Abstract
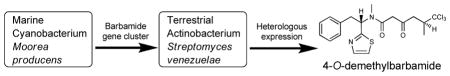
Heterologous expression of the barbamide biosynthetic gene cluster, obtained from the marine cyanobacterium Moorea producens, in the terrestrial actinobacterium Streptomyces venezuelae, resulted in the production of a new barbamide congener 4-O-demethylbarbamide, demonstrating the potential of this approach for investigating the assembly and tailoring of complex marine natural products.
Marine invertebrates and bacteria are an extraordinarily rich source of novel bioactive secondary metabolites.1 Several marine natural products and their derivatives are now used as clinical therapeutics, and many others have shown potential for the treatment of cancer, inflammation, pain, and other diseases.2 However, a general impediment to the development of many of these exciting molecules is the limited quantities obtained from Nature as well as the difficulty in culturing the source organisms, including invertebrates, algae or associated symbiotic microorganisms. Moreover, total chemical synthesis of structurally complex metabolites is economically impracticable in many cases. Therefore, in order to harness the therapeutic potential of these natural product lead compounds, reliable methods must be developed for their re-supply. Cloning and heterologous expression of the biosynthetic gene clusters represents one such solution that can also facilitate the generation of insightful analog structures through metabolic engineering. To date, the majority of biosynthetic studies of marine secondary metabolites have been limited to those isolated from marine actinobacteria and cyanobacteria.3 Most genetic and enzymatic studies of cyanobacterial secondary metabolites have been conducted with the marine genus Moorea producens (previously classified as Lyngbya majuscula),4 a prolific producer of structurally diverse natural products5 including the cancer cell cytotoxin curacin A6 and the molluscicidal agent barbamide (1; Figure 1).7 A draft genome was recently reported for the M. producens 3L strain,8 which revealed the genetic basis for its unique spectrum of natural products. However, an inability to genetically manipulate this filamentous cyanobacterium along with its extremely slow growth rate (doubling time = 6 days or more)9 motivated us to develop a robust heterologous expression platform for cyanobacterial secondary metabolite gene clusters.
Figure 1.
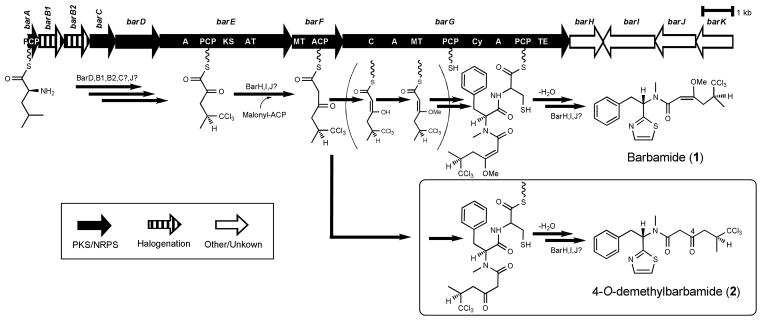
Barbamide gene cluster and the proposed biosynthetic pathways for 4-O-demethylbarbamide (2) and barbamide (1).11 A, adenylation domain; ACP, acyl carrier protein; AT, acyltransferase domain; C, condensation domain; Cy, cyclization domain; KS, ketosynthase domain; MT, methyltransferase domain; PCP, peptidyl carrier protein; TE, thioesterase domain.
Although cyanobactin ribosomal peptides from cyanobacteria were heterologously produced and engineered successfully in Escherichia coli,10 no polyketides or hybrid polyketide-nonribosomal peptides from marine cyanobacteria have been produced in a heterologous host to date. Barbamide (1) attracted our attention because its biosynthetic gene cluster encoding for a NRPS/PKS (nonribosomal peptide synthetase/polyketide synthase) hybrid was the first identified from a marine cyanobacterium,11 and its assembly involves several unique biochemical features such as trichlorination, a one-carbon truncation during chain extension, formation of an E-double bond, and thiazole ring formation (Figure 1; see the detailed biosynthetic pathway in Figure S1, Supporting Information). Currently, only the trichlorination of leucine has been biochemically characterized.12 The 26 kb barbamide gene cluster contains twelve ORFs, designated barA–barK (GenBank accession no. AF516145).11
Streptomyces spp. are known to produce an enormous range of secondary metabolites. Therefore, this genus has been used as a heterologous expression host for a variety of secondary metabolites including polyketides and nonribosomal peptides.13 One of the advantages of Streptomyces for the heterologous expression of PKS or NRPS pathways is its unique capability to accommodate these large genes compared to other heterologous hosts, such as E. coli.13 Recently, heterologous expression was applied in Streptomyces coelicolor M512 for the approximately 11 kb biosynthetic gene cluster of the indole alkaloid lyngbyatoxin A from M. producens.9 Despite large differences in the %GC content and codon usage between cyanobacteria and actinobacteria, two nonmodular proteins, a cytochrome P450 monooxygenase LtxB and a reverse prenyltransferase LtxC were successfully expressed, thus showing the potential of Streptomyces as a useful heterologous host. However, premature transcriptional termination of the ltxA gene product, a large bimodular NRPS, was observed and thus neither lyngbyatoxin A nor its derivatives were produced. Among Streptomyces, pikromycin-producing S. venezuelae has been developed as a promising host for the production of polyketides, hybrid polyketide-nonribosomal peptides as well as aminoglycosides due to its rapid growth and relative ease of genetic manipulation.14–16 Here, we demonstrate heterologous expression of the entire barbamide biosynthetic gene cluster in an engineered strain of S. venezuelae DHS 2001 from which the pikromycin PKS gene cluster has been deleted.17 These efforts resulted in the production of 4-O-demethylbarbamide (2; Figure 1), a new barbamide derivative that only lacks an O-methyl group relative to the parent structure but shows several-fold improved molluscicidal activity compared to 1. To our knowledge, this is the first successful functional heterologous expression of a marine cyanobacterial NRPS/PKS gene cluster in a genetically amenable terrestrial host.
As a starting point, the barbamide biosynthetic genes barA–barK were cloned into a replicative E. coli–Streptomyces shuttle vector pDHS702,18 containing the pikAI promoter of the pikromycin PKS from cosmid PLM4911 in a series of sub-cloning steps (Figure S2, Tables S1 and S2, Supporting Information). The resulting expression plasmid pYJ1614 was designed to express the barA–barH genes under the control of the pikAI promoter and to transcribe barI–barK genes convergently from a native promoter in the barbamide cluster. The native intergenic regions and ribosome-binding site of each gene were maintained in this construct. The engineered plasmid was introduced into S. venezuelae DHS2001 to provide the S. venezuelae YJ348 strain. HPLC-ESI-MS/MS analysis of the organic extract of YJ348 grown on R2YE19 solid medium at 30°C for 6 days revealed a peak that was consistent with a barbamide (1) derivative, although 1 was not detected (Figure S3, Supporting Information). Analysis of the extracts of the cultured recombinant S. venezuelae YJ348 strain revealed a new peak that eluted at 42.8 min with an m/z 448. This product was only detected in the YJ348 strain and not from extracts obtained from S. venezuelae DHS2001 possessing an empty vector. The m/z 448 molecular ion cluster showed the characteristic isotope signature for three chlorine atoms, and the MS/MS spectrum deriving from it yielded fragment ions at m/z 231, 219, 188 and 134. The presence of fragments at m/z 219, 188 and 134 in common to those of 1, as well as the production of a fragment at m/z 231 corresponding to the loss of 14 Da from the diagnostic fragment of 1 at m/z 245 (Figure S4, Supporting Information), suggested that it was a close structural analog of 1. These mass differences relative to 1 suggested that the new compound was deficient in a methyl group located between the C-1 and C-4 positions. Based on the proposed barbamide biosynthetic pathway and precursor incorporation studies,20 elimination of the C-1 methyl group deriving from the leucine starter unit seemed unlikely. Therefore, the new barbamide derivative detected in the extract of this engineered YJ348 strain was predicted to be 4-O-demethylbarbamide (2).
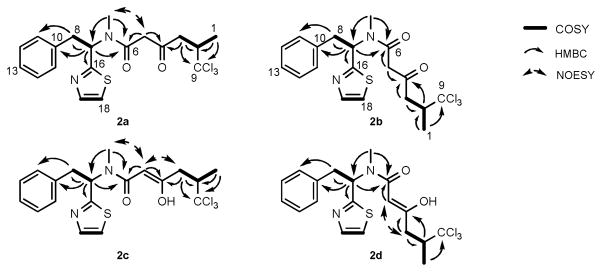
Interestingly, we found this same peak as a minor compound in partially purified M. producens fractions by HPLC-ESI-MS analysis, suggesting that the demethyl analog is a naturally occurring derivative of 1 (Figure S5, Supporting Information). Hence, the new analog was purified in larger scale from a M. producens extract by reversed-phase HPLC, and its chemical structure fully elucidated by detailed spectroscopic analyses (Figure S6 to S11, Supporting Information). The 1H NMR spectrum of the O-demethyl derivative of barbamide was complicated by the presence of four interconverting isomers (2a–2d), present in an equilibrium ratio of ca. 17:7:2:1. However, 1D and 2D NMR methods were used to assign almost all shifts of the four isomers (2a–2d, Figure 2 and Table S3). In 2a, HMBC correlations from H-2/H2-3/H2-5 to C-4 (δC 201.7) allowed assignment of a ketone at the C-4 position, a key point of departure from the structure of 1. Additionally, a NOESY correlation between the NCH3 group and H2-5 revealed that this major compound was the trans amide isomer. The 1H NMR signals of the second isomer (2b) were almost identical to those of 2a, except that the chemical shift of H-7 and C-7 of 2b were located at δH 5.59 and δC 59.8, suggesting that this was the cis amide conformer. Consistent with this proposal (i.e. the cis amide isomer with a 4-keto functionality), a NOESY correlation between the NCH3 and H2-5 was not observed. The NMR signals of the third isomer (2c) were slightly different from those of 2a. In the 1H NMR, H-7 of 2c was observed at δH 6.27, indicating a trans amide isomer as found in 2a. However, the peak for H-5 in 2c was found as a broad singlet at δH 5.47 and the 13C NMR shift of C-5 of 2c was located as δC 88.8, both of which are inconsistent with a 4-keto functionality. Furthermore, the H-5 singlet proton showed HMBC correlations with two deshielded quaternary carbons (C-4 at δC 174.4 and C-6 at δC 171.8). These data indicated that 2c was present in the 4,5-enolic form (i.e. 2a and 2c are tautomers). The geometry of the Δ4,5 double bond was established as Z by observation of a NOESY correlation between H-5 and H2-3. Finally, the chemical shifts for H-5 and C-5 of minor isomer 2d were determined as δH 5.83 and δC 88.2, respectively, and HMBC correlations from H-5 to C-4 and C-6 again indicated the presence of a Δ4,5 double bond. The configuration of this olefin was determined as Z by observation of a NOESY correlation between H-5 and H2-3. However, no NOESY correlation was observed between the NCH3 and H-5 in 2d; combined with the chemical shifts of H-7 and C-7 at δH 6.00 and δC 58.0, respectively, these data indicated isomer 2d possesses a cis amide bond. A comparison of the specific rotation of barbamide with this new barbamide analog, present as a mixture of these four isomers, allowed assignment of the absolute configuration as 2S, 7S.
Figure 2.
Simultaneous structure elucidation of four isomers of 4-O-demethylbarbamide (2a–2d) based on key COSY, HMBC and NOESY correlations.
Compound 2 was found to be a potent molluscicidal agent against the snail Biomphalaria glabrata (LD50 3.9 μM, 95% CI 2.7–5.5 μM, Figure S12, Supporting Information), and is several fold more potent than 1 itself (revised LD100 22 μM).7 Indeed, we had long recognized that there was a more potent molluscicide in the extract of this cyanobacterium, but had been unable to complete its structure elucidation as 2 due to the small quantity and multiple isomers in solution.
It is plausible that 2 is a biosynthetic congener of 1, which results from skipping of the BarF-catalyzed O-methylation reaction (Figures 1 and S1, Supporting Information). However, it is at present unclear why 2 is generated as the sole product in the heterologous host. Semi-quantitative RT-PCR analysis of the transcript levels in the engineered YJ348 strain demonstrated that most of the bar genes are transcribed, including barF that encodes for the O-methyltransferase (Figure S13, Supporting Information). Therefore, it is possible that the barF transcript is unstable in S. venezuelae and fails to be translated. Alternatively, other gene product(s) located outside of the barbamide gene cluster might facilitate the unfavourable energetics involved in forming the E enol substrate which appears to be required for the O-methyl transfer reaction to form 1. The detection of barJ transcripts suggests that the native Moorea promoter can be recognized in Streptomyces, as previously reported,9 although its expression level appears to be lower as compared to genes transcribed from the Streptomyces promoter. Therefore, placement of a Streptomyces promoter in front of the barI–barK operon could increase production of 2. However, truly significant enhancement of production would not be expected because even the expression levels of those cyanobacterial genes driven by the pikAI promoter were low compared to Streptomyces PKS genes heterologously expressed in S. venezuelae,17 probably due to codon usage bias between cyanobacteria and actinobacteria.
In the absence of a gene disruption system for M. producens, identification of the barbamide gene cluster was supported by ATP-PPi exchange assay results using the BarE NRPS adenylation domain as well as the halogenase activity of both BarB1 and BarB2 using recombinant enzymes expressed in E. coli.8,9 The production of 2 by heterologous expression of the 26 kb barbamide gene cluster (barA–barK) unequivocally confirms the identity of this gene cluster. In addition, our results demonstrate that the barA–barK genes constitute the minimal gene set required for formation of 2, which includes the unusual one-carbon truncation. However, significant challenges remain in the heterologous production of 2 in S. venezuelae, most dramatically the very low production yields (less than 1 μg/L) which may be due to suboptimal codon usage in the cyanobacteria DNA compared to the ‘high %GC content’ actinobacteria. Our previous studies demonstrated that heterologous expression of tylosin PKS genes derived from S. fradiae produced approximately 0.5 mg/L of tylactone using the same host and vector system employed in this study.17 However, heterologous expression of epothilone NRPS-PKS genes obtained from the myxobacterium Sorangium cellulosum produced very low levels of epothilone (less than 1 μg/L).15 Synthesis and expression of codon-optimized bar biosynthetic genes corresponding to the S. venezuelae codon bias would likely improve production of 2, as observed for production of plant-derived phenylpropanoids in S. venezuelae.21
The results presented herein provide an important precedent and approach for the biosynthetic study of marine natural products derived from genetically intractable organisms, such as sheath-containing filamentous cyanobacteria. In the current work, we demonstrated heterologous production and identification of a new barbamide derivative 2, which had not been previously isolated from the natural producer. This new compound is several fold more potent than 1 as a molluscicide, and given its lack of other toxicities,7 may be a superb candidate for treating snail-infested waterways which pose health risks for human populations. Moreover, we report here the first functional heterologous expression of a complete marine cyanobacterial NRPS/PKS gene cluster using a readily manipulable antibiotic-producing Streptomyces species, suggesting new opportunities for the large-scale production of valuable marine natural products. Continued advances using cloning-independent next-generation sequencing-based metagenomic approaches22 will certainly provide numerous biosynthetic gene clusters from unculturable symbionts or environmental metagenomic DNA. Heterologous gene expression technologies will thus play a critical role in understanding their products and advancing their utility in drug discovery programs.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation (NRF) grant funded by the Korea government (MEST) (20110018617 and 20100001487), the Intelligent Synthetic Biology Center of Global Frontier Project funded by MEST (20110031961), the Marine and Extreme Genome Research Center Program of the Ministry of Land, Transportation and Maritime Affairs, Republic of Korea, and the National Institutes of Health grant CA108874 (D.H.S and W.H.G). We thank the Korea Basic Science Institute for recording 900 MHz NMR spectra.
Footnotes
Supporting Information Available: Experimentals, biosynthetic details, spectroscopic data of 2, RT-PCR, and molluscicidal assay. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
William H. Gerwick, Email: wgerwick@ucsd.edu.
Yeo Joon Yoon, Email: joonyoon@ewha.ac.kr.
References
- 1.Salomon C, Magarvey N, Sherman D. Nat Prod Rep. 2004;21:105. doi: 10.1039/b301384g. [DOI] [PubMed] [Google Scholar]
- 2.Gerwick W, Moore B. Chem Biol. 2012;19:85. doi: 10.1016/j.chembiol.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane A, Moore B. Nat Prod Rep. 2011;28:411. doi: 10.1039/c0np90032j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engene N, Rottacker E, Kastovsky J, Byrum T, Choi H, Ellisman M, Komarek J, Gerwick W. Int J Syst Evol Microbiol. 2012;62:1171. doi: 10.1099/ijs.0.033761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones A, Monroe E, Eisman E, Gerwick L, Sherman D, Gerwick W. Nat Prod Rep. 2010;27:1048. doi: 10.1039/c000535e. [DOI] [PubMed] [Google Scholar]
- 6.Gerwick W, Proteau P, Nagle D, Hamel E, Blokhin A, Slate D. J Org Chem. 1994;59:1243. [Google Scholar]
- 7.Orjala J, Gerwick W. J Nat Prod. 1996;59:427. doi: 10.1021/np960085a. [DOI] [PubMed] [Google Scholar]
- 8.Jones A, Monroe E, Podell S, Hess W, Klages S, Esquenazi E, Niessen S, Hoover H, Rothmann M, Lasken R, Yates J, Reinhardt R, Kube M, Burkart M, Allen E, Dorrestein P, Gerwick W, Gerwick L. Proc Natl Acad Sci USA. 2011;108:8815. doi: 10.1073/pnas.1101137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones A, Ottilie S, Eustaquio A, Edwards D, Gerwick L, Moore B, Gerwick W. FEBS J. 2012;279:1243. doi: 10.1111/j.1742-4658.2012.08517.x. [DOI] [PubMed] [Google Scholar]
- 10.(a) Schmidt E, Nelson J, Rasko D, Sudek S, Eisen J, Haygood M, Ravel J. Proc Natl Acad Sci U S A. 2005;102:7315. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tianero M, Donia M, Young T, Schultz P, Schmidt E. J Am Chem Soc. 2012;134:418. doi: 10.1021/ja208278k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang Z, Flatt P, Gerwick W, Nguyen V, Willis C, Sherman D. Gene. 2002;296:235. doi: 10.1016/s0378-1119(02)00860-0. [DOI] [PubMed] [Google Scholar]
- 12.Galonić D, Vaillancourt F, Walsh C. J Am Chem Soc. 2006;128:3900. doi: 10.1021/ja060151n. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Boghigian B, Armando J, Pfeifer B. Nat Prod Rep. 2011;28:125. doi: 10.1039/c0np00037j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Han A, Ban Y, Yoo Y, Kim E, Yoon Y. Appl Microbiol Biotechnol. 2010;85:1227. doi: 10.1007/s00253-009-2326-8. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Park J, Jung W, Han A, Ban Y, Kim E, Sohng J, Sim S, Yoon Y. Appl Microbiol Biotechnol. 2008;81:109. doi: 10.1007/s00253-008-1674-0. [DOI] [PubMed] [Google Scholar]
- 16.Park J, Park S, Nepal K, Han A, Ban Y, Yoo Y, Kim E, Kim E, Kim D, Sohng J, Yoon Y. Nat Chem Biol. 2011;7:843. doi: 10.1038/nchembio.671. [DOI] [PubMed] [Google Scholar]
- 17.Jung W, Lee S, Hong J, Park S, Jeong S, Han A, Sohng J, Kim B, Choi C, Sherman D, Yoon Y. Appl Microbiol Biotechnol. 2006;72:763. doi: 10.1007/s00253-006-0318-5. [DOI] [PubMed] [Google Scholar]
- 18.Xue Y, Sherman D. Metab Eng. 2001;3:15. doi: 10.1006/mben.2000.0167. [DOI] [PubMed] [Google Scholar]
- 19.Kieser T, Bibb M, Buttner M, Chater K, Hopwood D. Practical Streptomyces Genetics. John Innes Centre; Norwich, England: 2000. [Google Scholar]
- 20.(a) Flatt P, O’Connell S, McPhail K, Zeller G, Willis C, Sherman D, Gerwick W. J Nat Prod. 2006;69:938. doi: 10.1021/np050523q. [DOI] [PubMed] [Google Scholar]; (b) Sitachitta N, Rossi J, Roberts M, Gerwick W, Fletcher M, Willis C. J Am Chem Soc. 1998;120:7131. [Google Scholar]
- 21.Park S, Yoon J, Paik J, Park J, Jung W, Ban Y, Kim E, Yoo Y, Han A, Yoon Y. J Biotechnol. 2009;141:181. doi: 10.1016/j.jbiotec.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Rath C, Janto B, Earl J, Ahmed A, Hu F, Hiller L, Dahlgren M, Kreft R, Yu F, Wolff J, Kweon H, Christiansen M, Hakansson K, Williams R, Ehrlich G, Sherman D. ACS Chem Biol. 2011;6:1244. doi: 10.1021/cb200244t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.