Abstract
Microgels with two interpenetrating polymer networks of poly-N-isopropylacrylamide and poly-acrylic acid (PNIPAM-IPN-PAAc) were synthesized using a seed method. The IPN microgels in water have an average hydrodynamic radius of about 85 nm at 21 °C, measured by dynamic light scattering method. The atomic force microscope image showed that the particles were much smaller after they were dried but remain their spherical shape. The storage and loss moduli G' and G' ' of dispersions of IPN microgels were measured in the linear stress regime as functions of temperature and frequency at various polymer concentrations using a stress-controlled rheometer. For dispersions with high polymer concentration (3.5 and 6.0 wt%) and at high temperatures (34 and 38 °C), the samples behave as viscoelastic solids and the storage modulus was larger than the loss modulus over the entire frequency range. The loss tangent was measured at various frequencies as a function of temperature. The gelation temperature was determined to be 33 °C at the point where a frequency-independent value of the loss tangent was first observed.
Using an animal implantation model, the biocompatibility and drug release properties of the IPN microgl dispersion were evaluated. Fluorescein as a model drug was mixed into an aqueous microgel dispersion at ambient temperature. This drug loaded liquid was then injected subcutaneously in Balb/C mice from Taconic Farms. The test results have shown that the IPN microgels were biocompatible in this acute implantation model and the presence of gelled microgel dispersion substantially slowed the release of fluorescein.
Introduction
Poly-N-isopropylacrylamide polymer and its derivatives have been studied extensively because they exhibit a sharp thermo-reversible phase transition and easily accessible, tunable lower critical solution temperature (LCST) near physiological temperature.[1–4] Copolymers of N-isopropylacrylamide (NIPAAm) and polyacrylic acid (PAAc) have been prepared using the reversible addition fragmentation chain transfer (RAFT) polymerization method and they are useful in a variety of molecular switching and drug delivery applications where responses to small pH changes are relevant.[5] Injectable copolymers of PNIPAAm-co-AAc [6] and PNIPAM-co-AAc hydrogel scaffolds with proteolytic degradability by incorporation of an oligopeptide cross-linker [7] were prepared for biomedical applications. Intramolecular complex formation of PNIPAM with Human Serum Albumin was investigated for enhancing a better understanding of the mechanisms of biomacromolecular interactions available in nature.[8]
Recently, we have developed microgels consisting of polymer interpenetrating networks (IPN) of PNIPAM and PAAc (PNIAPM-IPN-PAAc).[9–12] One of the major advantages of the PNIPAM-IPN-PAAc over the copolymers of PNIPAM-co-PAAc is that the phase transition temperature of the PNIPAM remains the same, while the random copolymerization results in the increasing Tc of the system upon the increase of PAAc.[3] Even more unusual, we have found that aqueous dispersion of PNIPAM-IPN-PAAc microgels can transfer from a liquid at room temperature to a physically bonded microgel network above 33 °C [9–10] and has been explored for controlled drug release.[11–12] Many polymeric systems can form gels via normal thermoreversible sol-gel transitions without chemical reaction such as gelatin and polysaccharides,[13–14] that are a liquid at a higher temperature and become a gel at an lower temperature. Some polymeric solutions with an inverse thermoreversible gelation have been studied including poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers (Pluronic or Poloxamer) [15] and degradable triblock copolymers [16]. However, there are few reports on microgels dispersions that undergo an inverse thermo-reversible gelation .
In this paper, we report the study of viscoelastic behavior and controlled release in vivo of the PNIPAM-IPN-PAAc microgel dispersion. Viscoelastic behavior is one of the most important properties of colloidal or polymer dispersions and related to biomacromolecule applications.[17–18] Previously, viscoelastic behavior of concentrated colloidal dispersions of a core-shell latex with a poly(styrene) (PS) core and a cross-linked, temperature sensitive PNIPAM shell have been studied.[19] It is found that by chemically fixing a thermo-sensitive PNIPAM shell onto the surface of a charge-stabilized PS core, the rheological properties of which can be controlled by temperature and which is stable against flocculation even at elevated temperatures. On the other hand, rheological behavior of spherical PNIPAM microgels were investigated.[20] The dispersion of these microgels are liquid but becomes physically close-packed below the LCST of the PNIPAM.[20] The dispersion of PNIPAM-IPN-PAAc microgels studied here should be an good model system for investigation of the inverse thermoreversible gelation. We then study the biocompatibility of drug loaded microgel dispersion in the subcutaneous cavity of mice and controlled release in vivo usig a fluorescent dye as a model drug.
Experimental Section
Materials
N-Isopropylacrylamide was purchased from Polysciences, Inc. Dodecyl sulfate sodium salt 98%, potassium persulfate, acrylic acid 99%, N,N’-methylenebisacrylamide 99%, tetramethylethylenediamine, and ammonium persulfate were purchased from Aldrich. Distilled and deionized water was used through all the experiments.
IPN Microgel Preparation
PNIPAM-IPN-PAAc microgels were synthesized according to previous reports.[9–10] First, we prepared PNIPAM microgels using precipitation polymerization. 3.8g of N-isopropylacrylamide, 0.132g of N,N’-methylenebisacrylamide, and 0.6 g of sodium dodecyl sulfate were dissolved in 240 g of distilled and deionized water under continuous stirring for 1 h. After the solution was bubbled for 40 min at 70 °C using nitrogen gas, 0.166 g of potassium persulfate was dissolved in 20mL of DI water and added to start the polymerization. The reaction lasted for 4 h at 70 °C under nitrogen atmosphere. The PNIPAM microgels were then dialyzed (Spectra/Por 7 dialysis membrane, MWCO 14 000, VWR) against DI water for two weeks at room temperature to remove the unreacted monomers and surfactant. The final PNIPAM microgel concentration was adjusted to 13 mg/mL.
Second, we used PNIPAM microgels as seeds to prepare PNIPAM-IPN-PAAc microgels. 35g of the as-prepared PNIPAM microgel solution was diluted to 350g with DI water. 0.5g of N,N’ - methylenebisacrylamide and 2.3 g of acrylic acid were added. The solution was stirred for 120 min at 22°C with nitrogen gas purging. 0.2 g of ammonium persulfate and 0.2 g of tetramethylethylenediamine separately dissolved in 10g of DI water were added rapidly to the solution. The reaction mixture was kept at 22°C for 40 min under nitrogen atmosphere. The obtained PNIPAM-IPN-PAAc microgels were purified through dialysis.
Particle size measurements
The sizes of PNIPAM and IPN microgels in water were measured using dynamic light scattering spectrometer (ALV, Germany), which was equipped with an ALV-5000 digital time correlator and a helium-neon laser (Uniphase 1145P, output power of 22 mW and wavelength of 632.8 nm). All the measurements were carried out at scattering angle of 90°. The sample temperature was controlled with a refrigerator circulating water bath.
Rheological characterization
Dynamic rheological analysis was performed with a stress-controlled rheometer (ATS Viscoanalyser) equipped with a solvent trap to prevent water evaporation. The parallel plate geometry with the plate diameter of 25 cm was used and the sample gap was adjusted to 0.5 mm. All the rheological experiments were performed within the linear viscoelastic region. The results were analyzed with Rheoexplorer v5.0 software.
Atomic Force Microscopy (AFM)
AFM images were obtained with a Dimension 3100 scanning probe microscope (xxx) operating in tapping mode with a drive frequency 257 kHz. Samples were prepared by casting 4–5 drops of dispersion onto a glass slide, and they were allowed to dry.
In vivo testing
Animal implantation model was used to determine the biocompatibility and drug slow release properties of PNIPAM-IPN-PAAc microgel dispersion. Briefly, fluorescein, as a model drug (with final concentration of 0.04%), was added into an aqueous dispersion of PNIPAM-IPN-PAAc microgels with polymer concentration of 6 wt% at ambient temperature. Because the microgel dispersion is a free-flowing liquid, fluorescein can be uniformly mixed with the dispersion under stirring. This drug loaded liquid was then injected subcutaneously in Balb/C mice (25 grams body weight) from Taconic Farms (Germantown, NY, USA). After implantation for 24 hours, implant-bearing mice were sacrificed and the implants and the surrounding tissues were then frozen in OCT embedding media (Polysciences Inc., Warrington, PA) at −80°C. Ten micrometers thick sections were sliced using a Leica Cryostat (CM1850) and placed on poly-L-lysine coated slides. To assess the tissue responses to microgel implants, some of these slides were H&E stained. To determine the amount of residual fluorescein, the slide sections were observed using a Leica fluorescence microscope (Leica Microsystems) equipped with a Nikon E500 Camera (8.4V, 0.9A, Nikon Corp., Japan).
Results and Discussion
A. Characterization of IPN microgels
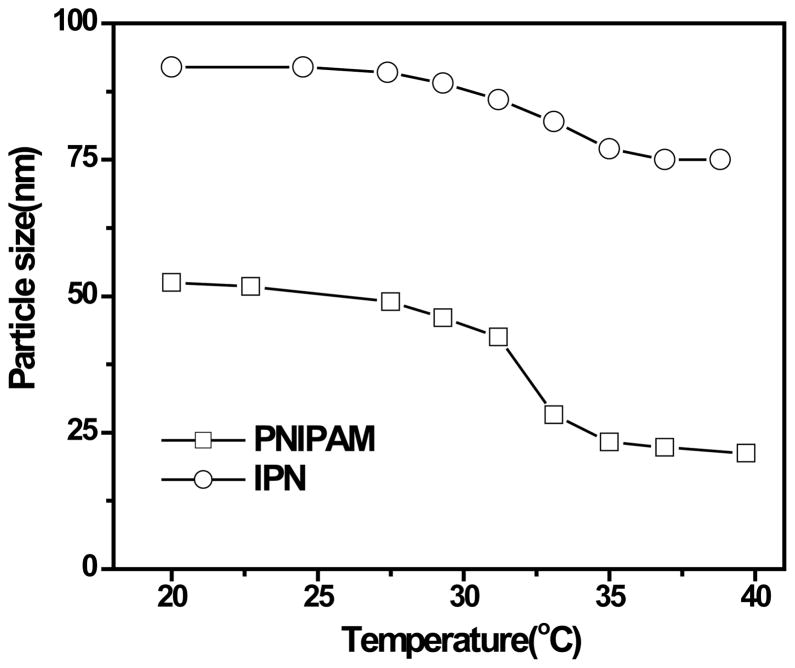
Figure 1 shows the size distributions of hydrodynamic radii of PNIPAM and IPN microgels at 21 °C. The polymer concentration of the PNIPAM and IPN microgel dispersions were the same as 5.0 × 10−6 g/mL with pH 6.5–7.0. The radius of the IPN microgel is lager than the one for PNIPAM microgel because of the addition of hydrophilic PAAc polymer network making the microgel to absorb more water at room temperature.
Figure 1.
The distribution of hydrodynamic radius of PNIPAM and IPN microgels at 21 °C. The polymer concentration of the PNIPAM and IPN microgel dispersions were the same as 5.0 × 10−6g/mL with pH 7.0.
One atomic force microscope image of the IPN microgelsis presented in Figure 2. Comparing with dynamic light scattering spectrum in Figure 1, one can see that the average particle diameter of microgels is reduced considerably to about 50 nm in the AFM image. This is because the microgel was dried for AFM measurements while the microgel was swollen in water for light scattering measurements. As shown in Figure 2, although the size of microgels is reduced during the drying process, the particles remain spherical essentially.
Figure 2.
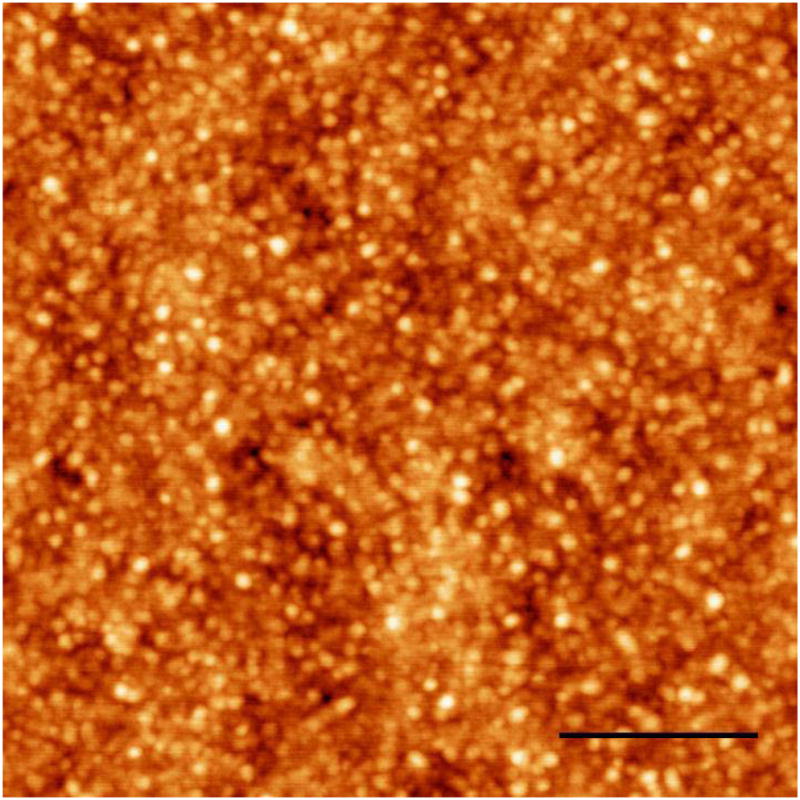
AFM image of PNIPAM-IPN-PAAc microgels. The microgels were dried after the IPN dispersion was cast in a glass slide. The driven frequency is 276 kHz. The sale bar is 0.5 microns.
Figure 3 shows temperature-induced volume phase transition. The IPN microgel undergoes a volume phase transition at 33 °C that is the same as the one for the PNIPAM microgel. For a randomly copolymerized PAAc/PNIPAM gel, the volume phase transition temperature increases with PAAc concentration.[3] The volume change of the IPN below and above the volume transition temperature is smaller than that of the PNIPAM. This is because the PAAc network in the IPN is not sensitive to the temperature change and can reduce the shrinkage of the PNIPAM network.
Figure 3.
Temperature dependence of hydrodynamic radii for the diluted PNIPAM and IPN microgel dispersions at pH 7.
B. Viscoelastic behavior of the IPN microgel dispersions
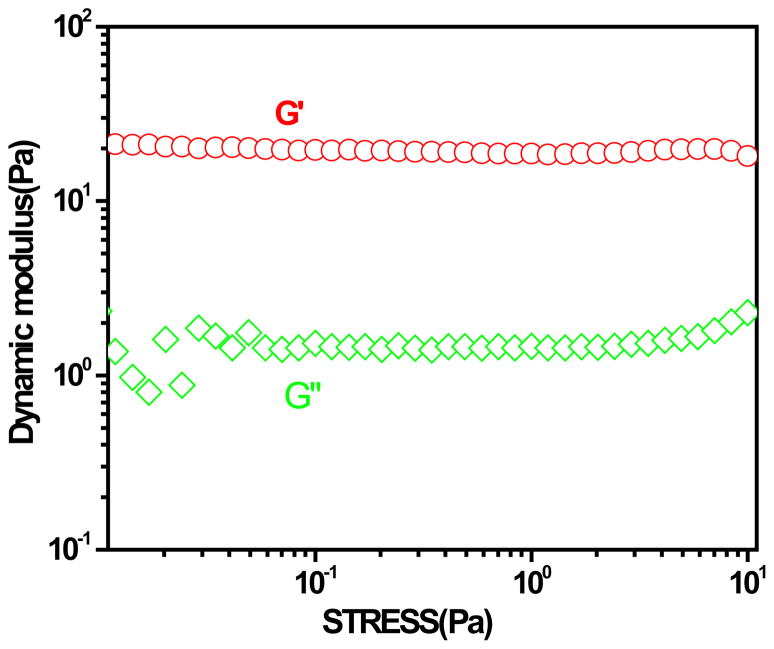
In order to ensure that the rheological measurements were performed within linear viscoelastic region, a stress sweep experiment at a frequency of 1.0 Hz was carried out to define this region. Figure 4 shows the stress dependence of storage and loss modulus, G' and G' ' of the dispersions of PNIPAM-IPN-PAAc microgels at a temperature above the gelation temperature. There is no significant change in G' and G'' until the stress is more than 10 Pa. To avoid possible stress induced sliding between the microgels [20] and obtain good signals, we have used the stress of 2 Pa for all the data taken by the Rheometer.
Figure 4.
Stress dependence of storage and loss moduli (G' and G' ') of the dispersion of IPN microgels at 21 °C. The polymer concentration of the dispersion is 2× 10−2g/mL.
The storage and loss moduli G' and G' ' of dispersions of IPN microgels are shown in Figure 5 as a function of temperature at various polymer concentrations. At a low polymer concentration of 1.5 wt %, G’ is always larger than G’’ over the entire temperature range studied. This indicates that the dispersion remains in a liquid state. When the temperature increases from 25°C to 32°C, both G' and G'' decrease with the temperature increase. This is the common behavior of a polymer solution.[21-23] For polymer solutions, dynamic modulus decrease can be ascribed to increase in chain flexibility and compactness of polymer molecules in solution with increasing temperature. Therefore, rheological parameters decrease with increasing temperature. However, for our samples which consist of the microgels dispersed in the water, the decrease in the dynamic modulus can result from the decrease of particle size and hydrogen-bonded hydration water with increasing temperature. When temperature further increases, both G' and G'' increase sharply and the growth rate of G' is much more than that of G'' . This indicates that there are interactions among microgels upon the temperature increases above 33 °C. However, these interactions are not large enough to make the fluid to become a gel.
Figure 5.
Evolution of dynamic modulus of PNIPAM-IPN-PAAc microgels (pH 7) at 37 °C with temperature increase at different polymer concentrations: (a) 1.5, (b) 3.0 and (c) 6.0 wt%, respectively.
However, as polymer concentration increases from 1.5 to 3.5 wt%, there is an intercept between G’ and G’’ at 33 °C(Figure 5b) . Below the intercept temperature, G’ is larger than G’’ but G’has much higher values than G’’ and the elastic response dominates above this point. For a higher polymer concentration of 6 wt%, the interception point remains the same as one as for the 3.5 wt% sample(Figure 5c) .
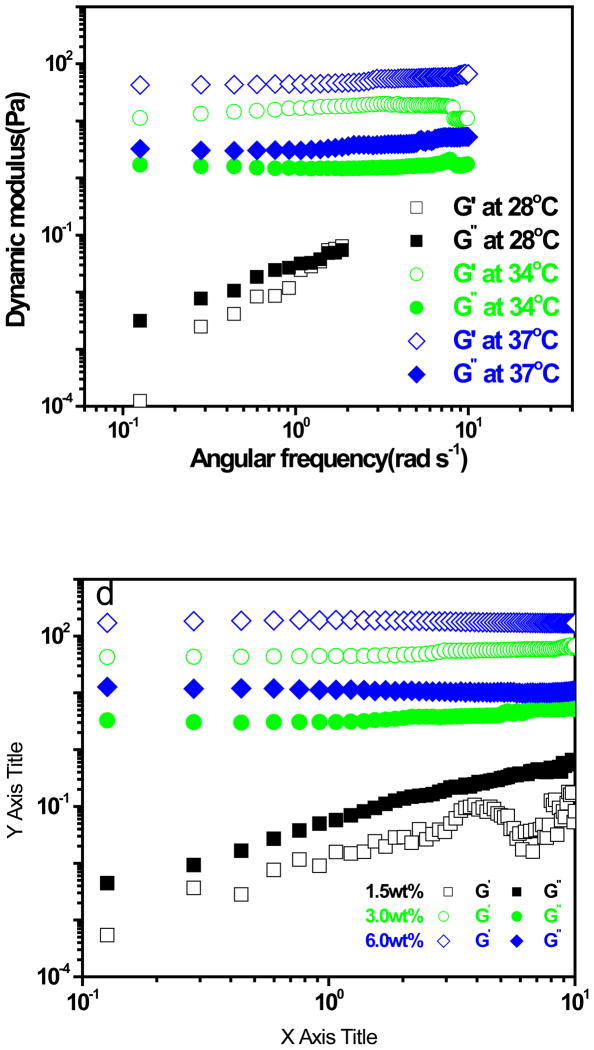
The frequency dependence of storage and loss moduli, G' and G' ', respectively, was determined in low amplitude oscillatory shear experiments as shown in Figure 6a for IPN microgel dispersions at polymer concentration 3.0 wt% for three different temperatures. At high temperatures (38 and 34 °C), the samples behave as viscoelastic solids and the storage modulus is larger than the loss modulus over the entire frequency range. The IPN microgel particles possess strong interparticle interactions that hold the particles together. G' is independent of frequency and corresponds to the plateau modulus, Gp. G’ at 38 C is higher than G’ at 34 C indicates that the gel particles becomes stronger as the temperature is increased higher from the gelation temperature 33. Figure 6b showed frequency dependencies of G’ and G’’ for the IPN with different concentrations at 37°C. One can see that for 1.5 wt% dispersion, G’ was less than G’’ and frequency dependencies of G’ and G’’ was found. This is viscoelastic solution behavior. However, when the concentration of IPN were increase to 3.0 and 6.0 wt%, respectively, G’ was much larger than G’’ and frequency independencies of G’ and G’’ was observed, which is characteristic of solid-like state.
Figure 6.
Storage modulus, G', and loss modulus, G' ', as a function of angular frequency (ω). (a) varying tmperature: 28, 34, and 37 °C, while the polymer concentration remains the same at 3.0 wt%. (b) varying polymer concentrations: 1.5, 3.0 and 6.0 wt%, while the temperature remains the same at 28 °C .
To determine the gelation temperature, we have used Winter and Chambon’s method [24] by measuring loss tangent at various frequencies as shown in Figure 6 for IPN microgel dispersion at polymer concentration of 3 wt% with stress of 2 Pa. Here tan(delta)=G’/G’’. For a fluid state, tan(δ) →∞ and tan(δ) →0 after the gelation is complete.[20] It can be seen from Figure 6 that the loss tangent is frequency dependent and decreases during the gel formation, indicating that the dispersion become more and more elastic. The gel temperature is identified at the point where a frequency-independent value of the loss tangent is first observed.[25] We thus determined that the gelation temperature for our system is 33 °C for our sample. It is noted this gelation temperature is exactly the LCST temperature for PNIPAM polymer. Above the LCST, the PNIPAM changes from hydrophilic state to hydrophobic state. We thus conclude that the connectivity among microgels during the gelation was provided by hydrophobic interactions among the microgels. In contrast pure PNIPAM microgels that shrink above the LCST, the PNIPAM-IPN-PAAc microgels do not shrink significantly due to the support of non-shrinking PAAc component of the IPN. As a result, these particles can still remain contact even above the LCST. Below the LCST, the hydrophobic interaction was disrupted so that the gel is transformed into a sol. The unusual gelation capabilities of PNIPAM-IPN-PAAc microgel dispersions come from the contributions of both interpenetrated networks of PNIPAM and PAAc: the PNIPAM provides physical crosslinking bonds between particles via a temperature-dependent interparticle potential, while PAAc in the neutral pH provides repulsive ionic charges that are temperature-independent and prevent the collapse of the particles into an aggregate.
C. Biocompatibility and in vivo drug release experiments
One of the most interesting applications of newly developed microgels dispersions with thermal gelling capabilities may be in the area of controlled drug release. To demonstrate this potential application, we have studied the biocompatibility of the PNIPAM-IPN-PAAc microgel dispersion and its use for entrapping a drug in mice.
The loading of the microgel dispersion with a drug has been achieved by simple mixing of the drug with already prepared dispersion. After subcutaneous implantation in mice, this liquid quickly gelled inside animals because the mice body temperature (~37 °C) was higher than the gelation temperature of 33 °C as revealed by viscoelastic study above. After implantation for 24 hours, the biocompatibility and drug slow release characteristics of PNIPAM-IPN-PAAc microgel were evaluated.
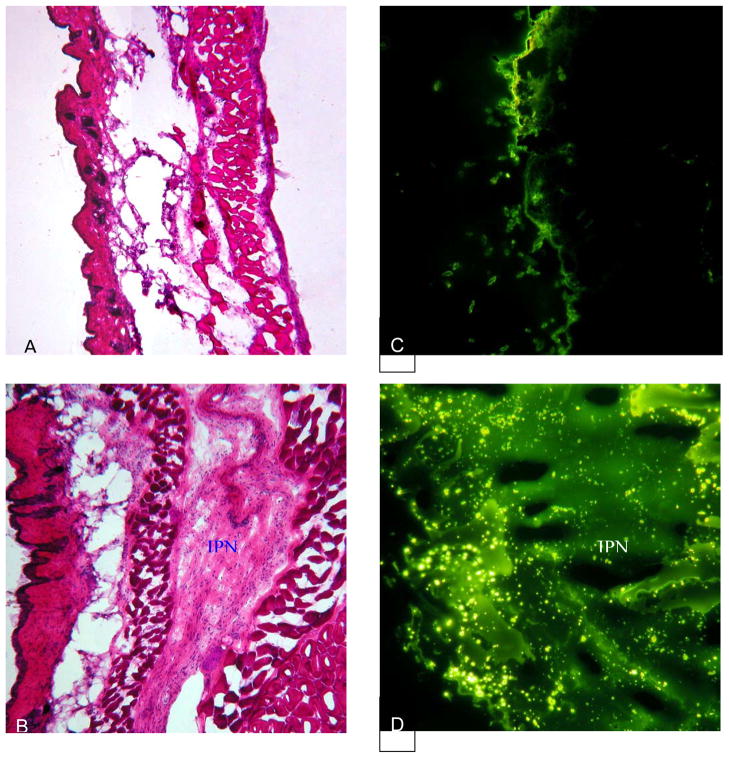
There was no animal fatality which suggest no acute toxicity of the PNIPAM-IPN-PAAc microgel has no acute toxicity to animals. H&E stain of the implants show mild accumulation of the inflammatory cells inside and surrounding implants by compared with saline control (Figure 8a&b). By injecting fluorescein alone, we find that most of the fluorescein dissipates from the implantation site indicating small retention rate of fluorescein in tissue (Figure 8c). On the other hand, large amount of fluorescein is found in the microgel-implanted tissue supporting that the presence of gelled microgel dispersion substantially reduces the diffusion and thus causes the slow release of fluorescein (Figure 8d). These results have shown that the PNIPAM-IPN-PAAc microgel is biocompatible at least in this acute implantation model. Equally important, our findings also support the slow release property of the gelled microgel dispersion.
Figure 8.
Biocompatibility and slow release property of IPN hydrogel were assessed in vivo. Balb/c mice were subcutaneously implanted with either fluorescein or fluorescein loaded IPN gel. At 24 hours post implantation, the animals were sacrificed and the implant-surrounding tissues were recovered for histological analyses. H&E stain shows that (A) fluorescein injection triggers minimal tissue response whereas (B) hydrogel implant prompt mild inflammatory responses. On the other hand, fluorescence images of the tissues reveal that (C) a small amount of residual fluorescein was present in the fluorescein-injected tissue though (D) large amount of fluorescein still reside in the IPN hydrogel implanted tissue.
D. Conclusion
PNIPAM-IPN-PAAc microgels were synthesized by first preparing PNIPAM microgels using precipitation polymerization method and then using PNIPAM microgels as seeds to form an interpenetrating PAAc polymer network. The IPN microgels in water have an average hydrodynamic radius of about 85 nm at 21 °C, measured by dynamic light scattering method. The atomic force miscrocope image shows that the particles are much smaller with a diameter about 50 nm after they are dried but remain their spherical.
The storage and loss moduli G' and G' ' of dispersions of IPN microgels are measured in the linear stress regime as a function of temperature at various polymer concentrations using a stress-controlled rheometer equipped with a solvent trap to prevent water evaporation. At a low polymer concentration of 1.5 wt %, G’ is always larger than G’’ over the entire temperature range studied. This indicates that the dispersion remains in a liquid state. However, as polymer concentration increases from 1.5 to 3.5 or to 6 wt%, there is an intercept between G’ and G’’ at 33 °C. Below the intercept temperature, G” is larger than G’ but above this temperature, G’ has much higher values than G”, indicating the elastic response dominates. The frequency dependence of storage and loss moduli, G' and G' ', respectively, was determined in low amplitude oscillatory shear experiments for IPN microgel dispersions. For dispersions with high polymer concentration (3.5 and 6.0 wt%) and at high temperatures (34 and 38 °C), the samples behave as viscoelastic solids and the storage modulus is larger than the loss modulus over the entire frequency range. To determine the gelation temperature, we have used Winter and Chambon’s method by measuring loss tangent at various frequencies as a function of temperature. The gelation temperature is found to be 33 °C at the point where a frequency-independent value of the loss tangent is first observed.
Via an animal implantation model (Hu et al., 2005; Weng et al., 2004), we have observed that PNIPAM-IPN-PAAc microgels show mild accumulation of the inflammatory cells inside and surrounding implants similar to PNIPAM particles and better than poly-L lactic acid particles and polystyrene particles found in our recent study (Weng et al., 2004). PNIPAM-IPN-PAAc microgels are also found to be non-toxic to test subjects. These results suggest that the gelation process is biocompatible and does not adversely promote foreign body reactions at least in this animal model. On the other hand, our recent study has shown that PNIPAM-IPN-PAAc microgels prolong drug release in vitro. The slow release is likely associated with the gelation procedure which creates diffusion barrier and thus leads to slow release.. This assumption is further supported by our in vivo findings that most of the fluorescein, injecting alone, dissipates from the implantation site quickly, while large amount of fluorescein remains in the microgel-implanted tissue.
In this study, we further investigated a new group of microgels which are produced with two interpenetrating polymer networks of poly-N-isopropylacrylamide and poly-acrylic acid (PNIPAM-IPN-PAAc). These microgels are found to be biocompatible. The temperature dependent gelation properties are proved to be useful for loading a variety of drugs and proteins at room temperature and slowly releasing the loaded agents at body temperature. These findings may lead to the development of novel slow release devices with improved safety and efficacy.
Figure 7.
Temperature dependence of loss tangent (tan(δ)=G’/G’’) at various oscillatory frequencies of 0.28, 0.60, 1.0, 3.12, and 6.28 rad/s. The stress applied is 2 Pa and the polymer concentration of IPN microgel dispersion is 4.91 × 10 −2g/mL at pH 7 .
Acknowledgments
This work is partly supported by the National Science Foundation under grant no. DMR-0507208 (Z. H), Texas Advanced Research Program (Z. H & L. T),and the National Institute of Health grant GM074021 (L.T.).
References
- 1.Schild HG. Prog Polym Sci. 1992;17:163. [Google Scholar]
- 2.Pelton RH, Chibante P. Colloids Surf. 1986;20:247. [Google Scholar]
- 3.Hirotsu Y, Hirokawa T, Tanaka T. J Chem Phys. 1987;87:1392. [Google Scholar]
- 4.Hoffman AS. J Controlled Release. 1987;6:297. [Google Scholar]
- 5.Yin X, Hoffman AS, Stayton PS. Biomacromolecules. 2006;7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 6.Han CK, Bae YH. Polymer. 1998;39:2809. [Google Scholar]
- 7.Stile RA, Healy KE. Biomacromolecules. 2002;3:591–600. doi: 10.1021/bm0101466. [DOI] [PubMed] [Google Scholar]
- 8.Matsudo T, Ogawa K, Kokufuta E. Biomacromolecules. 2003;4:728–735. doi: 10.1021/bm034033t. [DOI] [PubMed] [Google Scholar]
- 9.Hu ZB, Xia XH. Advanced Materials. 2004;16:305–308. [Google Scholar]
- 10.Xia XH, Hu ZB. Langmuir. 2004;20:2094–2098. doi: 10.1021/la0354483. [DOI] [PubMed] [Google Scholar]
- 11.Xia XH, Hu ZB, Marquez M. Journal of Controlled Release. 2005;103:21. doi: 10.1016/j.jconrel.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Hu ZB, Xia XH, Marques M, Weng H, Tang LP. Macromolecular Symposia. Biological and Synthetic Polymer Networks and Gels. 2005;227:275. [Google Scholar]
- 13.Guenet JM. Thermoreversible Gelation of Polymers and Biopolymers. Academic Press; London: 1992. [Google Scholar]
- 14.Franz G. Adv Polym Sci. 1986;76:1. [Google Scholar]
- 15.Alexandridis P, Hatton TA. Colloids Surfaces A: Physicochem Eng Aspects. 1995;96:1. [Google Scholar]
- 16.Jeong B, Kim SW, Bae YH. Adv Drug Del Rev. 2002;54:37. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 17.Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, Leroux JC, Atkinson BL, Selmani FA. Biomaterials. 2000;21:2155. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 18.Silioc C, Maleki A, Zhu KZ, Kjøniksen AL, Nystrom B. Biomacromolecules. 2007;8:719–728. doi: 10.1021/bm061090o. [DOI] [PubMed] [Google Scholar]
- 19.Senff H, Richtering W. Langmuir. 1999;15:102–106. [Google Scholar]
- 20.Zhao Y, Cao Y, Yang Yl, Wu C. Macromolecules. 2003;36:855–859. [Google Scholar]
- 21.Cho J, Heuzey MC, Begin A, Carreau PJ. Biomacromolecules. 2005;6:3267–3275. doi: 10.1021/bm050313s. [DOI] [PubMed] [Google Scholar]
- 22.Amin S, Kermis TW, van Zanten RM, Dees SJ, van Zanten JH. Langmuir. 2001;17:8055–8061. [Google Scholar]
- 23.Seetapan N, Maingam K, Plucktaveesak N, Sirivat A. Rheologica Acta. 2005:45. [Google Scholar]
- 24.Winter and Chambon’s method [24]
- 25.Nystrom, B.
References
- Hu ZB, Xia XH, Marques M, Weng H, Tang L. Hydrogel nanoparticles dispersions and their tissue response. Macromolecular Symposia. 2005;227:275–284. [Google Scholar]
- Weng H, Zhou J, Tang L, Hu Z. Tissue responses to thermally responsive hydrogel nanoparticles. Journal of Biomaterials Science Polymer Edition. 2004;15(9):1167–1180. doi: 10.1163/1568562041753106. [DOI] [PubMed] [Google Scholar]