Abstract
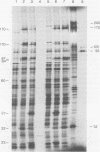
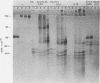
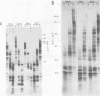
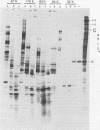
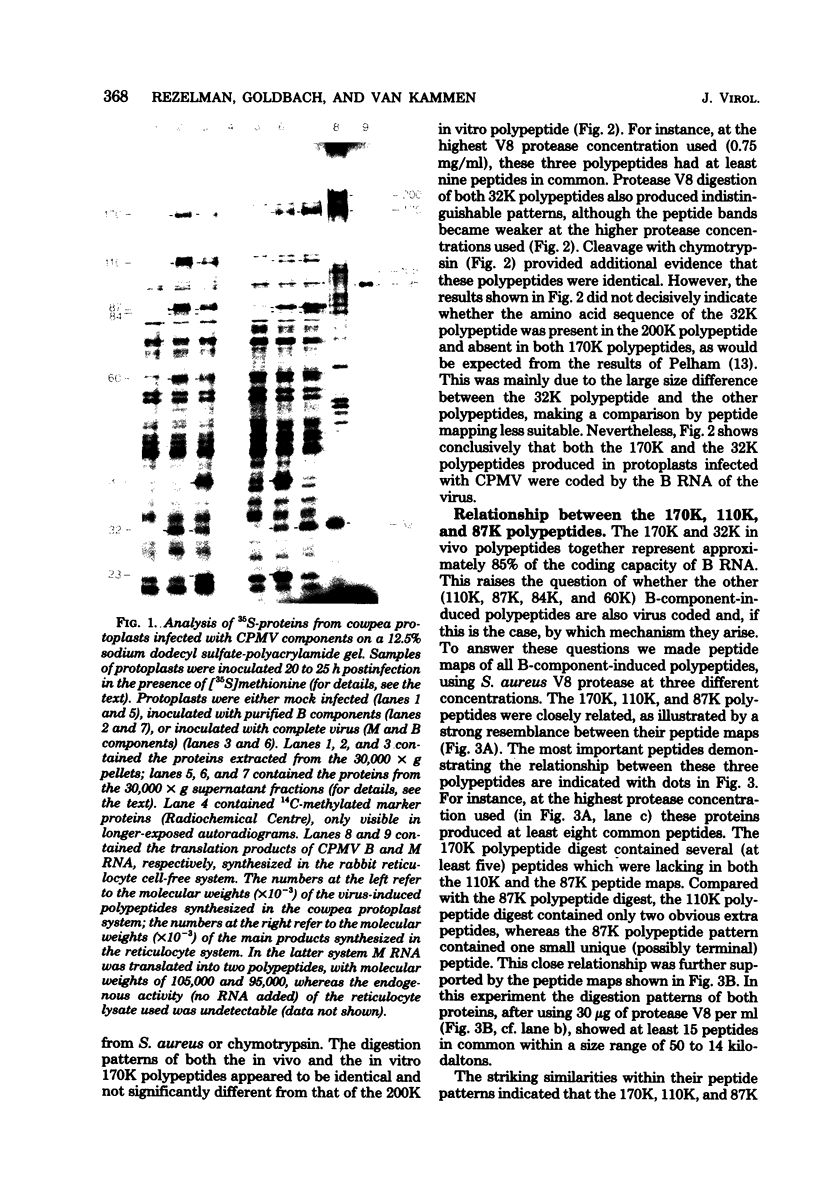
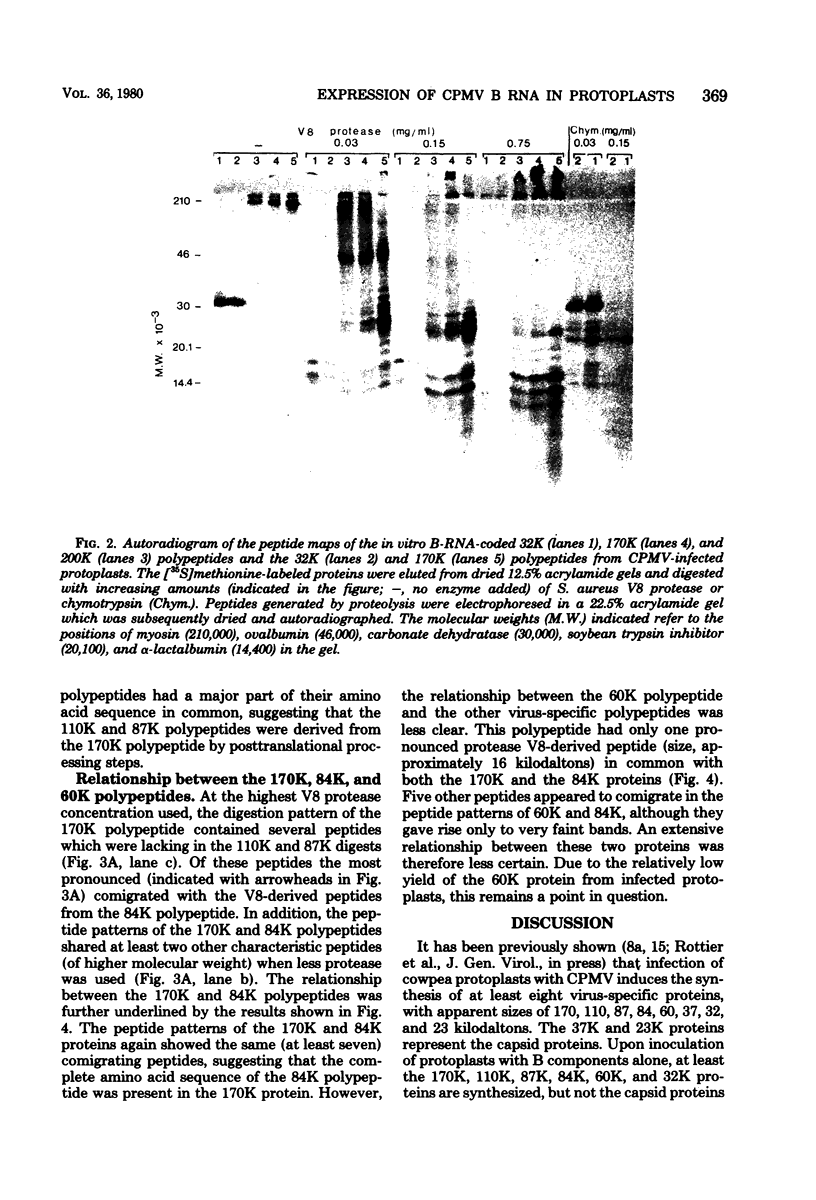
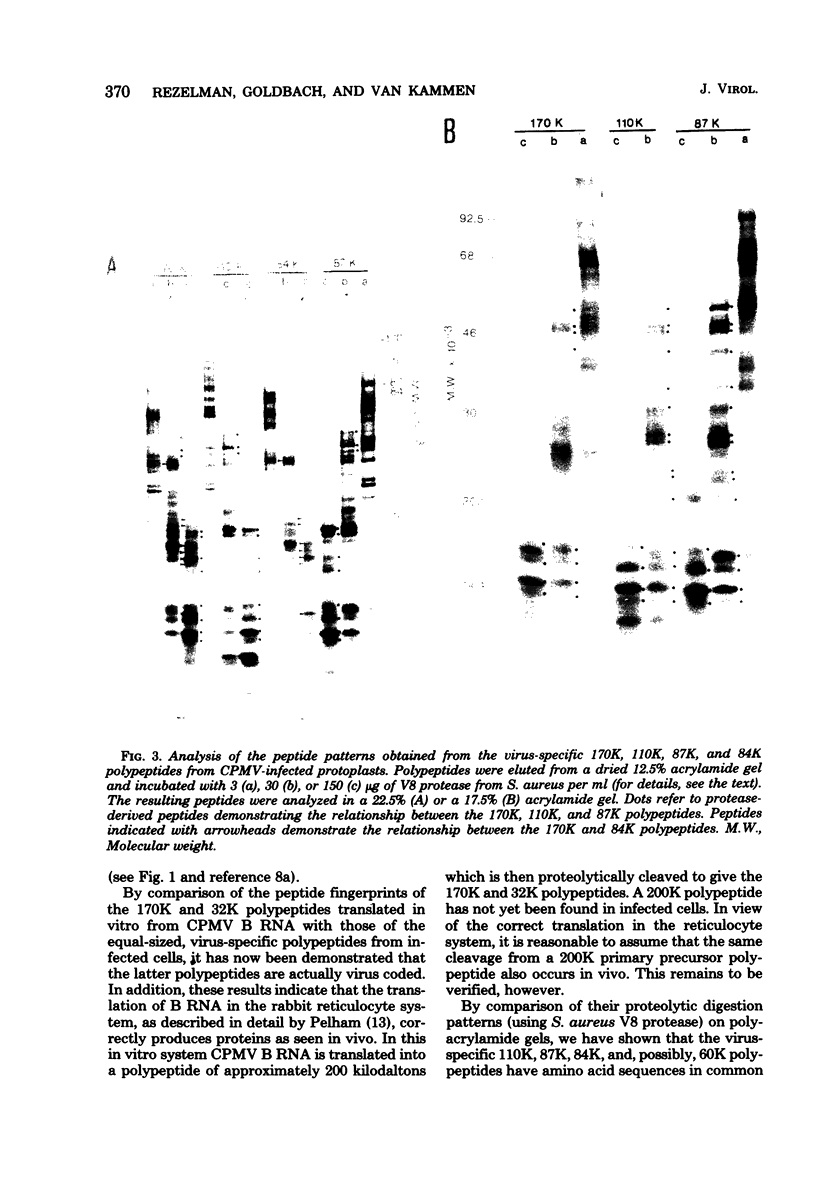
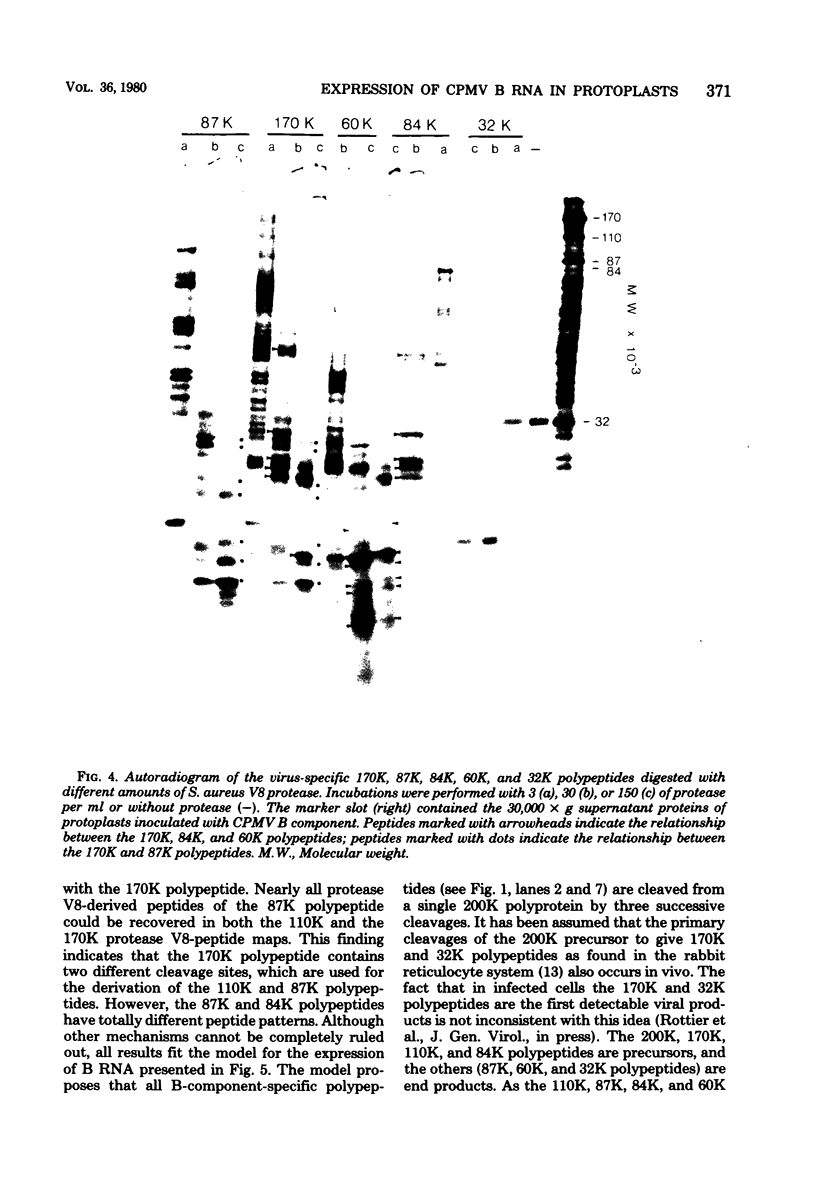
Upon inoculation of cowpea protoplasts with the bottom component of cowpea mosaic virus, at least six virus-induced proteins (with sizes of 170, 110, 87, 84, 60, and 32 kilodaltons) are synthesized, but not the capsid proteins (37 and 23 kilodaltons). These bottom-component-induced proteins were studied with respect to their genetic origin and mode of synthesis. The analyses were based on their electrophoretic peptide patterns resulting from partial digestion with Staphylococcus aureus protease V8. Comparison of the peptide patterns of the virus-induced proteins with those of the cowpea mosaic virus RNA-coded polypeptides produced in rabbit reticulocyte lysate showed that the 170- and 32-kilodalton polypeptides, which are the first viral products in cowpea mosaic virus-infected cells, were actually coded by the bottom component RNA of the virus. The 110-, 87-, and 84-kilodalton polypeptides, and possibly the 60-kilodalton polypeptide, appeared to have amino acid sequences in common with the 170-kilodalton polypeptide, demonstrating that they were virus coded as well. The results indicated that cowpea mosaic virus bottom component RNA was translated in vivo into a single 200-kilodalton polyprotein from which probably all bottom-component-specific proteins arose by three successive cleavages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Daubert S. D., Bruening G., Najarian R. C. Protein bound to the genome RNAs of cowpea mosaic virus. Eur J Biochem. 1978 Dec 1;92(1):45–51. doi: 10.1111/j.1432-1033.1978.tb12721.x. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Verver J. W., Goldbach R. W., Van Kammen A. Efficient reverse transcription of cowpea mosaic virus RNAs. Nucleic Acids Res. 1978 Dec;5(12):4643–4661. doi: 10.1093/nar/5.12.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager C. P. Genetic analysis of cowpea mosaic virus mutants by supplementation and reassortment tests. Virology. 1976 Mar;70(1):151–163. doi: 10.1016/0042-6822(76)90245-2. [DOI] [PubMed] [Google Scholar]
- Doel T. R., Sangar D. V., Rowlands D. J., Brown F. A re-appraisal of the biochemical map of foot-and-mouth disease virus RNA. J Gen Virol. 1978 Nov;41(2):395–404. doi: 10.1099/0022-1317-41-2-395. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Borst P., Bollen-de Boer J. E., van Bruggen E. F. The organization of ribosomal RNA genes in the mitochondrial DNA of Tetrahymena pyriformis strain ST. Biochim Biophys Acta. 1978 Nov 21;521(1):169–186. doi: 10.1016/0005-2787(78)90260-5. [DOI] [PubMed] [Google Scholar]
- Hibi T., Rezelman G., Van Kammen A. Infection of cowpea mesophyll protoplasts with cowpea mosaic virus. Virology. 1975 Apr;64(2):308–318. doi: 10.1016/0042-6822(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Jaspars E. M. Plant viruses with a multipartite genome. Adv Virus Res. 1974;19:37–149. doi: 10.1016/s0065-3527(08)60659-4. [DOI] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Zabel P., van Kammen A. Cowpea mosaic virus RNAs have neither m7GpppN ... nor mono-, di- or triphosphates at their 5' ends. Cell. 1977 May;11(1):73–82. doi: 10.1016/0092-8674(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Aalbers A. M., van Kammen A., Thuring R. W. Molecular weights of plant viral RNAs determined by gel electrophoresis under denaturing conditions. Virology. 1974 Aug;60(2):515–521. doi: 10.1016/0042-6822(74)90345-6. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Rezelman G., van Kammen A. The inhibition of cowpea mosaic virus replication by actinomycin D. Virology. 1979 Jan 30;92(2):299–309. doi: 10.1016/0042-6822(79)90135-1. [DOI] [PubMed] [Google Scholar]
- Stanley J., Rottier P., Davies J. W., Zabel P., Van Kammen A. A protein linked to the 5' termini of both RNA components of the cowpea mosaic virus genome. Nucleic Acids Res. 1978 Dec;5(12):4505–4522. doi: 10.1093/nar/5.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Manna M. M., Bruening G. Polyadenylate sequences in the ribonucleic acids of cowpea mosaic virus. Virology. 1973 Nov;56(1):198–206. doi: 10.1016/0042-6822(73)90299-7. [DOI] [PubMed] [Google Scholar]
- van Kammen A. Purification and properties of the components of cowpea mosaic virus. Virology. 1967 Apr;31(4):633–642. doi: 10.1016/0042-6822(67)90192-4. [DOI] [PubMed] [Google Scholar]
- van Kammen A. The relationship between the components of cowpea mosaic virus. I. Two ribonucleoprotein particles necessary for the infectivity of CPMV. Virology. 1968 Feb;34(2):312–318. doi: 10.1016/0042-6822(68)90241-9. [DOI] [PubMed] [Google Scholar]