), the NIMH Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (VS). Support from the Lennon Family Foundation to MGC for the initial part of this work is also greatly appreciated. WBS was the recipient of a NRSA postdoctoral fellowship (F32-MH-083404) and BDS is the recipient of a Minority Supplement award from the National Institutes of Health (MH79201-03S1). JPRJ is the grateful recipient of an individual grant from The Lundbeck Foundation of Denmark. We thank Wendy Roberts for skillful technical assistance and Dr Thomas Cremers for advice and making the SymDAQ HPLC-MS technology available. We thank Dr Garth Brown of Perkin-Elmer and Dr Hank F Kung of the University of Pennsylvania for advice and supplying [125I]p-MPPI.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–1264. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- 2.Shaw DM, Camps FE, Eccleston EG. 5-Hydroxytryptamine in the hind-brain of depressive suicides. Br J Psychiatry. 1967;113:1407–1411. doi: 10.1192/bjp.113.505.1407. [DOI] [PubMed] [Google Scholar]
- 3.Lacasse JR, Leo J. Serotonin and depression: a disconnect between the advertisements and the scientific literature. PLoS Med. 2005;2:e392. doi: 10.1371/journal.pmed.0020392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer H. Serotonergic dysfunction in depression. Br J Psychiatry Suppl. 1989;8:25–31. [PubMed] [Google Scholar]
- 5.Asberg M. Neurotransmitters and suicidal behavior. The evidence from cerebrospinal fluid studies. Ann NY Acad Sci. 1997;836:158–181. doi: 10.1111/j.1749-6632.1997.tb52359.x. [DOI] [PubMed] [Google Scholar]
- 6.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 7.Mann JJ, Malone KM, Psych MR, Sweeney JA, Brown RP, Linnoila M, et al. Attempted suicide characteristics and cerebrospinal fluid amine metabolites in depressed inpatients. Neuropsychopharmacology. 1996;15:576–586. doi: 10.1016/S0893-133X(96)00102-9. [DOI] [PubMed] [Google Scholar]
- 8.Fadda F, Cocco S, Stancampiano R. A physiological method to selectively decrease brain serotonin release. Brain Res. 2000;5:219–222. doi: 10.1016/s1385-299x(00)00016-7. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, et al. Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology. 1998;19:26–35. doi: 10.1016/S0893-133X(97)00198-X. [DOI] [PubMed] [Google Scholar]
- 10.Coccaro EF, Kavoussi RJ, Cooper TB, Hauger R. Acute tryptophan depletion attenuates the prolactin response to d-fenfluramine challenge in healthy human subjects. Psychopharmacology. 1998;138:9–15. doi: 10.1007/s002130050639. [DOI] [PubMed] [Google Scholar]
- 11.Kohmoto K, Tsunasawa S, Sakiyama F. Complete amino acid sequence of mouse prolactin. Eur J Biochem. 1984;138:227–237. doi: 10.1111/j.1432-1033.1984.tb07905.x. [DOI] [PubMed] [Google Scholar]
- 12.Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23:91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- 13.Lin MT, Tsay HJ, Su WH, Chueh FY. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol. 1998;274(5 Part 2):R1260–R1267. doi: 10.1152/ajpregu.1998.274.5.R1260. [DOI] [PubMed] [Google Scholar]
- 14.Licht CL, Knudsen GM, Sharp T. Effects of the 5-HT(4) receptor agonist RS67333 and paroxetine on hippocampal extracellular 5-HT levels. Neurosci Lett. 2010;476:58–61. doi: 10.1016/j.neulet.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol. 2008;22(2 Suppl):12–19. doi: 10.1177/0269216307087148. [DOI] [PubMed] [Google Scholar]
- 16.Emiliano AB, Fudge JL. From galactorrhea to osteopenia: rethinking serotonin-prolactin interactions. Neuropsychopharmacology. 2004;29:833–846. doi: 10.1038/sj.npp.1300412. [DOI] [PubMed] [Google Scholar]
- 17.Shapira B, Newman ME, Gelfin Y, Lerer B. Blunted temperature and cortisol responses to ipsapirone in major depression: lack of enhancement by electroconvulsive therapy. Psychoneuroendocrinology. 2000;25:421–438. doi: 10.1016/s0306-4530(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 18.Lesch KP. 5-HT1A receptor responsivity in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:723–733. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- 19.Cowen PJ, Power AC, Ware CJ, Anderson IM. 5-HT1A receptor sensitivity in major depression. A neuroendocrine study with buspirone. Br J Psychiatry. 1994;164:372–379. doi: 10.1192/bjp.164.3.372. [DOI] [PubMed] [Google Scholar]
- 20.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- 21.Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- 22.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther. 1999;291:999–1007. [PubMed] [Google Scholar]
- 24.Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT1A receptor function in normal subjects on clinical doses of fluoxetine: blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20:628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura Y, Akiyama K, Hashimoto S, Kitagawa K, Kawasaki H, Shibata K, et al. Effects of imipramine on extracellular serotonin and noradrenaline concentrations in ACTH-treated rats. Eur J Pharmacol. 2007;566:113–116. doi: 10.1016/j.ejphar.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Ghisi V, Ramsey AJ, Masri B, Gainetdinov RR, Caron MG, Salahpour A. Reduced D2-mediated signaling activity and trans-synaptic upregulation of D1 and D2 dopamine receptors in mice overexpressing the dopamine transporter. Cell Signal. 2009;21:87–94. doi: 10.1016/j.cellsig.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai K, Yokota N, Yamawaki S. Effect of chronic tryptophan depletion on the circadian rhythm of wheel-running activity in rats. Physiol Behav. 1994;55:1005–1013. doi: 10.1016/0031-9384(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 28.Compan V, Segu L, Buhot MC, Daszuta A. Differential effects of serotonin (5-HT) lesions and synthesis blockade on neuropeptide-Y immunoreactivity and 5-HT1A, 5-HT1B/1D and 5-HT2A/2C receptor binding sites in the rat cerebral cortex. Brain Res. 1998;795:264–276. doi: 10.1016/s0006-8993(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 34.Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, et al. AAPS-FDA Workshop White Paper: microdialysis principles, application, and regulatory perspectives. J Clin Pharmacol. 2007;47:589–603. doi: 10.1177/0091270006299091. [DOI] [PubMed] [Google Scholar]
- 35.Rossi DV, Burke TF, McCasland M, Hensler JG. Serotonin-1A receptor function in the dorsal raphe nucleus following chronic administration of the selective serotonin reuptake inhibitor sertraline. J Neurochem. 2008;105:1091–1099. doi: 10.1111/j.1471-4159.2007.05201.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacobsen JP, Nielsen EO, Hummel R, Redrobe JP, Mirza N, Weikop P. Insensitivity of NMRI mice to selective serotonin reuptake inhibitors in the tail suspension test can be reversed by co-treatment with 5-hydroxytryptophan. Psychopharmacology. 2008;199:137–150. doi: 10.1007/s00213-008-1142-7. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawasai O, Arai Y, Satoh SE, Satoh N, Neda M, Hozumi M, et al. Monoamine oxidase and head-twitch response in mice. Mechanisms of alpha-methylated substrate derivatives. Neurotoxicology. 2004;25:223–232. doi: 10.1016/S0161-813X(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 38.Kung MP, Frederick D, Mu M, Zhuang ZP, Kung HF. 4-(2′-Methoxy-phenyl)-1-[2′-(n-2″-pyridinyl)-p-iodobenzamido]-ethyl-piperazine ([125I]p-MPPI) as a new selective radioligand of serotonin-1A sites in rat brain: in vitro binding and autoradiographic studies. J Pharmacol Exp Ther. 1995;272:429–437. [PubMed] [Google Scholar]
- 39.Hensler J, Durgam H. Regulation of 5-HT(1A) receptor-stimulated [35S]-GtpgammaS binding as measured by quantitative autoradiography following chronic agonist administration. Br J Pharmacol. 2001;132:605–611. doi: 10.1038/sj.bjp.0703855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology. 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- 42.Hirano K, Kimura R, Sugimoto Y, Yamada J, Uchida S, Kato Y, et al. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi T, Hayashi E, Shimamura M, Kinoshita M, Murphy NP. Neurochemical responses to antidepressants in the prefrontal cortex of mice and their efficacy in preclinical models of anxiety-like and depression-like behavior: a comparative and correlational study. Psychopharmacology. 2008;197:567–580. doi: 10.1007/s00213-008-1070-6. [DOI] [PubMed] [Google Scholar]
- 44.Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, et al. Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry. 1982;139:741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- 45.Correa H, Duval F, Mokrani M, Bailey P, Tremeau F, Staner L, et al. Prolactin response to D-fenfluramine and suicidal behavior in depressed patients. Psychiatry Res. 2000;93:189–199. doi: 10.1016/s0165-1781(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 46.Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, et al. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–132. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- 47.Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cowen PJ. Psychopharmacology of 5-HT(1A) receptors. Nucl Med Biol. 2000;27:437–439. doi: 10.1016/s0969-8051(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 50.Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- 51.Blier P. Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J Psychiatry Neurosci. 2001;26(Suppl):S3–S10. [PMC free article] [PubMed] [Google Scholar]
- 52.Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 53.Allers KA, Dremencov E, Ceci A, Flik G, Ferger B, Cremers TI, et al. Acute and repeated flibanserin administration in female rats modulates monoamines differentially across brain areas: a Micro-dialysis Study. J Sex Med. 2010;7:1757–1767. doi: 10.1111/j.1743-6109.2010.01763.x. [DOI] [PubMed] [Google Scholar]
- 54.Delorme R, Durand CM, Betancur C, Wagner M, Ruhrmann S, Grabe HJ, et al. No human tryptophan hydroxylase-2 gene R441H mutation in a large cohort of psychiatric patients and control subjects. Biol Psychiatry. 2006;60:202–203. doi: 10.1016/j.biopsych.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, et al. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. 2005;62:1109–1118. doi: 10.1001/archpsyc.62.10.1109. [DOI] [PubMed] [Google Scholar]
- 56.Baehne CG, Ehlis AC, Plichta MM, Conzelmann A, Pauli P, Jacob C, et al. Tph2 gene variants modulate response control processes in adult ADHD patients and healthy individuals. Mol Psychiatry. 2009;14:1032–1039. doi: 10.1038/mp.2008.39. [DOI] [PubMed] [Google Scholar]
- 57.Cichon S, Winge I, Mattheisen M, Georgi A, Karpushova A, Freudenberg J, et al. Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum Mol Genet. 2008;17:87–97. doi: 10.1093/hmg/ddm286. [DOI] [PubMed] [Google Scholar]
- 58.Sheehan K, Lowe N, Kirley A, Mullins C, Fitzgerald M, Gill M, et al. Tryptophan hydroxylase 2 (TPH2) gene variants associated with ADHD. Mol Psychiatry. 2005;10:944–949. doi: 10.1038/sj.mp.4001698. [DOI] [PubMed] [Google Scholar]
- 59.Tsai SJ, Hong CJ, Liou YJ, Yu YW, Chen TJ, Hou SJ, et al. Tryptophan hydroxylase 2 gene is associated with major depression and antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:637–641. doi: 10.1016/j.pnpbp.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 60.Van Den Bogaert A, Sleegers K, De Zutter S, Heyrman L, Norrback KF, Adolfsson R, et al. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and bipolar disorder in a Northern Swedish, isolated population. Arch Gen Psychiatry. 2006;63:1103–1110. doi: 10.1001/archpsyc.63.10.1103. [DOI] [PubMed] [Google Scholar]
- 61.Tzvetkov MV, Brockmoller J, Roots I, Kirchheiner J. Common genetic variations in human brain-specific tryptophan hydroxylase-2 and response to antidepressant treatment. Pharmacogenet Genomics. 2008;18:495–506. doi: 10.1097/FPC.0b013e3282fb02cb. [DOI] [PubMed] [Google Scholar]
- 62.Mann JJ, McBride PA, Malone KM, DeMeo M, Keilp J. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 63.Romero L, Hervas I, Artigas F. The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neurosci Lett. 1996;219:123–126. doi: 10.1016/s0304-3940(96)13199-2. [DOI] [PubMed] [Google Scholar]
- 64.Hjorth S, Westlin D, Bengtsson HJ. WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto)receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology. 1997;36:461–465. doi: 10.1016/s0028-3908(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 65.Ishiwata T, Saito T, Hasegawa H, Yazawa T, Otokawa M, Aihara Y. Changes of body temperature and extracellular serotonin level in the preoptic area and anterior hypothalamus after thermal or serotonergic pharmacological stimulation of freely moving rats. Life Sci. 2004;75:2665–2675. doi: 10.1016/j.lfs.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 66.Hillegaart V. Effects of local application of 5-HT and 8-OH-DPAT into the dorsal and median raphe nuclei on core temperature in the rat. Psychopharmacology. 1991;103:291–296. doi: 10.1007/BF02244281. [DOI] [PubMed] [Google Scholar]
- 67.Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hjorth S, Sharp T. Effect of the 5-HT1A receptor agonist 8-OH-DPAT on the release of 5-HT in dorsal and median raphe-innervated rat brain regions as measured by in vivo microdialysis. Life Sci. 1991;48:1779–1786. doi: 10.1016/0024-3205(91)90216-x. [DOI] [PubMed] [Google Scholar]
- 69.Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berry SA, Shah MC, Khan N, Roth BL. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol. 1996;50 :306–313. [PubMed] [Google Scholar]
- 71.Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17(Suppl 1):2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 74.Blier P, Szabo ST. Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J Clin Psychiatry. 2005;66(Suppl 8):30–40. [PubMed] [Google Scholar]
- 75.Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, et al. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.