A link between the autonomic nervous system and sudden cardiac death (SCD) is well recognized (1,2). However, the mechanisms underlying this relationship remain incompletely understood. Proarrhythmic changes occur at the level of the myocardium and presumably at higher levels of the cardiac neuraxis. Intramyocardial nerve sprouts in border zones of infarcts and remote normal tissue have been observed in animals and humans and have been correlated with ventricular arrhythmias (3–5). In animal models of ischemia-reperfusion injury, neural remodeling has been associated with expression and release neurotrophic agents including nerve growth factor, which may be transported to the stellate ganglia via nerve tracts (6,7). Several therapeutic strategies for reducing arrhythmias by reducing sympathetic output to the heart have been proposed. These have been both pharmacologic and nonpharmacologic (neuraxial modulation).
There are limited data on the actual remodeling of neuronal structures that give rise to nerve fibers that are destined for the heart (specifically, the stellate ganglia). Further, direct physical evidence of neural remodeling and increased sympathetic nerve activity is also limited.
In this issue of the Journal, Han et al. (8) describe electroanatomic remodeling of the left stellate ganglion (LSG) in a canine model of ischemia-reperfusion injury. They observed increased stellate ganglion nerve activity (SGNA) immediately after myocardial infarction, which dramatically continued over the following 8 weeks. Increased SGNA was associated with intramyocardial nerve sprouts and increased neuronal size and synaptic density in the LSG, as well as the right stellate ganglion (RSG). These results provide a possible mechanistic link between neural remodeling (nerve sprouting within the myocardium and remodeling of the stellate ganglion) and ventricular arrhythmias. Although SCD was not observed in this study, the group previously showed that sympathetic nerve discharges tended to precede ventricular arrhythmias (3,4,9).
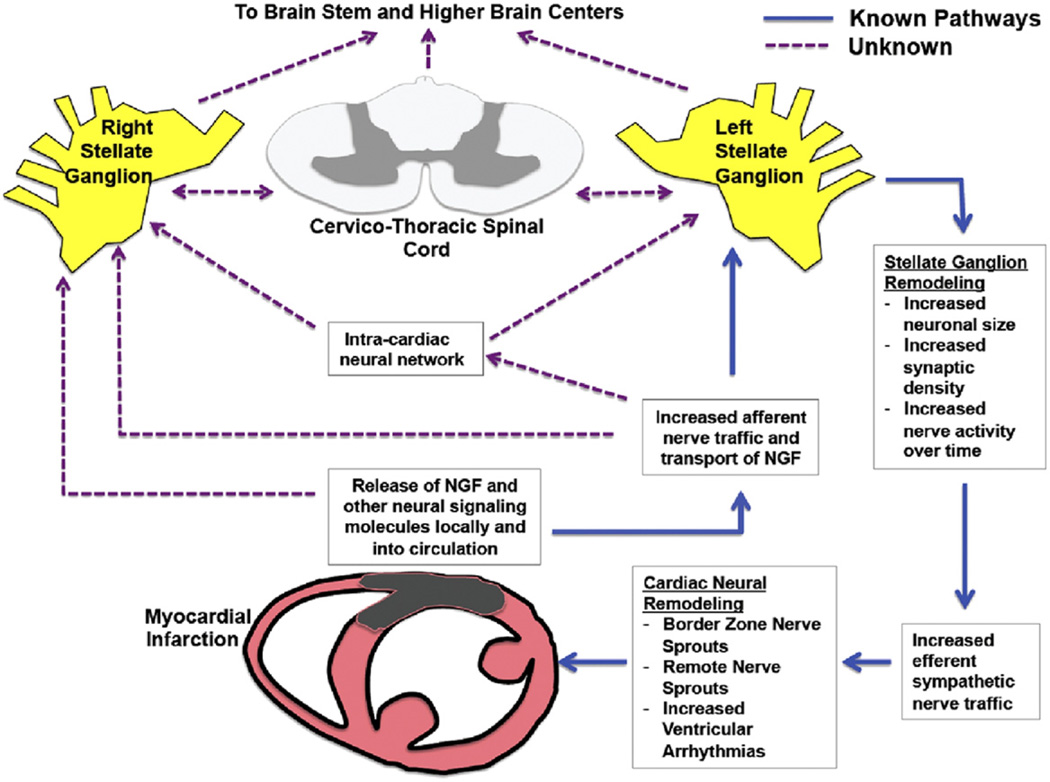
This work is important on multiple fronts. For the first time, there is direct physiologic evidence that neural remodeling within the stellate ganglia is associated with increased SGNA. This point had previously only been inferred. The increase in size and density of neurons within the stellate ganglia indicate that the adverse remodeling caused by ischemia extends beyond the cardiac borders. The observed increase in nerve activity is likely a combination of afferent and efferent neural signals, suggesting an ongoing deleterious communication between the heart and its neural axis (Fig. 1). That these signals persist for 8 weeks (and likely beyond) may explain why ventricular arrhythmias develop in patients well beyond the ischemic episode, presumably after some threshold of sympathovagal imbalance is crossed. Whether these signals are able to affect regions of the nervous system higher than the stellate ganglion represents an important question for investigation (Fig. 1) because behavioral changes have been associated with myocardial infarctions (MIs) in humans.
Figure 1. Cardiac-Neuraxial Pathways of Sympathetic Nerve Signaling and Remodeling After Myocardial Infarction.
Major elements of the cardiac neural axis with known and unknown directions of nerve traffic after myocardial infarction. Intramyocardial nerve injury signals reach the cardiac neural axis via direct neural impulses, nerve growth factor (NGF) (or other signaling molecules) transported via axons or circulatory system. These afferent signals reach the left (and right) stellate ganglion, resulting in anatomic remodeling within the stellates and increased efferent nerve signals back to the heart via intracardiac neurons and the cardiac neuronal network. It is unknown whether the spinal cord and higher brain centers also participate in this process and remodel after significant cardiac injury.
These data also highlight the value of therapies such as beta-blockers after an MI because increased SGNA occurs, which may result in greater intramyocardial catecholamine release, compared with normal conditions. Further, it underscores the importance of continued beta-blocker therapy because remodeling of neural structures will continue to adversely affect sympathovagal imbalance. Arrhythmias seen in the chronic phase, well after the ischemic event, could be related to such adverse neural remodeling. Deleterious neural remodeling in the peri-infarct region likely contributes to the electrophysiologic heterogeneity of the myocardium, altering excitability and creating a substrate that facilitates ventricular arrhythmias. Heart failure is known to induce intramyocardial transdifferentiation of sympathetic nerves to cholinergic (10), a process that may create electrophysiologic heterogeneity leading to arrhythmias. The present study highlights changes both at the level of the organ and at the level of the ganglion that controls sympathetic fibers destined for the heart.
The pathophysiologic changes in the stellate ganglion support the rationale for cardiac sympathetic denervation in the treatment of ventricular arrhythmias refractory to beta-blockade and other conventional therapies. Cardiac sympathetic denervation is increasingly applied for managing ventricular arrhythmias when all other therapies fail (11,12). The present study also provides direct evidence linking sympathovagal imbalance to heart rate variability (HRV). HRV is routinely used as parameter to assess vagosympathetic tone. The association between HRV and sympathovagal imbalance has not previously been made in a model of long-term direct ambulatory recordings of both the stellate ganglion and vagus nerve.
A major limitation of the present study is the lack of arrhythmias beyond 2 days in this chronic model of ischemia-reperfusion, despite significant neural remodeling in both the stellate ganglion and left ventricular myocardium. Had SCD been observed in this model, it would have provided irrefutable proof of the link between infarct-induced neural remodeling and SCD. One potential explanation for the lack of SCD in this model is the period of follow-up. The SGNA continued to gradually increase of over the following 8 weeks. It is plausible that the critical threshold of sympathovagal imbalance had not been crossed. Another possible explanation is the extent of myocardial damage because the left ventricular ejection fraction remained at 51% two months after the MI.
Another limitation is the lack of association between ventricular tachycardia and increased stellate ganglion nerve traffic. The same group previously showed that high-amplitude spike discharge activity and low-amplitude burst discharge activity (4,9) tend to precede ventricular arrhythmias; therefore, absence of this link in the present study is surprising. The presence of ventricular arrhythmias in this model would have provided direct proof that increased high-amplitude spike discharge activity and low-amplitude burst discharge activity, in the setting of stellate ganglion remodeling and intramyocardial nerve sprouts, result in ventricular arrhythmias. The link between ventricular arrhythmias and stellate ganglion remodeling in this ischemia-reperfusion model is therefore inferred but not proved.
Neural remodeling in both LSG and RSG in response to infarction in the anterior and lateral left ventricle is a striking finding. Early studies on the territory of innervation of nerve fibers originating from the LSG and RSG suggest distinct regions of innervation within the heart, with some areas of overlap (13–15). Anatomic dissections of cardiothoracic neural tracts suggest mixing of sympathetic fibers from both the LSG and RSG, as well as parasympathetic fibers from the vagus nerve (16). Remodeling in both ganglia suggest that fibers from both ganglia innervate the anterolateral left ventricle or possibly indicate evidence of cross-talk between both ganglia via mixed fibers or a systemic release of neurotrophic agents, which then reach multiple components of the cardiac neural axis via the circulatory system (Fig. 1).
The findings in this study underscore the importance of improving our understanding of the cardiac-neuraxis links and its regulatory interaction with the heart. Innervation of the heart exerts profound control on cardiac function, but may trigger malignant ventricular arrhythmias and lead to SCD in both structurally normal and abnormal hearts. The present study supports targeting pathophysiologic neural remodeling after MI in the prevention of SCD. Studies have shown the value of neuraxial modulation in preventing death in a ventricular tachycardia/ventricular fibrillation storm (11,12).
In conclusion, deleterious neural remodeling at the level of the stellate ganglion in response to myocardial injury and the subsequent increase in sympathetic nerve activity may be critical elements in the arrhythmogenicity of sympathetic nerves. However, the direct link between increased SGNA and SCD remains to be seen. The present study is an important step in this direction. Further studies are warranted to improve our understanding of this complex system because autonomic modulation therapies hold promise for cardiac therapeutics.
Acknowledgments
This study is supported by the NHLBI (R01HL084261 to K.S.).
Footnotes
Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology.
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Zipes DP, Barber MJ, Takahashi N, Gilmour RF., Jr Influence of the autonomic nervous system on the genesis of cardiac arrhythmias. Pacing Clin Electrophysiol. 1983;6:1210–1220. doi: 10.1111/j.1540-8159.1983.tb04459.x. [DOI] [PubMed] [Google Scholar]
- 2.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50:404–409. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.cir.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Jung B-C, Tan AY, et al. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5:131–139. doi: 10.1016/j.hrthm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Vracko R, Thorning D, Frederickson RG. Nerve fibers in human myocardial scars. Hum Pathol. 1991;22:138–146. doi: 10.1016/0046-8177(91)90035-n. [DOI] [PubMed] [Google Scholar]
- 6.Oh YS, Jong AY, Kim DT, et al. Spatial distribution of nerve sprouting after myocardial infarction in mice. Heart Rhythm. 2006;3:728–736. doi: 10.1016/j.hrthm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Chen LS, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 8.Han S, Kobayashi K, Joung B, et al. Electroanatomic remodeling of the left stellate ganglion after myocardial infarction. J Am Coll Cardiol. 2012;59:954–961. doi: 10.1016/j.jacc.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa M, Zhou S, Tan A, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Kanazawa H, Ieda M, Kimura K, et al. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J Clin Invest. 2010;120:408–421. doi: 10.1172/JCI39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourke T, Vaseghi M, Michowitz Y, et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation. 2010;121:2255–2262. doi: 10.1161/CIRCULATIONAHA.109.929703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajijola OA, Lellouche N, Bourke T, et al. Bilateral cardiac denervation for the management of electrical storm. J Am Coll Cardiol. 2012;59:91–92. doi: 10.1016/j.jacc.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res. 1966;18:416–428. doi: 10.1161/01.res.18.4.416. [DOI] [PubMed] [Google Scholar]
- 14.Ueda H, Yanai Y, Murao S, et al. Electrocardiographic and vectorcardiographic changes produced by electrical stimulation of the cardiac nerves. Jpn Heart J. 1964;28:359–372. doi: 10.1536/ihj.5.359. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez RJ, Ajijola OA, Zhou W, et al. A new electrocardiographic marker for sympathetic nerve stimulation: modulation of repolarization by stimulation of stellate ganglia. J Electrocardiol. 2011;44:694–699. doi: 10.1016/j.jelectrocard.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol. 2005;209:425–438. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]