Abstract
Wnt/β-catenin signaling initiates taste papilla development in mouse embryos, however, its involvement in taste cell turnover in adult mice has not been explored. Here we used the BATGAL reporter mouse model, which carries an engineered allele in which the LacZ gene is expressed in the presence of activated β-catenin, to determine the responsiveness of adult taste bud cells to canonical Wnt signaling. Double immunostaining with markers of differentiated taste cells revealed that a subset of type I, II and III taste cells express β-galactosidase. Using in situ hybridization, we showed that β-catenin activates the transcription of the LacZ gene mainly in intragemmal basal cells that are immature taste cells, identified by their expression of Sonic Hedgehog (Shh). Finally, we showed that β-catenin activity is significantly reduced in taste buds of 25 week-old mice compared to 10 week-old animals. Our data suggest that Wnt/β-catenin signaling may influence taste cell turnover by regulating cell differentiation. Reduced canonical Wnt signaling in older mice could explain in part the loss of taste sensitivity with aging, implicating a possible deficiency in the rate of taste cell renewal. More investigations are now necessary to understand if and how Wnt signaling regulates adult taste cell turnover.
Keywords: Taste cell, Wnt/β-catenin, Sonic Hedgehog (Shh), cell renewal, BATGAL, aging
Introduction
The sense of taste is responsible for early detection of nutrients in the oral cavity during feeding, and thus helps the body to discriminate beneficial versus noxious foods. The peripheral taste system on the mammalian tongue is composed of taste buds embedded in gustatory papillae, including anterior fungiform, and more posteriorly located foliate and circumvallate papillae. Taste buds, regardless of location, are multicellular onion shaped structures comprising three differentiated taste cell types. Type I cells express the ectoATPase nucleoside triphosphate diphosphohydrolase 2 or NTPdase2 (Bartel et al., 2006), the Glutamate-Aspartate Transporter (GLAST) (Lawton et al., 2000), and have cytoplasmic projections that wrap around other taste cell types (Lawton et al., 2000; Pumplin et al., 1997). Because of these molecular and morphological characteristics type I cells are thought to be glial-like with a supportive function, but they also express functional amiloride-sensitive epithelial sodium channels, i.e., ENaCs, suggesting type I cells may be involved in salt taste detection as well (Vandenbeuch et al., 2008). Type II cells express proteins involved in the detection of sweet, bitter and umami tastants, including the apical receptor proteins Taste Receptor 1 (T1R or Tas1R) (Kim et al., 2003b; Montmayeur et al., 2001) and Taste Receptor 2 (T2R or Tas2R) (Adler et al., 2000; Matsunami et al., 2000), and intracellular transduction proteins such as the G-protein α-gustducin (Boughter et al., 1997; Yang et al., 2000b), phospholipase C β2 (PLCβ2) (Clapp et al., 2004) and type 3 inositol-triphosphate receptor (IP3R3) (Clapp et al., 2001). Type III cells are sour detectors (Huang et al., 2006; Huang et al., 2008), and are the only taste cell type that establishes conventional synaptic connections with afferent nerve fibers (Clapp et al., 2004; Yang et al., 2000a; Yang et al., 2004; Yee et al., 2003). They express proteins involved in synaptic function, including a synaptic membrane protein SNAP25 (Yang et al., 2000a), Neural Cell Adhesion Molecule (NCAM) (Nelson and Finger, 1993; Takeda et al., 1992), and the neurotransmitter serotonin (Huang et al., 2005; Yee et al., 2001).
During adult life, renewal of the three differentiated cell types within rat taste buds is thought to occur on average every 10 days (Beidler and Smallman, 1965; Farbman, 1980); however, how this process of cell turnover is regulated at the cellular and molecular levels has not been defined. The taste progenitor pool includes proliferating perigemmal basal cells, i.e., cells residing at the basement membrane and outside of taste buds proper (Hamamichi et al., 2006; Miura et al., 2004; Nguyen and Barlow, 2010), as well as morphologically defined edge cells, which flank taste buds at more apical positions (Farbman, 1965). These proliferative cells give rise to immature taste cells, which are postmitotic and enter taste buds within 12–24 hours of cell birth (Asano-Miyoshi et al., 2008; Hamamichi et al., 2006; Miura et al., 2004; Nguyen and Barlow, 2010), and differentiate within an additional 2–4 days post-birth (Cho et al., 1998; Hamamichi et al., 2006; Oike et al., 2006).
Several signaling pathways known to regulate embryonic development of taste buds are likely to be involved in regulation of taste cell renewal in adults. For example, Shh, a secreted morphogen, is expressed in embryonic taste bud progenitors (Hall et al., 1999; Jung et al., 1999; Thirumangalathu and Barlow, 2009; Thirumangalathu et al., 2009), and in vitro, negatively regulates initial fungiform papilla development (Hall et al., 2003; Mistretta et al., 2003). Shh is also expressed in the taste buds of adult mice, specifically in intragemmal basal cells (also called type IV cells) (Miura et al., 2001). These Shh-expressing cells are thought to represent immature taste cells in the process of differentiating once they have entered the taste bud (Miura and Barlow, 2010; Miura et al., 2004), raising the question of the function of Shh in taste bud cell renewal. Similarly, several other factors known to regulate embryonic taste system development, such as Bone Morphogenetic Proteins or BMPs (Beites et al., 2009; Jung et al., 1999; Kim et al., 2003a; Zhou et al., 2006), Sox2 (Okubo et al., 2006) and the Notch pathway (Ota et al., 2009), are also thought to be involved in taste cell renewal in adult mice based on gene expression patterns (Kusakabe et al., 2002; Miura et al., 2005; Nguyen and Barlow, 2010; Okubo et al., 2009; Seta et al., 2003; Seta et al., 2006; Suzuki, 2008).
Wnt/β-catenin signaling is a key pathway in development, adult tissue homeostasis and disease (Clevers, 2006), e.g. controlling stem cell proliferation and differentiation in the nervous system (Chenn and Walsh, 2002; Ding et al., 2003; Galceran et al., 2000; Hirabayashi et al., 2004; Zhou et al., 2004) and in hair follicles (Huelsken et al., 2001; Lowry et al., 2005; Van Mater et al., 2003; Watt and Jensen, 2009; Watt et al., 2006). Wnt/β-catenin is also required for embryonic taste bud development (Iwatsuki et al., 2007; Liu et al., 2007), however, its involvement in taste bud cell renewal in adult mice has not been explored.
Our present work investigates the pattern of Wnt/β-catenin signaling in taste buds of the fungiform and circumvallate papillae of adult mice to determine how this pathway may regulate taste bud renewal. We used the BATGAL mouse line (Maretto et al., 2003) that expresses β-galactosidase in the presence of activated β-catenin and thus reports activation of canonical Wnt signaling. We find that the BATGAL allele reveals Wnt/β-catenin activity in a subset of each of the 3 differentiated taste cell types, as well as in immature Shh-expressing cells. Moreover, while Wnt/β-catenin activity is robust in 10 week-old mice, in older (25–30 week-old) animals we find BATGAL reporting is significantly reduced.
Results
Because Wnt/β-catenin signaling is involved in cell proliferation and differentiation during normal development in a variety of tissues (Clevers, 2006), including embryonic taste epithelium (Iwatsuki et al., 2007; Liu et al., 2007), we investigated whether the pattern of activation of this pathway is consistent with a function in taste cell turnover in adult mice. As reported by others, BATGAL-driven β-galactosidase is present in a subset of cells in taste buds in fungiform papillae (Fig. 1b; Schneider et al., 2010), as well as in circumvallate taste epithelium (Fig. 1a).
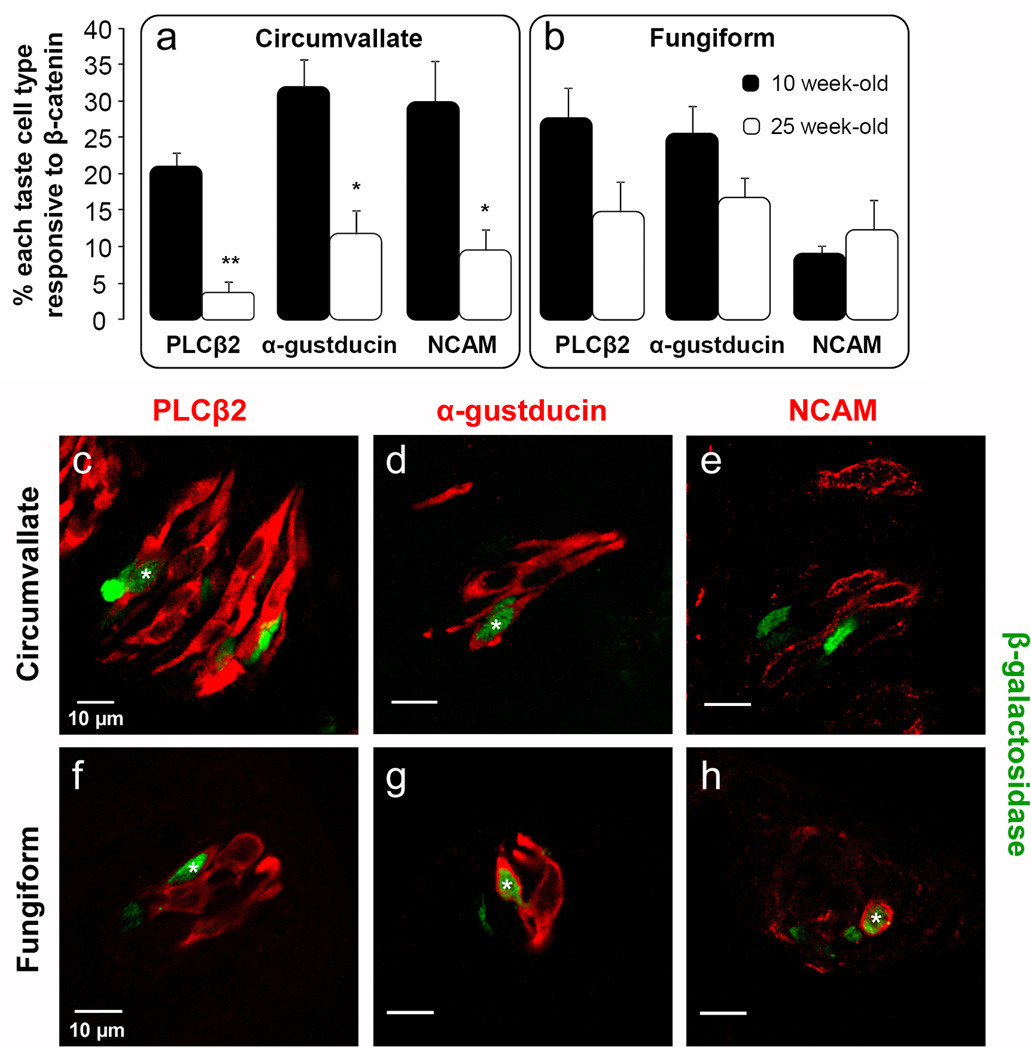
Fig. 1. A subset of each fusiform taste cell type is responsive to β-catenin in both the circumvallate and fungiform papillae of adult mice.
Double labeled cells are identified with a white asterisk on 0.75 µm optical section micrographs of co-labeling of β-galactosidase (green nuclei) and cell type markers (Type I NTPdase2; Type II PLCβ2 and α-gustducin; and Type III NCAM, red) in (a) the circumvallate papilla and (b) fungiform papillae from 10 week-old BATGAL reporter mice. Data express the percentage of β-catenin responsive cells within each taste cell type per taste bud section. N = 3 mice. Scale bars = 10 µm in all panels.
Circumvallate Taste Buds
To define which taste cell type(s) are Wnt responsive, double immunostaining for β-galactosidase and antisera for markers of each of the three specific taste cell types was performed on circumvallate sections of 10 week-old BATGAL mice. Using antiserum against the ecto-ATPase, NTPdase2 (Bartel et al., 2006), we found examples of NTPDase2-immunoreactive (IR) type I cells with β-galactosidase-IR nuclei (example shown in Fig. 1a, white asterisk). However, because NTPdase2 localizes predominantly to cell membranes and type I cells have extensive and complex cellular processes (Lawton et al., 2000; Pumplin et al., 1997), and because β-galactosidase is targeted to the nucleus in BATGAL mice (Maretto et al., 2003), precise quantification of double-labeled type I cells was not possible.
βeta-galactosidase-IR was also readily detected in α-gustducin- and PLCβ2- (type II), and NCAM- (type III) immunopositive cells (Fig. 1a). These markers, along with the more simple fusiform profiles of type II and III cells (Murray, 1986; Pumplin et al., 1997; Yoshie et al., 1990), allowed us to quantify what proportion of each of these taste cell types is Wnt-responsive. Specifically, 21% of PLCβ2 positive cells, 32% of α-gustducin positive cells, and 30% of NCAM positive cells per circumvallate taste bud section also had β-galactosidase-IR nuclei (Fig. 1a). Thus a subset of each of the 3 fusiform taste cell types within circumvallate taste buds is Wnt/β-catenin responsive.
Fungiform Taste Buds
As several recent reports have shown significant differences in gene product expression between circumvallate and fungiform taste buds (Kim et al., 2003b; Nguyen and Barlow, 2010; Stone et al., 2007; Tizzano et al., 2008), we also examined the extent and pattern of BATGAL-driven β-galactosidase expression in fungiform taste buds. We show here that all 3 differentiated taste cell types are also Wnt/β-catenin responsive in fungiform taste buds (Fig. 1b). However, a significantly smaller proportion of fungiform NCAM-IR type III cells were BATGAL positive (9%) compared to type III cells double labeled in circumvallate taste buds (Fig. 1b; Student’s t-test, n=3 mice, p<0.05). It is noteworthy that we did not detect β-galactosidase-IR in extragemmal cells outside taste buds in either the circumvallate or fungiform papillae (Fig. 1b). In sum, our results demonstrate Wnt/β-catenin responsiveness for all three differentiated taste cell types in both anterior (fungiform) and posterior (circumvallate) taste buds, and suggest that this signaling pathway may be involved in the regulation of aspects of taste cell turn-over in adult mice.
Type IV basal cells are Wnt/β-catenin responsive
Based on gene expression patterns, the Shh pathway has been proposed to function in taste cell renewal, and in particular is thought to be expressed in immature taste cells prior to their differentiation (Miura et al., 2004). Thus we performed double fluorescence in situ hybridization with antisense probes for Shh and LacZ to determine the extent of Wnt/β-catenin responsiveness and Shh ligand co-expression. As published previously (Miura et al., 2004), we found that Shh transcripts are restricted to type IV basal cells, which reside in the bottom compartment of taste buds (Fig. 2a–b). Some but not all Shh-positive basal cells also express BATGAL-driven LacZ (Fig. 2a). While most Wnt/β-catenin responsive cells within taste buds are intragemmal basal cells (Fig. 2b), not all of these LacZ positive cells expressed Shh (Fig. 2b). As with the expression of β-galactosidase protein in a subset of each of the 3 differentiated taste cell types (Fig. 1), BATGAL-driven LacZ was detected in fusiform taste cells, but only sporadically (Fig. 2a). However, we also found occasional extragemmal basal cells that were LacZ-positive (Fig. 2b), in contrast to the lack of β-galactosidase protein expression by cells outside of taste buds.
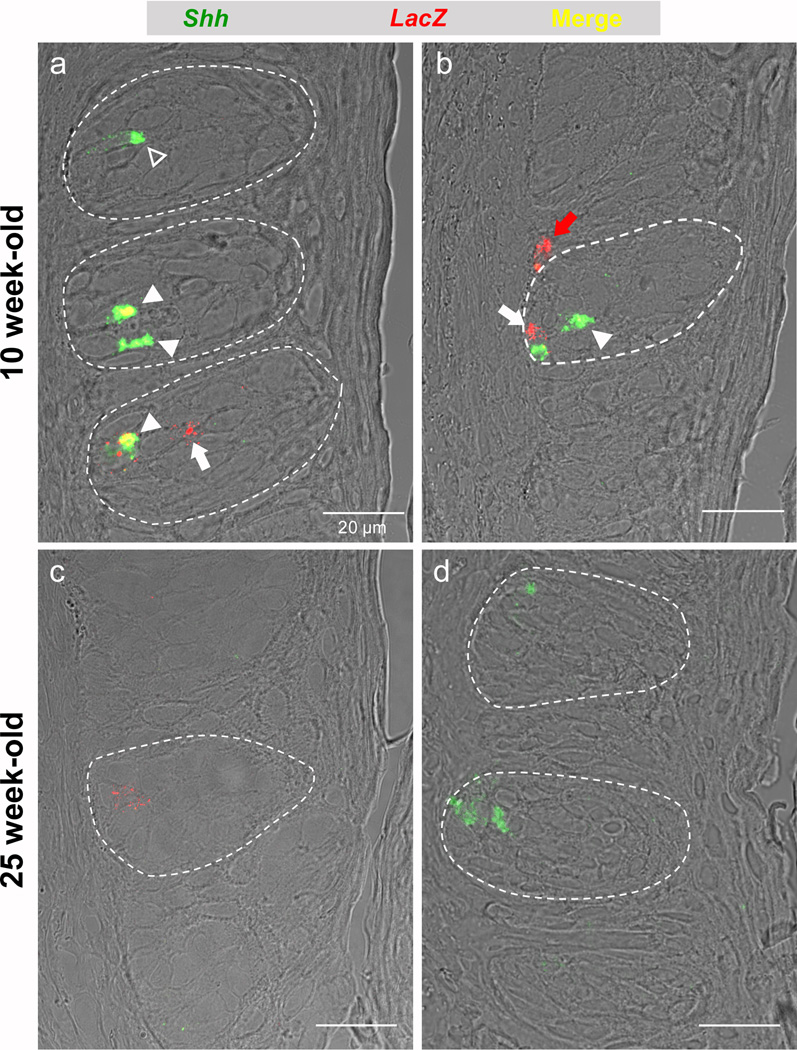
Fig. 2. In situ hybridization reveals that β-catenin responsive cells are primarily basal cells and often express Sonic Hedgehog (Shh).
Representative 0.75 µm optical sections of taste buds (encircled by white dash) in the circumvallate papilla reveal both Shh (green), and LacZ expressing (red) cells within taste buds; co-labeled cells are yellow. a) At 10 weeks of age, numerous double labeled cells are evident in taste buds of young mice (white arrowheads), as are cells singly labeled for Shh (open arrowhead: Shh-only expressing type IV cell), and cells labeled only for LacZ (white arrow: LacZ-expressing fusiform cell). b) In addition to Shh/LacZ double labeled cells within taste buds (white arrowhead), occasional basal cells outside of taste buds express LacZ (yellow arrow); Blue arrow: LacZ expressing intragemmal basal cell. c) At 25 weeks, LacZ expression is greatly diminished in circumvallate taste buds; we detected only sparse and very week expression of the reporter transcript (dim red; blue arrow), and sparsely encountered cells also double labeled for Shh. d) Shh expression (green; white arrowhead) in taste buds of 25 week-old mice, by contrast, was comparable to that detected in taste buds from mice at 10 weeks of age. Scale bars = 20 µm in all panels.
Wnt/β-catenin signaling is reduced in taste buds of older mice
Because taste function may decline with age (Fukunaga et al., 2005; Hays and Roberts, 2006; Mojet et al., 2001; Schiffman, 1997), we next compared the pattern of BATGAL expression in young adult mice (10 weeks postnatal), to that of older mice (25 weeks old), reasoning that if Wnt/β-catenin signaling regulates renewal, then we might expect changes in this pathway with age. Indeed, we found that in older mice, expression of LacZ is scarce; by contrast the distribution of Shh transcripts in 25–30 week old mice, that are still normally detected (Fig. 2c–d), was comparable to that of younger mice (Fig 2a–b). Moreover, the proportion of each differentiated taste cell type that was responsive to β-catenin was significantly decreased in circumvallate taste buds of 25 week-old mice (Fig. 3a, c–e; Student’s t-test, n=3 mice, p<0.01 for PLCβ2, p<0.05 for α-gustducin and NCAM). However, no such difference was detected in fungiform taste buds of 25 week-old compared to 10 week-old mice, although there was a downward trend in double labeling (Fig. 3b, f–h). The smaller proportion of co-labeled cells in circumvallate papilla of older mice was attributable to a global decrease in the number of BATGAL cells (Fig. 4a), and not to an overall decrease in differentiated taste cells. In the circumvallate papilla, roughly 7 cells per bud were BATGAL-positive in young mice, and that number declined significantly (2 cells per bud) in older mice. A significant, yet less dramatic reduction (4 versus 3 β-galactosidase-IR cells per bud in young versus old BATGAL mice) was also evident in fungiform taste buds. By contrast, the number of type II and III taste cells per bud was unchanged in both circumvallate and fungiform papillae (Fig. 4b). Despite the reduction in the number of β-catenin responsive cells, the proportion of β-galactosidase-IR cells double labeled for each taste cell type marker was not altered in old mice (Fig. 4c). In sum, our data suggest that aging causes a reduction in overall Wnt/β-catenin signaling in taste buds, but does not affect the pattern of β-catenin responsiveness across differentiated taste cell types.
Fig. 3. Aging significantly reduces β-galactosidase expression in differentiated taste cells in circumvallate but not fungiform taste buds.
a) The proportion of β-galactosidase positive type II (PLCβ2 and α-gustducin) and type III (NCAM) taste cells is significantly reduced in circumvallate taste buds of older mice (black bars: 10 week-old; white bars: 25 week-old mice). b) In fungiform taste buds, a similar trend of fewer β-galactosidase immunoreactive type II and III taste cells is evident, but this shift is not significant. Examples of taste buds from 25 week-old with limited β-galactosidase expressing type II (c,d,f,g) and type III (e,h) cells in 0.75 µm optical sections from circumvallate (c,d,e) and fungiform (f,g,h) papillae. Student’s t-test, n = 3 mice. *, p<0.05; **, p<0.01. Scale bars = 10 µm in all panels.
Fig. 4. Aging reduces the number of β-catenin responsive cells per taste bud, but not the number of each taste cell type, nor the proportion of each cell type expressing β-galactosidase.
a) Left: The number of β-galactosidase positive cells per taste bud section of circumvallate and fungiform papilla from 10 week-old is significantly greater than that of 25 week-old mice. Right: representative stacks of confocal optical sections reveal the much greater extent of BATGAL reporting (green) in young versus old mice. Student’s t-test, n=5–6 mice. *, p<0.05; ***, p<0.001. Scale bars = 20 µm in all panels. b) By contrast, the number of differentiated type II and III cells per taste bud section does not differ between 10 and 25 week-old mice. Student’s t-test, n=3 mice. c) The proportion of β-galactosidase positive cells expressing each of the cell type markers per taste bud section also does not differ between 10 and 25 week-old mice.
Discussion
Wnt/β-catenin signaling regulates the expression of genes involved in cell proliferation and differentiation during development and homeostatic maintenance of various tissues (Clevers, 2006). Wnt/β-catenin signaling is also necessary for embryonic taste bud development (Liu et al., 2007), but its involvement in taste bud cell renewal in adult mice has not been explored. Using BATGAL reporter mice, which express β-galactosidase in the presence of nuclear β-catenin (Maretto et al., 2003), the present work suggests new hypotheses concerning a contribution of this signaling pathway to taste cell turn-over in adult mice.
Canonical Wnt signaling may be related to the renewal of type I, II and III taste cells
Double labeling for β-galactosidase and specific taste cell type markers revealed here that a subset of every taste cell type in both circumvallate and fungiform papillae of adult mice is responsive to β-catenin, suggesting a role for this pathway in taste cell differentiation. When quantifying the proportion of β-catenin responsive cells within each cell type population, values did not exceed one third, implying either that β-catenin does not control the renewal of the whole population of a given cell type, or that the β-galactosidase marks a particular, and perhaps early, stage of taste cell differentiation. Considering that β-galactosidase half-life is reported to be approximately 13 hours (Hall et al., 1983; Jacobsen and Willumsen, 1995) and that cells inside taste buds renew about every 10 days (Beidler and Smallman, 1965; Farbman, 1980), the second hypothesis is more plausible and infers that β-galactosidase immunostaining likely reveals young cells, and that β-galactosidase expression ceases, and the protein is degraded as cells complete differentiation. On every tissue section, the intensity of β-galactosidase staining is highly variable, from bright to faint; this also may reflect the age of the cells, with younger cells expressing β-galactosidase more robustly. Consistent with the idea that BATGAL reporting is restricted to younger cells, we found that Shh expressing type IV basal cells were often LacZ positive in young adult mice. These basal cells are considered transient taste cell progenitors, as they express Shh within 12 hours of leaving the cell cycle, and expression peaks at 48 hours (Miura et al., 2004; Miura et al., 2006), prior to when these cells fully differentiate a day or more later into one or more of the three taste cell types (Hamamichi et al., 2006; Miura et al., 2006).
It is noteworthy that in fungiform taste buds, for example, 28% of PLCβ2-IR and 26% of α-gustducin-IR type II cells versus only 9% of NCAM expressing type III cells are positive for β-galactosidase. Since all β-galactosidase positive cells were counted independently of the brightness intensity of their nuclei, it would suggest that NCAM (type III) cells renew less frequently than PLCβ2 or α-gustducin (type II) cells in fungiform but not in circumvallate taste buds. Different taste cell type-specific turnover rates have been proposed in the past (Beidler and Smallman, 1965) and in 1980, Farbman showed that the lifespan of type I cells was 9 days and estimated that type II cells persisted much longer (Farbman, 1980). The longer lifespan of type II cells has been supported by an electron microscopic autoradiographic study (Delay et al., 1986), and more recently using the specific type II cell marker PLCβ2 (Hamamichi et al., 2006). However, the produrance of cell types I and III within taste buds remains to be explored.
How could Wnt/β-catenin signaling contribute to the renewal of mature taste cell?
While β-galactosidase protein was found in differentiated cell types inside taste buds, LacZ mRNA was also observed in type IV basal cells within taste buds; these cells are assumed to be immature taste cells undergoing differentiation (Miura et al., 2004). Therefore, β-catenin likely activates transcription of target genes in basal cells to induce their differentiation into types I, II and/or III. Shh is expressed specifically in a subpopulation of the type IV cells (Miura et al., 2004), and we find that many of these Shh positive basal cells are also responsive to β-catenin. This observation is interesting since the Shh pathway and Wnt/β-catenin signaling are known to interact to regulate fungiform papilla development in embryos. Indeed, blocking Shh signaling up-regulates the Wnt/β-catenin pathway and enhances fungiform papilla formation in embryonic mouse tongues in culture, whereas activation of Wnt/β-catenin up-regulates Shh (Iwatsuki et al., 2007; Liu et al., 2007). In adult mice, Shh-dependent inhibition of β-catenin also occurs in tongue epithelium, as does β-catenin-dependent up-regulation of Shh (Schneider et al., 2010), although this interaction was not explored specifically in taste buds. Altogether, these data suggest that Shh and Wnt/β-catenin may regulate type IV basal cell differentiation into mature taste cells. One question raised here is how these reciprocal interactions can decide the fate of the basal cells, i.e., to become a type I and/or type II and/or type III cell. Our in situ hybridization experiments revealed that some Shh-positive cells are not responsive to β-catenin and conversely, some β-catenin responsive cells do not express Shh. Therefore, these diverse expression configurations could contribute to distinct differentiation patterns, especially as Shh-expressing taste placodes in embryos give rise to type I and II, but not type III, taste cells postnatally (Thirumangalathu and Barlow, 2009; Thirumangalathu et al., 2009). Fate mapping of Shh expressing cells and β-catenin responsive basal cells, or conditionally inactivating β-catenin in Shh expressing cells will test these hypotheses.
While many LacZ expressing cells were identified as intragemmal basal cells suggesting a possible role for β-catenin in the differentiation of this cell population into mature taste cells, LacZ expression was also occasionally found in basal epithelial cells outside taste buds. These latter cells are part of the proliferative population (Hamamichi et al., 2006; Miura et al., 2004; Nguyen and Barlow, 2010) that gives rise to immature taste cells (Asano-Miyoshi et al., 2008; Hamamichi et al., 2006; Miura et al., 2004; Nguyen and Barlow, 2010). Consequently, β-catenin signaling may have a dual role within the gustatory epithelium by regulating both proliferation of progenitor cells outside of taste buds, and differentiation of immature cells within taste buds. Considering that taste buds have both epithelial and neuronal characteristics, this assumption is reminiscent of the functions of β-catenin signaling within stem cell niches, controlling both proliferation of stem cells and differentiation of post-mitotic cells during neurogenesis (Chenn and Walsh, 2002; Ding et al., 2003; Galceran et al., 2000; Hirabayashi et al., 2004; Zhou et al., 2004) and cyclical growth of hair follicles (Huelsken et al., 2001; Lowry et al., 2005; Van Mater et al., 2003; Watt and Jensen, 2009; Watt et al., 2006).
Additional molecular factors also likely contribute to regulation of the fate of type IV basal cells. In the intestine, BMPs inhibit Wnt/β-catenin signaling, and are associated with a reduction in stem cell renewal (He et al., 2004). BMP4 is expressed in type I, II, III and IV cells in circumvallate papillae and is co-expressed with Sox2 in immature taste cells (Nguyen and Barlow, 2010).
In sum, the mechanisms involved in taste cell renewal seem to be complex, implicate different signaling pathways that can interact and may depend on the taste field (i.e. fungiform versus circumvallate).
Wnt/β-catenin signaling in taste buds is reduced in older mice
While β-catenin signaling is robust in the taste buds of young mice, we found that signaling was dramatically reduced in older mice in both fungiform and circumvallate taste buds. Interestingly, while the percentage of each circumvallate taste bud cell type responsive to β-catenin is reduced in 25 week-old mice compared to their 10 week-old counterparts, the proportion of β-catenin responsive cells that express a cell type marker was not affected by aging. Hence, the distribution of the β-catenin reactive cells among the different taste cell types does not appear to be influenced by aging, but rather the absolute level of β-catenin signaling is diminished. We hypothesize that this reduction may slow the pace of cell renewal, as transcription of β-catenin target genes, as shown by sparse LacZ expression, is specifically reduced in intragemmal basal cells of 25 week-old mice; since these type IV basal cells are thought to be immature cells undergoing differentiation (Miura and Barlow, 2010; Miura et al., 2004), our expression data suggest that aging may affect the rate of differentiation of immature cells into elongate cells reflected in reduction of canonical Wnt signaling.
These observations could lead to uncovering cellular and molecular mechanisms responsible for the loss of taste sensitivity in the elderly (Fukunaga et al., 2005; Hays and Roberts, 2006; Mojet et al., 2001; Schiffman, 1997), especially as authors have thought for years that an alteration or slowing in the rate of taste cell turn-over leading to a disruption of taste cell structure and/or function could contribute to declining taste sensitivity (Beidler and Smallman, 1965; Fukunaga et al., 2005; Hays and Roberts, 2006; Mistretta and Baum, 1984). Indeed, since we show here that the number of taste cells remains the same, it may be that cells may live longer in older animals, and these aged taste cells would have reduced functional capabilities (Markovska et al., 1990; Nagy et al., 1985; Scott et al., 1988).
Changes in the level of β-catenin signaling with age have not been extensively studied. Nevertheless, an age-related increase in oxidative stress in bones has been shown to divert β-catenin from the Lymphoid Enhancer Factor/T Cell Factor (LEF/TCF) mediated transcription of target genes to the Forkhead Box O (FOXO) mediated pathway (Almeida et al., 2007a) inducing loss of bone mass (Almeida et al., 2007b). Hence, a similar diversion may occur in taste buds when mice get older: β-catenin might be redirected to FOXO mediated pathway to protect cells against oxidative stress at the expense of taste cell renewal and taste sensitivity, but this assumption remains to be tested.
Further investigations are now necessary to understand precisely how Wnt/β-catenin signaling regulates taste cell renewal in adult mice.
Methods
Animals and procedures
BATGAL reporter mice (B6.Cg-Tg(BAT-lacZ)3Picc/J) (Maretto et al., 2003) were purchased from the Jackson Laboratory (Bar Harbor ME, USA) and were maintained on a C57BL/6 background. Mice were housed in compliance with the Guide for the Care and Use of Laboratory Animals, Animal Welfare Act and Public Health Service Policy, and all procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus.
For immunohistochemistry, mice were anesthetized by ip injection of 400 mg/kg body weight Avertin (2,2,2-tribromoethanol, Sigma-Aldrich, St-Louis MO, USA). Mice were transcardially perfused with ice cold 0.9% sodium chloride with 0.1% heparin to clear blood and then with periodate-lysine-paraformaldehyde (PLP) fixative (75mM L-lysine monohydrochloride / 1.6% paraformaldehyde / 10mM sodium periodate, Sigma-Aldrich, St-Louis MO, USA). Tongues were harvested, post-fixed for 3h in PLP at 4°C, and cryoprotected in 20% sucrose (Fisher Scientific, Pittsburgh PA, USA) in 0.1 M phosphate buffer overnight at 4°C. Samples were embedded in O.C.T Compound (Tissue-Tek 4583, Sakura Finetek, Torrance CA, USA), frozen on dry ice and stored at −80°C.
For in situ hybridization, mice were euthanized by carbon dioxide inhalation. Tongues were harvested, rinsed with 0.1M phosphate buffered saline, embedded in O.C.T Compound, frozen on dry ice and stored at −80°C.
Immunohistochemistry
Double immunolabelling for β-galactosidase and different taste cell type markers was performed on 12 µm cryostat sections collected on Superfrost Plus Slides (Fisher Scientific, Pittsburgh PA, USA). Sections were incubated in blocking solution (5% normal goat serum, 1% bovine serum albumin, 0.3% Triton X100 in 0.1 M phosphate buffered saline, pH 7.3) for 1.5 h at room temperature, and then incubated with primary antisera in blocking solution overnight at 4°C. Sections were rinsed, incubated with secondary antisera in blocking solution for 1h at room temperature, rinsed again, and coverslipped using Fluoromount G (SouthernBiotech, Birmingham AL, USA). Antisera and dilutions used are listed in Table 1. Immunoreactivity for each antigen listed was abolished when primary antibodies were omitted.
Table 1.
| Primary antibody | Source | Dilution | Secondary Antibody |
Source | Dilution |
|---|---|---|---|---|---|
| Guinea pig anti-β-galactosidase |
T. Finger, UC Denver, USA (Yee et al., 2003) |
1/1000 | Alexa Fluor® 488 goat anti-guinea pig IgG |
Invitrogen A11073 |
1/1000 |
| Rabbit anti-NTPdase2 |
J. Sévigny, Université Laval, Canada (Bartel et al., 2006) |
1/1000 | Alexa Fluor® 546 goat anti-rabbit IgG |
Invitrogen A11010 |
1/1000 |
| Rabbit anti-PLCβ2 |
Santa Cruz sc-206 |
1/800 | |||
| Rabbit anti-α-gustducin |
Santa Cruz sc-395 |
1/1000 | |||
| Rabbit anti-NCAM |
Chemicon International AB5032 |
1/1000 |
In situ hybridization
Five micrometer cryostat sections were collected on Superfrost Plus Slides. Double detection of mRNA encoding for LacZ and Shh was performed as previously described (Miura et al., 2004). Antisense RNA probes were transcribed in vitro from a linearized plasmid containing a LacZ cDNA insert (Gilbert et al., 2005) or Shh cDNA insert (Kitamura et al., 1997) using digoxigenin-conjugated UTP or FITC-conjugated UTP, respectively. Sections were incubated overnight at 65°C in a moist chamber with the RNA probes in hybridization solution (50% formamide, 5× SCC, 5× Denhardt’s solution, 500 µg/ml salmon sperm DNA and 250 µg/ml tRNA). Sections were washed 90 min at 65°C in 0.2× SSC, then incubated overnight at 4°C in a moist chamber with peroxidase-coupled anti-digoxigenin antibody and alkaline phosphatase-coupled anti-FITC antibody. To detect Shh mRNA, sections were treated with Streptavidin-Alexa 488 (Invitrogen, Carlsbad, CA, USA) for 30 min following a 30 min tyramide-biotin treatment (TSA™ Biotin Tyramide Reagent, PerkinElmer, Waltham, MA, USA). Finally, the LacZ transcript was detected by incubating sections with HNPP/Fast Red reagent (HNPP Fluorescent Detection Set, Roche Applied Science, Mannheim, Germany).
Image acquisition and analysis
Confocal images were acquired using a laser-scanning Olympus Fluoview confocal microscope and FluoView Software, or Leica TCS SP5 II confocal system and LASAF software. Counting was performed on 0.75 µm optical sections. Beta-galactosidase immunopositive nuclei were counted independently of their size and of the intensity of the fluorescence signal. Three mice were used for each marker and papilla. The following numbers of taste bud profiles were tallied per taste cell type marker for fungiform and circumvallate papillae in 10 week-old (vs. 25 week-old) mice: anti-α-gustducin 35 and 107 (vs. 44 and 110); anti-NCAM: 26 and 97 (vs. 38 and 106); and anti-PLCβ2: 24 and 65 (vs. 23 and 74).
Statistical analysis
Data are represented as means ± SEM. Statistical analysis were performed using SigmaStat (Systat Software). Normal distribution and equal variances between groups were assessed with a p value set at 5%, to run a Student’s t-test. Statistical differences were established with a confidence interval of 95%.
Acknowledgements
The authors thank Brooke Baxter, Elizabeth Harvey, Jason Nealy and Jennifer Liang for expert technical support, and Tom Finger, Ha M. Nguyen and Shoba Thirumangalathu for critical comments on an earlier version of the manuscript. We also thank the Rocky Mountain Taste and Smell Center (NIH P30 DC004657) for imaging, genotyping and animal housing support. This work is supported by NIH/NIDCD DC008373 to LAB.
Abbreviations
- BMP
Bone Morphogenetic Protein
- ENaC
amiloride-sensitive Epithelial Sodium Channels
- FOXO
Forkhead Box O
- GLAST
Glutamate-Aspartate Transporter
- IR
immunoreactive
- LEF/TCF
Lymphoid Enhancer Factor/T Cell Factor
- NCAM
Neural Cell Adhesion Molecule
- NTPdase2
nucleoside triphosphate diphosphohydrolase 2
- PLCβ2
phospholipase C β2
- PLP
periodate-lysine-paraformaldehyde
- Shh
Sonic Hedgehog
- SNAP25
synaptic membrane protein 25
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A Novel Family of Mammalian Taste Receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative Stress Antagonizes Wnt Signaling in Osteoblast Precursors by Diverting β-Catenin from T Cell Factor- to Forkhead Box O-mediated Transcription. Journal of Biological Chemistry. 2007a;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal Involution by Age-associated Oxidative Stress and Its Acceleration by Loss of Sex Steroids. Journal of Biological Chemistry. 2007b;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano-Miyoshi M, Hamamichi R, Emori Y. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Histol. 2008;39:193–199. doi: 10.1007/s10735-007-9151-0. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Hollenbeck PLW, Kim J, Lovell-Badge R, Lander AD, Calof AL. Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development. 2009;136:2187–2197. doi: 10.1242/dev.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD, Jr, Pumplin DW, Yu C, Christy RC, Smith DV. Differential expression of alpha-gustducin in taste bud populations of the rat and hamster. J Neurosci. 1997;17:2852–2858. doi: 10.1523/JNEUROSCI.17-08-02852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Cho YK, Farbman AI, Smith DV. The Timing of {alpha}-Gustducin Expression during Cell Renewal in Rat Vallate Taste Buds. Chem. Senses. 1998;23:735–742. doi: 10.1093/chemse/23.6.735. [DOI] [PubMed] [Google Scholar]
- Clapp T, Stone L, Margolskee R, Kinnamon S. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neuroscience. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/[beta]-Catenin Signaling in Development and Disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Delay RJ, Kinnamon JC, Roper SD. Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J Comp Neurol. 1986;253:242–252. doi: 10.1002/cne.902530210. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci U S A. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman AI. Fine Structure of the Taste Bud. J Ultrastruct Res. 1965;12:328–350. doi: 10.1016/s0022-5320(65)80103-4. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Uematsu H, Sugimoto K. Influences of Aging on Taste Perception and Oral Somatic Sensation. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:109–113. doi: 10.1093/gerona/60.1.109. [DOI] [PubMed] [Google Scholar]
- Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- Gilbert MM, Weaver BK, Gergen JP, Reich NC. A novel functional activator of the Drosophila JAK/STAT pathway, unpaired2, is revealed by an in vivo reporter of pathway activation. Mech Dev. 2005;122:939–948. doi: 10.1016/j.mod.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Hall CV, Jacob PE, Ringold GM, Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2:101–109. [PubMed] [Google Scholar]
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263–277. doi: 10.1016/s0012-1606(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Hall JM, Hooper JE, Finger TE. Expression of sonic hedgehog, patched, and Gli1 in developing taste papillae of the mouse. J Comp Neurol. 1999;406:143–155. doi: 10.1002/(sici)1096-9861(19990405)406:2<143::aid-cne1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–2138. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Hays NP, Roberts SB. The anorexia of aging in humans. Physiol Behav. 2006;88:257–266. doi: 10.1016/j.physbeh.2006.05.029. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Liu H-X, Gründer A, Singer MA, Lane TF, Grosschedl R, Mistretta CM, Margolskee RF. Wnt signaling interacts with Shh to regulate taste papilla development. Proceedings of the National Academy of Sciences. 2007;104:2253–2258. doi: 10.1073/pnas.0607399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KD, Willumsen BM. Kinetics of expression of inducible beta-galactosidase in murine fibroblasts: high initial rate compared to steady-state expression. J Mol Biol. 1995;252:289–295. doi: 10.1006/jmbi.1995.0496. [DOI] [PubMed] [Google Scholar]
- Jung HS, Oropeza V, Thesleff I. Shh, Bmp-2, Bmp-4 and Fgf-8 are associated with initiation and patterning of mouse tongue papillae. Mech Dev. 1999;81:179–182. doi: 10.1016/s0925-4773(98)00234-2. [DOI] [PubMed] [Google Scholar]
- Kim JY, Mochizuki T, Akita K, Jung HS. Morphological evidence of the importance of epithelial tissue during mouse tongue development. Exp Cell Res. 2003a;290:217–226. doi: 10.1016/s0014-4827(03)00319-7. [DOI] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun. 2003b;312:500–506. doi: 10.1016/j.bbrc.2003.10.137. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Yanazawa M, Miyashita T, Kato K. Expression patterns of Brx1 (Rieg gene), Sonic hedgehog, Nkx2.2, Dlx1 and Arx during zona limitans intrathalamica and embryonic ventral lateral geniculate nuclear formation. Mech Dev. 1997;67:83–96. doi: 10.1016/s0925-4773(97)00110-x. [DOI] [PubMed] [Google Scholar]
- Kusakabe Y, Miura H, Hashimoto R, Sugiyama C, Ninomiya Y, Hino A. The Neural Differentiation Gene Mash-1 has a Distinct Pattern of Expression from the Taste Reception-related Genes gustducin and T1R2 in the Taste Buds. Chem. Senses. 2002;27:445–451. doi: 10.1093/chemse/27.5.445. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;12:3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, Andl T, Taketo MM, Dlugosz AA, Moon RT, Barlow LA, Millar SE. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–112. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/Beta-catenin signaling during mouse development and in colorectal tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovska TT, Petkova DH, Momchilova-Pankova AB, Koumanov KS. Age-related changes in rat liver phospholipid transfer activity. Exp Gerontol. 1990;25:55–60. doi: 10.1016/0531-5565(90)90009-q. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur J-P, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Baum BJ. Quantitative study of taste buds in fungiform and circumvallate papillae of young and aged rats. J Anat. 1984;138(Pt 2):323–332. [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM, Liu HX, Gaffield W, MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol. 2003;254:1–18. doi: 10.1016/s0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Miura H, Barlow LA. Taste bud regeneration and the search for taste progenitor cells. Arch Ital Biol. 2010;148:107–118. [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kato H, Kusakabe Y, Ninomiya Y, Hino A. Temporal changes in NCAM immunoreactivity during taste cell differentiation and cell lineage relationships in taste buds. Chem Senses. 2005;30:367–375. doi: 10.1093/chemse/bji031. [DOI] [PubMed] [Google Scholar]
- Miura H, Kato H, Kusakabe Y, Tagami M, Miura-Ohnuma J, Ninomiya Y, Hino A. A Strong Nerve Dependence of Sonic hedgehog Expression in Basal Cells in Mouse Taste Bud and an Autonomous Transcriptional Control of Genes in Differentiated Taste Cells. Chem. Senses. 2004;29:823–831. doi: 10.1093/chemse/bjh248. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006;69:209–225. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Sugiyama C, Kawamatsu M, Ninomiya Y, Motoyama J, Hino A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 2001;106:143–145. doi: 10.1016/s0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- Mojet J, Christ-Hazelhof E, Heidema J. Taste Perception with Age: Generic or Specific Losses in Threshold Sensitivity to the Five Basic Tastes? Chemical Senses. 2001;26:845–860. doi: 10.1093/chemse/26.7.845. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Murray RG. The mammalian taste bud type III cell: a critical analysis. J Ultrastruct Mol Struct Res. 1986;95:175–188. doi: 10.1016/0889-1605(86)90039-x. [DOI] [PubMed] [Google Scholar]
- Nagy I, Toth S, Lustyik G. Verification of the membrane hypothesis of aging on the identified giant neurons of the snail Lymnaea stagnalis L. (Gastropoda, Pulmonata) by a combined application of intracellular electrophysiology and X-ray microanalysis. Arch Gerontol Geriatr. 1985;4:53–66. doi: 10.1016/0167-4943(85)90018-4. [DOI] [PubMed] [Google Scholar]
- Nelson GM, Finger TE. Immunolocalization of different forms of neural cell adhesion molecule (NCAM) in rat taste buds. J Comp Neurol. 1993;336:507–516. doi: 10.1002/cne.903360404. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Barlow LA. BMP4 expression differs in anterior fungiform versus posterior circumvallate taste buds of mice. BMC Neurosci. 2010;11:129. doi: 10.1186/1471-2202-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike H, Matsumoto I, Abe K. Group IIA phospholipase A2 is coexpressed with SNAP-25 in mature taste receptor cells of rat circumvallate papillae. The Journal of Comparative Neurology. 2006;494:876–886. doi: 10.1002/cne.20848. [DOI] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Pevny LH, Hogan BLM. Sox2 is required for development of taste bud sensory cells. Genes & Development. 2006;20:2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota MS, Kaneko Y, Kondo K, Ogishima S, Tanaka H, Eto K, Kondo T. Combined in silico and in vivo analyses reveal role of Hes1 in taste cell differentiation. PLoS Genet. 2009;5:e1000443. doi: 10.1371/journal.pgen.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Taste and smell losses in normal aging and disease. Jama. 1997;278:1357–1362. [PubMed] [Google Scholar]
- Schneider FT, Schanzer A, Czupalla CJ, Thom S, Engels K, Schmidt MH, Plate KH, Liebner S. Sonic Hedgehog Acts as a Negative Regulator of {beta}-Catenin Signaling in the Adult Tongue Epithelium. Am J Pathol. 2010;177:404–414. doi: 10.2353/ajpath.2010.091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B, Leu J, Cinader B. Effects of aging on neuronal electrical membrane properties. Mech Ageing Dev. 1988;44:203–214. doi: 10.1016/0047-6374(88)90022-x. [DOI] [PubMed] [Google Scholar]
- Seta Y, Seta C, Barlow LA. Notch-associated gene expression in embryonic and adult taste papillae and taste buds suggests a role in taste cell lineage decisions. J Comp Neurol. 2003;464:49–61. doi: 10.1002/cne.10787. [DOI] [PubMed] [Google Scholar]
- Seta Y, Stoick-Cooper CL, Toyono T, Kataoka S, Toyoshima K, Barlow LA. The bHLH transcription factors, Hes6 and Mash1, are expressed in distinct subsets of cells within adult mouse taste buds. Arch Histol Cytol. 2006;69:189–198. doi: 10.1679/aohc.69.189. [DOI] [PubMed] [Google Scholar]
- Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and gustducin in palatal taste buds of mice. Chem Senses. 2007;32:255–262. doi: 10.1093/chemse/bjl053. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Expression of Sox2 in mouse taste buds and its relation to innervation. Cell Tissue Res. 2008;332:393–401. doi: 10.1007/s00441-008-0600-1. [DOI] [PubMed] [Google Scholar]
- Takeda M, Suzuki Y, Obara N, Nagai Y. Neural Cell Adhesion Molecule of Taste Buds. Journal of Electron Microscopy. 1992;41:375–380. [PubMed] [Google Scholar]
- Thirumangalathu S, Barlow LA. In vivo fate tracing studies of mammalian taste cell progenitors. Ann N Y Acad Sci. 2009;1170:34–38. doi: 10.1111/j.1749-6632.2009.04371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136:1519–1528. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano M, Dvoryanchikov G, Barrows J, Kim S, Chaudhari N, Finger T. Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue. BMC Neuroscience. 2008;9:110. doi: 10.1186/1471-2202-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000a;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Stoick CL, Kinnamon JC. Synaptobrevin-2-like immunoreactivity is associated with vesicles at synapses in rat circumvallate taste buds. J Comp Neurol. 2004;471:59–71. doi: 10.1002/cne.20021. [DOI] [PubMed] [Google Scholar]
- Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol. 2000b;425:139–151. doi: 10.1002/1096-9861(20000911)425:1<139::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. "Type III" cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Yoshie S, Wakasugi C, Teraki Y, Fujita T. Fine structure of the taste bud in guinea pigs. I. Cell characterization and innervation patterns. Arch Histol Cytol. 1990;53:103–119. doi: 10.1679/aohc.53.103. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu HX, Mistretta CM. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol. 2006;297:198–213. doi: 10.1016/j.ydbio.2006.05.022. [DOI] [PubMed] [Google Scholar]