Abstract
There is increasing evidence that age-associated chronic low-grade inflammation promotes the development of both large-vessel disease (myocardial infarction, stroke, peripheral arterial disease) and small-vessel pathologies (including vascular cognitive impairment) in older persons. However, the source of age-related chronic vascular inflammation remains unclear. To test the hypothesis that cell-autonomous mechanisms contribute to the proinflammatory changes in vascular phenotype that accompanies advancing age, we analyzed the cytokine secretion profile of primary vascular smooth muscle cells (VSMCs) derived from young (∼13 years old) and aged (∼21 years old) Macaca mulatta. Aged VSMCs cultured in the absence of systemic factors exhibited significantly increased secretion of interleukin-1β, MCP-1, and tumor necrosis factorα compared with young control cells. Secretion of interleukin-6 also tended to increase in aged VSMCs. This age-associated proinflammatory shift in the cellular secretory phenotype was associated with an increased mitochondrial O2 − production and nuclear factor κ-light-chain-enhancer of activated B cells activation. Treatment of aged VSMCs with a physiologically relevant concentration of resveratrol (1 μM) exerted significant anti-inflammatory effects, reversing aging-induced alterations in the cellular cytokine secretion profile and inhibiting nuclear factor κ-light-chain-enhancer of activated B cells. Resveratrol also attenuated mitochondrial O2 − production and upregulated the transcriptional activity of Nrf2 in aged VSMCs. Thus, in non-human primates, cell-autonomous activation of nuclear factor κ-light-chain-enhancer of activated B cells and expression of an inflammatory secretome likely contribute to vascular inflammation in aging. Resveratrol treatment prevents the proinflammatory properties of the aged VSMC secretome, an effect that likely contributes to the demonstrated vasoprotective action of resveratrol in animal models of aging.
Keywords: Vascular aging; Inflammation; Oxidative stress; Cytokine; 3,5,4’-trihydroxy-trans-stilbene
Aging-induced low-grade inflammation is increasingly recognized as a causal factor in the development of both large-vessel disease (myocardial infarction, stroke, peripheral arterial disease) and small-vessel pathologies (including vascular cognitive impairment) in older persons (1,2). The source of age-related chronic inflammation was originally attributed to the progressive activation of immune cells with age. However, studies during the past decade have shown that with advancing age there is a striking increase in the secretion of proinflammatory mediators, including various cytokines and chemokines, in the vascular wall even in the absence of infiltrating immunocytes (2). Previous studies have indicated that in aged endothelial cells production of inflammatory cytokines is significantly increased (2). We have also provided evidence that aging is associated with an increased expression of tumor necrosis factor (TNF)α in the vascular smooth muscle cells (VSMCs) in rat arteries (2). However, a detailed analysis of aging-induced changes in a broad range of secreted factors by VSMCs has not yet been performed. Moreover, there is no information available of age-related alterations in the secretome of VSMCs in non-human primates.
The mechanisms underlying age-related increases in the cellular production of proinflammatory cytokines are likely multifaceted. Potential non–cell-autonomous causes of vascular inflammation in aging include activation of toll-like receptors by circulating microbial factors, changes in the hemodynamic environment (such as higher-than-normal systolic blood pressure in aging), and neurohormonal changes. In addition, recent studies suggest that cell-autonomous factors (eg, activation of oxidative stress-induced pathways) have an important role in chronic activation of vascular inflammatory processes (3). The effect of aging per se on the secretome in vascular cells (independent of age-related changes in systemic factors), however, has not been well documented.
Pharmacological interventions that attenuate proinflammatory cytokine expression have been shown to confer significant vasoprotective effects in various animal models of cardiovascular diseases in humans. Germane to the present discussion, the naturally occurring polyphenolic compound, resveratrol (3,5,4’-trihydroxy-trans-stilbene), has been shown to confer vasoprotection and to improve the general health of aged laboratory animals (4–10). Resveratrol was shown to inhibit cellular pathways involved in inflammatory processes (11,12), but its effects on the cytokine expression profile of aged vascular cells have not been characterized.
The present study was undertaken to test the hypothesis that aging-induced cell-autonomous mechanisms promote proinflammatory alterations in the secretome of VSMCs of non-human primates. We also sought to determine whether treatment of aged VSMCs with a physiologically relevant concentration of resveratrol exerts anti-inflammatory effects by reversing aging-induced changes in the secretory profile of these cells. We chose to study primary VSMCs derived from young and aged Macaca mulatta because this non-human primate model has the advantage of being phylogenetically close to humans while exhibiting few of the complicating effects of the cardiovascular diseases (eg, diabetes and hypertension) associated with aging that may modulate the vascular aging phenotype (13–15). By studying the cells in culture, the age-related cell-autonomous changes in the secretome could be elucidated without the confounding effects of systemic factors.
Methods
Cell Cultures
Early-passage (passage 4) arterial VSMCs derived from young (∼13 years old) and aged (∼21 years old) rhesus monkeys (M mulatta) were used, as reported (13). The species longevity record for M mulatta in captivity is 40 years, according to the AnAge database (http://genomics.senescence.info/species/) compiled by de Magalhaes and coworkers (16). VSMCs were isolated and cultured as previously described (13). VSMCs were grown in Smooth Muscle Cell Growth Medium (Cell Applications Inc., San Diego, CA) in six-well plates (at 37°C, 20% O2). Upon reaching 50% confluence, the medium was changed to serum-free Minimum Essential Medium. The cells were treated with resveratrol (1 μmol/L) or vehicle (Dimethyl sulfoxide) for 48 hours, and then the conditioned media were collected for analysis of secreted cytokines and growth factors. The cells were harvested, and sample protein content was determined for normalization purposes by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co., Rockford, IL).
Analysis of Secreted Cytokines in Conditioned Media
Profiling of cytokines secreted by cultured VSMCs was conducted using a magnetic multiplex bead array (MILLIPLEX MAP Non-Human Primate Cytokine Magnetic Bead Panel; Millipore, Billerica, MA). The concentration of a range of cytokines and growth factors involved in vascular physiology and pathophysiology (Granulocyte-macrophage colony-stimulating factor, interferon-γ, interleukin [IL]-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 [p40], IL-13, IL-15, IL-17, MCP-1, MIP-1β, sCD40L, TNFα and vascular endothelial growth factor [VEGF]) was measured in the conditioned media collected after 48 hours of culture in serum-free Minimum Essential Medium in the presence or absence of resveratrol (1 μmol/L). The cells were harvested, and sample protein content was determined by a spectrophotometric quantitation method using BCA reagent (Pierce Chemical Co.). Secreted cytokines are reported as pmol/mg cellular protein/day.
Measurement of Mitochondrial O2 − Production in Cultured VSMCs
Mitochondrial O2 − production in VSMCs from young and aged M mulatta was measured using MitoSox Red (Invitrogen, Carlsbad CA), a mitochondrion-specific hydroethidine-derivative fluorescent dye, as previously reported (3,11,17,18). The cells were treated with resveratrol (1 μmol/L) or vehicle for 24 hours and then loaded with MitoSox (4 μmol/L, for 20 minutes). MitoSox fluorescence was measured using a Tecan Infinite M200 plate reader (Tecan U.S., Research Triangle Park, NC). Hoechst 33258 fluorescence, representing cellular DNA content, was used for normalization.
Transient Transfection, nuclear factor κ-light-chain-enhancer of activated B cells and Nrf2 Reporter Gene Assays
Transcriptional activity of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) was tested in VSMCs derived from young and old M mulatta by a reporter gene assay as described (13). We used an NF-κB reporter composed of an NF-κB response element upstream of firefly luciferase (NF-κB-Luc, Stratagene/Agilent Technologies, Santa Clara, CA) and a renilla luciferase plasmid under the control of the CMV promoter. The effects of resveratrol (1 μmol/L, for 24 hours) on NF-κB activity in aged VSMCs were also determined.
The effects of resveratrol (from 0.3 to 10 μmol/L, for 24 hours) on transcriptional activity of Nrf2 were tested in aged VSMCs by a reporter gene assay as described (13,19,20). We used an antioxidant responsive element reporter composed of tandem repeats of the antioxidant responsive element transcriptional response element upstream of firefly luciferase (SA Biosciences, Frederick, MD) and a renilla luciferase plasmid under the control of the CMV promoter (as an internal control). All transfections in VSMCs were performed using the Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported (21–23). Firefly and renilla luciferase activities were assessed after 24 hours using the Dual Luciferase Reporter Assay Kit (Promega, Madison, WI) and a Tecan Infinite M200 plate reader.
Data Analysis
Cytokine secretion was expressed as pg/mg cellular protein/day. Data were expressed as means ± SEM. Statistical analyses of data were performed by one-way analysis of variance followed by Tukey’s post hoc test (24–27). p < .05 was considered statistically significant (28–37).
Results
Age-Related Changes in Cytokine Secretion Profile in VSMCs From M mulatta
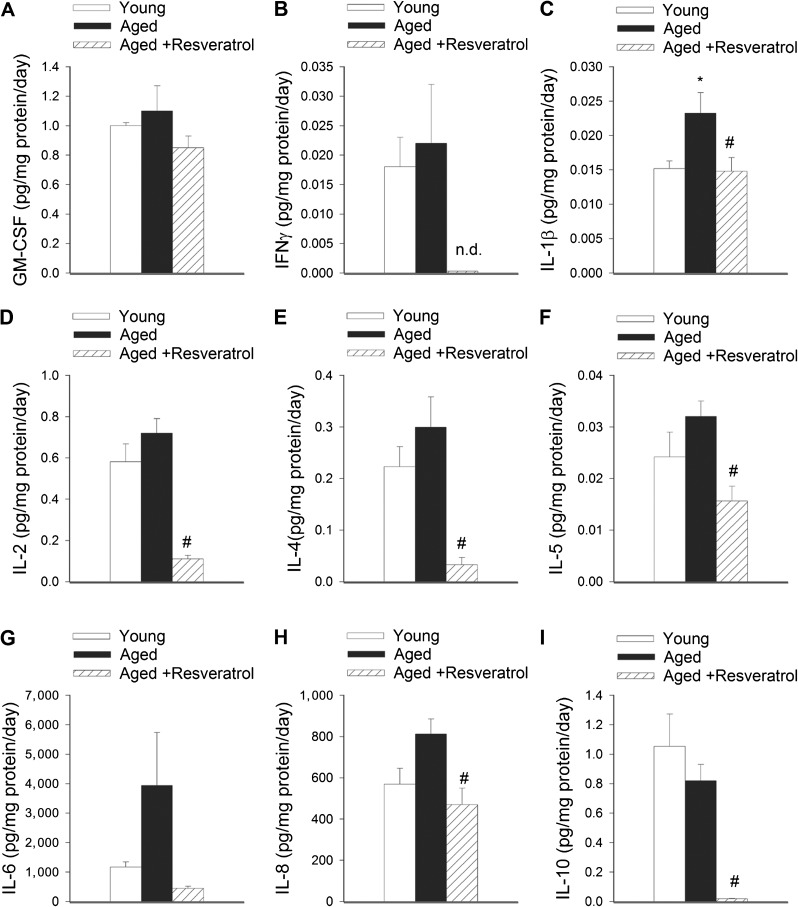
We have demonstrated previously that aging in laboratory rodents is associated with a proinflammatory shift in cytokine expression profile of VSMCs (2). To determine whether aging in the non-human primate M mulatta also promotes proinflammatory alterations in the VSMC secretome, we have utilized a multiplex bead array to simultaneously measure the level of a range of cytokines, chemokines, and growth factors in the conditioned media. Of the cytokines tested, the secretion of IL-1β (Figure 1C), TNFα (Figure 2H), MCP-1 (Figure 2E), and VEGF (Figure 2I) was significantly increased in aged VSMCs as compared with young VSMCs. Secretion of IL-6 (Figure 1G), IL-8 (Figure 1H), and IL-17 (Figure 2D) also tended to increase in VSMCs derived from aged animals, although these differences did not reach statistical significance.
Figure 1.
Secretory profiles of vascular smooth muscle cells (VSMCs) derived from young and aged Macaca mulatta. Soluble Granulocyte-macrophage colony-stimulating factor (A), interferon-gamma (B), interleukin (IL)-1β (C), IL-2 (D), IL-4 (E), IL-5 (F), IL-6 (G), IL-8 (H), and IL-10 (I) secreted by these cells were detected by a magnetic multiplex bead array (see Methods section). The effects of treatment with resveratrol (1 μmol/L for 48 h) are also shown. Data are mean ± SEM. *p < .05 vs young; #p < .05 vs untreated.
Figure 2.
Secretion of interleukin (IL)-12/23 (A), IL-13 (B), IL-15 (C), IL-17 (D), MCP-1 (E), MIP-1β (F), sCD40L (G), tumor necrosis factorα (H), and vascular endothelial growth factor (VEGF) (I) by vascular smooth muscle cells (VSMCs) derived from young and aged Macaca mulatta. Soluble factors secreted by these cells were detected as in Figure 1. The effects of treatment with resveratrol (1 μmol/L for 48 h) are also shown. Data are mean ± SEM. *p < .05 vs young; #p < .05 vs untreated.
Resveratrol-Induced Changes in the Cytokine Secretion Profile in VSMCs From Aged M mulatta
We have shown previously that resveratrol confers significant anti-inflammatory effects in laboratory rodents (3,11,12,38), including a downregulation of inflammatory cytokines in aged mouse arteries (6). In addition, resveratrol exerts diverse antiaging effects (4,7,8,29,39,40). Here, we report that treatment of VSMCs from aged M mulatta with resveratrol reverses most of the age-related changes in the secretory phenotype of these cells. Resveratrol significantly decreased the secretion of IL-1β (Figure 1C), IL-8 (Figure 1H), TNFα (Figure 2H), MCP-1 (Figure 2E), and VEGF (Figure 2I) and also tended to attenuate secretion of IL-6 (Figure 1G) and IL-17 (Figure 2D). In addition, resveratrol significantly decreased the secretion of interferon-gamma (Figure 2B), IL-2 (Figure 1D), IL-4 (Figure 1E), IL-5 (Figure 1F), IL-10 (Figure 1I), IL-12/23 (Figure 2A), MIP-1β (Figure 2F), and sCD40L (Figure 2G), below the levels observed in young VSMCs.
Correlation Between Age-Related Changes in the Secretory Profile of VSMCs and the Senescence-Associated Secretory Phenotype
We also aimed to determine whether the age-related changes in the secretory profile of VSMCs resemble the senescence-associated secretory phenotype (SASP) described in various cell types at replicative senescence (41). We found a significant correlation between the secretory profiles of VSMCs derived from aged M mulatta and the secretory profiles of mouse (Figure 3A) or human (Figure 3B) fibroblasts expressing an SASP. The SASP data used for these analyses were taken from recent publications by Judith Campisi, PhD (41).
Figure 3.
Correlation between the secretory profiles of vascular smooth muscle cells (VSMCs) derived from aged Macaca mulatta and the secretory profiles of mouse (A) or human (B) fibroblasts expressing a senescence-associated secretory phenotype. The VSMC data are from the data sets presented in Figures 1 and 2 and are expressed as fold changes, using the corresponding secretory profiles of VSMCs derived from young M mulatta as baseline. The fibroblast data are taken from recent publications (41). The fibroblasts were rendered senescent by x-ray irradiation (XRA). Baselines for the senescent profiles of fibroblasts are the corresponding profiles of dividing presenescent fibroblasts (PRE; data are expressed as log2 [fold changes]) (41).
Effect of Resveratrol on Mitochondrial O2 − Production in VSMCs From Aged M mulatta
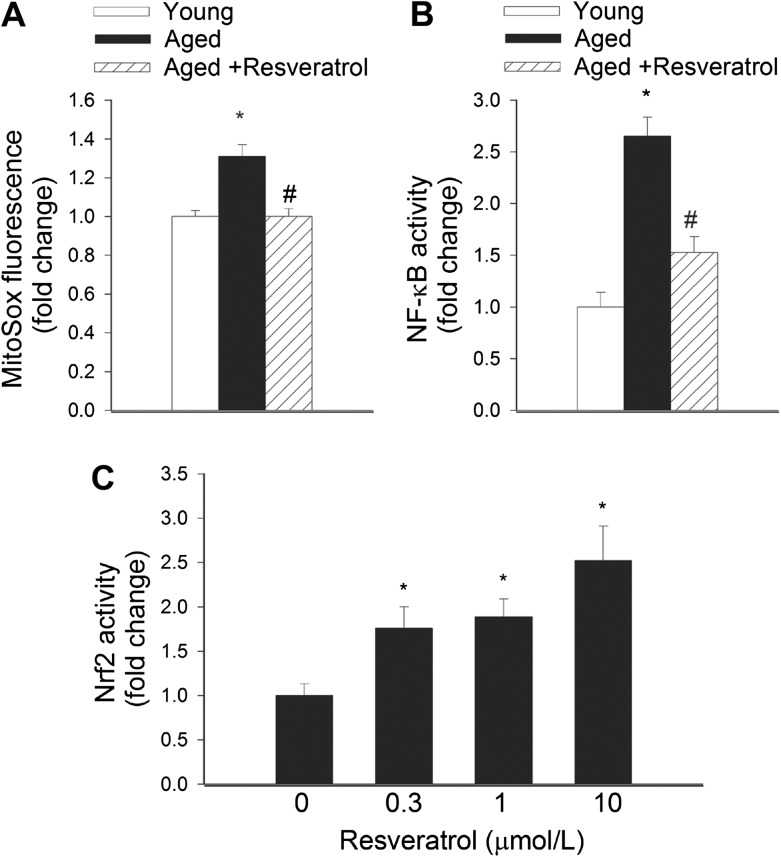
We have shown previously that resveratrol confers mitochondrial protective effects in vascular cells (11,18,42,43). To determine whether resveratrol attenuates mitochondrial oxidative stress in aged VSMCs, we have utilized a MitoSox fluorescence-based approach. We found that in VSMCs derived from aged M mulatta, MitoSox fluorescence (Figure 4A), indicating mitochondrial O2 − generation, was significantly increased compared with that in cells from young animals. Treatment with resveratrol significantly decreased MitoSox fluorescence in aged VSMCs (Figure 4A).
Figure 4.
(A) Mitochondrial O2 − production, assessed using the MitoSox Red fluorescence method, was increased in primary vascular smooth muscle cells (VSMCs) derived from aged Macaca mulatta compared with VSMCs derived from young animals. Treatment of aged VSMCs with resveratrol (1 μmol/L) significantly attenuated mitochondrial O2 − production. *p < .05 vs young VSMCs; #p < .05 vs untreated. Data are mean ± SEM. (B) Reporter gene assay demonstrating that primary VSMCs derived from aged M mulatta exhibit increased transcriptional activity of nuclear factor κ-light-chain-enhancer of activated B cells and is significantly inhibited by treatment with resveratrol (1 μmol/L). *p < .05 vs young VSMCs; #p < .05 vs untreated. Data are mean ± SEM. (C) Reporter gene assay showing the effects of resveratrol on Nrf2/antioxidant responsive element reporter activity in cultured VSMCs derived from aged M mulatta. Data are mean ± SEM. Resveratrol significantly increased Nrf2 in a dose-dependent manner.
Effect of Resveratrol on Transcriptional Activity of NF-κB in VSMCs From Aged M mulatta
Previously, we have found that age-related vascular inflammation in laboratory rodents is associated with increased NF-κB activation (3,44). We found that in VSMCs derived from aged M mulatta, transcriptional activity of NF-κB was, in the present study also, significantly increased compared with VSMCs from young monkeys (Figure 4B). Resveratrol treatment significantly inhibited NF-κB activation in VSMCs from aged M mulatta (Figure 4B).
Resveratrol-Induced Nrf2 Activation in VSMCs From Aged M mulatta
Recent studies suggest that the antioxidant responsive element/Nrf2 pathway is an important molecular target of resveratrol in multiple cell types (19,45–47). To determine whether resveratrol can activate Nrf2 in aged VSMCs of non-human primates, we performed a reporter gene assay. We found that resveratrol, even at submicromolar concentrations, significantly increased transcriptional activity of Nrf2 in VSMCs of aged M mulatta (Figure 4C).
Discussion
The principal new finding of this study is that aging in non-human primates significantly alters the secretome of VSMCs. Characterization of this secretome highlights an increased production of proinflammatory cytokines, including several factors (ie, IL-6, IL-1β, TNFα) previously identified in the cytokine expression profile in blood vessels from aged rodents (2,48–50). These observations support the concept that the age-associated changes in the secretome of vascular cells (previously described as age-associated arterial secretory phenotype [51]) are, at least in part, due to cell-autonomous mechanisms. Similar conclusions have been reached previously using VSMCs obtained from aged rodents (52). It is possible that VSMCs in the aged arterial wall are phenotypically heterogeneous, thus future studies should characterize different VSMC subpopulations on the basis of their cytokine expression profile. Increased levels of inflammatory cytokines, when chronically present, can disrupt tissue structure and promote the development of age-related cardiovascular diseases (1,44,53). Inflammatory cytokines released from the aged vasculature, like VSMCs from an artery providing blood supply to the brain in the present study, may also negatively affect the function of the supplied organs and promote the development of a wide range of inflammation-related diseases of aging (ie, Alzheimer disease). Recent studies suggest that a higher-than-normal level of inflammatory cytokines may also promote malignant phenotypes in nearby premalignant cells (54). It should be noted that age-related changes in the cellular secretome are likely very complex (eg, the Secreted Protein Discovery Initiative identified >1,000 different proteins secreted by cells [55]) and aging likely affects a large number of secreted proteins in addition to the cytokines assessed. Because VSMCs play a crucial role in the development of cardiovascular diseases, the primate VSMC secretome may be a valuable source of potential biomarkers for age-related cardiovascular diseases in humans. To promote vascular disease, aged VSMCs likely interact with endothelial cells. Thus, in future studies, it would be interesting to assess the endothelial effects of the conditioned media obtained from young and aged VSMCs.
To delineate the mechanisms that may contribute to the age-related changes in the cellular secretome, we measured the transcriptional activity of NF-κB in VSMCs derived from young and aged M mulatta. Our present findings and data from previous studies (13) show that aged VSMCs exhibit increased transcriptional activity of NF-κB, as compared with that in cells derived from young animals. Consistent with these ex vivo data, previously, we reported that blood vessels isolated from aged M mulatta also exhibit increased nuclear translocation of NF-κB (13). Increased NF-κB activation appears to be a hallmark of vascular aging as it has been repeatedly demonstrated both in aged laboratory rodents (3,56) and elderly humans (57). Although there is strong evidence that increased NF-κB activation promotes proinflammatory changes in the cellular cytokine expression profile in the vasculature (58), further studies are warranted to establish the causal link between age-related induction of NF-κB and changes in the secretome of VSMCs. The cell-autonomous mechanisms responsible for increased NF-κB activation in the aged vascular cells likely include an increased release of H2O2 from the mitochondria (3,13). This conclusion is supported by the findings that mitochondrial antioxidant responsive element generation is significantly increased in VSMCs of aged M mulatta even in the absence of circulating factors and that increased NF-κB activation in VSMCs of aged M mulatta can be attenuated by scavenging of H2O2 (13). In addition to cell-autonomous pathways, changes in circulating factors (ie, age-related increases in the plasma levels of angiotensin II and TNFα [44,49]) may also contribute to increased NF-κB activation in the aged vasculature in vivo, which may exacerbate vascular inflammation in aging.
Interestingly, cultured cells, that are growth-arrested by multiple passaging or x-ray irradiation, also exhibit distinctive changes in the profile of secreted cytokines, a phenotype that has been termed the SASP (41,54,59,60). Previous studies in laboratory rodents suggest that SASP shares many features with age-associated arterial secretory phenotype (51). Recent studies suggest that the expression of SASP (and likely age-associated arterial secretory phenotype [52]) is evolutionarily conserved (41) and that these changes have a role in the development of age-related pathophysiological alterations in many organs (54,61). Interestingly, the SASPs described in senescent mouse and human fibroblasts (41) and the age-associated arterial secretory phenotype described in rats (52) overlap substantially with secretory profiles of VSMCs derived from aged M mulatta that (in contrast to prior studies of SASP) were not growth-arrested (Figure 3). Cellular replicative senescence is characterized by an increased activation of NF-κB, which has been causally linked to the expression of SASP both in human fibroblasts and in porcine coronary arterial endothelial cells after multiple passaging (62,63). Whether replicative senescence has a causative role in organismal aging is controversial, yet, the aforementioned observations raise the possibility that common cellular pathways, including NF-κB activation, may underlie the proinflammatory phenotypic changes observed in the cells derived from aged organisms (including expression of age-associated arterial secretory phenotype (51) and the expression of SASP in senescent cells in vitro). Interestingly, VSMCs derived from both aged M mulatta and non-dividing senescent cells secrete increased amounts of VEGF (41,64,65), suggesting that, in addition to NF-κB activation, multiple pathways are activated both in cells of aged animals and in cells at replicative senescence.
Here, we show for the first time that in VSMCs derived from aged non-human primates resveratrol treatment confers significant anti-inflammatory effects, reversing the age-induced alterations in the cellular secretome. The available evidence suggests that in vivo treatment with resveratrol also exerts similar anti-inflammatory effects on the cytokine expression profile in the vascular wall of aged animals. For example, treatment of aged mice with resveratrol downregulates vascular expression of a range of proinflammatory cytokines (6) and attenuates LPS-induced secretion of IL-1β and other paracrine mediators (66). Resveratrol treatment also inhibits expression of proinflammatory cytokines in vessels of animals with pathological conditions associated with oxidative stress (38) and accelerated vascular aging (67). Interestingly, resveratrol also reversed the age-related increases of the secretion of noninflammatory factors, including VEGF. Finally, resveratrol also inhibits the secretion of cytokines, which does not markedly increase with age, including that of interferon-gamma and IL-10. Future studies should determine whether targeting subclinical changes in the vascular secretome may reduce the incidence and progression of age-associated arterial diseases, including hypertension and atherosclerosis.
The inhibitory effects of resveratrol on cellular expression of proinflammatory cytokines, chemokines, and other paracrine mediators are likely not cell type specific. For example, treatment with resveratrol has been reported to inhibit expression of IL-6, IL-17, and MCP-1 in endothelial cells (12,68), cardiac fibroblasts (69), and adipocytes (70), respectively. In addition to altering vascular secretion of chemokines, resveratrol treatment may also inhibit monocyte chemotaxis to the vascular wall by interfering with Rho kinase-dependent signaling pathways in the leukocytes themselves (71). Resveratrol was also shown to inhibit expression of IL-8, Granulocyte-macrophage colony-stimulating factor, and/or VEGF in human airway smooth muscle cells (72) and human retinal cells (73). Treatment of aged mice with resveratrol also inhibits proinflammatory cytokine expression in immune cells (74). Recent studies demonstrate that treatment of human subjects with resveratrol and/or resveratrol-containing extract of Polygonum cuspidatum decreases plasma concentrations of TNFα, IL-1β, and IL-6 (75,76). Recent studies also suggest that resveratrol treatment in vivo exerts vasoprotective effects in a non-human primate model of accelerated vascular aging (metabolic arterial inflammation; Z. Ungvari, MD, PhD, A. Csiszar, MD, PhD, R. de Cabo, PhD, and E. G. Lakatta, MD, PhD, manuscript in preparation, 2011). Further studies are warranted to determine whether treatment with resveratrol also attenuates aging-induced vascular inflammation in humans and/or non-human primates.
The molecular mechanisms underlying the anti-inflammatory effects of resveratrol are likely multifaceted. We propose that the ability of resveratrol to attenuate mitochondrial oxidative stress and to inhibit NF-κB activity contributes significantly to its ability to attenuate secretion of proinflammatory cytokines by VSMCs from aged non-human primates. This view is supported by results of previous studies demonstrating that resveratrol treatment effectively decreases oxidative stress and inhibits NF-κB–driven gene expression in arteries of aged rodents (3,6). Further, resveratrol-induced attenuation of mitochondrial antioxidant responsive element production (18) and inhibition of NF-κB (12) are also associated with downregulation of inflammatory cytokines in vascular endothelial cells as well. The mechanisms by which resveratrol inhibits NF-κB likely include activation of SIRT1 (11,77). In addition, recent studies suggest that resveratrol is a potent activator of Nrf2 (19) and that a cross talk exists between Nrf2 and NF-κB signaling. Recent studies suggest that aging results in dysregulation of Nrf2 signaling in the vasculature, impairing the ability of aged cells to mount an effective antioxidant defense in response to an increased production of antioxidant responsive element (13,78,79). Because we found that the ability of resveratrol to activate Nrf2 in VSMCs is preserved in aging (Figure 4C), we propose that activation of Nrf2 by resveratrol contributes to the inhibition of NF-κB in vascular cells of non-human primates. Importantly, resveratrol was recently shown to inhibit neointimal formation by an Nrf2-dependent pathway in mice (80). The aforementioned pathways may also contribute to the effects of resveratrol on replicative senescence–associated cellular alterations (62,81). The posttranscriptional regulation of cytokine genes via the destabilizing activity of microRNAs likely plays a role in regulation of the half-life of many cytokines and achieving the temporal and spatial distributions required for regulation of these genes. Resveratrol has been shown to affect microRNAs in the cardiovascular system (82,83). Thus, it is possible that regulation of microRNAs by resveratrol may also contribute to the downregulation of inflammatory cytokines in aged VSMCs.
Taken together, resveratrol in physiologically relevant concentrations reverses the proinflammatory properties of the VSMC secretome of aged non-human primates. When viewed within the broader context of prior studies, the findings presented herein provide additional support for the concept that resveratrol confers beneficial effects in the aged cardiovascular system (and possibly other organs, including the skeletal muscle (7,8) and the brain [84]) by attenuating age-associated chronic low-grade inflammation. These effects are likely independent of the effects of resveratrol on life span (40). Future studies will be necessary to elucidate the role of resveratrol-sensitive pathways (including Nrf2 activation) on the regulation of the secretome in the aged vasculature, to open new avenues for therapeutic intervention for the prevention/treatment of cardiovascular diseases in the elderly population.
Funding
American Diabetes Association to Z.U.; American Federation for Aging Research to A.C.; Oklahoma Center for the Advancement of Science and Technology to A.C. and Z.U.; University of Oklahoma College of Medicine Alumni Association to A.C.; American Heart Association to A.C.; National Institutes of Health (AG031085 to A.C.; AT006526 to Z.U.; AG038747, NS056218, and P01 AG11370 to W.E.S.); The Ellison Medical Foundation to W.E.S.; Intramural Research Program of National Institutes of Health to E.L. and M.W.
Acknowledgments
The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 3.Ungvari ZI, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 4.Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr062. doi:10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan MJ, Jackson JR, Hao Y, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barger JL, Kayo T, Vann JM, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 13.Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-{kappa}B activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 15.Shi Q, Aida K, Vandeberg JL, Wang XL. Passage-dependent changes in baboon endothelial cells—relevance to in vitro aging. DNA Cell Biol. 2004;23:502–509. doi: 10.1089/1044549041562294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Magalhaes JP, Costa J, Church GM. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J Gerontol A Biol Sci Med Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ungvari Z, Labinskyy N, Mukhopadhyay P, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ungvari Z, Bagi Z, Feher A, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungvari Z, Bailey-Downs L, Gautam T, et al. Adaptive induction of NF-E2–related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:1133–1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csiszar A, Ahmad M, Smith KE, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 23.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 24.Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungvari Z, Philipp EE. Comparative gerontology—from mussels to man. J Gerontol A Biol Sci Med Sci. 2011;66:295–297. doi: 10.1093/gerona/glq198. [DOI] [PubMed] [Google Scholar]
- 26.Ungvari Z, Ridgway I, Philipp EE, et al. Extreme longevity is associated with increased resistance to oxidative stress in Arctica islandica, the longest-living non-colonial animal. J Gerontol A Biol Sci Med Sci. 2011;66(7):741–750. doi: 10.1093/gerona/glr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci. 2011;66(5):501–510. doi: 10.1093/gerona/glr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behrens MI, Silva M, Schmied A, et al. Age-dependent increases in apoptosis/necrosis ratios in human lymphocytes exposed to oxidative stress. J Gerontol A Biol Sci Med Sci. 2011;66:732–740. doi: 10.1093/gerona/glr039. [DOI] [PubMed] [Google Scholar]
- 29.Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66(12):965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- 30.Chung E, Diffee GM. Effect of aging on power output properties in rat skinned cardiac myocytes. J Gerontol A Biol Sci Med Sci. 2011;66(12):1267–1273. doi: 10.1093/gerona/glr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dekker P, de Lange MJ, Dirks RW, et al. Relation between maximum replicative capacity and oxidative stress-induced responses in human skin fibroblasts in vitro. J Gerontol A Biol Sci Med Sci. 2011;66:45–50. doi: 10.1093/gerona/glq159. [DOI] [PubMed] [Google Scholar]
- 32.Endt H, Sprung CN, Keller U, Gaipl U, Fietkau R, Distel LV. Detailed analysis of DNA repair and senescence marker kinetics over the life span of a human fibroblast cell line. J Gerontol A Biol Sci Med Sci. 2011;66:367–375. doi: 10.1093/gerona/glq197. [DOI] [PubMed] [Google Scholar]
- 33.Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol A Biol Sci Med Sci. 2011;66:385–392. doi: 10.1093/gerona/glq205. [DOI] [PubMed] [Google Scholar]
- 34.McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. J Gerontol A Biol Sci Med Sci. 2011;66(12):1309–1317. doi: 10.1093/gerona/glr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sloane LB, Stout JT, Vandenbergh DJ, Vogler GP, Gerhard GS, McClearn GE. Quantitative trait loci analysis of tail tendon break time in mice of C57BL/6J and DBA/2J lineage. J Gerontol A Biol Sci Med Sci. 2011;66:170–178. doi: 10.1093/gerona/glq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith DL, Jr, Mattison JA, Desmond RA, et al. Telomere dynamics in rhesus monkeys: no apparent effect of caloric restriction. J Gerontol A Biol Sci Med Sci. 2011;66(11):1163–1168. doi: 10.1093/gerona/glr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu M, Hu J, Perez E, et al. Effects of long-term cranberry supplementation on endocrine pancreas in aging rats. J Gerontol A Biol Sci Med Sci. 2011;66(11):1139–1151. doi: 10.1093/gerona/glr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csiszar A, Labinskyy N, Olson S, et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labbe A, Garand C, Cogger VC, et al. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- 40.Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coppe JP, Patil CK, Rodier F, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(1):H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A. Mitochondrial protection by resveratrol. Exerc Sport Sci Rev. 2011;39(3):128–132. doi: 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh TC, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem. 2006;2:275–285. doi: 10.2174/157340606776930709. [DOI] [PubMed] [Google Scholar]
- 46.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 47.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 48.Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 51.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang L, Wang M, Zhang J, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark HF, Gurney AL, Abaya E, et al. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 2003;13:2265–2270. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–498. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- 57.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 62.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee MY, Wang Y, Vanhoutte PM. Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of NFkB. J Vasc Res. 2010;47:287–298. doi: 10.1159/000265563. [DOI] [PubMed] [Google Scholar]
- 64.Ksiazek K, Jorres A, Witowski J. Senescence induces a proangiogenic switch in human peritoneal mesothelial cells. Rejuvenation Res. 2008;11:681–683. doi: 10.1089/rej.2008.0736. [DOI] [PubMed] [Google Scholar]
- 65.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 66.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rius C, Abu-Taha M, Hermenegildo C, et al. Trans- but not cis-resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-kappaB activation and peroxisome proliferator-activated receptor-gamma upregulation. J Immunol. 2010;185:3718–3727. doi: 10.4049/jimmunol.1001043. [DOI] [PubMed] [Google Scholar]
- 69.Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2008;294:H2078–H2087. doi: 10.1152/ajpheart.01363.2007. [DOI] [PubMed] [Google Scholar]
- 70.Zhu J, Yong W, Wu X, et al. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun. 2008;369:471–477. doi: 10.1016/j.bbrc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 71.Cicha I, Regler M, Urschel K, Goppelt-Struebe M, Daniel WG, Garlichs CD. Resveratrol inhibits monocytic cell chemotaxis to MCP-1 and prevents spontaneous endothelial cell migration through Rho kinase-dependent mechanism. J Atheroscler Thromb. 2011 doi: 10.5551/jat.8136. doi:10.5551/jat.8136. [DOI] [PubMed] [Google Scholar]
- 72.Knobloch J, Hag H, Jungck D, Urban K, Koch A. Resveratrol impairs the release of steroid-resistant cytokines from bacterial endotoxin-exposed alveolar macrophages in chronic obstructive pulmonary disease. Basic Clin Pharmacol Toxicol. 2011;109:138–143. doi: 10.1111/j.1742-7843.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- 73.Dugas B, Charbonnier S, Baarine M, et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur J Nutr. 2010;49:435–446. doi: 10.1007/s00394-010-0102-2. [DOI] [PubMed] [Google Scholar]
- 74.Wong YT, Gruber J, Jenner AM, Tay FE, Ruan R. Chronic resveratrol intake reverses pro-inflammatory cytokine profile and oxidative DNA damage in ageing hybrid mice. Age (Dordr) 2011;33(3):229–246. doi: 10.1007/s11357-010-9174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghanim H, Sia CL, Abuaysheh S, et al. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–E8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghanim H, Sia CL, Korzeniewski K, et al. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96:1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ungvari Z, Bailey-Downs L, Sosnowska D, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2011 doi: 10.1093/gerona/glr164. doi:10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim JW, Lim SC, Lee MY, et al. Inhibition of neointimal formation by trans-resveratrol: role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol Nutr Food Res. 2010;54:1497–1505. doi: 10.1002/mnfr.201000016. [DOI] [PubMed] [Google Scholar]
- 81.Giovannelli L, Pitozzi V, Jacomelli M, et al. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:9–18. doi: 10.1093/gerona/glq161. [DOI] [PubMed] [Google Scholar]
- 82.Mukhopadhyay P, Mukherjee S, Ahsan K, Bagchi A, Pacher P, Das DK. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS One. 2010;5:e15705. doi: 10.1371/journal.pone.0015705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukhopadhyay P, Pacher P, Das DK. MicroRNA signatures of resveratrol in the ischemic heart. Ann N Y Acad Sci. 2011;1215:109–116. doi: 10.1111/j.1749-6632.2010.05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]