Abstract
Purpose
To estimate radiation therapy planning margins based on inter- and intrafractional uncertainty for pediatric brain and head and neck tumor patients at different imaging frequencies.
Methods
Pediatric patients with brain (n = 83) and head and neck (n = 17) tumors (median age = 7.2 years) were enrolled on an internal review board–approved localization protocol and stratified according to treatment position and use of anesthesia. Megavoltage cone-beam CT (CBCT) was performed before each treatment and after every other treatment. The pretreatment offsets were used to calculate the interfractional setup uncertainty (SU), and posttreatment offsets were used to calculate the intrafractional residual uncertainty (RU). The SU and RU are the patient-related components of the setup margin (SM), which is part of the planning target volume (PTV). SU data was used to simulate four intervention strategies using different imaging frequencies and thresholds.
Results
The SM based on all patients treated on this study was 2.1 mm (SU = 0.9 mm, RU = 1.9 mm) and varied according to treatment position (supine = 1.8 mm, prone = 2.6 mm) and use of anesthesia (with = 1.7 mm, without = 2.5 mm) because of differences in the RU. The average SU for a 2-mm threshold based on no imaging, once per week imaging, initial five images, and daily imaging was 3.6, 2.1, 2.2, and 0.9 mm, respectively.
Conclusion
On the basis of this study, the SM component of the PTV may be reduced to 2 mm for daily CBCT compared with 3.5 mm for weekly CBCT. Considering patients who undergo daily pretreatment CBCT, the SM is larger for those treated in the prone position or smaller for those treated under anesthesia because of differences in the RU.
Keywords: Pediatric brain tumor, Patient motion, CBCT, Target localization, Margins
INTRODUCTION
The trend to irradiate young children with brain tumors and the promise of recent advancements has increased the acceptance of radiation therapy (RT) for pediatric patients. Because RT has wide ranging side effects in pediatric patients (1–5), investigators have focused on reducing normal tissue irradiation. One method is to reduce the target volume margins that are used in RT planning.
The volume and margin definitions of the International Commission on Radiation Units and Measurements Reports 50 and 62, (6, 7) have been adopted in pediatric clinical trials for brain and head and neck tumors—namely, gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV). The purpose has been to use three-dimensional treatment planning and delivery systematically in clinical trials and study ways to reduce treatment effects using disease-specific target volume margins. Only the CTV should receive the prescription dose; however, because of temporal variation in the position, shape, and size of the CTV, an internal margin (IM) must be added. In addition, because of uncertainties in the daily patient positioning, patient intrafractional motion, dose calculation, and beam delivery, a setup margin (SM) is also required. The combination of the IM and SM define the PTV. The need to estimate and minimize the IM and SM in a variety of clinical settings and for children of all ages has become increasingly important as highly conformal radiation therapy, including intensity modulated radiation therapy (IMRT) and proton therapy, have entered the mainstream.
The PTV margins currently specified for children with brain and head and neck tumors enrolled on institutional and cooperative group trials are largely empiric. As the definition of the GTV is refined and the CTV is systematically reduced for specific diseases, the margin chosen for the PTV will become an increasingly important means to minimize dose to normal tissues and the potential risk factor for treatment failure. Given the importance and complexity in determining the appropriate PTV margin, a number of studies have proposed patient population-based formulas for the patient-related portion of the setup margin (8–11) to allow for systematic quantification of setup uncertainties based on statistical methods. Among the sites under study, intracranial (12, 13) and head and neck (14–17) sites are of particular interest; however, only two small studies have been published focusing on pediatric localization (18, 19).
We developed a protocol to quantitatively assess localization and refine PTV margin definitions for pediatric patients with head and neck and intracranial tumors. The goal was to estimate the patient-related components of the SM and provide guidelines for target volume definitions in clinical trials. In this report, we estimated setup uncertainty (SU) and residual uncertainty (RU). The SU represented interfractional positioning differences, and the RU represented intrafractional patient motion. We used daily pretreatment cone-beam computed tomography (CBCT) and alternate day posttreatment CBCT to calculate SU and RU. The acquired data were modeled using different imaging regimens (20, 21) to determine the influence of imaging frequency on the SM.
METHODS AND MATERIALS
Patient cohort
One hundred patients with brain tumors (n = 83) and tumors involving the head and neck region (n = 17) were included in this study. The cohort included 54 male and 46 female patients with a median age of 7.2 years (range, 1.0–25.3 years). Only two patients were older than 21 years of age at the time of irradiation. They were included because they were diagnosed with pediatric brain and musculoskeletal tumors. General anesthesia (GA) was required for 46 (median age, 4.8 years; range, 1.0–19.1 years) and not required for 54 (median age, 12.5 years; range, 5.7–25.3 years). The treatment position was prone for 25 (median age, 8.9 years; range, 1.6–17.6 years) and supine for 75 (median age, 7.2 years; range, 1.0–25.3 years). For 62 patients, the treatment method was considered three-dimensional conformal radiation therapy. IMRT was used for 38 patients. The patients were treated with 1.8 Gy per day. The median number of treatment fractions was 29 (range, 7–35).
CBCT device
Patients were prospectively enrolled on an internal review board–approved clinical trial to study treatment localization using imaging beam line CBCT (IBL-CBCT). The IBL-CBCT is a modification of Siemens MVision CBCT (Siemens Medical USA, Concord, CA). The major modifications consisted of replacing the tungsten target with a carbon target, removing the flattening filter, and decreasing the beam energy by 30% to 4.2 MeV. This results in a photon beam with a mean energy of approximately 800 keV. The details of the modifications are explained by Faddegon et al. (22) The imaging and dosimetric properties are detailed elsewhere (23). Depth dose, profiles, and output for various field sizes of the IBL were measured and modeled in the PlanUNC (University of North Carolina, Chapel Hill; http://www.planunc.radonc.unc.edu) treatment planning system (TPS). Dose calculations from the TPS were verified with ion chamber measurements. Daily image quality and weekly output and energy checks are routinely performed on the IBL system to ensure proper functionality. The dose to isocenter for each IBL-CBCT was 1 cGy, verified by an IBL-CBCT treatment plan for each patient.
CBCT procedure to obtain SU and RU
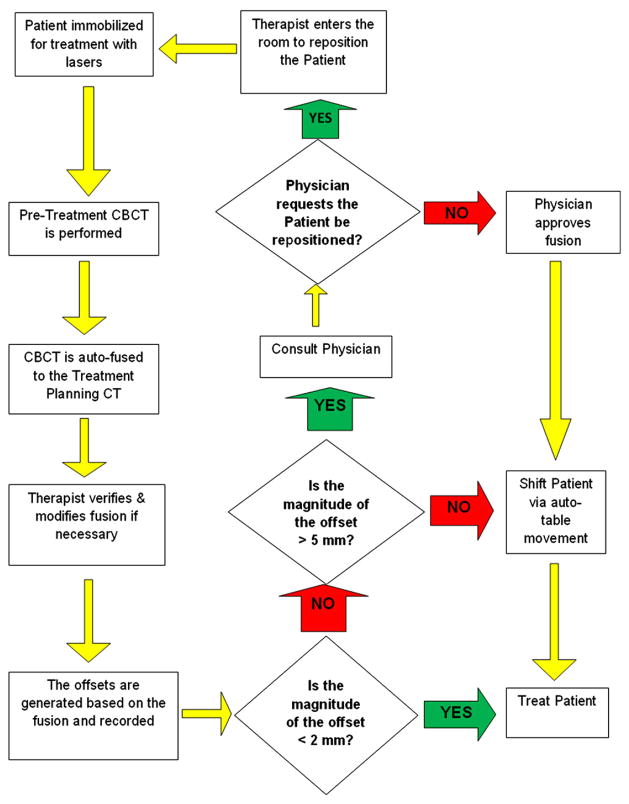
Each patient was evaluated by IBL-CBCT at the start of each daily treatment fraction and the completion of every other fraction. The pre-treatment IBL-CBCT was used to assess the setup uncertainty (SU). The post-treatment IBL-CBCT was used to quantify the residual uncertainty (RU). For purposes of analysis, the SU and RU were meant to correspond to the interfraction and intrafraction variability, respectively. On a daily basis, the therapists positioned the patient according to external visual markings before acquiring the CBCT. The CBCT was registered to the treatment planning CT using automated software with a mutual information algorithm. The therapist examined the registration and modified when necessary following the instructions of the treating radiation oncologist on the first day of treatment. The bony anatomy in the vicinity of the target was critical to the registration process. Coordinates were obtained automatically corresponding to the lateral, longitudinal, and vertical offsets. If the calculated magnitude of the three-dimensional offset vector was less than 2 mm, no shift was applied. If the offset vector was ≥2 mm but ≤5 mm, the corrective translational shifts were applied using automated treatment table movement. When the three-dimensional offset vector was >5 mm or the registered CBCT did not match visually to the treatment planning CT, a physician was consulted, and the patient repositioned before the acquisition of an additional CBCT. When necessary, the procedure was repeated until the offset vector met criteria for no intervention. All offsets were recorded in a database at the time of treatment and used to determine SU. Figure 1 is a flow chart that outlines the process.
Fig. 1.
A flow chart outlining the pretreatment cone-beam CT procedure.
The differences between the offsets of the posttreatment CBCT and the pre-treatment CBCT were used to determine RU. If the patient was shifted on the basis of pretreatment CBCT, their during-treatment offsets were assumed to be (0, 0, 0). If the patient was not shifted, the offset of the pretreatment CBCT was used. For example, if the pretreatment CBCT offsets were (1, 1, 1) and the post-treatment CBCT was (−1, −1, −1), the RU for that treatment was (−1–1, −1–1, −1–1) = (−2, −2, −2) because the during-treatment offset was assumed to be (1, 1, 1). If the pretreatment CBCT was (2, 2, 2) and the posttreatment CBCT was (−1, −1, −1), the RU for that treatment was (−1–0, −1–0, −1–0) = (−1, −1, −1) because the during-treatment offset was assumed to be (0, 0, 0). In the first case, the patient was not shifted because the pretreatment offset vector was <2 mm; in the second case, the patient was shifted because the pretreatment offset vector was >2 mm.
SU, RU, and SM analysis
To be included in this report, the patients must have had a minimum of seven pretreatment CBCT and three posttreatment CBCT evaluations. The geometric margin formula developed by van Herk et al. (11), 2.5Σ+ 0.7σ, where Σrepresents the systematic or preparation errors and σ is represents the execution or random error, served as an estimation of the setup uncertainty (SU) due to interfraction motion and residual uncertainty (RU) caused by intrafraction motion. The SU and RU were combined by adding the respective errors in quadrature giving the patient related setup margin (SMpat).
| Eq. 1 |
The patient cohort was subgrouped according to treatment position (supine with thermoplastic facemask vs. prone with customized vacuum bag) and use of general anesthesia and compared on the basis of SU and RU.
SU simulation by imaging frequency and action threshold
To demonstrate the usefulness of daily CBCT localization, we determined the differences in SMpat by simulating four CBCT localization conditions (L1-4) and five action thresholds (1–5mm, 1-mm increments) for all patients. The number of systematic shifts, which would involve changing the setup position on the patient, was calculated on the basis of a 6-week (30-fraction) treatment.
-
L1
The first localization condition was based on visual marks alone. The patient was assumed to have been treated in the initial setup position documented by the CBCT without shifts applied. There was no action threshold for this condition because no imaging would apply.
-
L2
The second localization condition assumed the patient was imaged once a week and that a systematic shift was applied if the offset vector was greater than a given action threshold (1–5 mm). A systematic shift implied that new setup marks were placed on the patient for future treatments.
-
L3
The third localization condition assumed imaging every day for the first week and that a systematic shift based on the average offset was applied for future treatments. Following the first week, the weekly imaging procedure as described in L2 was applied.
-
L4
The fourth localization condition was similar to the one that was actually followed in our experimental design. The patient was shifted on the basis of daily CBCT if the offset vector was greater than a given threshold (1–5 mm). A systematic shift, meaning new setup marks placed on the patient, was applied if the difference between the absolute value of the average offset and the standard deviation of the offsets for the previous five fractions in any direction was greater than the given threshold. For example, if the average offset for the previous five fractions for a particular patient was 3 mm anterior–posterior, −3 mm right/left, and 0 mm superior/inferior and the standard deviation of the previous five fractions was 1 mm anterior/posterior, 1 mm right/left, and 1 mm superior/inferior, the value of this metric would be would be 2 mm, 2 mm, −1 mm. If the action threshold was ≤2 mm, a systematic shift would be applied; if the action threshold was >2 mm, then a systematic shift would not be applied. The purpose of using the difference between the absolute value and the standard deviations from the prior treatments was to ensure that a true systematic error was used for remarking the patient.
RESULTS
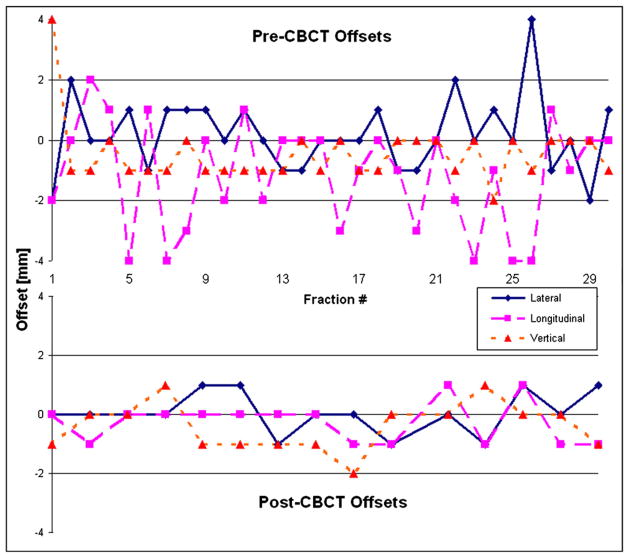
For the 100 patients included in this report, 2,362 pretreatment and 1,092 posttreatment localization CBCTs were acquired. The median (± SD) number per patient was 29 ± 8.7 and 12.5 ± 4.1, respectively. Figure 2 shows images from a 1-cGy IBL-CBCT and treatment planning CT for a 4-year-old patient. Figure 3 shows the offsets for the pretreatment CBCT and posttreatment CBCT of the patient.
Fig. 2.
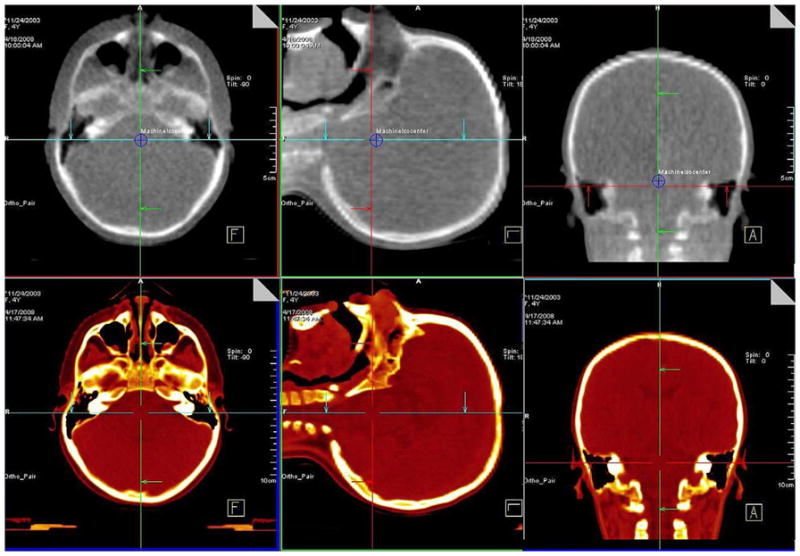
A 1-cGy imaging beam line cone-beam CT (CBCT) for a 4-year-old patient (top) and the simulation CT (bottom).
Fig. 3.
A graph of the offset in each direction for the pretreatment cone-beam CT (CBCT) (top) and the posttreatment CBCT (bottom) for the 4-year-old patient shown in Fig. 2. This patient was treated in the supine position with general anesthesia.
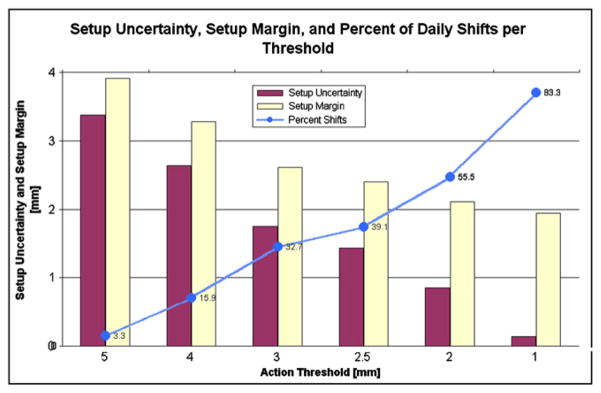
The average setup uncertainty (SU) of the three directions for the L1 localization was 3.6 mm. For the 2-mm threshold, the average SU for L2 was 2.1 mm, L3 was 2.2 mm, and L4 was 0.9 mm. The average RU was 1.9 mm. The resulting SM for these conditions was L1 = 4.1 mm, L2 and L3 = 2.9 mm, and L4 = 2.1 mm. Figure 4 displays the percent of fractions that would require a shift based on daily imaging, the average setup uncertainty, and the average setup margin for each threshold level.
Fig. 4.
A graph of the percent shifts that are needed for each action threshold of the daily localization condition, the resulting average setup uncertainty, and the setup margin.
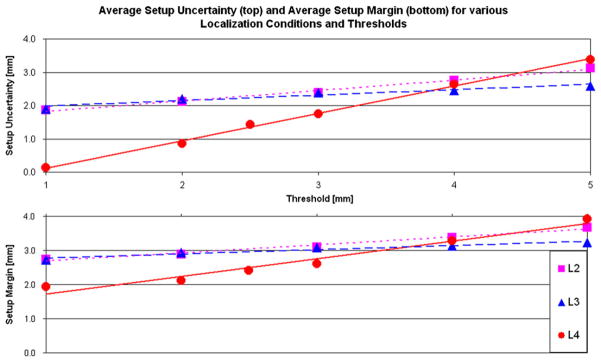
Table 1 lists the details for the SU in each direction for the various localization methods given each action threshold, the RU, and the combined setup margin (SMpat). The number of systematic shifts required for each localization method, L1–L4, for a given action threshold are also listed in Table 1. The decrease in the average SU was directly related to a decrease in the action threshold and was inversely related to the imaging frequency. These relationships are demonstrated in Fig. 5, which shows that for the 5-mm threshold, the L2 (weekly) and L4 (daily) setup uncertainties are almost identical and differ by 0.2 mm. However, L3, which includes imaging for the first full week, is capable of assessing the systematic error. When corrected for the entire treatment, this leads to a smaller margin of 0.7 mm.
Table 1.
The values of the mean offset, systematic error (Σ), random error (σ), and resulting setup uncertainty in millimeters for each direction for the various localization methods and action thresholds.
| Localization schema | Action threshold | Direction | Mean (mm) | Systematic error Σ | Random error σ | Setup uncertainty | Setup margin | Systematic shifts |
|---|---|---|---|---|---|---|---|---|
| L1 (visual marks) | — | Lat | −0.4 | 1.0 | 1.1 | 3.3 | 3.8 | — |
| Long | −0.3 | 1.1 | 1.5 | 3.8 | 4.2 | |||
| Vert | 1.0 | 1.1 | 1.2 | 3.6 | 4.2 | |||
| L2 (weekly) | 5 mm | Lat | −0.1 | 0.8 | 1.5 | 3.1 | 3.6 | 0.5 |
| Long | 0.0 | 0.8 | 1.8 | 3.3 | 3.8 | |||
| Vert | 0.4 | 0.8 | 1.5 | 3.1 | 3.7 | |||
| 4 mm | Lat | −0.1 | 0.7 | 1.5 | 2.8 | 3.4 | 1.0 | |
| Long | −0.1 | 0.7 | 1.7 | 2.9 | 3.5 | |||
| Vert | 0.3 | 0.6 | 1.5 | 2.6 | 3.3 | |||
| 3 mm | Lat | 0.0 | 0.4 | 1.4 | 2.0 | 2.8 | 2.1 | |
| Long | −0.2 | 0.7 | 1.7 | 2.9 | 3.5 | |||
| Vert | 0.2 | 0.5 | 1.4 | 2.2 | 3.0 | |||
| 2 mm | Lat | 0.0 | 0.4 | 1.4 | 2.0 | 2.8 | 3.7 | |
| Long | −0.1 | 0.5 | 1.7 | 2.4 | 3.1 | |||
| Vert | 0.1 | 0.4 | 1.3 | 1.9 | 2.8 | |||
| 1 mm | Lat | 0.0 | 0.3 | 1.4 | 1.7 | 2.6 | 5.3 | |
| Long | 0.0 | 0.4 | 1.7 | 2.2 | 2.9 | |||
| Vert | 0.0 | 0.3 | 1.3 | 1.7 | 2.6 | |||
| L3 | 5 mm | Lat | −0.1 | 0.6 | 1.3 | 2.4 | 3.1 | 0.2 |
| (full first week) | Long | −0.1 | 0.7 | 1.6 | 2.9 | 3.4 | ||
| Vert | 0.4 | 0.6 | 1.4 | 2.5 | 3.2 | |||
| 4 mm | Lat | −0.1 | 0.5 | 1.3 | 2.2 | 2.9 | 0.4 | |
| Long | −0.1 | 0.7 | 1.5 | 2.8 | 3.4 | |||
| Vert | 0.4 | 0.6 | 1.3 | 2.4 | 3.1 | |||
| 3 mm | Lat | −0.1 | 0.5 | 1.2 | 2.1 | 2.8 | 0.9 | |
| Long | −0.1 | 0.7 | 1.4 | 2.7 | 3.3 | |||
| Vert | 0.2 | 0.6 | 1.2 | 2.3 | 3.1 | |||
| 2 mm | Lat | 0.0 | 0.5 | 1.1 | 2.0 | 2.8 | 2.0 | |
| Long | 0.0 | 0.6 | 1.4 | 2.5 | 3.1 | |||
| Vert | 0.1 | 0.5 | 1.2 | 2.1 | 2.9 | |||
| 1 mm | Lat | 0.0 | 0.4 | 1.1 | 1.8 | 2.6 | 4.0 | |
| Long | 0.0 | 0.5 | 1.3 | 2.2 | 2.9 | |||
| Vert | 0.0 | 0.4 | 1.1 | 1.8 | 2.7 | |||
| L4 (daily) | 5 mm | Lat | −0.3 | 1.0 | 1.0 | 3.2 | 3.7 | 0.0 |
| Long | −0.3 | 1.1 | 1.3 | 3.7 | 4.1 | |||
| Vert | 0.9 | 1.0 | 1.1 | 3.3 | 3.9 | |||
| 4 mm | Lat | −0.3 | 0.7 | 1.0 | 2.5 | 3.1 | 0.1 | |
| Long | −0.2 | 0.8 | 1.1 | 2.8 | 3.4 | |||
| Vert | 0.7 | 0.8 | 1.0 | 2.7 | 3.4 | |||
| 3 mm | Lat | −0.1 | 0.4 | 0.8 | 1.6 | 2.4 | 0.5 | |
| Long | −0.1 | 0.5 | 0.9 | 1.9 | 2.7 | |||
| Vert | 0.3 | 0.5 | 0.8 | 1.8 | 2.7 | |||
| 2.5 mm | Lat | 0.0 | 0.3 | 0.7 | 1.2 | 2.3 | 1.1 | |
| Long | 0.0 | 0.4 | 0.8 | 1.6 | 2.4 | |||
| Vert | 0.2 | 0.4 | 0.7 | 1.5 | 2.5 | |||
| 2 mm | Lat | 0.0 | 0.2 | 0.5 | 0.9 | 2.1 | 2.0 | |
| Long | 0.0 | 0.2 | 0.5 | 0.9 | 2.1 | |||
| Vert | 0.1 | 0.2 | 0.5 | 0.9 | 2.2 | |||
| 1 mm | Lat | 0.0 | 0.0 | 0.2 | 0.1 | 1.9 | 4.0 | |
| Long | 0.0 | 0.0 | 0.2 | 0.1 | 1.9 | |||
| Vert | 0.0 | 0.0 | 0.2 | 0.1 | 2.0 | |||
| Residual uncertainty | – | Lat | 0.0 | 0.5 | 0.9 | 1.9 | — | – |
| Long | −1.0 | 0.5 | 0.9 | 1.9 | — | |||
| Vert | 0.4 | 0.5 | 1.1 | 2.0 | — |
Also listed is the patient-related component of the setup margin, which is the combination of the setup uncertainty for the particular localization method and the residual uncertainty (listed at the bottom of the table). The required number of systematic shifts per patient, assuming a 30-fraction treatment, is shown
Abbreviations: Lat = lateral; Long = longitudinal; Vert = vertical.
Fig. 5.
The relationship between localization condition (L2, L3, and L4) that corresponds to an increase in imaging frequency, the action threshold, the setup uncertainty, and the setup margin.
The average SU based on L1 for prone patients was 3.9 mm and 3.5 mm for supine patients. The average SU was 3.7 mm with GA and 3.5 mm without GA. The average RU for prone patients was 2.4 mm and 1.6 mm for supine patients. The average RU for patients who received general anesthesia was 1.5 mm and was 2.3 mm for those who did not. There was no difference in the SU for the various subgroups given daily localization with a 2-mm threshold (0.9 mm). The details for SU based on L1, RU, and SMpat for L1 and L4 with 2-mm threshold for these subgroups are listed in Table 2. The parameter that has the greatest impact on the various uncertainties is the standard deviation of the mean offsets, referred to here as Σ(Eq. 1). Therefore an analysis of variance F test on the mean offsets was used to measure whether there was a significant difference in the Σfor prone vs. supine and GA vs. no GA for both the SU and the RU. For SU, only the lateral direction for supine vs. prone reached statistical significance (p < 0.01). For RU, both the lateral and longitudinal directions for supine vs. prone had a p value of <0.01; vertical did not reach statistical significance. All directions were statistically significant for GA vs. no GA, with p < 0.01 for lateral and p = 0.02 for longitudinal and vertical. The values of Σfor these various cohorts can be found in Table 2.
Table 2.
Listed are the values for the mean offset, systematic error (Σ), random error (σ), and resulting setup uncertainty in millimeters for each direction given the L1 localization condition (visual marks) of the various subgroups based on the pretreatment CBCT. Also shown is the residual uncertainty for each subgroup based on the posttreatment CBCT. The final column lists the patient-related component of the setup margin for the L1 and L4 with 2-mm threshold localization condition, which is the combination of the setup and residual uncertainty for a particular subgroup. The L4 setup uncertainty is the same for all subgroups (see Table 1)
| Subgroup (count) | CBCT | Direction | Mean (mm) | Systematic error Σ | Random error σ | Setup and residual uncertainty | Setup margin L1 (top) L4 (bottom) |
|---|---|---|---|---|---|---|---|
| Prone (25) | Pre (setup) | Lat | −0.3 | 1.5 | 1.3 | 4.7 | 5.5 |
| Long | −0.1 | 0.9 | 1.3 | 3.2 | 3.9 | ||
| Vert | 0.7 | 1.2 | 1.0 | 3.7 | 4.3 | ||
| Post (residual) | Lat | 0.1 | 0.8 | 1.2 | 2.8 | 3.0 | |
| Long | 0.4 | 0.6 | 1.2 | 2.3 | 2.5 | ||
| Vert | 0.7 | 0.6 | 1.0 | 2.2 | 2.4 | ||
| Supine (75) | Pre | Lat | −0.4 | 0.9 | 1.1 | 3.0 | 3.3 |
| Long | −0.4 | 1.2 | 1.5 | 4.1 | 4.3 | ||
| Vert | 1.1 | 1.0 | 1.2 | 3.3 | 4.0 | ||
| Post | Lat | 0.0 | 0.3 | 0.8 | 1.3 | 1.6 | |
| Long | −0.3 | 0.3 | 0.9 | 1.4 | 1.6 | ||
| Vert | 0.4 | 0.5 | 1.2 | 2.1 | 2.3 | ||
| GA (46) | Pre | Lat | −0.3 | 1.0 | 1.1 | 3.3 | 3.6 |
| Long | −0.2 | 1.2 | 1.4 | 4.0 | 4.3 | ||
| Vert | 0.8 | 1.2 | 1.1 | 3.8 | 4.1 | ||
| Post | Lat | 0.0 | 0.3 | 0.8 | 1.3 | 1.6 | |
| Long | −0.1 | 0.4 | 0.7 | 1.5 | 1.7 | ||
| Vert | 0.3 | 0.4 | 0.8 | 1.6 | 1.8 | ||
| No-GA (54) | Pre | Lat | −0.4 | 1.1 | 1.2 | 3.6 | 4.2 |
| Long | −0.4 | 1.0 | 1.5 | 3.6 | 4.2 | ||
| Vert | 1.1 | 1.0 | 1.2 | 3.3 | 4.2 | ||
| Post | Lat | 0.1 | 0.6 | 1.0 | 2.2 | 2.4 | |
| Long | 0.0 | 0.6 | 1.1 | 2.3 | 2.4 | ||
| Vert | 0.6 | 0.6 | 1.3 | 2.4 | 2.6 | ||
| All (100) | Pre | Lat | −0.4 | 1.0 | 1.1 | 3.3 | 3.8 |
| Long | −0.3 | 1.1 | 1.5 | 3.8 | 4.2 | ||
| Vert | 1.0 | 1.1 | 1.2 | 3.6 | 4.2 | ||
| Post | Lat | 0.0 | 0.5 | 0.9 | 1.9 | 2.1 | |
| Long | −1.0 | 0.5 | 0.9 | 1.9 | 2.1 | ||
| Vert | 0.4 | 0.5 | 1.1 | 2.0 | 2.2 |
Abbreviations: GA = general anesthesia; Lat = latitudinal; Long = longitudinal; Vert = vertical.
DISCUSSION
On the basis of current U.S. treatment standards, we estimate more than 1,000 children with primary brain, head and neck, and orbital tumors will receive fractionated external beam radiation therapy (RT) each year as part of their initial management. The treatment of these patients and the requirements for daily localization and verification is complicated by the extremes of age, level of cooperation, and clinical and treatment factors associated with their tumor type, tumor location, and treatments preceding or administered during RT.
Using a 1-cGy IBL-CBCT daily for each patient, we quantified the patient related portion of the setup margin required for pediatric patients with intracranial lesions for various localization conditions. The 1-cGy IBL-CBCT provides sufficient quality for bony anatomy localize as evident by Fig. 1. The IBL-CBCT has previously been shown (23) to deliver a lower dose to critical structures compared with traditional portal images.
Regarding which localization condition, including action threshold, one should use, many items must be considered. First, we state that the maximum margin of the three directions should be considered as the margin for a particular localization condition because this is what will likely be done in a clinical situation (use of symmetric margin). If margin size alone is the fundamental parameter, then the 1-mm threshold is the ideal for each method. However, this is impractical because it would require a systematic shift almost every week. The number of required systematic shifts is important because potential errors and confusion may arise as the number of these shifts increase. Therefore, the combination of margin size and number of systematic shifts should be considered.
If at most two systematic shifts and a margin of ~0.5 mm greater than the ideal for a particular method are allowed, then the choice is made easier. Assuming that an average pediatric brain tumor patient will receive 30 fractions, the optimal choice for weekly imaging (six images) is the 4-mm threshold, which requires one systematic shift per patient and a setup margin of 3.5 mm. For full first week imaging (10 images), the 5-mm threshold is optimal, requiring one systematic shift for every five patients in addition to the systematic shift after the first week and a margin of 3.4 mm. There are two choices for daily imaging (30 images), the 2.5-mm threshold requires one systematic shift, 12 daily shifts, and a margin of 2.5 mm. The 2-mm threshold requires two systematic shifts, 16.5 daily shifts, and a 2.2-mm margin. The true limitation is the residual uncertainty (intrafraction motion), because of its 2.0-mm margin. If the effort of daily imaging is going to be put forth, then the 2-mm threshold is the logical choice. If daily imaging is not used, then the weekly imaging with the 4-mm threshold is an appropriate choice.
Comparing the prone to supine patients, we see that the prone patients generally have a larger setup and residual uncertainty. Of note is the large difference between the lateral setup margins of the prone patients (5.5 mm) vs. the supine patients (3.3 mm). With daily imaging, the difference in setup uncertainty can be erased, but the large residual uncertainty remains—for example, 2.8 vs. 1.3 mm in the lateral direction. This is most likely due to the type of immobilization used for prone patients and requires further investigation. Considering the use of general anesthesia, an interesting result is that the average residual uncertainty for GA patients is 1.5 mm vs. 2.3 mm for those without, which leads one to conclude that conscious patients move within their immobilization device more than those treated with GA, which suggests there is room for improvement.
The setup uncertainties for adult head and neck has been shown to average approximately 3 mm (14, 16), which is similar to the results presented here. This margin is based on a similar geometric formula (11) to that used here; however, it has been proposed that because of biological response (24) and the existence of a dose cloud (25) in photon RT, that the margin may be reduced by approximately 3 mm while maintaining proper coverage. This is an interesting possibility and is being investigated for various pediatric brain tumors through motion simulation studies.
It is important to remember the PTV margin comprises a patient-specific component of the setup margin (inter- and intrafraction motion) that has been discussed here, as well as a process-specific component, which includes treatment machine, image registration, and treatment planning uncertainties, not detailed here. In addition, the PTV should include an internal margin. The process-related uncertainties are currently being investigated to establish their impact on the SM for pediatric patients with brain and head and neck tumors, and preliminary data show that an addition of at most 1 mm may be appropriate. Interobserver delineation uncertainties are not included in this uncertainty estimation. The internal margin accounts for changes in the target itself and cannot reasonably be accounted for with geometric margins. A good example is craniopharyngioma, a cystic pediatric brain tumor, which changes shape and size during therapy (26). In this case, the internal margin can only reasonably be accounted for with adaptive planning.
The last issue that warrants discussion is rotational uncertainty. If patient rotation was observed, the therapist manually repositioned then reimaged the patient. In most circumstances, this corrected the error and treatment continued. If a satisfactory correction was not obtained, the physician was notified, and in some circumstances, new immobilization devices, and hence new plans, were required. Our system did not allow for online quantification of the rotational error. We are currently evaluating the magnitude and dosimetric effect of rotation.
CONCLUSION
CBCT can be used to minimize positional uncertainty in pediatric patients and reduce dose to normal tissue. When daily CBCT is used for pediatric patients with brain and head and neck tumors, a SM of 2 mm appears to be appropriate. The SM may be increased for patients treated in the prone position or further reduced when general anesthesia is used. When weekly CBCT is used, the SM should be increased to 3.5 mm.
Although additional uncertainties in the SM may be attributed to the hardware and software used in planning and delivery, these should be relatively small compared with the patient contribution estimated in this study but should be considered along with the IM when considering the margin chosen for the PTV. Because this was a single-institution study, it is important to note that there may be differences in immobilization effectiveness and setup errors at different institutions; therefore, the values presented here should be verified at each institution.
Acknowledgments
This research was partially funded by a grant from Siemens Medical Systems and by support from the American Lebanese Syrian Associated Charities (ALSAC). This work was presented in part at the 2009 AAPM conference in Anaheim CA.
Footnotes
Conflict of interest: none.
References
- 1.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 2.Hua C, Bass JK, Khan R, et al. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72:892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 3.Krasin MJ, Xiong X, Wu S, et al. The effects of external beam irradiation on the growth of flat bones in children: Modeling a dose–volume effect. Int J Radiat Oncol Biol Phys. 2005;62:1458–1463. doi: 10.1016/j.ijrobp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE, Kiehna EN, Li C, et al. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–1554. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Kiehna EN, Miles MA, et al. Acute effects of irradiation on cognition: Changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. Int J Radiat Oncol Biol Phys. 2002;53:1271–1278. doi: 10.1016/s0360-3016(02)02828-6. [DOI] [PubMed] [Google Scholar]
- 6.International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy. Bethesda, MD: ICRU; 1993. ICRU Report 50. [Google Scholar]
- 7.International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50) Bethesda, MD: ICRU; 1999. ICRU Report 62. [Google Scholar]
- 8.Stroom JC, de Boer HC, Huizenga H, et al. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol Biol Phys. 1999;43:905–919. doi: 10.1016/s0360-3016(98)00468-4. [DOI] [PubMed] [Google Scholar]
- 9.Stroom JC, Heijmen BJ. Geometrical uncertainties, radiotherapy planning margins, and the ICRU-62 report. Radiother Oncol. 2002;64:75–83. doi: 10.1016/s0167-8140(02)00140-8. [DOI] [PubMed] [Google Scholar]
- 10.Stroom JC, Heijmen BJ. Limitations of the planning organ at risk volume (PRV) concept. Int J Radiat Oncol Biol Phys. 2006;66:279–286. doi: 10.1016/j.ijrobp.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.van Herk M, Remeijer P, Rasch C, et al. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 12.Hoogeman MS, Nuyttens JJ, Levendag PC, et al. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70:609–618. doi: 10.1016/j.ijrobp.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 13.Verellen D, Soete G, Linthout N, et al. Optimal control of set-up margins and internal margins for intra- and extracranial radio-therapy using stereoscopic kilovoltage imaging. Cancer Radiother. 2006;10:235–244. doi: 10.1016/j.canrad.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys M, Guerrero Urbano MT, Mubata C, et al. Assessment of a customised immobilisation system for head and neck IMRT using electronic portal imaging. Radiother Oncol. 2005;77:39–44. doi: 10.1016/j.radonc.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Linthout N, Verellen D, Tournel K, et al. Six dimensional analysis with daily stereoscopic x-ray imaging of intrafraction patient motion in head and neck treatments using five points fixation masks. Med Phys. 2006;33:504–513. doi: 10.1118/1.2165417. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Nishimura Y, Nakamatsu K, et al. Analysis of inter-fractional set-up errors and intrafractional organ motions during IMRT for head and neck tumors to define an appropriate planning target volume (PTV)- and planning organs at risk volume (PRV)-margins. Radiother Oncol. 2006;78:283–290. doi: 10.1016/j.radonc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Bai S, Chen N, et al. The clinical feasibility and effect of online cone beam computer tomography-guided intensity-modulated radiotherapy for nasopharyngeal cancer. Radiother Oncol. 2009;90:221–227. doi: 10.1016/j.radonc.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Beltran C, Trussell J, Merchant TE. Dosimetric impact of intra-fractional patient motion in pediatric brain tumor patients. Med Dosim. 2010;35:43–48. doi: 10.1016/j.meddos.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Stovall J, Jr, Butler L, et al. Comparison of two immobilization techniques using portal film and digitally reconstructed radiographs for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys. 2000;48:1233–1240. doi: 10.1016/s0360-3016(00)00733-1. [DOI] [PubMed] [Google Scholar]
- 20.de Boer HCJ, Heijmen BJM. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys. 2001;50:1350–1365. doi: 10.1016/s0360-3016(01)01624-8. [DOI] [PubMed] [Google Scholar]
- 21.Zeidan OA, Langen KM, Meeks SL, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67:670–677. doi: 10.1016/j.ijrobp.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Faddegon B, Wu V, Pouliot J, et al. Low dose megavoltage cone beam computed tomography with an unflattened 4 MV beam from a carbon target. Med Phys. 2008;35:5777–5786. doi: 10.1118/1.3013571. [DOI] [PubMed] [Google Scholar]
- 23.Beltran C, Lukose R, Gangadharan B, et al. Image quality & dosimetric property of an investigational Imaging Beam Line MV-CBCT. J Appl Clin Med Phys. 2009;10:3023. doi: 10.1120/jacmp.v10i3.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol Biol Phys. 2002;52:1407–1422. doi: 10.1016/s0360-3016(01)02805-x. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JJ, Siebers JV. Evaluation of dosimetric margins in prostate IMRT treatment plans. Med Phys. 2008;35:569–575. doi: 10.1118/1.2826558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkfield KM, Linsenmeier C, Yock TI, et al. Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:716–721. doi: 10.1016/j.ijrobp.2008.05.010. [DOI] [PubMed] [Google Scholar]