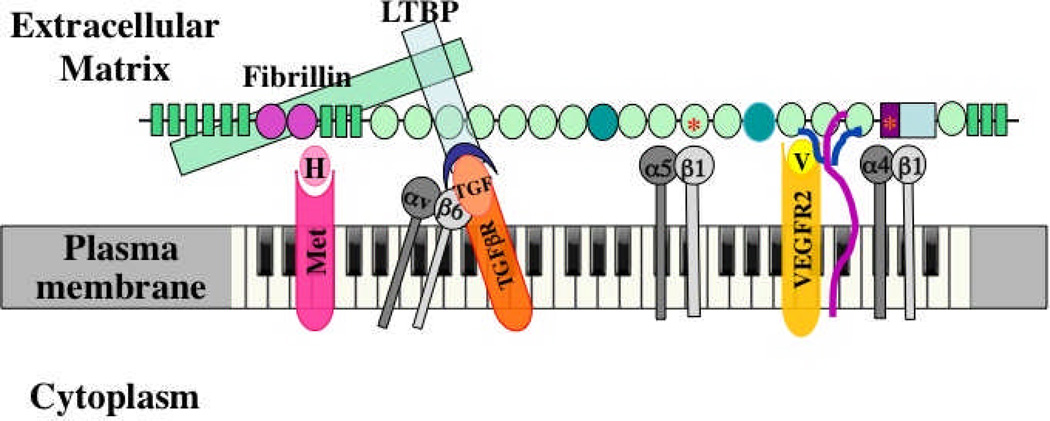
Figure 3. Multidomain interactions of ECM proteins with cells.
The example shown is fibronectin40. Multiple domains are known to bind to integrins, to other ECM proteins and to growth factors, as shown. Integrins α5β1 and α4β1 bind, respectively, to RGD and LDV motifs; heparan sulfate chains of syndecan (purple/blue) bind to FN3-13 as does VEGF. Evidence suggests that VEGF (V, yellow) signals through its own receptor (VEGFR2) more effectively when bound to fibronectin16. The same is proposed here for HGF (H) and its receptor (Met, pink). As shown in Figures 1 and 2, fibrillin (green) binds to an N-terminal region of fibronectin and in turn binds LTBP (blue), which recruits TGFβ in a latent complex with LAP (blue crescent). αvβ6 integrin can bind an RGD site in LAP, activating TGFβ, so that it can bind its own receptors (orange). The proposal is that fibronectin organizes and integrates all these signals at two levels. First, by recruiting growth factors to the ECM, fibronectin localizes those signals at the cellular level. Second, the close juxtaposition of the domains in fibronectin brings the different receptors together into an organized submicron patch in the cell surface membrane. Each domain is 2–4 nm in diameter and the entire fibronectin subunit shown is 60–70 nm long, so the receptors will be brought into close apposition such that their signals provide complex, integrated information to the cell – metaphorically generating a melodies and chords in contrast with the “single notes” generated by each receptor. Fibronectin is essential for angiogenesis, and most of the bound receptors and ligands have been shown to play roles in angiogenesis. This model suggests that fibronectin and its associated ECM proteins orchestrate and integrate these signals. In addition, alternatively spliced domains of fibronectin (darker green ovals) are also necessary for proper vascular development and it is a reasonable hypothesis that they introduce additional ligands and/or receptors into the mix.