Abstract
We describe here the analysis of Nanodisc complexes by using native mass spectrometry (MS) to characterize their molecular weight (MW) and polydispersity. Nanodiscs are nanoscale lipid bilayers that offer a platform for solubilizing membrane proteins. Unlike detergent micelles, Nanodiscs are native-like lipid bilayers that are well defined and potentially monodisperse. Their mass spectra allow peak assignment based on differences in the mass of a single lipid per complex. Resultant masses agree closely with predicted values and demonstrate conclusively the narrow dispersity of lipid molecules in the Nanodisc. Fragmentation with collisionally activated dissociation (CAD) or electron-capture dissociation (ECD) shows loss of a small number of lipids and eventual collapse of the Nanodisc with release of the scaffold protein. These results provide a foundation for future studies utilizing Nanodiscs as a platform for launching membrane proteins into the gas phase.
Although membrane proteins are of high biological and pharmaceutical importance, membrane protein structural biology has lagged behind that of cytosolic proteins owing to the difficulty of expressing and solubilizing membrane proteins for analysis. Nanodiscs, which are nanoscale lipid bilayers encircled and held intact by engineered membrane scaffold proteins (MSP), can solubilize membrane proteins for analysis.1,2 The scaffold protein defines the diameter of the Nanodisc complex while the type of lipid defines how many lipids are required to pack the bilayer area inside the MSP perimeter.3 Nanodiscs were used previously in several MS applications, but in those studies, the researchers disassembled the Nanodiscs prior to analysis.4–7 We report here the first evidence that intact Nanodisc complexes can be introduced to the gas phase of a mass spectrometer using native electrospray MS for direct measurement of their molecular weight and polydispersity. These measurements provide important fundamental characterization of Nanodiscs and will serve as a basis for future studies using Nanodiscs as a platform for membrane protein native MS.
We describe the analysis of Nanodiscs containing the scaffold protein MSP1D1(−) and lipids 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) or 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). DMPC and POPC were chosen for this study because they were previously characterized2 and are commonly used in Nanodisc preparation. The preparation of these Nanodiscs was described previously,1,3 and a detailed description can be found in Supporting Information. The Nanodiscs were purified on a Superdex 200 size-exclusion column with elution in 0.1 M ammonium acetate (pH = 6.8). Eluted fractions containing Nanodiscs were pooled (final concentrations were between 5 and 10 μM) and infused, using nano-ESI, into a Bruker SolariX Fourier-transform ion cyclotron resonance (FTICR) mass spectrometer under native conditions. In-source, collisionally activated dissociation (CAD) was accomplished by increasing the voltage of the first skimmer cone of the source from 10 V to 190 V, thereby accelerating the ions to cause some dissociation upon collision with background gas. Electron capture dissociation (ECD) was performed with a voltage bias of 0.1 V for 10 ms. A complete list of instrumental parameters is in Supporting Information.
The native mass spectra for DMPC and POPC Nanodiscs as measured by FTICR mass spectrometry show a fine spacing within each broad peak (Figures 1 and 2 respectively with additional spectra shown Figure S-1 and S-2). We hypothesize that this spacing is due to slight differences in lipid packing, with each peak representing a Nanodisc with a defined number of lipids. Adjacent narrow peaks differ by the mass of a single lipid. Distributions in aggregation number were previously observed in native MS of detergent micelles,8,9 but this is the first direct observation of the lipid distribution (polydispersity) of Nanodiscs. Accepting the above hypothesis, we determined the charge for each species by dividing the mass of the lipid by the spacing between adjacent peaks. The three broad peaks, corresponding to the +22, +21, and +20 charge states, were fit to three overlapping Gaussian distributions to calculate the average MW and polydispersity (Figures S-3 and S-4). The mean and standard deviations of the three distributions were averaged, and errors are reported as one standard deviation.
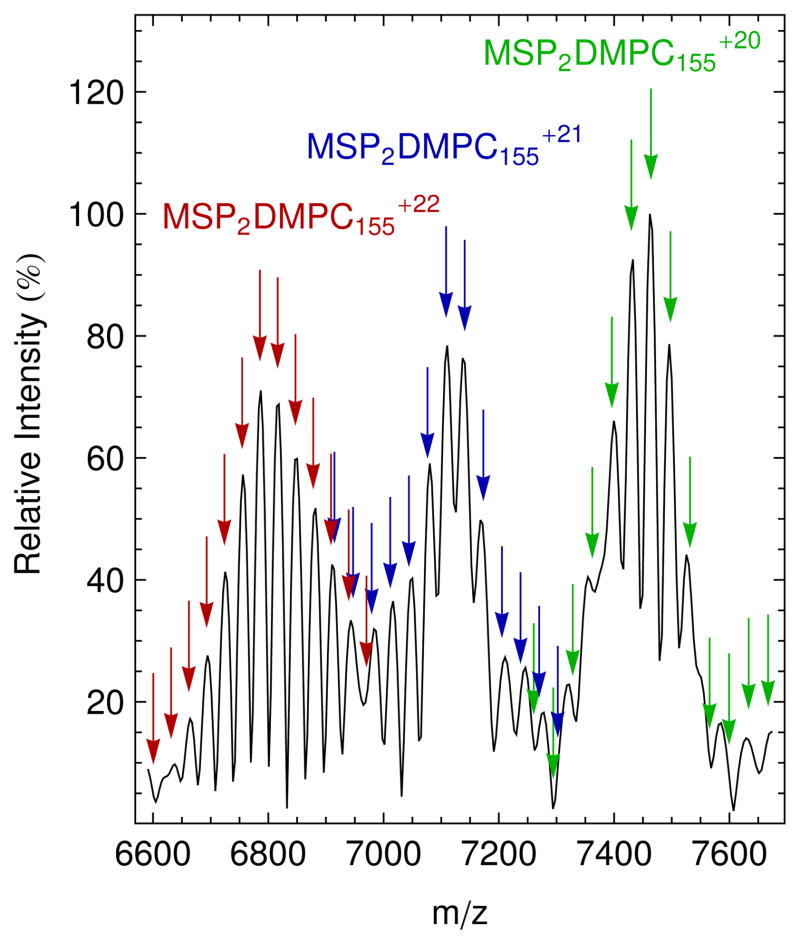
Figure 1.
Native mass spectrum at 70 V CAD of DMPC Nanodiscs (black) with simulated masses of the +22 (red), +21 (blue), and +20 (green) charge states for Nanodisc complexes with two MSP molecules, six bound water molecules, and an average of 155 DMPC molecules. Arrows of the same color show simulated values for Nanodiscs in the same charge state with a varying number of lipid molecules. The average, 155 lipids, is the highest peak in each charge state. Simulated m/z values are marked by the arrows, and their vertical position was matched with the intensities of mass spectrum.
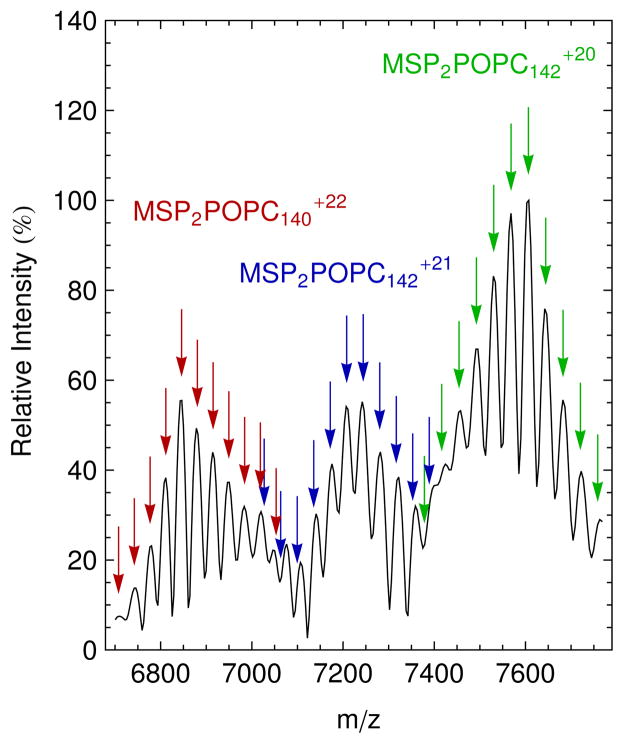
Figure 2.
Native mass spectrum at 70 V CAD of POPC Nanodiscs (black) with simulated masses of the +22 (red), +21 (blue), and +20 (green) charge states for Nanodisc complexes with two MSP molecules, five bound water molecules, and an average of 141 POPC molecules. Arrows of the same color show simulated values for Nanodiscs of the same charge state with a varying number of lipid molecules. The highest peak in each charge series is annotated. Simulated m/z values are marked by the arrows, and their vertical position was matched with the intensities of mass spectrum.
Fitting FTICR mass spectra to Gaussian distributions (Figure S-3) shows that Nanodiscs containing DMPC have an average mass of 149.5 ± 0.5 kDa and are sprayed with an average charge state of +21 at 70 V CAD. These DMPC Nanodiscs average 155 lipids per Nanodisc or 77.5 lipids per leaflet. This lipid total agrees well with the 77 lipids per leaflet, previously measured for DMPC by radioactive methods.2 The average lipid count has a standard deviation of 2.4 ± 0.5 lipids per leaflet.
The results for POPC Nanodiscs (Figure 2 and S-4) reveal that they can be native sprayed with an average charge of +21 at 70 V CAD to yield an average mass of 151.7 ± 0.3 kDa. There are on average 141 POPC lipids per Nanodisc or 70.5 lipids per leaflet. This is in acceptable agreement with lipid counts for POPC Nanodiscs, which were reported to be 62 lipids per leaflet1 and 67 lipids per leaflet.10 Similar to DMPC, the POPC lipid distribution is 2.61 ± 0.02 lipids per leaflet.
Informed by the charges determined from lipid spacing, we used a simple formula to simulate the expected native mass-to-charge ratio of a Nanodisc, m/zND, as a function of lipid number:
| (1) |
where MMSP is the mass of scaffold, 22044 Da; n is the number of lipid molecules; ML is the average lipid mass, 677.933 Da for DMPC and 760.076 Da for POPC; and z is the charge state. Because intact Nanodisc ions from the native ESI have relatively high m/z and are poorly desolvated compared to those from conventional ESI of denatured proteins, the corresponding peaks of intact Nanodisc complex were not isotopically resolved. Thus, average rather than exact values of lipid molecular masses were used. The value of n was simulated for a range around the average lipid number calculated above.2 The 18m term was included to account for tightly bound solvent molecules or counter-ions such as ammonium (both water and ammonium have molecular weights of 18 Da), which were observed in previous native mass spectrometry studies.11 This value was determined by adjusting m to achieve to minimal difference between the simulated peaks and the middle five peaks in the three distributions. The best fit was found when m was 6 for DMPC and 5 for POPC although the width of the peaks limits the precision of these numbers. After correcting for bound solvent, simulated masses for the middle five peaks (approximately one standard deviation) in each of the three charge states agreed with measured peaks to within 0.03% for DMPC and 0.02% for POPC. However, peaks at the edges of the distributions do not match as well owing to the overlapping of distributions from multiple charge states, as seen in Figures 1 and 2. The close fit between simulations and measured spectra confirm our assignments of the spectra to intact Nanodisc complexes.
The Nanodisc ions remain stable upon CAD from 10 to 70 V with a slight decrease in charge state as CAD energy increases (see Figures S-1 and S-2). Ionization does not occur with large amounts of bound solvent, indicating the ionization conditions are effective at desolvating the Nanodisc even at low CAD energies. Adding additional collisional energy by increasing the accelerating potential to 100 V and 130 V continued to shift the output to lower charge states.
Using the lipid spacing to deconvolute the charge states, we observed that both 100 V and 130 V CAD cause the average lipid count to decrease. This suggests that the Nanodiscs begin to release a small number of lipids without complete destruction. CAD at 100 V shows DMPC Nanodiscs with average lipid counts of 153, 148, and 146 (Figure S-5A). CAD at 130 V with an ECD bias of 0.1 V shows DMPC Nanodiscs with average counts of 143 and 139 lipids (Figure S-5B). These results are consistent with data on detergent complexes; those data demonstrate that lower aggregation numbers are observed at lower charge states.8 At these higher energies, different charge states and lipid distributions begin to overlap, and the peaks become too difficult to assign based on lipid distribution.
Finally, the mass spectrum of the MSP at charge states from +6 to +12 begins to appear in the low mass region at 130 V, suggesting the complete dissociation of some of the Nanodisc population (Figure S-6). At 160 V, higher mass peaks largely disappear, leaving only low mass peaks for MSP and lipid clusters. The appearance of bare MSP at high, but not low, collision energy further confirms the hypothesis that intact Nanodiscs are observed at low to moderate collision energy.
These spectra suggest a comprehensive hypothesis for the behavior of Nanodisc complexes in the gas-phase. At low to moderate CAD energies, Nanodisc complexes can be observed with a distribution of lipids. This represents a stable ionization region where the complexes are mostly desolvated but still intact. Increasing the source voltages (and CAD energies) begins to strip the complexes of lipids, leaving partially dissociated Nanodiscs. At high energies, the complex is entirely dismantled, leaving only bare MSP and lipid clusters at low m/z range.
Whether surfactant aggregates in the gas phase mirror solution-phase aggregates has been a matter of debate in the field of native mass spectrometry.12 These results demonstrate that Nanodisc complexes in the gas phase closely mirror the properties of Nanodiscs in solution. This is likely due to the constraint from the MSP belt, which maintains the defined structure of the Nanodisc complex. However, it is possible that ionization removes some loosely bound lipids, slightly down-shifting the distribution from its normal state in solution. We do not anticipate that the lipid bilayer has inverted as seen in some micelle systems.13 However, future studies will be necessary to examine the gas-phase structure of Nanodiscs and determine if any structural changes occur in the ionization process.
Native electrospray MS provides structural information on protein complex subunit identity, stoichiometry, and interactions.14–16 Native MS of membrane-bound proteins is currently accomplished by applying moderate collision energy to “shake off” detergent so that homogeneous membrane protein complexes emerge from heterogeneous detergent micelles.17–19 Given the important protective nature of these micelles, there has been significant interest in understanding the behavior of detergent and lipid complexes in the gas phase so as to improve the ionization.8,9,12,20
Native MS for analysis of Nanodiscs holds unique potential as a launch pad for studying membrane protein complexes. Given that Nanodiscs contain a native-like lipid bilayer, they provide a more physiologically relevant environment to study membrane protein complexes than do micelles. Nanodiscs are also more monodisperse than detergent micelles, and the lipid content of the Nanodisc can be tailored to permit investigation of protein-lipid interactions.
These results demonstrate the remarkable stability and homogeneity of Nanodiscs as well as the degree to which native MS can maintain large hydrophobic complexes in the gas-phase. Two previous reviews speculated that Nanodiscs could be used to solubilize membrane proteins for native mass spectrometry.21,22 This report provides the first experimental evidence for this possibility and lays the groundwork for characterizing various synthetic Nanodiscs and performing and interpreting future studies of Nanodisc-solubilized membrane complexes by top-down,23,24 ion mobility, and other gas-phase analysis techniques.
Supplementary Material
Acknowledgments
We thank Catherine Baker for assistance in designing the TOC graphic. This work was supported by the National Institutes of Health (R01-GM31756 and R01-GM33775 to S.G.S). Support was also provided by grants from the National Institute of General Medical Sciences (8 P41 GM103422-35 to M.L.G.) of the NIH and of National Science Foundation (IBDR 0964199 to M.L.G.). Partial salary support for H.Z. was from the Photosynthetic Antenna Research Center (PARC), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (Grant No. DESC 0001035 to R.E.B.). M.T.M. was supported by the Robert C. and Carolyn J. Springborn Endowment.
Footnotes
Supporting Information. Nanodisc preparation protocols, MS instrumental parameters, and supporting figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bayburt TH, Grinkova YV, Sligar SG. Nano Lett. 2002;2:853–856. [Google Scholar]
- 2.Bayburt TH, Sligar SG. FEBS Lett. 2010;584:1721–1727. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. J Am Chem Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 4.Hebling CM, Morgan CR, Stafford DW, Jorgenson JW, Rand KD, Engen JR. Anal Chem. 2010;82:5415–5419. doi: 10.1021/ac100962c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin VL, Bayburt TH, Sligar SG, Mrksich M. Angew Chem, Int Ed. 2007;46:8796–8798. doi: 10.1002/anie.200702694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marty MT, Das A, Sligar SG. Anal Bioanal Chem. 2012;402:721–729. doi: 10.1007/s00216-011-5512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan CR, Hebling CM, Rand KD, Stafford DW, Jorgenson JW, Engen JR. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.010876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borysik AJ, Robinson CV. Langmuir. 2012;28:7160–7167. doi: 10.1021/la3002866. [DOI] [PubMed] [Google Scholar]
- 9.Sharon M, Ilag LL, Robinson CV. J Am Chem Soc. 2007;129:8740–8746. doi: 10.1021/ja067820h. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AW, Pureza VS, Sligar SG, Morrissey JH. J Biol Chem. 2007;282:6556–6563. doi: 10.1074/jbc.M607973200. [DOI] [PubMed] [Google Scholar]
- 11.Freeke J, Robinson CV, Ruotolo BT. Int J Mass spectrom. 2010;298:91–98. [Google Scholar]
- 12.Ceraulo L, Giorgi G, Liveri VT, Bongiorno D, Indelicato S, Di Gaudio F, Indelicato S. Eur J Mass Spectrom. 2011;17:525–541. doi: 10.1255/ejms.1158. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Larsson DSD, van der Spoel D. Biochemistry. 2009;48:1006–1015. doi: 10.1021/bi801952f. [DOI] [PubMed] [Google Scholar]
- 14.Hilton GR, Benesch JLP. J R Soc Interface. 2012;9:801–816. doi: 10.1098/rsif.2011.0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharon M. J Am Soc Mass Spectrom. 2010;21:487–500. doi: 10.1016/j.jasms.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Loo JA. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Science. 2008;321:243–246. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- 18.Barrera NP, Isaacson SC, Zhou M, Bavro VN, Welch A, Schaedler TA, Seeger MA, Miguel RN, Korkhov VM, van Veen HW, Venter H, Walmsley AR, Tate CG, Robinson CV. Nat Methods. 2009;6:585–U49. doi: 10.1038/nmeth.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou M, Morgner N, Barrera NP, Politis A, Isaacson SC, Matak-Vinkovic D, Murata T, Bernal RA, Stock D, Robinson CV. Science. 2011;334:380–385. doi: 10.1126/science.1210148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friemann R, Larsson DSD, Wang Y, van der Spoel D. J Am Chem Soc. 2009;131:16606. doi: 10.1021/ja902962y. [DOI] [PubMed] [Google Scholar]
- 21.Barrera NP, Robinson CV. Annu Rev Biochem. 2011;80:247–271. doi: 10.1146/annurev-biochem-062309-093307. [DOI] [PubMed] [Google Scholar]
- 22.van der Spoel D, Marklund EG, Larsson DSD, Caleman C. Macromol Biosci. 2011;11:50–59. doi: 10.1002/mabi.201000291. [DOI] [PubMed] [Google Scholar]
- 23.Xie Y, Zhang J, Yin S, Loo JA. J Am Chem Soc. 2006;128:14432–3. doi: 10.1021/ja063197p. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Cui W, Wen J, Blankenship RE, Gross ML. Anal Chem. 2011;83:5598–606. doi: 10.1021/ac200695d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.